Summary
The human posterior cricoarytenoid (PCA) muscle is divided into two compartments, the vertical and horizontal bellies, which contain differences in their myosin heavy chain (MyHC) composition. Using immunohistochemical techniques on whole PCA samples, this study provides a more thorough description of the fiber type composition of entire bellies of the PCA. Four patients provided complete PCA samples containing both compartments of their right and left sides; two with unilaterally immobilized vocal folds. The horizontal belly had 80% slow (type I) fibers and 20% fast (type II) fibers. The vertical belly contained equal amounts of slow and fast fibers (~55%:45%); clearly distinguishing between two compartments. Atrophy of muscle fibers and fiber type grouping were also present in both normal and affected subjects; providing no clear confirmation of the clinical findings of vocal fold immobilization. Further study of the PCA muscle from patients with unilaterally immobilized vocal folds is needed.
Keywords: Posterior cricoarytenoid muscle compartments, Vocal fold immobilization, Myosin heavy chain
INTRODUCTION
Preliminary work has been done on human laryngeal muscles to characterize their myosin heavy chain (MyHC) isoform composition and fiber types. Previous studies have classified laryngeal muscle fibers as fast (type IIA or IIX), slow (type I), or a hybrid of these isoforms.1 Most reports conclude that human thyroarytenoid muscle contains a majority of fast fibers (about 65%), and the posterior cricoarytenoid about equivalent proportions for slow (type I) and fast (type II).2,3 The presence of unique MyHC isoforms, such as neonatal, α-cardiac, tonic, or extraocular, has yet to be thoroughly examined in these muscles. It is likely that some of these unusual MyHC isoforms are present in the PCA given the evidence of these isoforms in other specialized cranial muscles, and the unusual shortening velocities reported for these fibers.4,5 Anatomical studies have shown a reliable presence of horizontal and vertical components of the posterior cricoarytenoid (PCA) muscle, but minimal information exists regarding the composition and function of these muscle subcompartments.6 Innervation studies have demonstrated a distinct innervation pattern to different intrinsic laryngeal muscle subcompartments.7 However, the exact physical and physiologic composition of these PCA subcompartments is not known. The hypothesized function of the muscle bellies has been teleological, or based on information obtained in animal models that may not be directly related to human laryngeal muscles.8 Detailed biologic information regarding the vertical and horizontal bellies of the PCA muscle is essential information for understanding both normal voice production and a variety of vocal pathologies, such as vocal fold immobilization and spasmodic dysphonia. A teleological, biomechanical analysis of the different bellies of the PCA allows inference to completely different functions of these muscles. Knowledge regarding the exact composition and function of these muscles may be used in the future for both medical and surgical treatment options, including botox injection and laryngeal pacing. Further information on intrinsic laryngeal muscle characteristics may lead to different locations and combinations of Botox injections. Similarly, this information may lead to more sophisticated laryngeal pacemaker electrode placement and stimulation pattern.
There may also be phenotypic changes in laryngeal muscles that occur with age, and brought about by denervation and subsequent reinnervation.9 Subsequent studies have identified neonatal myosin heavy chain (MyHC) isoform presence with immunohistochemical staining as indicative of muscle regeneration after reinnervation has been established.10 Normally neonatal MyHC is expressed in adult muscle only in regenerating fibers,11 as a response to denervation (especially in type IIA fibers),12 or as a transient response from stretch-induced hypertrophy of muscle.13 However, neonatal MyHC is commonly expressed in a subpopulation of fibers in masseter muscles of normal human subjects,14 tensor tympani muscles in some species,15 and extraocular muscle in several species including man.16,17 One of the goals of this study is to determine if neonatal MyHC is expressed in fibers of both normal and affected PCA muscles. If so, the PCA shares a likeness to specialized cranial muscle, and the presence of neonatal myosin may not necessarily indicate pathology. Whether developmental isoforms are transiently expressed during pathologic processes or normally coexpressed with other myosins as a stable phenotype are important attributes in understanding normal variation versus disease incidence.
Another indication of a prior neuropathic event with subsequent restoration of muscle fiber contractile activity is “fiber type grouping.” Generally in this condition, a group of denervated muscle fibers are reinnervated by dendritic sprouts from a single motor neuron. Upon recovery, these fibers will obtain the same myosin expression and fiber type as those muscle fibers originally innervated by the α-motor neuron. Upon staining such areas differentially for fiber type on histological sections, these areas of muscle have fibers of the same type closely packed together or “grouped,” rather than the typical mosaic pattern with fibers from the same motor unit interspersed throughout an area of the muscle fasicle. Fiber type grouping has been reported previously in intrinsic laryngeal muscles,18 and it is intuitive that this condition would exist since the recurrent laryngeal nerve may be damaged by surgical intervention or viral infection. In this case, too, there is a parallel with another specialized cranial muscle, the human masseter muscle, where grouping of fibers is a common feature. Yet this phenotype is characteristic in normal young subjects.19 Direct EMG studies of masseter have shown that this may be the result of large numbers of motor units with small diameter fibers closely packed together, rather than grouping after deinnervation.20,21 Given the lack of EMG and direct histologic studies, the degree of low-intensity but chronic pathological process in the human intrinsic laryngeal muscle is presently uncertain. The clinical implications for more detailed study include a greater knowledge of the functional properties of these muscles to aid in the treatment of laryngeal dysfunction.
In this investigation we describe the fiber type properties of both the vertical and horizontal compartments of the human posterior cricoarytenoid muscle using immunohistochemical techniques, allowing us to examine the whole of the muscle. Previous work characterizing fiber types of the two PCA compartments was done by dissection of single fibers from the muscle, and analysis of fiber type using SDS-PAGE (sodium dodecyl sulfate-poly-acrylamide gel electrophoresis) techniques.1 Limitations of that study include an incomplete analysis of all muscle fibers present in the whole muscle, with only approximately 40 fibers taken from each sample; as well as a potentially skewed result toward dissection of larger diameter fibers. Our previous work determining shortening velocities of single fibers has proven this tendency. Larger diameter fibers are dissected with greater ease and frequency, giving an inappropriately inflated number of these fibers.5,22 Therefore, single fiber analysis has significant limitations for quantifying fiber types in a specific muscle, which must be kept in mind when comparing this study’s techniques with previously published work in the literature. Immunohistochemical techniques allow a complete representation of all fiber types present along the entire length of muscle.
In this study, we determined the expression of MyHC isoforms and the morphology of the muscle samples as further description of the PCA muscle. In addition, we characterized the differences between normally and abnormally functioning muscles sampled from patients with both normal and immobilized vocal folds.
