Abstract
Background
Cardiac progenitor cells (CPCs) derived from human embryonic stem cells (hESCs) can multiply and generate cardiomyocytes offering their tremendous potential for cardiac regenerative therapy. However, poor survival under stressful conditions is a major hurdle in the regeneration. We investigated whether isoflurane-induced preconditioning can increase hESC-derived CPCs survival under oxidative stress.
Methods
Undifferentiated hESCs were cultured in suspension with 20% FBS and 20 ng/ml of BMP-4 to form embryoid bodies and grown onto Matrigel-coated plates for 2-3 wks. To characterize the differentiated CPCs, immunostaining for Nkx2.5 and Isl-1 was performed. hESC-derived CPCs were exposed to oxidative stress induced by H2O2 and FeSO4. For anaesthetic preconditioning, CPCs were exposed to isoflurane (0.25, 0.5, 1.0 mM). CPCs survival was determined by trypan blue exclusion. A mitoKATP channels inhibitor, 5-hydroxydecanoic acid (200 μM) and an opener, diazoxide (100 μM) were used to investigate the involvement of mitoKATP channels.
Results
hESC-derived CPCs stained with Nkx2.5 were 95 ± 3% of total cell number. Isoflurane (0.5 and 1.0 mM)-preconditioned CPCs showed a significantly lower death rate compared with control (0.5 mM: 30.6 ± 10.7% and 1.0 mM: 28.5 ± 6.2% vs control: 43.2 ± 9.9%). Inhibition of mitoKATP channels with 5-HD completely abolished the protective effects of isoflurane. Diazoxide significantly decreased CPC death (29.5 ± 12.4%). However, when diazoxide was applied to CPC preconditioned with isoflurane, CPC death did not decrease further (28.7 ± 10.9%).
Conclusion
Isoflurane increased hESC-derived Nkx2.5+ CPCs survival under oxidative stress and mitoKATP channels may be involved in the protective effect.
Introduction
Loss of myocardium or dysfunction during myocardial infarction is mostly irreversible and may lead to heart failure.1 Given the limited supply of transplant hearts, cardiac cell engraftment is being considered as a potential means of treating patients with myocardial infarction or cardiac failure. Although several cell types including human embryonic stem cells (hESCs), skeletal myoblasts, bone marrow-derived progenitor cells and cardiomyocyte progenitor cells have been proposed,2-5 hESCs offer an attractive stem cell-based strategy for cardiac repair. Advantages of hESCs over some of these other cells for the cardiac restoration are their proliferative capacity and pluripotency, allowing them to give rise to differentiated cells such as cardiomyocytes.6, 7 In addition, cardiomyocytes derived from hESCs showed robust proliferative capacity and electromechanical integration with host myocardium after transplantation.8-10
Despite the great promise in the cardiac repair, the transplantation of hESC-derived cardiomyocyte has some major obstacles in the way to its clinical application. They include poor graft survival, potential development of tumors, immune rejection and cardiac arrhythmias after hESC-derived cardiomyocyte engraftment.8-11 Among them, the improved survival of new cardiomyocyte immediately after transplantation may play a key role to lead the new strategy in the field of cardiac repair and regenerative medicine. The poor survival rate in the infarcted heart suggests that most of the grafted cardiomyocytes die after grafting. Grafted cardiomyocytes in infarcted myocardium are subject to be surrounded by hostile circumstances such as ischemia, anoikis, apoptosis and inflammatory factors.9, 12
Volatile anaesthetics are well known to have beneficial effects on ischemic myocardium, whereby a transient exposure to an anaesthetic protects the heart against subsequent ischemic damage.13-15 Volatile anaesthetics provides equally effective cardiac protection compared to that elicited by ischemic preconditioning, and the two modes of preconditioning share a number of signaling pathways. Recently, we demonstrated that isoflurane protected hESC-derived cardiomyocytes from oxidative stress suggesting a protective mechanism via opening mitoKATP channels and delaying mitochondrial permeability transition pore opening.16 The results first showed the possibility that isoflurane can increase the survival of hESC-derived cardiomyocytes for engraftment. The cardiomyocytes were from beating mature cardiomyocytes 12 weeks after treatment with growth factors. However, fully differentiated cardiomyocytes have a limited number of divisions, and an enormous number of cells would be required to replace the infarcted myocardium.17 Accordingly, using progenitor cells capable of dividing during differentiation could be a better choice. Cardiac progenitor cells (CPCs) express nonspecific transcriptional markers such as Nkx2.5, Isl-1, Flk1, SSEA-1 or MESP-1 and are capable of generating cardiomyocyte, endothelial cell and smooth muscle.18-20 Some recent studies showed that CPCs positive for Nkx2.5, Flk1, or SSEA-1 showed proliferative activity even after transplantation.18, 21, 22 Therefore, it is worth verifying a hypothesis that isoflurane can protect immature CPCs derived from hESC.
