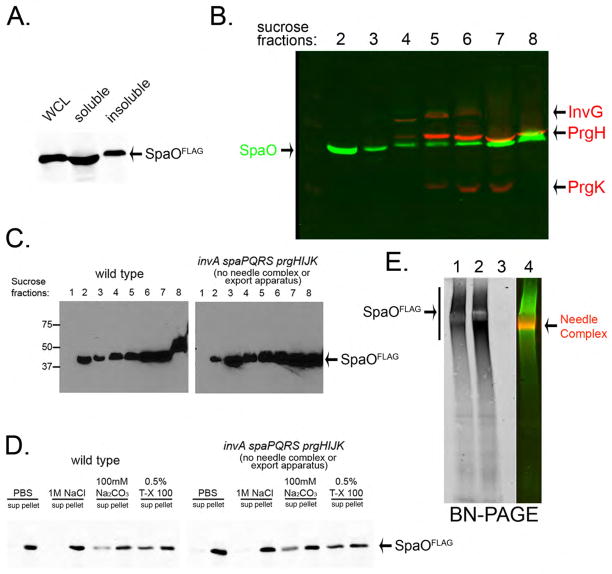
Fig. 1. SpaO forms a large molecular weight complex.
(A, B) Subcellular fractionation of SpaO (A) A whole cell lysate (WCL) of a S. Typhimurium strain expressing FLAG-epitope-tagged SpaO was separated into soluble and pelletable fractions by high-speed centrifugation and the presence of SpaO in the different fractions was probed by immuno blotting with an anti FLAG antibody. (B) The pellet fraction was subsequently separated by sucrose gradient centrifugation and the different gradient fractions were probed for the presence of SpaO (green) and needle complex components (red) by immuno blotting and imaging with the Odyssey system (Li-Cor Bioscience). (C) Comparison of the distribution of SpaO in wild type and the ΔinvA ΔspaPQRS ΔprgHIJK isogenic mutant derivative, which lacks the needle complex and membrane protein components of the export apparatus. The distribution of SpaO in the different fractions of a sucrose gradient was probed as indicated above. (D) Localization of SpaO after different treatments. Pellet fractions of SpaO obtained from the indicated strains were subjected to different treatments (as indicated) and its localization of high-speed centrifugation was determined by immuno blot analysis of the pellet and soluble fractions. (E) Analysis of the SpaO complex by BN-PAGE. Pellet fractions from a wild-type S. Typhimurium encoding a FLAG-epitope-tagged SpaO (1), a ΔinvA ΔspaPQRS ΔprgHIJK mutant derivative (2), or the untagged wild-type strain (3) were separated by BN-PAGE and analyzed by immuno blot for the presence of SpaO or the needle complex. The simultaneous detection of the SpaO (green) and needle complexes (red) from the sample shown on lane 1 is shown in lane 4. Only the SpaO channel is shown in lanes 1, 2, and 3.