MATERIALS AND METHODS
Posterior cricoarytenoid and infrahyoid muscles were excised from patients undergoing total laryn-gectomy at the University of Pittsburgh Medical Center. The research protocol and informed consent process for human subjects was followed as approved by the University of Pittsburgh Internal Review Board (IRB #991280). For this study, the patient population consisted of 3 males and 1 female. The age range of the population was 48–71 years old. Laryngeal function was determined prior to obtaining the muscle harvest by flexible laryngoscopy.
Following removal of the laryngectomy specimen from the patient, the PCA muscles were exposed by resection of the postcricoid mucosa. A suture was placed through the muscular process of the arytenoid cartilage (insertion point of the PCA). The fibrous tissue dividing the horizontal and vertical bellies of the PCA was identified.6 This suture was then used to bisect both the horizontal and vertical bellies of the PCA. The lateral portion of the bisected vertical belly of the PCA (from origin to insertion) and the medial (superior) portion of the bisected horizontal belly of the PCA were harvested. One surgeon (CR) collected all the muscle according to this protocol to provide consistency in muscle belly sampling.
Four patients provided complete PCA samples in the manner described above. This resulted in a total of 16 samples (vertical and horizontal bellies of the right and left sides) to be analyzed individually. Two of the patients (1 male/1 female) had normal functioning vocal folds; the other 2 patients (both males) had a unilaterally immobile vocal fold (see Tables 1 and 2).
TABLE 1.
Estimated Percentage of Fiber Types Present in the Vertical Compartment of the PCA
| Patient | Sex | Age | Side | Paralyzed | Slow (%) Type I |
Fast (%) |
Atrophic Fibers | Fiber Type Grouping |
|
|---|---|---|---|---|---|---|---|---|---|
| IIA | IIX | ||||||||
| 1 | M | 66 | Right | No | 20 | 60 | 20 | Few | Yes |
| Left | Yes | 40 | 45 | 15 | No | Yes | |||
| 2 | M | 48 | Right | Yes | 90 | 5 | 5 | Yes | No |
| Left | No | 60 | 30 | 10 | Few | Yes | |||
| 3 | M | 63 | Right | No | 60 | 30 | 10 | No | No |
| Left | No | 60 | 35 | 5 | No | No | |||
| 4 | F | 71 | Right | No | 70 | 25 | 5 | No | No |
| Left | No | 50 | 45 | 5 | Few | No | |||
TABLE 2.
Estimated Percentage of Fiber Types Present in the Horizontal Compartment of the PCA
| Patient | Sex | Age | Side | Paralyzed | Slow (%) Type I |
Fast (%) |
Atrophic Fibers | Fiber Type Grouping |
|
|---|---|---|---|---|---|---|---|---|---|
| IIA | IIX | ||||||||
| 1 | M | 66 | Right | No | 80 | 15 | 5 | Yes | Yes |
| Left | Yes | 80 | 15 | 5 | Yes | Yes | |||
| 2 | M | 48 | Right | Yes | 90 | 15 | 5 | Yes | Yes |
| Left | No | 90 | 15 | 5 | Yes | Yes | |||
| 3 | M | 63 | Right | No | 90 | 8 | 2 | Few | No |
| Left | No | 90 | 8 | 2 | Yes | Yes | |||
| 4 | F | 71 | Right | No | 50 | 45 | 5 | No | Yes |
| Left | No | 60 | 35 | 5 | No | Yes | |||
The muscle samples were processed for cryosectioning by snap freezing in 2-methylbutane cooled by dry ice. Infrahyoid muscle was frozen alongside of the posterior cricoarytenoid muscle to provide a control for the staining procedures. The muscle blocks were serially sectioned, cross-sectional to the fibers, at 10 µm thickness. Slides were stored at −70°C until staining procedures were carried out. Approximately 100−200 10-µm sections were taken from each sample. The length of the PCA muscle belly was estimated based on the number of sections.
Immunohistochemistry was completed by indirect immunoperoxidase method, using either a peroxidase-conjugated secondary antibody or a biotin-labeled secondary antibody visualized by extravidin peroxidase. The following myosin heavy chain (MyHC)-specific antibodies were used: antitype I, IIA, II (all fast isoforms), neonatal, α-cardiac, IIB, IIM (type II masticatory), and tonic. Antibodies against neural cell adhesion molecule (N-CAM) and desmin were also used to determine reinnervation or degeneration in any of the biopsies. The specificity of the antibodies and references of their use is given in Table 3.
TABLE 3.
Myosin Isoform-Specific Antibodies Used in This Study
| Name | Type* | Antibody | Myosin Isoform Specificity | Source† | References |
|---|---|---|---|---|---|
| Anti-I | mc | BA-F8 | type I (slow) HC‡ | DSM§ | 18 |
| Anti-II | mc | MY-32 | type IIA, IIB, IIX, neonatal | Sigma | 11 |
| Anti-IIA | mc | SC-71 | type IIA HC | DSM | 19 |
| Anti-IIB | mc | BF-F3 | type IIB HC | DSM | 20 |
| Anti-IIM | pc | type IIM HC | — | 21 | |
| Anti-α cardiac | mc | MAS366p | α cardiac HC | Sera-lab | 11 |
| Anti-tonic | pc | tonic HC | — | 22 | |
| Anti-NE | pc | neonatal HC | — | 12 | |
| Nonmyosin antibodies used in this study | |||||
| Anti-desmin | mc | 1698 | desmin | Chemicon | 23 |
| Anti-N-CAM | mc | OB11 | N-CAM | Sigma | 24 |
mc, monoclonal (mouse); pc = polyclonal (rabbit).
Commercial source for monoclonals; polyclonal antibodies as used previously by authors.
HC, heavy chain.
DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany.
Hematoxylin and eosin staining was done for each sample to examine the general morphology of the tissue. Additionally, the presence of centralized nuclei may be observed as an indicator of regenerating fibers, and as a comparison for any desmin or N-CAM antibody staining.
The fixed sections were viewed using an Olympus BX40 light microscope with an MTI CCD72 (Olympus Corporation, Melville, NY) digital video camera interfaced to a computer. The tissue image was projected on to the monitor through I-Cube image analysis software (Image Pro-Plus, Silver Spring, MD). This software allowed for image capturing, saving, and printing for direct comparison of serial sections. Fiber type composition of each sample was based on the differential staining of the fibers by immunohistochemistry. Amounts of each fiber type present were estimated by observation of photomicrographs.