The aims of the present study were to determine whether isoflurane increases the survival rate of hESC-derived Nkx2.5+ cardiac progenitor cells (2-3weeks after treatment with BMP-4) under oxidative stress and to investigate that the protective mechanism is involved in mitoKATP channel opening.
Materials and Methods
hESC culture
H1 hESC (WA01) was cultured on mitomycin C inactivated STO (ATCC, CRL-1503) feeder cell layer. The culture medium consists of Dulbecco’s modified Eagle medium (DMEM-F12 with L-glutamate and 15 mM HEPES), 20% KnockOut Serum Replacement (Invitrogen, Carlsbad, CA), 1% nonessential amino acids, 1mM glutamine, 0.1 mM 2-mercaptoethanol, and 4 ng/ml bFGF. The colonies of hESCs were passaged every 7 days using a mechanical micro-dissection method.
Generation of hESC -derived cardiac progenitor cells (CPCs)
Cells were maintained in the undifferentiated state until two thirds of bottom of the plates were covered with the cell colonies (up to 7 days). hESC -derived CPCs were generated using a modified protocol which was recently reported for efficient cardiogenesis.23 In brief, the undifferentiated cells were disbursed into small clumps by incubation in the collagenase solution. The cells were then transferred to suspension culture in a 24-well non-treated dish with a differentiation medium containing 80% knockout DMEM, 100 μM nonessential amino acids, 2 mM L-glutamine, 100 μM 2-mercaptoethanol, and 20% FBS. The suspension cultures were maintained for 4 days to form embryoid bodies (EBs). 20 ng/ml of BMP-4 (R&D Systems, Minneapolis, MN) was added to the differentiation medium during the suspension culture. EBs were then replated onto Matrigel-coated 35 mm-plates and expanded in the differentiation medium for 2-3 wks.
Characterization of differentiated CPCs
To characterize the differentiated CPCs, immunostaining was performed with antibodies directed against human Nkx2.5 (mouse monoclonal, R&D Systems, Minneapolis, MN) and Isl-1 (goat polyconal, R&D Systems, Minneapolis, MN). DAPI (Vector Laboratories, CA, USA) was used to stain all nuclei. DAPI-positive cells were counted and the number of Nkx2.5-positive cells was expressed as percent of total number of cells (indicated by DAPI staining). From each plate, images from 10 random microscopic fields were taken at X20 magnification.
