Abstract
Expression of isotopically labeled peptide standards as artificial concatamers (QconCATs) allows for the multiplex quantification of proteins in unlabeled samples by mass spectrometry. We have developed a generalizable QconCAT design strategy, which we term IQcat, wherein concatenated peptides are binned by isoelectric point to facilitate MS-sample enrichment by isoelectric focusing. Our method utilizes a rapid (~2 week), inexpensive and scalable purification of arg/lys labeled IQcat standards in the E. coli auxotroph AT713. With this pipeline we assess the fidelity of IQcat-based absolute quantification for 10 yeast proteins over a broad concentration range in a single information-rich isoelectric fraction. The technique is further employed for a quantitative study of androgen-dependent protein expression in cultured prostate cancer cells.
Keywords: absolute quantification, LNCaP, OFFGEL, prostate cancer, QconCAT
The success of targeted and quantitative MS proteomics will depend on the availability of inexpensive and robust isotopic peptide standards [1]. Various methods for the production of these standards, including chemical synthesis of individual peptides (AQUA peptides) [2] and recombinant expression of peptide concatamers (QconCATs) [3] have been compared and demonstrated to offer similar fidelity and quantitative accuracy [4]. While discrete peptide standards offer the most experimental flexibility, QconCATs are well suited for multiplex measurements, as they have been demonstrated to accommodate up to 59 concatenated signature peptides in a single construct [5]. However, in the absence of sample enrichment steps, the utility of QconCATs becomes limited. Targeted detection and quantitation of low abundance peptides within complex proteomic samples requires protein enrichment or fractionation steps that reduce the biological background in a sample prior to MS-analysis. Isoelectric focusing (IEF) at the peptide level is an enrichment strategy compatible with QconCAT methods and yields an order of magnitude gain in sensitivity [6–8]. We have developed an inexpensive and generalizable QconCAT design strategy that is compatible with IEF sample enrichment wherein the constituent peptides of individual QconCATs are binned by their predicted isoelectric point. This method, which we have named IQcat, allows for co-digestion of QconCAT proteins and the sample of interest prior to peptide enrichment by IEF and subsequent MS-analysis.
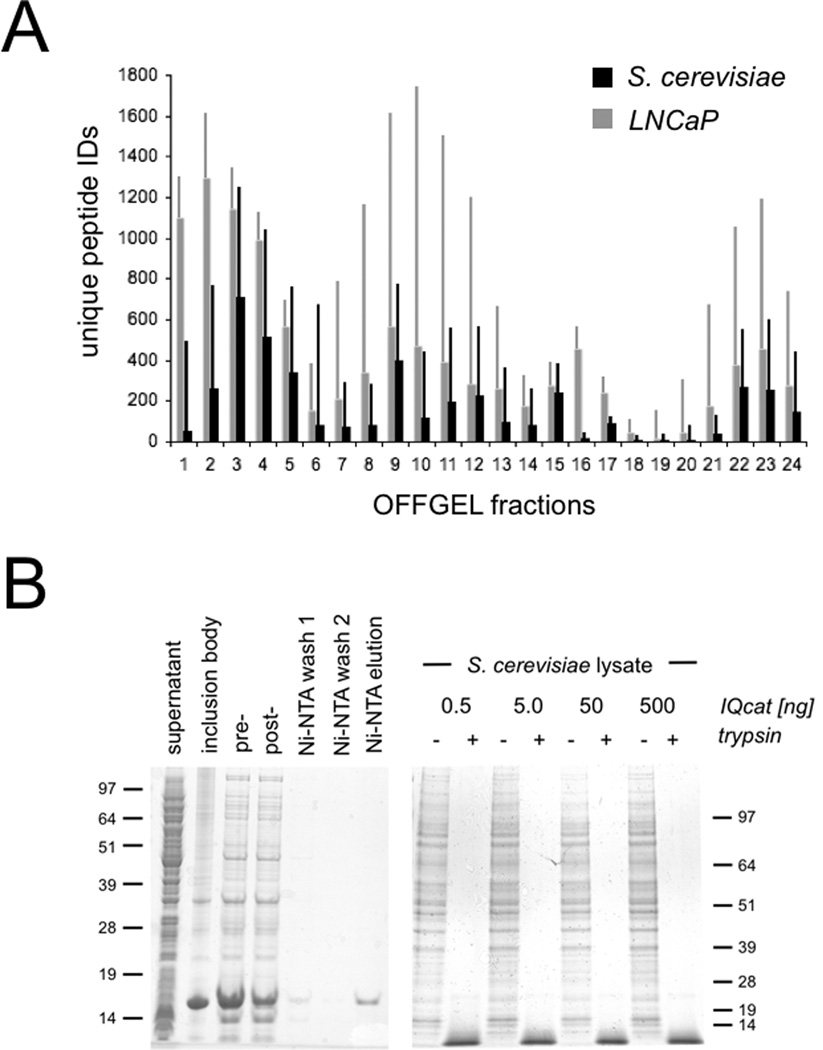
In testing the IQcat strategy, we chose to design QconCAT proteins specifically for pH ranges where the maximum number of tryptic peptides are resolved with the greatest isoelectric focusing power. We restricted our study to a commercially available OFFGEL pI fractionation instrument (Agilent), though our results are generalizable to other platforms. The focusing ability and pI fractionation of peptides by OFFGEL has been reported to be sample independent [8–10]. To confirm this