RESULTS
Fiber type characteristics of the infrahyoid muscle
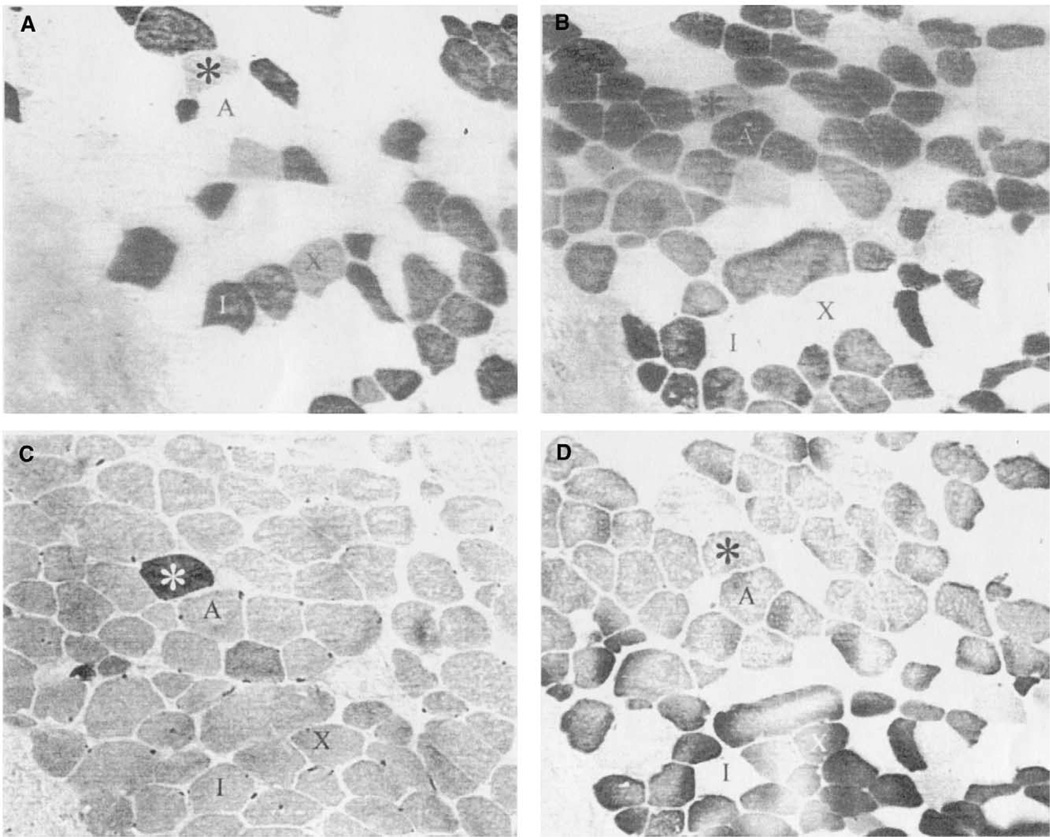
Staining of the infrahyoid muscle was used as a control to compare immunohistochemical results to that of the PCA muscle staining (Figure 1). This muscle showed antibody reactivity to slow type I (Figure 1A), IIA (Figure 1B), IIX (Figure 1D), and neonatal (Figure 1C) isoforms. The fiber types were similar to muscle of the abdomen and limb, oval in shape, and distributed in a typical mosaic pattern. Fiber type grouping was not apparent in the control muscle, and coexpresson of myosin isoforms (including neonatal MyHC) was rare.
FIGURE 1.
Photomicrographs of immunohistochemical staining of an Infrahyoid muscle. A. Anti-type I, fiber indicated by “I;” B. Anti-type IIA, fiber “A;” C. Anti-neonatal; D. Anti-type II, fiber “X” coexpressing with type I. Asterisk indicates fiber coexpressing I, IIA, and neonatal MyHCs. All slides are 400×.
Fiber type characteristics of the posterior cricoarytenoid muscle compartments
The four bellies of the PCA (right vertical and horizontal, and left vertical and horizontal) were considered independently of each other to analyze any differences among the fiber type composition. Four patients provided complete samples, resulting in sixteen separated bellies for individual analysis. Average lengths of the PCA compartments were: right horizontal: 8.1 mm; left horizontal: 9.0 mm; right vertical: 1.4 cm; and left vertical 1.6 cm. Consistency in fiber type composition is seen between the right and left sides of the PCA, even in cases of unilateral immobilization of the vocal folds (Tables 1 and 2). However, comparisons of the vertical and horizontal compartments of the PCA muscle revealed differences in the fiber type composition.
The vertical compartment
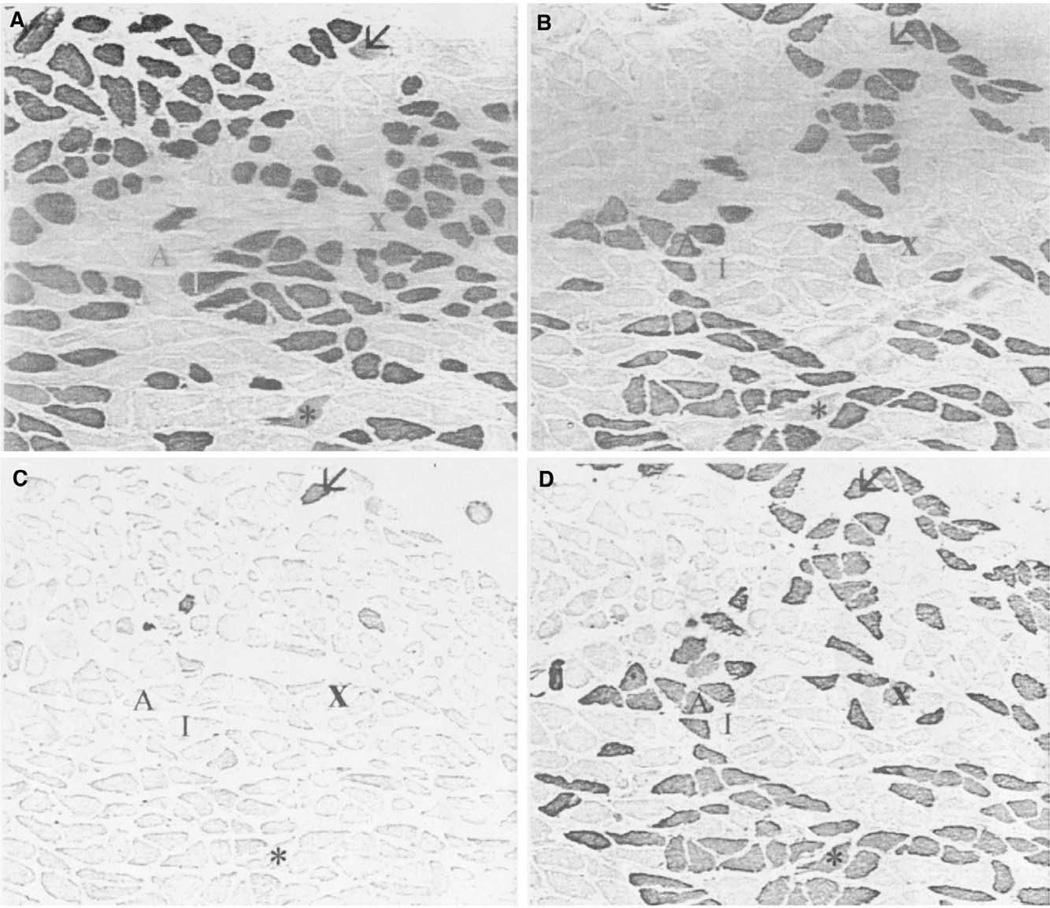
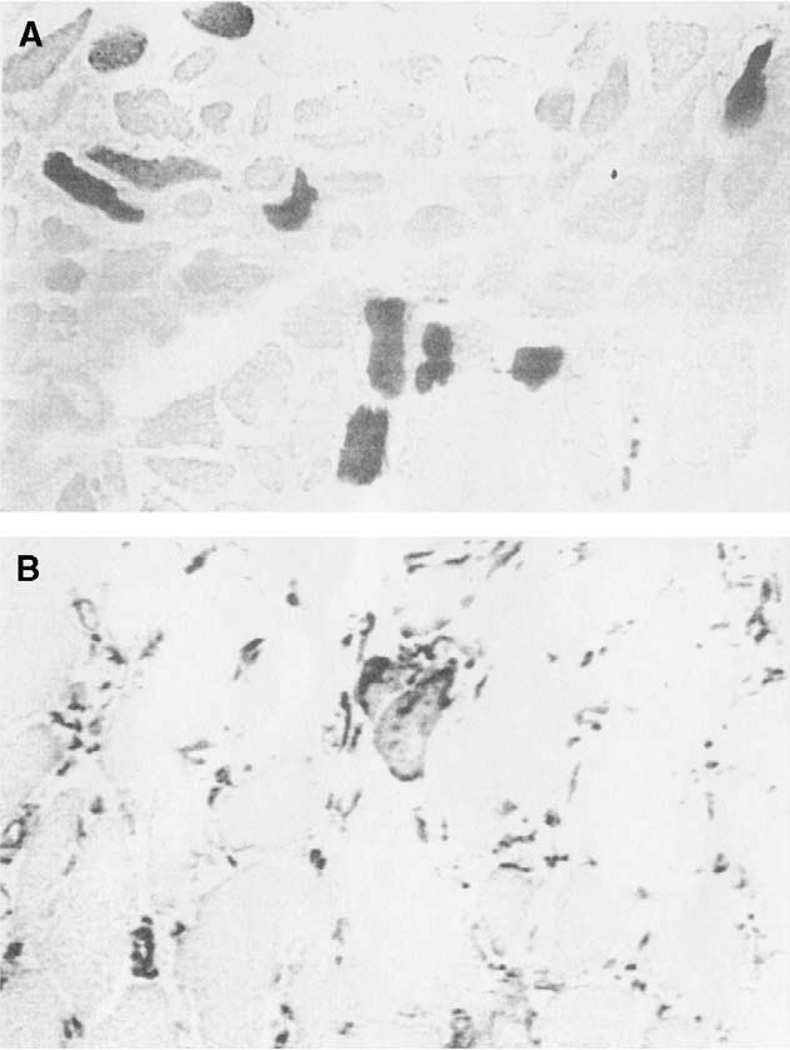
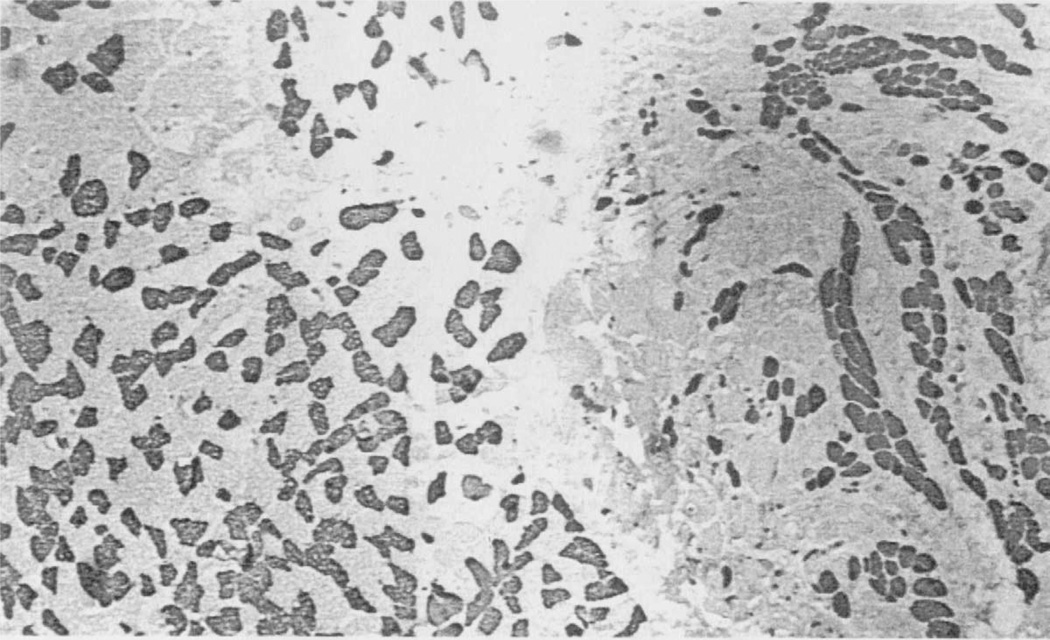
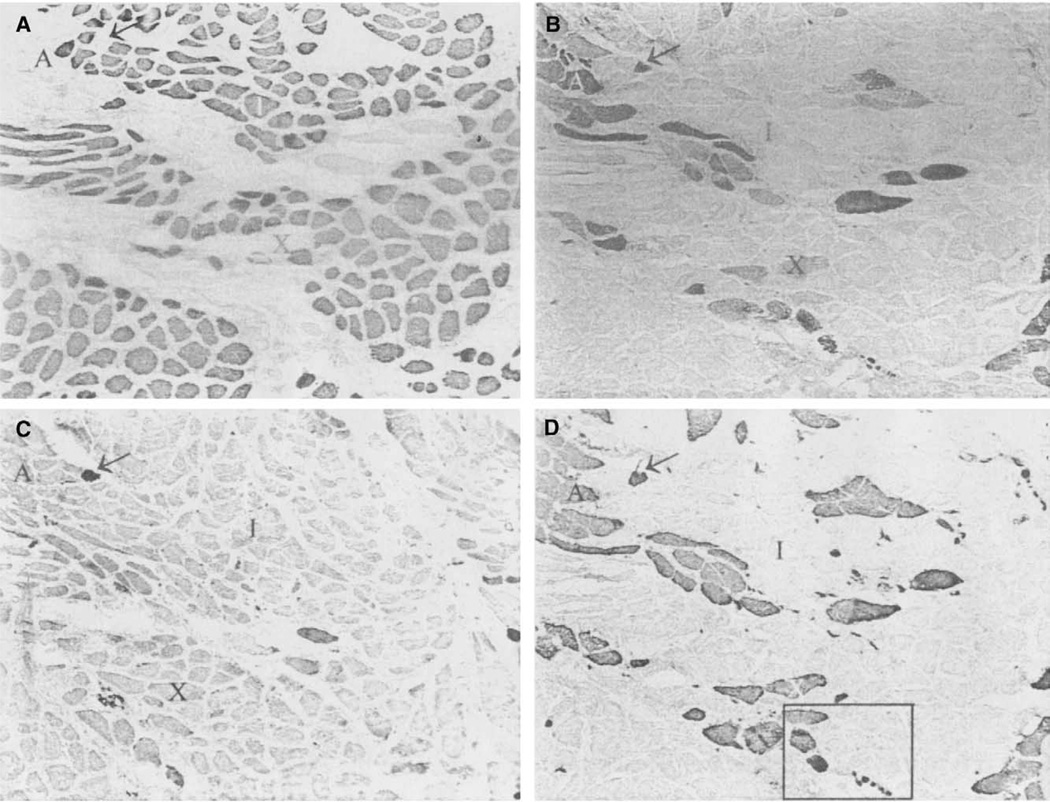