Cell survival study
Cell survival study was performed using a previously reported protocol.24 In brief, differentiated CPC clusters were enzymatically dissociated using 0.25% trypsin-EDTA solution for 5 min at room temperature and suspended in the differentiation medium. The suspension of dissociated CPCs (100 ul) was placed in a chamber on the stage of inverted microscope (ECLIPSE TS100; Nikon, Tokyo, Japan), and settled for 10 min. The CPCs were then stained with 0.5 ml trypan blue solution, 0.4% (Sigma-Aldrich), for 2 min followed by a dye washout with glucose-free Tyrode solution (132 mM NaCl, 10 mM, HEPES, 5 mM KCl, 1 mM CaCl2, and 1.2 mM MgCl2, adjusted to pH 7.4). Cells that were round-shaped and excluded trypan blue were considered living. The location of living cells was checked using a chamber bottom with a labeled grid and recorded on a paper marked with the same grids. This helped not only count the same cells before and after oxidative stress but also differentiate a missing cell (no trace after wash-out) from a dead one (stained blue with or without irregular margins). In each experiment, approximately 100 living CPCs were counted for 10 min. After cell counting, perfusion with glucose-free Tyrode was started. After 30 minutes, the CPCs were exposed to oxidative stress by perfusion with 200 μM H2O2 and 100 μM FeSO4·7H2O (Sigma-Aldrich) for 20 min. After oxidative stress, the CPCs were washed out with glucose-free Tyrode solution for 20 min. With the records of living cell location, the remaining living cells were counted after staining with trypan blue. Excepting the missing cells (under 5% of the living cells) from the cell count before oxidative stress, the percentage of living cells was calculated after oxidative stress. The time between cell counts before and after stress was exactly 70 min in all experiments. In the experimental groups that underwent anaesthetic preconditioning (APC), the CPCs were exposed to isoflurane for 20 min and 5 min anesthetic washout before oxidative stress. The protocols for the present experiment are illustrated in Figure 1.
Fig. 1.
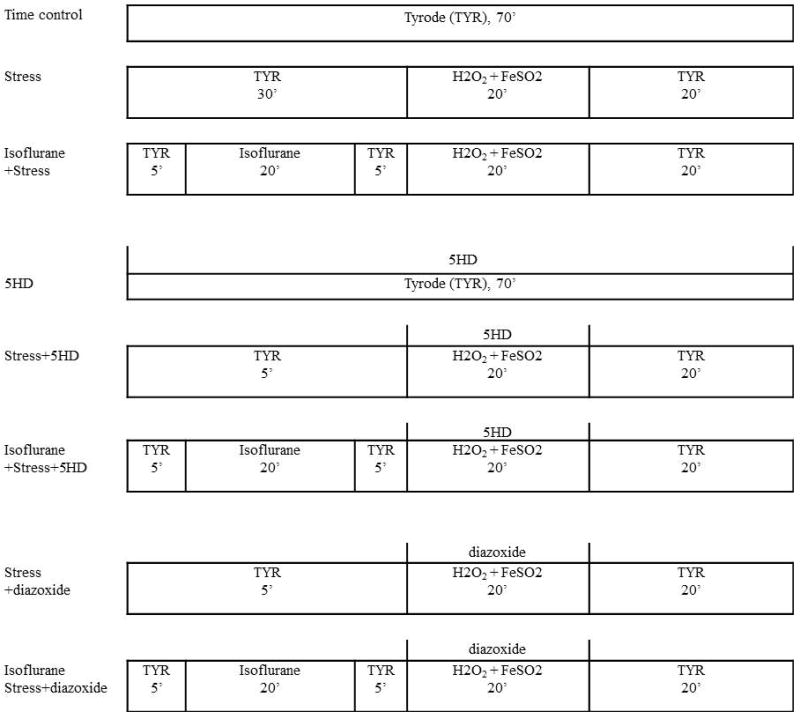
Summary of experiments (Cardiac progenitor cell survival study) performed in the study
TYR: perfusion with Tyrode solution; 5HD: 5-hydroxydecanoic acid
Drugs
Isoflurane was dispersed in glucose-free Tyrode solution by sonication and delivered to CPCs using the airtight syringes. hESC-derived CPCs were exposed to 0.25 mM (~0.5 minimal alveolar concentration: MAC), 0.5 mM (~1.0 MAC), and 1.0 mM (~2.0 MAC) isoflurane. At the end of each experiment, samples of isoflurane-containing solution were collected from the chamber, and the concentrations of isoflurane were analyzed by gas chromatography (Shimadzu, Kyoto, Japan), which varied ± 10% of the reported value. The specific mitoKATP channels inhibitor, 5-hydroxydecanoic acid (5-HD, 200 μM; Sigma-Aldrich, St. Louis, MO) or an opener, diazoxide (100 μM; Sigma-Aldrich, St. Louis, MO) was added to the superfusing solution during oxidative stress to illuminate the effects of the mitoKATP channels on cell survival. Diazoxide was dissolved in DMSO and diluted with Tyrode solution. Therefore, the effect of DMSO on CPC survival was checked at the concentration used in the experiment. DMSO alone showed no effect on CPC survival. 5-HD and diazoxide were kept as stock solution in double-distilled water. All stock solutions were diluted to required concentration in the superfusing buffer immediately before application.