observation and generate a MS dataset for targeted analysis, we performed parallel experiments on cell lysates from S. cerevisiae and the workhorse prostate cancer cell line, LNCaP. The protein content of cell lysates was quantified by Bradford assay and samples were reduced, alkylated, and trypsinized using an established SDS/urea-based denaturing protocol [11]. Detergent was removed from the tryptic digest by multiple-cation-exchange (Oasis MCX cartridge, Waters) and peptides were separated across a pH gradient by OFFGEL fractionation (Agilent 3100 OFFGEL; 24 cm pH 3–10 IPG gel strips; 2 µA/ 500 V, 24 hours). Twenty-four isolated fractions were acidified, desalted, and prepared for LTQ-Orbitrap MS using previously described methods [12]. The pIs of identified peptides were calculated using the algorithm reported by Bjellqvist et al [13] and these values were plotted versus OFFGEL-specified pH range to determine the empirical pH of each fraction (Tables S1, S2). Information-rich regions of the pH spectrum were visualized by a plot of peptide IDs within the 24 OFFGEL fractions (Fig 1a). Consistent with previous reports, the plots of total peptide identifications across the pH range showed a characteristic tri-modal profile with coverage gaps near pH 5.5 and 7.5 corresponding to OFFGEL fractions 6 – 7 and 18 – 19, respectively. The abundance of acidic peptides represented in the leftward balance of these profiles is due to the bias of MS-identification, which does not query peptides shorter than 6 amino acids that are more likely to carry a net positive charge. The IEF resolution, defined as the fraction of peptides identified in a single well that are unique to that well shows focusing optima in fractions 2 – 5 (pH 4.0 – 5.0) and fractions 15 – 17 (pH 6.4 – 7.1) (Figs S1, S2). The peptide residues glutamic and aspartic acid (pKa 3.9 – 4.1) and histidine (pKa 7.5) are likely responsible for this phenomenon, as the pKa’s of these amino-acid side chains correspond to the high-resolution fractions and these residues are observed at an increased incidence per peptide in the respective wells.
Figure 1.
IQcat Design and Measurement A) Total peptide IDs (thin bars) and peptide IDs unique to an individual OFFGEL fraction (thick bars) were determined from shotgun analysis of LNCaP and S. cerevisiae lysates. B) Isotopically labeled IQcat was purified from inclusion bodies by Ni-NTA (left), and co-digested in spike-in samples with 0.5, 5, 50, or 500 ng heavy IQcat to 25 µg S. cerevisiae lysate (right). Proteins are visualized by SDS-PAGE Coomassie stain of 12% and 4–12% gradient gels. C) OFFGEL fractionation of spike-in samples measured by LC-MS as the number of unique heavy IQcat peptide identifications per fraction. D) Log/Log plots of peptide heavy to light ratios give linear and overlapping correlations for the ALNEEAEAR peptide in both IQcat constructs (left). Linear fits for IQcat 1 peptides show a range of endogenous peptide concentration spanning two-log orders.