The vertical belly of the PCA contained relatively equal amounts of fast and slow MyHC protein, with a typical ratio estimated approximately to be 55% type I (Figure 2A) and 45% type II (Figure 2D), calculated by visual observation (Table 1). Immunohistochemistry, using SC-71 (anti-IIA) (Figure 2B) allowed direct observation of type IIA fibers in the samples. The MY-32 (anti-type II) (Figure 2D) antibody detected the presence of general type II fibers. Comparison of the SC-71 staining to the staining of the MY-32 antibody allowed the indirect determination of the presence of type IIX fibers. Several fibers coexpress the IIX isoform with type I isoform (Figure 2, fiber indicated by *). Half (4 of the 8 samples) of the vertical bellies contained a few neonatal fibers (Figure 2C), which showed coexpression with the type I and IIX isoforms. No positive staining was seen in sections stained with antibodies reactive for the tonic, type IIB, or IIM myosins. One vertical muscle compartment from patient 1 reacted positively to the α-cardiac antibody (Figure 3A). This antibody did not react in any other samples, horizontal or vertical. Apparent fiber type grouping was seen in 3 of the vertical samples. Figure 4 shows fiber type grouping in a PCA sample and the normal fiber distribution pattern in an infrahyoid control muscle. Patient 1 with unilateral vocal fold immobility showed grouping in both sides of the vertical compartment. Patient 2, also with unilateral vocal fold immobility showed grouping on the normal functioning side but showed a unique shift in fiber type toward mostly slow on the immobilized side (grouping undetermined). Patients 3 and 4 showed no apparent fiber type grouping in the vertical compartments.
FIGURE 2.
Photomicrographs of immunohistochemical staining of a nonimmobilized side of a vertical PCA compartment. A. Anti-type I; B. Anti-type IIA; C. Anti-neonatal; D. Anti-type II. Arrow indicates neonatal coexpressing fiber. “I” is a type I fiber, “A” type IIA, and “X” type IIX. Asterisk is a fiber coexpressing type I and IIX MyHC. All slides are 100×.
FIGURE 3.
A. α-cardiac antibody staining in a vertical belly of the PCA muscle. Magnification: 200×. B. Positive desmin antibody staining in a vertical belly of the PCA muscle (normal-functioning vocal folds) Magnification: 400×.
FIGURE 4.
Low-power photomicrograph of an infrahyoid muscle (on left) and PCA muscle (on right) stained with anti-type II (MY-32). Typical mosaic pattern distribution of fast fibers seen in the infrahyoid muscle, and fiber type grouping seen in the PCA. Magnification: 40×.