Statistical analysis
In all experimental groups, n indicates the number of CPCs plates. Results are presented as means ± SD. Each experimental group was composed of hESC-derived CPCs from at least five separate differentiations. PASW statistics 18 (SPSS inc., Chicago, IL) was used for the statistical analyses. Data were analyzed using one-way analysis of variance with Scheffé post hoc test. P values are from two-tailed tests. Differences were considered significant when the P value was less than 0.05.
Results
Differentiation and characterization of hESC-derived CPCs
The embryoid bodies replated onto Matrigel-coated plate expanded horizontally changing their shape into single-layered membrane. Five plates were used for confocal microscopic examination to identify CPCs derived from hESCs with early cardiac markers (Nkx2.5 and Isl-1) and determine differentiation efficiency (Fig. 2). The cells that stained with Nkx2.5 was very high (95 ± 3%) and consistent with the previous report.23 The cells positive for Isl-1 was 84 ± 6%.
Fig. 2.
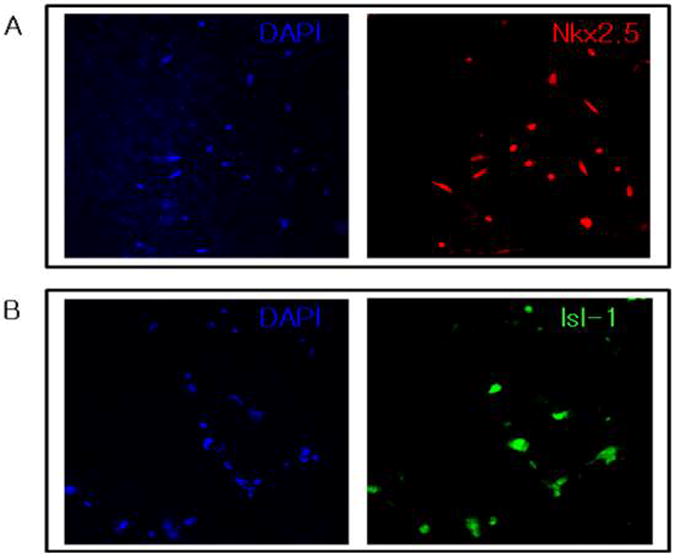
Cardiac differentiation of human embryonic stem cells by BMP4 treatment
Immunostaining with DAPI and anti-Nkx2.5 (A) or anti-Isl-1 (B) revealed a high efficiency of cardiac differentiation from human embryonic stem cells 2 weeks after BMP-4 treatment. Original magnification × 20.
Protective effects of isoflurane on hESC-derived CPCs under oxidative stress
Perfusion with glucose-free Tyrode solution throughout the experiment (70 min) did not significantly affect CPC death in the time control group (7.8 ± 2.6%, n = 5). To evaluate whether isoflurane can protect hESC-derived CPCs from oxidative stress-induced cell death, the preconditioned and non-preconditioned (control) CPCs were exposed to a mixture of FeSO4 and H2O2. At first, preconditioned CPCs with 0.5 mM isoflurane showed a significantly lower death rate compared with control, 30.6 ± 10.7% (n = 13) versus 43.2 ± 9.9% (n = 13), respectively (Fig. 3). These results are consistent with our recent results obtained in hESC-derived cardiomyocytes.16 Then, to evaluate the dose-response for isoflurane, CPCs were exposed to two additional concentrations of isoflurane (0.25 mM and 1.0 mM). Although CPCs death rate was not reduced after preconditioning with 0.25 mM isoflurane (38.3 ± 13.2%; n = 6), 1.0 mM isoflurane significantly decreased CPCs death rate (28.5 ± 6.2%; n = 6, Fig. 3). However, there was no difference of death rate between 0.5 mM and 1.0 mM isoflurane.
Fig. 3.