Based on peptide abundance and focusing optima, OFFGEL fractions 2 – 5 (pH 4.0 – 5.0) were identified as information-rich, pI-specific fractions. We chose to design an IQcat test series compatible with peptide enrichment in fraction 3. To do this, we selected 10 proteotypic yeast peptides identified from the shotgun experiments that were unique to fraction 3 and which lacked amino acid sequences that could lead to degenerate peptide digestion. This peptide set was concatenated into two random IQcat “scrambles”, IQcat 1 and IQcat 2, and synthetic IQcat genes were designed as codon-optimized constructs for expression in E. coli (Blue Heron).
To express IQcat proteins uniformly labeled with heavy arginine and lysine we looked to a recent report from Hanke et al, demonstrating the use of an auxotrophic strain of E. coli (AT713, CGSC New Haven) for the production of Absolute SILAC protein standards [14]. AT713 E. coli is deficient for ArgA and LysA biosynthetic genes (argA21, lysA22) and therefore capable of generating protein with uniform heavy arginine/lysine label when grown in isotopic media. To test the expression of IQcats in AT713, we cloned synthetic genes into the Ptrc promoter based pDW363-H6 expression vector [15]; a his-tagged bicistronic plasmid that expresses recombinant protein in tandem with biotin holoenzyme synthetase allowing protein biotinylation in E. coli [16]. We tested the compatibility of the Ptrc promoter based pDW363-H6 expression vector with the AT713 cell line by expressing the 17 kDa IQcats along with additional QconCATs ranging in size from 16 – 24 kDa as well as the 45 kDa G protein subunit Gαs(s) (Fig S3). Biotinylated soluble protein was expressed at a high level (>100 mg/L) and displayed uniform incorporation of isotopic label (>98% heavy by MS) when cultured in M9 minimal media supplemented with heavy arginine and lysine. A large percentage of the unstructured IQcat protein was observed to precipitate with the inclusion body (IB) fraction; we were able to take advantage of this phenomenon to simplify our protein purification protocol (Fig 1b). The purification steps include 1) cell lysis with a commercially available detergent B-PER (Thermo), 2) IB separation and resolubilization in denaturing buffer [8 M urea, 300 mM NaCl, 0.1% Triton, 50 mM Tris pH 8, 50 mM sodium phosphate pH 7.4], and 3) His-tag purification on Ni-NTA agarose (Qiagen) to yield gram/liter quantities of purified protein standard. Though not employed in our standard purification, the biotin label offers a secondary moiety for tandem affinity purification where increased protein purity is desired [17]. The production of purified IQcat standards entails less than 2 weeks of labor and a material cost of roughly $250, largely attributable to the cost of gene synthesis. This preparation can be scaled to yield microgram quantities of semi-pure IQcat from 2 mL cultures. A detailed description of these methods is included in the supporting information.