The horizontal compartment
The horizontal belly of the PCA was found to contain an approximate average of 80% type I fibers (Figure 5A) and 20% type II(Figure 5D), determined by visual observation (Table 2). Presence of type IIX fibers was deduced by comparison of the type IIA staining (Figure 5B) to the general type II antibody (Figure 5D). Three of the horizontal samples contained neonatal fibers (Figure 5C). These fibers showed evidence of coexpression with type IIA and I. No positive staining was found for α-cardiac, tonic, and type IIB and IIM fibers in the horizontal bellies that were sampled.
FIGURE 5.
Photomicrographs of immunohistochemical staining of a horizontal PCA from a immobilized vocal fold. A. Anti-type I; B. Anti-type IIA; C. Anti-neonatal; D. Anti-type II. Arrow indicates fiber expressing neonatal and IIA myosin. “I” is a type I fiber, “A” type IIA, and “X” type IIX. The black box in D is the area that is magnified in Figure 6A. All slides are 100×.
Variability in fiber type grouping was present in the horizontal compartment. Patient 1 and 2, both with unilaterally immobilized vocal folds, exhibited fiber type grouping on both the right and left sides of the horizontal belly. Patient 3 with normal functioning vocal folds had fiber type grouping on one side but not the other. Patient 4 also with normal functioning vocal folds exhibited fiber type grouping on both horizontal sides (no grouping was seen in vertical compartments).
Distinctions of immobilization
Morphological indications of the effects of vocal fold immobilization on the PCA muscles were inconclusive. Reactivity to myosin antibodies in the PCA was not affected by the unilateral immobilization of the vocal folds. Neonatal MyHC was present in PCA muscle fibers from both normal functioning and immobilized samples. The few fibers reactive for neonatal MyHC contained a combination of type I or IIA as well. Any effects of the immobilized condition did not generally alter fiber diameter and morphology. Apparent fiber type grouping occurred in both normal functioning and abnormal bellies, making no distinction between side, compartment, or immobilization status.