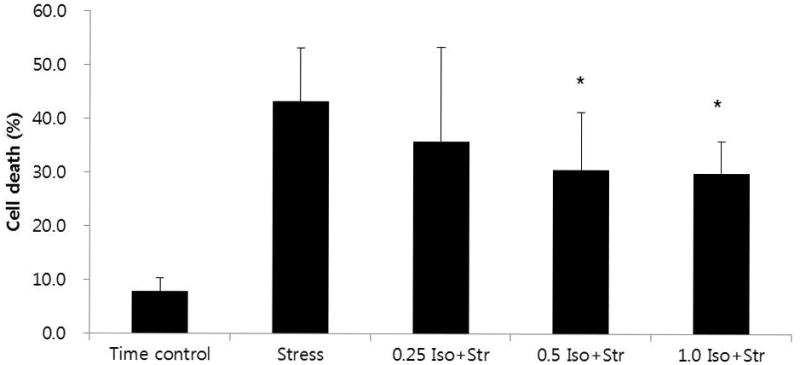
Death rate of hESC-derived cardiac progenitor cells during the course of experiment.
Isoflurane-induced preconditioning decreased death rate under oxidative stress.
* P < 0.05 versus stress. Other values were significantly different from time control.
0.25 Iso+Str: 0.25 mM isoflurane plus stress; 0.5 Iso+Str: 0.5 mM isoflurane plus stress; 1.0 Iso+Str: 1.0 mM isoflurane plus stress
Effects of 5-HD on isoflurane-induced CPC protection
To elucidate the role of mitoKATP channels in isoflurane-induced CPC protection, the selective mitoKATP channel inhibitor, 5-HD (200 μM) was used during the stress period. 5-HD applied throughout the experiment had no effect on CPC death in the time control group (9.6 ± 5.4%, n = 5). However, 5-HD completely abolished the protective effects of isoflurane, and cell death rate was 46.4 ± 10.4% (n = 8; Fig. 4). Addition of 5-HD did not potentiate the deleterious effects of oxidative stress, and CPC death in this group was 44.6 ± 6.6% (n = 8).
Fig. 4.
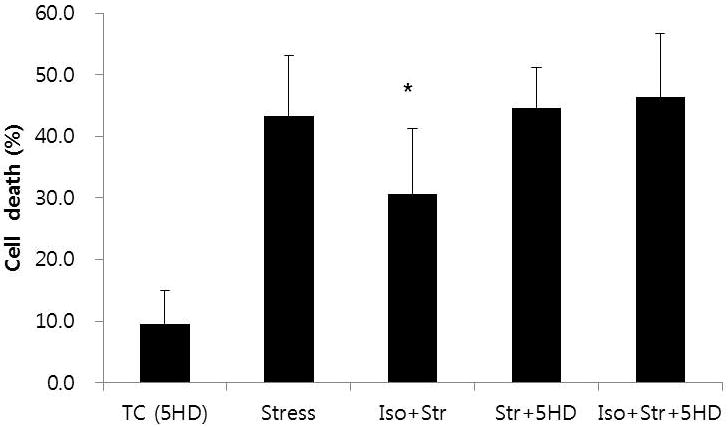
Effects of mitoKATP channel inhibition on isoflurane-induced preconditioning
Cardiac progenitor protection by isoflurane (Iso+Str vs Stress) was abolished in the presence of 5-hydroxydecanoic acid (Iso+Str+5HD).
* P < 0.05 versus Stress, Str+5HD, and Iso+Str+5HD. All values were significantly different from TC (5HD). TC (5HD): time control with 5-hydroxydecanoic acid; Iso+Str: isoflurane plus stress; Str+5HD: stress plus 5-hydroxydecanoic acid; Iso+Str+5HD: isoflurane plus stress plus 5-hydroxydecanoic acid
Effects of diazoxide on hESC-derived CPCs
Diazoxide (100 μM), an opener of mitoKATP channel did not have any effect on CPC death in the time control (9.2 ± 2.5%, n = 6; Fig. 5). However, diazoxide applied during the stress period significantly decreased CPC death (29.5 ± 12.4%, n = 8). When diazoxide was applied to CPC preconditioned with isoflurane, CPC death did not decrease further (28.7 ± 10.9%, n = 8). In addition, the CPC death rate among the Isoflurane plus stress, isoflurane plus stress plus diazoxide, and stress plus diazoxide group was not different. Therefore, it appears that there is no additive or synergistic interaction between isoflurane effect and diazoxide effect on CPC death, suggesting that they improve CPC survival rate through the same mechanism.