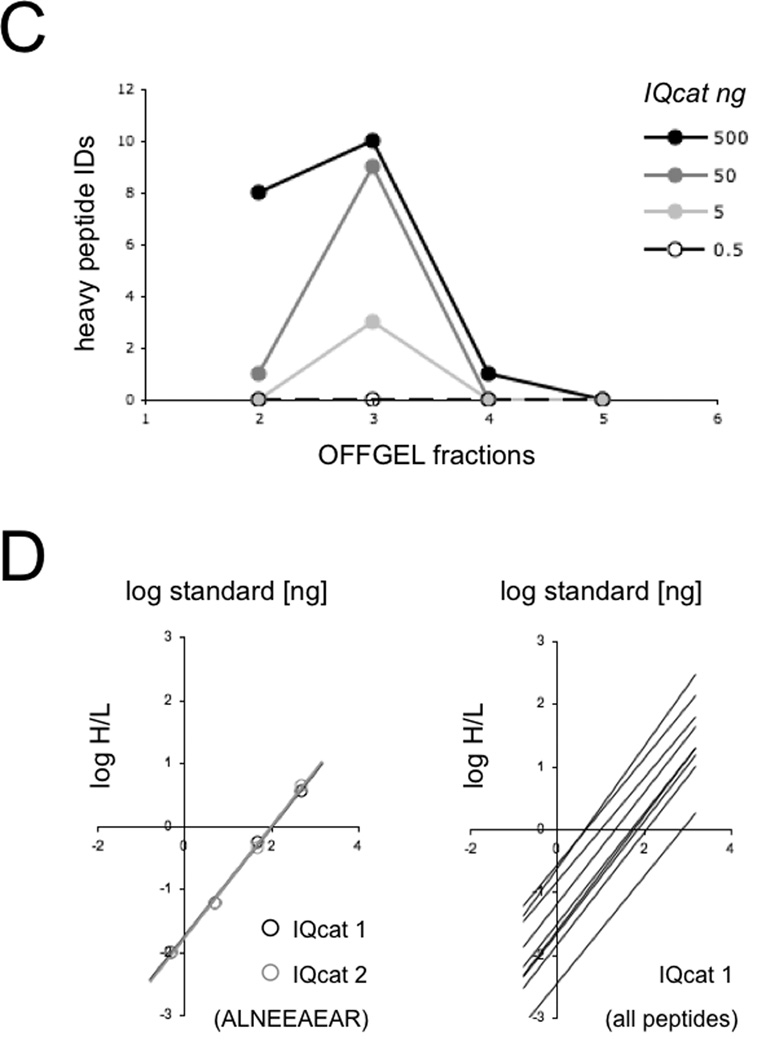
Due to the limited quantitative window of MS, absolute quantification measurements require that external IQcat standards be titrated into samples across the expected concentration range of all the included target peptides [18]. To perform an absolute quantification of yeast target proteins, lysate derived from a known number of S. cerevisiae cells was spiked with IQcat standard at titrations of 0.5, 5, 50, and 500 ng IQcat per 25 µg lysate (10.9 × 106 yeast cells per 25 µg lysate). The kinetics of trypsinization are known to differ significantly between QconCAT standards and folded endogenous proteins [19, 20]. To achieve uniform proteolysis, reduced and alkylated samples were denatured with strong detergent (0.1% SDS) in 8 M urea prior to complete trypsinization, monitored by SDS-PAGE (Fig 1b). Detergent was removed from the tryptic digest using an Oasis MCX extraction cartridge (Waters), and samples were fractionated by OFFGEL with individual isoelectric fractions screened by LC-MS (ThermoFinnigan LTQ linear ion trap) for the presence of heavy IQcat standards. IQcat standards were observed to focus to OFFGEL fraction 3 as expected, with a broadened distribution across fractions at the highest spike-in titration where the 10 IQcat peptides represented ~2% of total protein in the lysate (Fig 1c). LTQ-Orbitrap MS of the fractionated spike-in samples gave complete coverage identification for both IQcat constructs. MS data was searched for the presence of heavy proline, a potential conversion product of isotopically labeled arginine, but this modification was not observed.
The isotopic ratios of heavy IQcat standard to light endogenous peptide were quantified using XPRESS [21] and titrations plotted to determine target peptide concentration (Fig 1d). Identical peptides from both IQcat scrambles gave similar titration plots. Individual plots showed a limit of linearity spanning 2 orders of magnitude, in-line with the dynamic range of the instrument and the isotopic purity of standards. The absolute concentrations of the 10 peptides determined from these plots gave an average variation of 22% between IQcat1 and IQcat2 samples, consistent with sequence independent proteolysis of the concatamers. Notably, the peptide standard AADETAAAFYPSK was a relative outlier, exhibiting a 3-fold measurement difference between IQcat1 and IQcat2. In addition to Orbitrap-MS, spike-in samples were also measured by high sensitivity targeted SRM-MS. Absolute quantifications from the two instruments were similar, with an average discrepancy of 13% (Tables S3, S4).