Recent degeneration or reinnervation was not present, as determined by antibody staining. Antibodies against desmin and neural cell adhesion molecule (N-CAM) were used to detect any recent reinnervation or denervation in the muscle.23,24 Anti–N-CAM did not result in positive staining in any of the samples. The small atrophic fibers also did not react positively to the N-CAM antibody. Desmin was detected in a very small number of fibers in half of the total samples (Figure 3B). The small amount of desmin that was detected did not provide conclusive evidence for regeneration; nor did the desmin-stained fibers show the presence of centralized nuclei by H&E staining. The presence and absence of desmin positive fibers varied between immobilized and normal functioning samples.
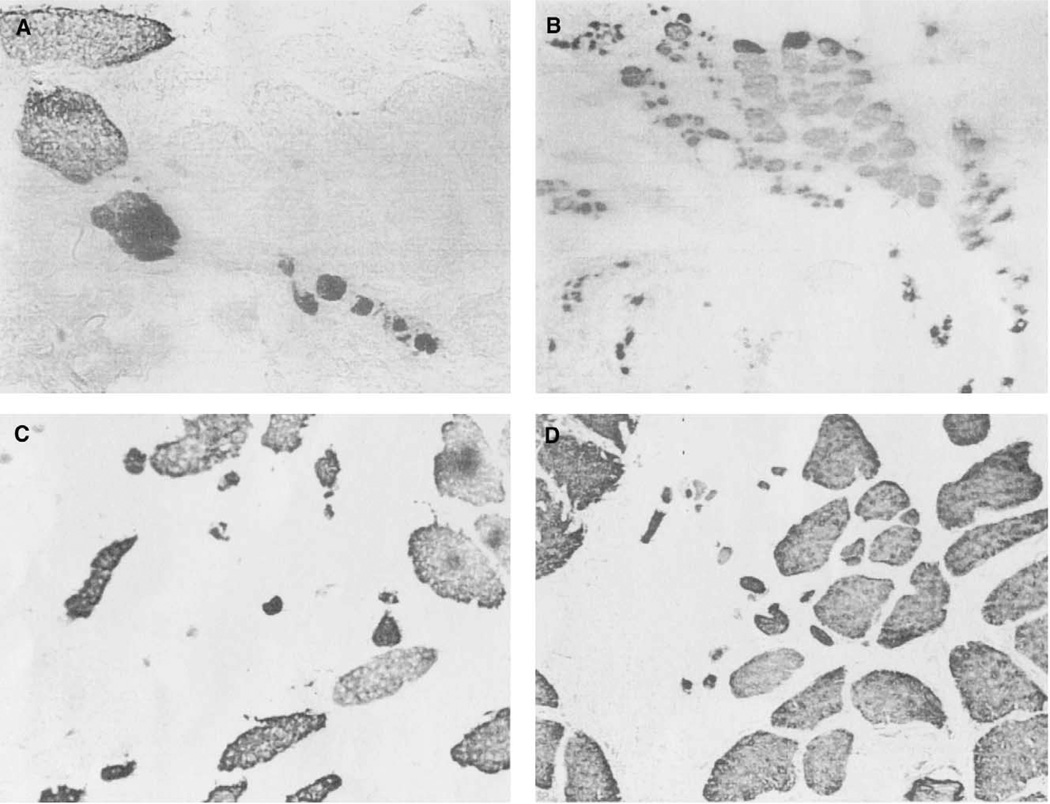
Atrophic fibers were also present in ten bellies, regardless of immobilization (Figure 6). Atrophic fibers stain positive with MY-32, indicating a fast fiber type (Figure 5D and Figure 6A). Figure 2 represents a vertical belly from a normal functioning side of the PCA. This sample lacks atrophic fibers, but other normal functioning vertical samples did contain them (Figure 6D). Examples of atrophic fibers found in vertical PCA muscles from both normal functioning and immobilized vocal folds are shown in Figure 6B and 6D. Figure 5 represents an immobilized horizontal side of the PCA, containing atrophic fibers. Figure 6A shows a higher power view (400×) of the fibers in the box of Figure 5D. However, the small atrophic fibers are also found in horizontal PCA muscle from normal functioning vocal folds as well (Figure 6C). Despite the small patient population, more muscle fiber damage seems to have occurred in the horizontal compartments. These results indicate that atrophy of muscle fibers in the PCA was present and variable in both unilaterally immobilized and normal functioning vocal folds (for a summary see Tables 1 and 2).
FIGURE 6.
Photomicrographs of atrophic fibers present in PCA bellies from both normal-functioning and unilaterally immobilized vocal folds. A. Horizontal PCA belly, from immobilized vocal fold; B. Horizontal PCA belly, from normal functioning side of the PCA; C. Vertical belly of the PCA, from immobilized vocal folds; D. Vertical belly of the PCA, from normal functioning vocal folds. All stained with anti-type II (MY-32). All taken at 400×.
DISCUSSION
Infrahyoid muscle was previously described by Korfage et al.25 Similar fiber typing results were seen, indicating the typical ratio of type I, IIA, and IIX fibers, with a small amount of hybrid (coexpressing) fibers. Additionally, α-cardiac MyHC was found in a small percentage of fibers, but not found in the four patients sampled here. These findings further characterize the infrahyoid as a fine control muscle to be used for comparison to specialized cranial muscles.
Separation of the PCA muscle into its horizontal and vertical compartments led to clear differences found in the MyHC composition in the each belly. Our findings for the MyHC isoforms that are present in the PCA muscle are consistent with previous reports. Wu et al1 separated the PCA into the two compartments in a similar fashion. Random single fibers that were dissected and run on SDS-PAGE gels for MyHC analysis led to findings that are consistent with our ratios determined through whole muscle analysis by immunohistochemistry.