Fig. 5.
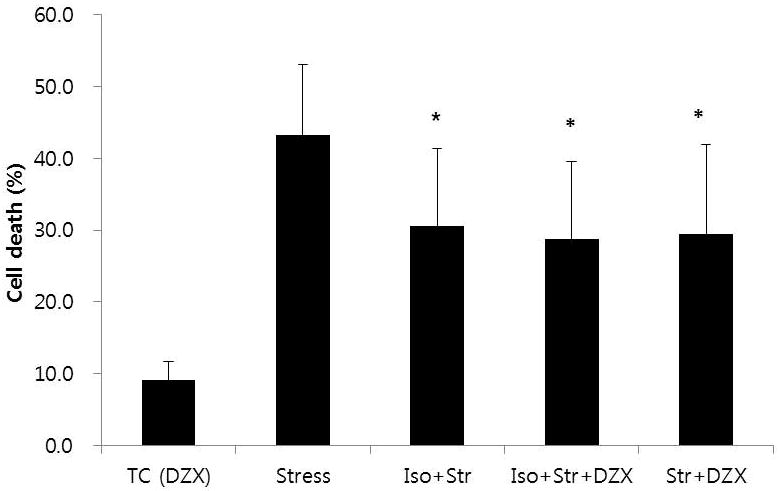
Effects of mitoKATP channel opening on oxidative stress and isoflurane-induced preconditioning
Diazoxide, a mitoKATP channel opener, significantly decreased cardiac progenitor death under oxidative stress (Str+DZX vs Stress). However, diazoxide applied to progenitor cells preconditioned with isoflurane, did not decrease death rate any further (Str+DZX vs Iso+Str+DZX).
* P < 0.05 versus Stress. All values were significantly different from TC (DZX). TC (DZX): time control with diazoxide; Iso+Str: isoflurane plus stress; Iso+Str+DZX: isoflurane plus stress plus diazoxide; Str+DZX: stress plus diazoxide
Discussion
The present study demonstrated that isoflurane preconditioning reduced hESC-derived CPC death rate under oxidative stress and suggested that mitoKATP channel might be involved in the protective mechanism.
One of the primary goals of the stem cell research may be transplantation. However, researches for hESC-derived cardiac cell engraftment have made limited progress because of inefficient cardiac differentiation, poor engraftment and low survival rate of engrafted cardiac cells within infarcted myocardium.2 Although a lot of interventions such as heat shock, allopurinol/uricase, anti-apoptotic proteins expression, and cardioprotective kinase over-expression 8, 25-27 have been tried to increase the engrafted cell survival, the trials only convinced us that single interventions had limited impact on engraftment of the cardiac cell. Recently, a cocktail containing multi-components was reported to improve survival of grafted hESC-derived cardiomyocyte and enhance function of the infarcted hearts.9 The prosurvival cocktail was composed of Matrigel, a cell-permeant peptide from Bcl-XL, cyclosporine A, KATP channel opener, IGF-1 and the caspase inhibitor ZVAD-fmk. Interestingly, most of the components are closely linked to the pathway which is considered to be involved in the cardiac protection by anaesthetic preconditioning (APC).15, 24, 28, 29 The report, therefore, strongly supports the potential protective effects of APC on the cardiomyocytes derived from hESCs. In this study, hESC-derived CPCs preconditioned with isoflurane (0.5 mM and 1.0 mM) showed significantly improved survival rate under oxidative stress. Although our study did not show a clear dose-dependent response of CPCs survival, these protective effects of isoflurane is consistent with some previous studies. 16, 30 Lucchinetti et al31 demonstrated that brief preconditioning with sevoflurane increased colony-forming capacity of stem cell-like endothelial progenitor cells also suggesting that volatile anaesthetics have protective effects on stem cells. Unlike the components of prosurvival cocktail, volatile anaesthetics such as isoflurane, desflurane and sevoflurane have been used in human approving their safety in clinical application.