The absolute concentration of targeted yeast peptides was determined from the IQcat titration plots and used to calculate peptide copies per yeast cell (Table 1). Peptide copy number is expected to match the cellular protein copy number because each constituent IQcat peptide is proteotypic for one yeast protein or set of protein isoforms. This is corroborated by a comparison of IQcat measurements with [35S]Met-based protein measurements previously reported in glucose cultured S. cerevisiae [22, 23]. All protein copy numbers are similar with the exception of the transcription elongation factor EF3, which is observed at significantly lower protein copy number by [35S]Met. Futcher et. al. did note, however, that EF3 was poorly resolved in their experiments and its abundance likely underestimated by radiolabel detection [22]. A similar comparison to quantitative proteomic measurements by Weissman and colleagues [24] has been included in supporting information (Table S6). A notable omission in our dataset is the 40S ribosomal peptide SNGLAPEIPEDLYYLIK, which failed to give quantifiable data for both IQcat scrambles due to poor signal. Because this peptide has a high hydrophobicity (SSRCalc = 41.01 determined by PeptideAtlas [25]) and is easily detected by LTQ-Orbitrap from in-gel digests (data not shown), we speculate that the peptide is lost to adsorption in the OFFGEL device. Based on this result we have incorporated a hydrophobicity criteria (SSRCalc < 40) in subsequent IQcat designs.
Table 1.
Absolute peptide/protein quantification in S. cerevisiae
| peptide copy/cell | protein copy/cell | ||||||
|---|---|---|---|---|---|---|---|
| Peptide | pI | Gene Name | Protein name, brief description | IQcat 1 | IQcat 2 | [35S]Meta | [35S]Metb |
| ALNEEAEAR | 4.25 | RPL19A(B) | 60S Ribosome (component) | 248,000 | 238,000 | ||
| TAGIQIVADDLTVTNPAR | 4.21 | ENO2 | Enolase II | 1,700,000 | 1,270,000 | 759,000 | 650,000 |
| FEDLNAALFK | 4.37 | SSB1(2) | Hsp70 chaperone | 111,000 | 102,000 | 146,500 | 270,000 |
| AGFAGDDAPR | 4.21 | ACT1 | Actin | 158,000 | 136,000 | 205,000 | |
| SNGLAPEIPEDLYYLIK | 4.14 | RPS13 | 40S Ribosome (component) | ||||
| TATYDGEEGILAAK | 4.14 | ARO2 | Chorismate synthase | † 9,090 | † 6,160 | ||
| LVEDPQVIAPFLGK | 4.37 | YEF3 | EF-3, translation elongation | 129,000 | 128,000 | 14,000 | |
| QNPQAIGQDLFTDPR | 4.21 | STI1 | Hsp90 cochaperone | 22,200 | 17,000 | 13,100 | 25,000 |
| LEATDYATR | 4.37 | BAT1(2) | Branched chain a.a. transferase | 46,400 | 25,400 | 49,900 | |
| AADETAAAFYPSK | 4.37 | PRO3 | Proline biosynthesis enzyme | 9,220 | 29,400 | ||
Values were determined by targeted SRM-MS.
Gygi et al. Mol. Cell Biol. 1999, 19(3):1720,
Futcher et al. Mol. Cell. Biol. 1999, 19(11):7357 b. The S. cerevisiae lysates used in these experiments were obtained from mid-log phase batch growth on glucose-based media.