This study examined the effects of unilateral vocal fold immobilization on the PCA muscle describing the phenotype for three conditions: PCA muscle from normal functioning vocal folds, PCA muscle from unilaterally immobile vocal folds, and PCA muscle from opposite sides of the unilaterally immobile fold. The “clinical term” of vocal fold immobilization denotes no demonstrable motion of the vocal fold with both phonation and a variety of vegetative activities of the larynx. Clinical colleagues have reported anecdotal experience (Jim Netterville, personal communication, 1998) with laryngeal electromyography in patients with a clinical diagnosis of vocal fold immobilization and laryngeal examination of vocal fold immobility and have identified significant electrical activity, despite showing no motion on laryngeal examination. Thus, knowledge of the relationship between the neuromuscular status of the intrinsic laryngeal muscles and “clinical function” of the vocal folds is unknown. It appears that when an immobile vocal fold is present, it could be due to pathology of the cricoarytenoid joint (fixation or dislocation) or vocal fold immobilization with partial reinnervation or laryngeal synkinesis. Hypotheses regarding laryngeal synkinesis have stated that following acute injury to the recurrent laryngeal nerve, reinnervation of the adductor and abductor musculature of the intrinsic larynx results in poor coordination of these musculatures, resulting in no net motion of the vocal fold due to simultaneous contraction of the abductor and adductor musculature of the vocal fold.26
In the cases that were studied involving unilateral immobilization, distinct muscular pathology was difficult to identify. Reactivity to myosin antibodies was unaffected. Fiber type grouping and atrophic fibers were variably present in PCA muscle from both immobilized and normal functioning vocal folds. There were no clear signs of denervation or regeneration in the affected muscles, as determined by antibody staining. Positive reactivity for desmin in a small amount of fibers may indicate that these fibers are in the process of atrophy; but in our muscles this was a rare occurrence, restricted to a few isolated fibers rather than whole motor units. Desmin is typically present during the development of muscle fibers, or during recently occurring or active regeneration of muscle fibers.23 Desmin may be readily located by antibody staining during normal muscle development and during pathologic processes. In healthy, fully developed muscle cells, desmin is present, but highly organized into microfilaments such that most epitopes cannot be accessed and identified by antibody molecules. During development and cellular disruptions, desmin microfilaments become disorganized into individual desmin molecules which aid in cellular organization (development) or reorganization (repair from pathology). This is true of desmin in muscle fiber cells as well as fibroblasts. Further and even more important, desmin and neonatal MyHC together in the same fiber is a strong indication of pathology; and this was not found in any of the muscles we sampled. However, in these four patients desmin and neonatal MyHC were expressed in both nonaffected and affected tissue.
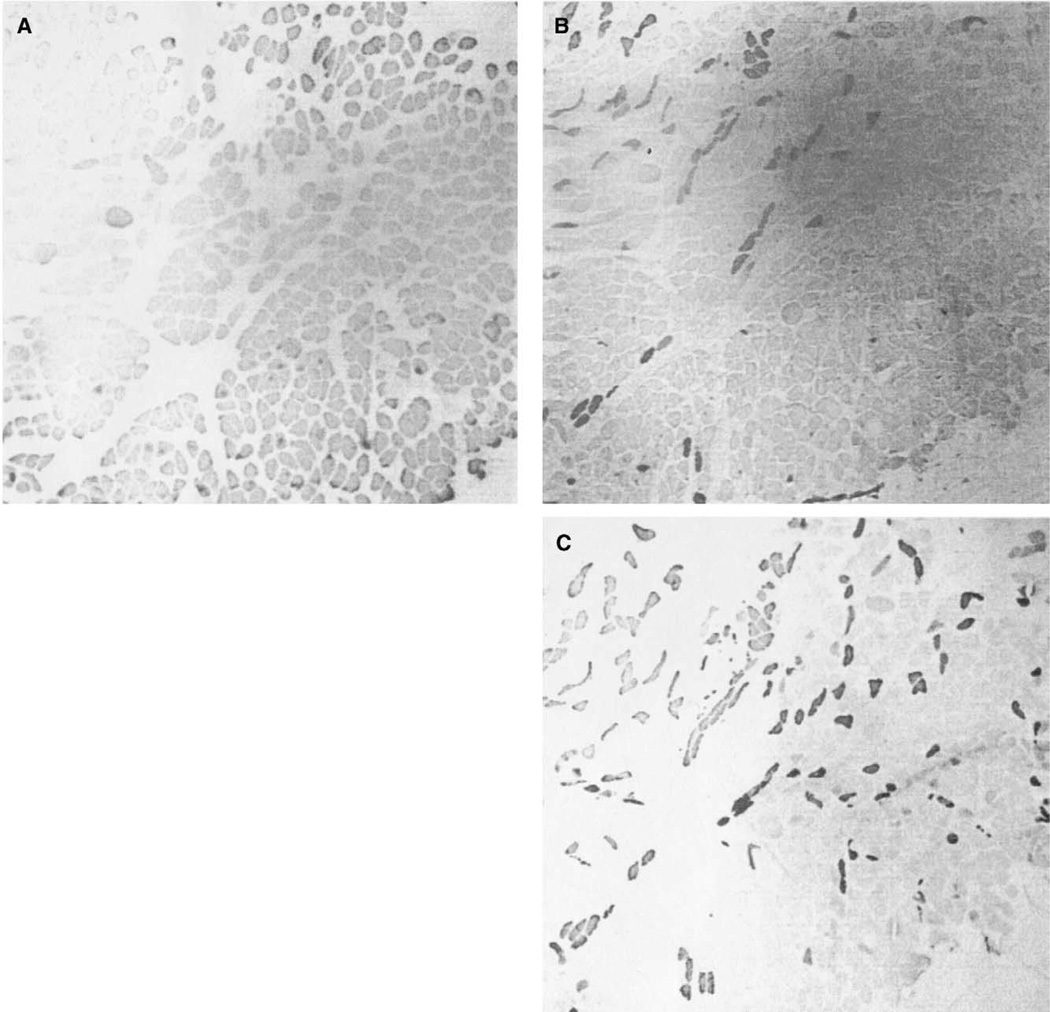
One interesting finding occurred in the right vertical belly of the PCA in patient 2. In this particular sample there was a shift toward more type I (slow) fibers (Figure 7A), replacing the typical 55:45 ratio of slow to fast fibers (Figures 7B and C). Notably this side of the larynx was also affected by immobilization. This is the only sample that showed a dramatic change in expected MyHC composition, and it is speculated to be a result of the vocal fold immobilization. However, there were no signs of recent reinnervation or regeneration, as determined by antibody staining; nor were there morphological changes, as thought to occur with abnormal functioning vocal folds. But it still remains to be determined why there are not consistent changes among the other samples that we have tested.
FIGURE 7.
Photomicrographs of immunohistochemical staining of a vertical belly of the PCA, from a unilaterally immobilized vocal fold. A. Anti-type I; B. Anti-type IIA; C. Anti-type II. Magnification: 40×.
Immobile vocal folds may be the result of one of three possible pathologies: tumor infiltration, joint dislocation, or nerve damage and subsequent synkinesis.26 EMG studies allow determination of the cause of vocal fold immobility, by determining if there is no neuropathy and only fixation, or the result of secondary innervation with synkinesis. Laryngeal EMG studies could not be completed on prelaryn-gectomy patients given the ethics of performing an invasive procedure without direct patient benefit. Therefore, to fully analyze the effects of vocal fold immobility on the fiber type composition and morphology of the PCA muscle, further studies should be completed using EMG techniques in an animal model. The goal of such studies would be to help elucidate the natural history of vocal fold immobility.