Several groups have engrafted hESC-derived cardiomyocytes into infarcted rat myocardium.9,32 However, some recent studies suggest that early cardiomyocytes or cardiac progenitor cells could be a good engraft cell resource for the infarced myocardium. Fetal liver kinase (Flk1)+ or stage-specific embryonic antigen 1 (SSEA-1)+ cardiac progenitor cells showed both proliferative activity and considerable differentiation to cardiomyocytes after transplantation.18, 21 Considering that up to 5 × 106 hESC-derived cardiomyocytes are required for the infarcted rat myocardium,9 proliferative activity of the cardiac progenitor cells offer a strong advantage over fully differentiated cardiomyocytes. More recently, van Laake et al. demonstrated that induced pluripotent (iPS)- and ESC-derived Nkx2.5-GFP+ progenitor cells can be isolated and have unexpectedly high similar cardiac differentiation leading to a successful transplantation.22 In our study, CPCs 2-3 wks after differentiation revealed ~95% positive for Nkx2.5, an early cardiac differentiation marker. Therefore, our results suggest that isoflurane could contribute to making competent cardiac progenitor cells derived from hESC and IPS cells for engraftment into infarcted hearts.
There are two types of KATP channels in cardiomyocyte: the mitoKATP channels located in the inner mitochondrial membrane and the sarcolemmal KATP channels located in the plasma membrane. Both of them have been considered essential components of cardioprotection by APC with isoflurane.24 In this study, 5-HD, a selective mitoKATP channel inhibitor, completely abolished protective effects of isoflurane on the CPCs. This result suggests that the protective mechanism of isoflurane on the CPCs is also associated with mitoKATP channels, which is consistent with our previous results in cardiomyocytes 16, 24. Diazoxide has been known as a mitoKATP channel opener, with “selectivity” toward the mitoKATP channels and only weak sarcolemmal KATP channel activation at high doses.33 Recently, Sellitto et al. showed that diazoxide protects cardiomyocytes and provides volume homeostasis via sulfonylurea receptor1 (SUR1) suggesting a protective mechanism distinct from sarcolemmal KATP channel.34 Our present study demonstrated that diazoxide could reduce the CPCs death rate induced by oxidative stress and that additional isoflurane did not decrease it further. Therefore, the results with diazoxide indirectly support that isoflurane protects the CPCs via mitoKATP channel opening. In a previous study by Zaugg et al30, however, isoflurane potentiated the cardio-protective effect of diazoxide suggesting its role of priming mitoKATP channel. There is no definite explanation for the discrepancy but it might be attributed to their experimental model (rat ventricular myocytes vs hESC-derived CPCs), stress condition (simulated ischemia vs oxidative stress) and finally time of diazoxide application (15 min before ischemia vs during the stress).
Although the present study has demonstrated some findings relevant or similar to our recent study16 such as the protective effect of isoflurane on hESC-derived cardiomyocytes and the mechanism of protection, there are also several unique differences between them. While the recent study has turned the spotlight onto hESC-derived cardiomyocytes (beating cardiomyocytes) in the field of anaesthetic preconditioning, the present work highlights the immature hESC-derived cardiac progenitor cells which could be a better potential candidate for the cell source than mature cardiomyocytes in the cardiac cell engraftment. In addition, the present experiment focuses on testing the possibility or feasibility of isoflurane treatment for the CPC protection rather than specifying the mechanism of the anesthetic preconditioning on human cardiomyocytes. Finally, our recent study using hESC-derived cardiomyocytes was performed by micro-dissection of contracting cell clumps on the plates to select differentiated cardiomyocytes.16 The labeling of the cells with the MLC2v-enhanced green fluorescent protein proved a high level (~ 85%) of differentiation of cardiomyocytes from hESCs in the dissected clusters. This selection, however, was not applicable to hESC-derived CPCs which show no- or scarce movement. In the present study, a new but modified differentiation protocol was used.23 According to our modified protocol, we have applied 20 ng/ml of BMP-4 to embryoid body suspension-cultured with 20% FBS. The embryoid bodies replated onto Matrigel-coated plate expanded horizontally and differentiated into a very high level of ~ 95 ± 3% of cells positive for the early CPC marker Nkx2.5. This highly efficient differentiation protocol made it possible to perform the CPC survival study without further selection of differentiated CPCs. Moreover, single-layered CPCs were suitable for confocal microscopic examination to identify CPCs derived from hESCs.