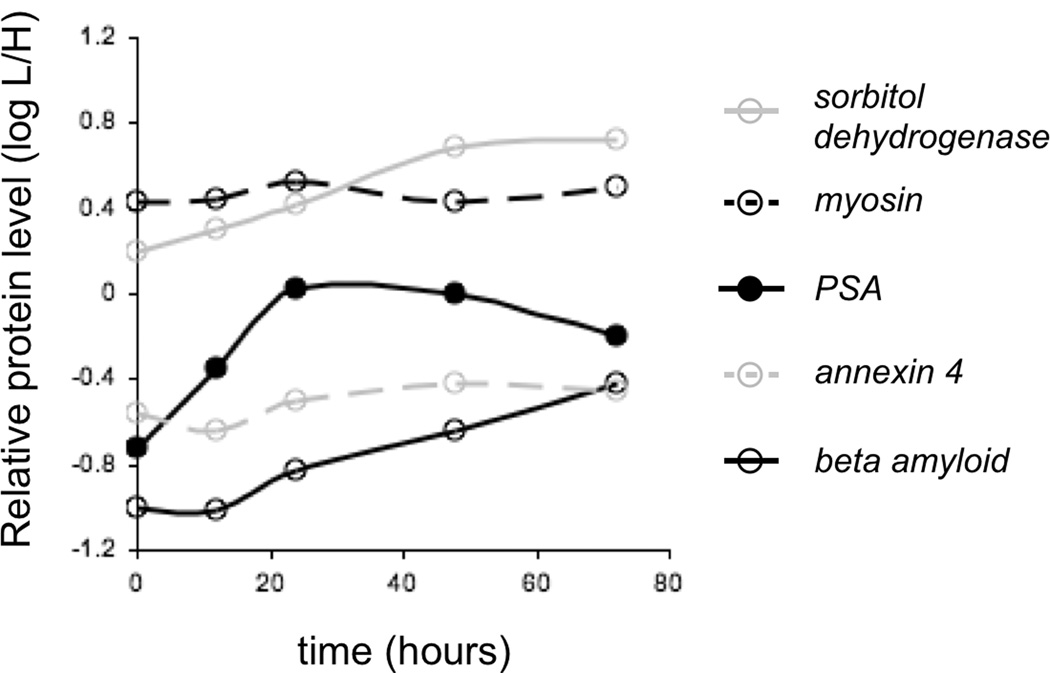
To test the utility of our strategy for studies of complex proteomic samples, we designed a pilot IQcat to quantify protein level changes in the androgen regulated proteome of human LNCaP cells (Fig 2). IQcat-AR, consisting of a concatamer of two proteotypic peptides for each of 4 proteins known to be up-regulated by androgen [26], as well as a myosin control protein, was designed to focus to pH 3.98 – 4.37. Isotopically labeled IQcat-AR was spiked into protein-normalized LNCaP lysates harvested at 0, 12, 24, 48, and 72 hour time points after stimulation with synthetic androgen (1 nM R1881). Quantification by SRM-MS demonstrated primary up-regulation of PSA at 12 hours, consistent with western blot analysis (Fig S4), and earlier than annexin and beta amyloid up-regulation. The LNCaP experiment demonstrates the ability to track changes in protein concentration in a system compatible with SILAC culture. While it is not necessary to use an IQcat spike-in method to perform protein quantification of in vitro samples that can be cultured using SILAC, numerous in vivo model systems are in use that cannot be approached with SILAC such as the human prostate LuCaP mouse xenografts [27]. These samples are more complex, as they can include murine plasma proteins, and stand to benefit tremendously from sample fractionation by IEF to increase depth of coverage. We plan to expand our analysis to the larger androgen regulome in cell lines, as well as xenograft samples.
Figure 2.
IQcat measurement of androgen regulated proteins in LNCaP. Stimulation of cells at 0 hours with 1 nM R1881 results in increased levels of androgen regulated proteins at 48 hours. PSA levels respond to stimulation faster than beta amyloid and annexin 4, consistent with a secondary mechanism of expression for these androgen regulated proteins. The level of myosin control protein oscillates slightly with a period corresponding to the doubling time of LNCaP cells (TD = 55 hours).