Acknowledgments
The authors wish to thank Drs. Jonas Johnson and Eugene Myers for surgical assistance; and Robin Wagner and Mike Tometsko for patient coordination and sample collection.
REFERENCES
- 1.Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ. New perspectives about human laryngeal muscle. Arch Otolaryngol Head Neck Surg. 2000;126:857–864. doi: 10.1001/archotol.126.7.857. [DOI] [PubMed] [Google Scholar]
- 2.Sadeh M, Kronenberg J, Gaton E. Histochemistry of human laryngeal muscles. Cell Mol Biol. 1981;27:643–648. [PubMed] [Google Scholar]
- 3.Teig E, Dahl HA, Thorkelson H. Actomyosin ATPase activity of human laryngeal muscles. Acta Otolaryngol (Stockh) 1978;85:272–281. doi: 10.3109/00016487809111935. [DOI] [PubMed] [Google Scholar]
- 4.Sciote JJ, Morris TJ, Brandon CA, Horton MJ, Rosen C. Unloaded shortening velocity and myosin heavy chain variations in human laryngeal muscle fibers. Ann Otol Rhinol Laryngol. 2002;46:821–827. doi: 10.1177/000348940211100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sciote JJ, Morris TJ, Brandon CA. Shortening speeds and myosin heavy chain content of skinned single fibres of human limb, jaw-closer, and laryngeal muscles. J Physiol. 2001;493:531P. [Google Scholar]
- 6.Bryant NJ, Woodson GE, Kaufman K, et al. Human posterior cricoarytenoid muscle compartments. Anatomy and mechanics. Arch Otolaryngol Head Neck Surg. 1996;122:1331–1336. doi: 10.1001/archotol.1996.01890240039009. [DOI] [PubMed] [Google Scholar]
- 7.Sanders I, Wu BL, Mu L, Biller HF. The innervation of the human posterior cricoarytenoid muscle: evidence for at least two neuromuscular junction compartments. Laryngoscope. 1994;104:880–884. doi: 10.1288/00005537-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Sanders I, Rao F, Biller HF. Arytenoid motion evoked by regional electrical stimulation of the canine posterior cricoarytenoid muscle. Laryngoscope. 1994;104:456–462. doi: 10.1288/00005537-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Malmgren LT, Fisher PJ, Bookman LM, Uno T. Age related changes in muscle fiber types in human thyroarytenoid muscle: an immunohistochemical and stereological study using confocal microscopy. Otolaryngol Head Neck Surg. 1999;121:441–451. doi: 10.1016/S0194-5998(99)70235-4. [DOI] [PubMed] [Google Scholar]
- 10.Malmgren LT, Lovice DB, Kaufman MR. Age related changes in muscle fiber regeneration in the human thyroary-tenoid muscle. Arch Otolaryngol Head Neck Surg. 2000;126:851–856. doi: 10.1001/archotol.126.7.851. [DOI] [PubMed] [Google Scholar]
- 11.Sartore S, Gorza L, Schiaffino S. Fetal myosin heavy chains in regenerating muscle. Nature. 1982;298:294–296. doi: 10.1038/298294a0. [DOI] [PubMed] [Google Scholar]
- 12.Schiaffino S, Gorza L, Pitton G, et al. Embryonic and neonatal myosin heavy chain in denervated and immobilized rat skeletal muscle. Dev Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- 13.Matthews W, Jenkins RR, Gonyea WJ. Myosin isozyme expression in response to stretch-induced hypertrophy in the Japanese quail. Anat Rec. 1990;228:225–261. doi: 10.1002/ar.1092280304. [DOI] [PubMed] [Google Scholar]
- 14.Sciote JJ, Rowlerson AM, Hopper C, Hunt NP. A fiber type classification scheme for the human masseter muscle. J Neurol Sci. 1994;126:15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scapolo PA, Rowlerson AM, Mascarello F, Veggetti A. Neonatal myosin in bovine and pig tensor tympani muscle fibers. J Anat. 1991;178:236–255. [PMC free article] [PubMed] [Google Scholar]
- 16.Sartore S, Mascarello F, Rowlerson A, Gorza L, Vianello M, Schiaffino S. Fibre types in extraocular muscles: new myosin isoform in the fast fibres. J Musc Res Cell Motil. 1987;8:161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- 17.Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Grinard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malmgren LT, Gacek RR. Histochemical characteristics of muscle fiber types in the posterior cricoarytenoid muscle. Ann Otol. 1981;90:423–429. doi: 10.1177/000348948109000503. [DOI] [PubMed] [Google Scholar]
- 19.Sciote JJ, Morris TJ. Skeletal muscle function and fiber types: Relationship between occlusal function and phenotype of jaw-closing muscles in humans. J Orthod. 2000;27:15–30. doi: 10.1093/ortho/27.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Yemm R. The orderly recruitment of motor units of the masseter and temporal muscles during voluntary isometric contraction in man. J Physiol. 1977;265:163–174. doi: 10.1113/jphysiol.1977.sp011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stalburgh E, Eriksson P-O. A scanning electromyographic study of the topography of human masseter single motor units. Arch Oral Biol. 1986;32:793–797. doi: 10.1016/0003-9969(87)90005-7. [DOI] [PubMed] [Google Scholar]
- 22.Morris TJ, Brandon CA, Horton MJ, Carlson DS, Sciote JJ. Maximum shortening velocity and myosin heavy chain isoform expression in human masseter muscle fibers. J Dent Res. 2001;80:1845–1848. doi: 10.1177/00220345010800091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bornemann A, Schmalbruch H. Desmin and vimentin in regenerating muscles. Mus Nerve. 1992;15:14–20. doi: 10.1002/mus.880150104. [DOI] [PubMed] [Google Scholar]
- 24.Neill JM, Barnstable CJ. Expression of the cell surface antigens RET-PE2 and N-CAM by rat retinal pigment epithelial cells during development and in tissue culture. Exp Eye Res. 1990;51:573–583. doi: 10.1016/0014-4835(90)90088-c. [DOI] [PubMed] [Google Scholar]
- 25.Korfage JAM, Schueler YT, Brugman P, Van Eijden TMGJ. Differences in myosin heavy-chain composition between human jaw-closing muscles and supra- and infrahyoid muscles. Arch Oral Biol. 2001;46:821–827. doi: 10.1016/s0003-9969(01)00042-5. [DOI] [PubMed] [Google Scholar]
- 26.Crumley RL. Laryngeal synkinesis: Its significance to the laryngologist. Ann Otol Rhinol Laryngol. 1989;98:87–92. doi: 10.1177/000348948909800201. [DOI] [PubMed] [Google Scholar]