We have designed and reduced to practice a novel, affordable, and technically straightforward system for targeted analysis of peptides in complex mixtures. The selection of peptides by isoelectric point in information-rich pI-specific fractions facilitates focused evaluation of a subset of proteins that may not be measurable without fractionation. IEF-enrichment has a low rate of peptide attrition and can be used for absolute or relative quantification particularly suited for complex biological samples not amenable to SILAC. Most proteins will have a peptide in one “well behaved” IEF fraction. Based on our LNCaP shotgun dataset, 26% of unique peptide IDs focus to OFFGEL fractions 2 – 5, representing 67% of all protein IDs (2163/3219 proteins). Adding a second pI range (e.g. pH 6.4 – 7.1) may increase protein coverage and facilitate robust multi-peptide measurements of proteins. The ability to quantify many different proteins within an enriched peptide fraction distinguishes the IQcat strategy from quantitative methods based on protein-enrichment such as Absolute SILAC [14] and PSAQ [28]. From a technical perspective, IQcat expression in auxotrophic E coli strains has several advantages which make this approach very competitive with peptide synthesis and in vitro translation of QconCATs . These include 1) the falling cost of gene synthesis, 2) simple QconCAT cloning methods which are rapid and multiplexable, and 3) expression and purification of QconCAT standards in small (and scalable) volumes sufficient for targeted mass spectrometry. In addition, QconCAT E. coli strains are archival and can be shared or reused as desired; whereas synthetic peptides have a shelf life and can oxidize and fragment, confounding quantitative studies. The improved sensitivity and speed of this IQcat method makes it an excellent partner for SRM-based studies of complex biological samples.
Supplementary Material
Acknowledgments
This work was supported by CDMRP training grant W81XWH-10-1-0220 to Ryan Austin; and NIH/NIGMS center grant P50 GM076547 to the Institute for Systems Biology (Lee Hood).
Abbreviations
- AQUA
absolute quantification
- IQcat
isoelectric QconCAT
- LTQ-MS
linear trap quandrupole-MS
- PSA
prostate-specific antigen
- PSAQ
protein standard absolute quantification
- QconCAT
artificial protein concatamer of quantitative peptides
- IB
inclusion body
- SSRCalc
sequence specific retention calculator
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
Supporting Information
Detailed experimental methods; design information for IQcat and QconCAT constructs; isoelectric calibration and focusing plots for S. cerevisiae (Table S1; Fig S1) and LNCaP (Table S2, Fig S2); QconCAT expression analysis (Fig S3); and tabulated quantitative MS data (Tables S3 – S7) are included as supplemental materials. Detailed MS data for Orbitrap and SRM-MS measurements of IQcats is compiled as a spreadsheet.
References
- 1.Mitchell P. Proteomics retrenches. Nature biotechnology. 2010;28:665–670. doi: 10.1038/nbt0710-665. [DOI] [PubMed] [Google Scholar]
- 2.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt JM, Simpson DM, Doherty MK, Rivers J, et al. Multiplexed absolute quantification for proteomics using concatenated signature peptides encoded by QconCAT genes. Nature protocols. 2006;1:1029–1043. doi: 10.1038/nprot.2006.129. [DOI] [PubMed] [Google Scholar]
- 4.Mirzaei H, McBee JK, Watts J, Aebersold R. Comparative evaluation of current peptide production platforms used in absolute quantification in proteomics. Mol Cell Proteomics. 2008;7:813–823. doi: 10.1074/mcp.M700495-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll KM, Simpson DM, Eyers EC, Knight CG, et al. Absolute quantification of a metabolic pathway in yeast: deployment of a complete QconCAT approach. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller M, Ye M, Michel PE, Morier P, et al. Added value for tandem mass spectrometry shotgun proteomics data validation through isoelectric focusing of peptides. Journal of proteome research. 2005;4:2273–2282. doi: 10.1021/pr050193v. [DOI] [PubMed] [Google Scholar]
- 7.Cargile BJ, Sevinsky JR, Essader AS, Stephenson JL, Jr, Bundy JL. Immobilized pH gradient isoelectric focusing as a first-dimension separation in shotgun proteomics. Journal of biomolecular techniques : JBT. 2005;16:181–189. [PMC free article] [PubMed] [Google Scholar]
- 8.Hubner NC, Ren S, Mann M. Peptide separation with immobilized pI strips is an attractive alternative to in-gel protein digestion for proteome analysis. Proteomics. 2008;8:4862–4872. doi: 10.1002/pmic.200800351. [DOI] [PubMed] [Google Scholar]
- 9.Chenau J, Michelland S, Sidibe J, Seve M. Peptides OFFGEL electrophoresis: a suitable pre-analytical step for complex eukaryotic samples fractionation compatible with quantitative iTRAQ labeling. Proteome science. 2008;6:9. doi: 10.1186/1477-5956-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horth P, Miller CA, Preckel T, Wenz C. Efficient fractionation and improved protein identification by peptide OFFGEL electrophoresis. Mol Cell Proteomics. 2006;5:1968–1974. doi: 10.1074/mcp.T600037-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Tian Y, Zhou Y, Elliott S, Aebersold R, Zhang H. Solid-phase extraction of N-linked glycopeptides. Nature protocols. 2007;2:334–339. doi: 10.1038/nprot.2007.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin R, Kuestner R, Chang D, Madden K, Martin D. A SILAC compatible strain of Pichia pastoris for expression of isotopically labeled protein standards and quantitative proteomics. manuscript under review. 2011 doi: 10.1021/pr200551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjellqvist B, Hughes GJ, Pasquali C, Paquet N, et al. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–1031. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- 14.Hanke S, Besir H, Oesterhelt D, Mann M. Absolute SILAC for accurate quantitation of proteins in complex mixtures down to the attomole level. Journal of proteome research. 2008;7:1118–1130. doi: 10.1021/pr7007175. [DOI] [PubMed] [Google Scholar]
- 15.Austin RJ, Ja WW, Roberts RW. Evolution of class-specific peptides targeting a hot spot of the Galphas subunit. Journal of molecular biology. 2008;377:1406–1418. doi: 10.1016/j.jmb.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao KL, DeBarbieri B, Michel H, Waugh DS. A versatile plasmid expression vector for the production of biotinylated proteins by site-specific, enzymatic modification in Escherichia coli. Gene. 1996;169:59–64. doi: 10.1016/0378-1119(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 17.Austin RJ, Smidansky H, Holstein C, Chang D, Martin D. Proteomic Analysis of the Androgen Receptor via MS-compatible Purification of Biotinylated Protein on Streptavidin Resin. 2011 doi: 10.1002/pmic.201100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brun V, Masselon C, Garin J, Dupuis A. Isotope dilution strategies for absolute quantitative proteomics. Journal of proteomics. 2009;72:740–749. doi: 10.1016/j.jprot.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Brownridge P, Beynon RJ. The importance of the digest: Proteolysis and absolute quantification in proteomics. Methods (San Diego, Calif. 2011;54:351–360. doi: 10.1016/j.ymeth.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kito K, Ota K, Fujita T, Ito T. A synthetic protein approach toward accurate mass spectrometric quantification of component stoichiometry of multiprotein complexes. Journal of proteome research. 2007;6:792–800. doi: 10.1021/pr060447s. [DOI] [PubMed] [Google Scholar]
- 21.Han DK, Eng J, Zhou H, Aebersold R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nature biotechnology. 2001;19:946–951. doi: 10.1038/nbt1001-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futcher B, Latter GI, Monardo P, McLaughlin CS, Garrels JI. A sampling of the yeast proteome. Molecular and cellular biology. 1999;19:7357–7368. doi: 10.1128/mcb.19.11.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Molecular and cellular biology. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaemmaghami S, Huh WK, Bower K, Howson RW, et al. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 25.Deutsch EW, Lam H, Aebersold R. PeptideAtlas: a resource for target selection for emerging targeted proteomics workflows. EMBO reports. 2008;9:429–434. doi: 10.1038/embor.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson PS, Clegg N, Arnold H, Ferguson C, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corey E, Quinn JE, Buhler KR, Nelson PS, et al. LuCaP 35: a new model of prostate cancer progression to androgen independence. The Prostate. 2003;55:239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 28.Huillet C, Adrait A, Lebert D, Picard G, et al. Accurate quantification of cardiovascular biomarkers in serum using protein standard absolute quantification (PSAQTM) and selected reaction monitoring. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.