A limitation of this study is the use of immature CPCs other than mature cardiomyocytes. As mentioned earlier, Nkx2.5 and Isl-1 used in this study are not specific markers of hESC-derived CPCs and undifferentiated or immature progenitor cells can develop into three germ layers forming teratomas after transplantation. A recent study, however, reported that ESC-derived CPCs positive for Nkx2.5-GFP successfully formed mature cardiomyocytes and survived on transplantation into the infarcted mouse heart without formation of teratomas.22 Another limitation is that this study was performed under oxidative stress which cannot fully mimic conditions after transplantation. However, oxidative stress is a widely used model for ischemia/reperfusion injury which might occurred after CPCs or cardiomyocytes engraft into ischemic or infarcted hearts.
In conclusion, our study demonstrated that isoflurane improved the survival of hESC-derived Nkx2.5+ CPCs under oxidative stress. This result suggests that APC could provide a potential method for decreasing the CPCs death rate after engraftment, thereby contributing to a successful regeneration of infarcted hearts.
Acknowledgments
Supported by grant (04-2008-007) from the SNUBH Research Fund and grant (SC1150) from Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea
References
- 1.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 2.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. 2006;577:467–78. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A. 2003;100:7808–11. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–71. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007 doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 10.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 11.Kehat I, Gepstein L. Human embryonic stem cells for myocardial regeneration. Heart Fail Rev. 2003;8:229–36. doi: 10.1023/a:1024709332039. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 13.Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–65. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–90. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 15.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007;21:212–9. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 16.Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–16. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 18.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagege A, Desnos M, Renaud JF, Menasche P, Puceat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–39. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 21.Baba S, Heike T, Yoshimoto M, Umeda K, Doi H, Iwasa T, Lin X, Matsuoka S, Komeda M, Nakahata T. Flk1(+) cardiac stem/progenitor cells derived from embryonic stem cells improve cardiac function in a dilated cardiomyopathy mouse model. Cardiovasc Res. 2007;76:119–31. doi: 10.1016/j.cardiores.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 22.van Laake LW, Qian L, Cheng P, Huang Y, Hsiao EC, Conklin BR, Srivastava D. Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ Res. 2010;107:340–7. doi: 10.1161/CIRCRESAHA.109.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takei S, Ichikawa H, Johkura K, Mogi A, No H, Yoshie S, Tomotsune D, Sasaki K. Bone morphogenetic protein-4 promotes induction of cardiomyocytes from human embryonic stem cells in serum-based embryoid body development. Am J Physiol Heart Circ Physiol. 2009;296:H1793–803. doi: 10.1152/ajpheart.01288.2008. [DOI] [PubMed] [Google Scholar]
- 24.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Kofidis T, Lebl DR, Swijnenburg RJ, Greeve JM, Klima U, Robbins RC. Allopurinol/uricase and ibuprofen enhance engraftment of cardiomyocyte-enriched human embryonic stem cells and improve cardiac function following myocardial injury. Eur J Cardiothorac Surg. 2006;29:50–5. doi: 10.1016/j.ejcts.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Kitabayashi K, Siltanen A, Patila T, Mahar MA, Tikkanen I, Koponen J, Ono M, Sawa Y, Kankuri E, Harjula A. Bcl-2 expression enhances myoblast sheet transplantation therapy for acute myocardial infarction. Cell Transplant. 2010;19:573–88. doi: 10.3727/096368909X486048. [DOI] [PubMed] [Google Scholar]
- 27.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–87. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–9. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–14. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lucchinetti E, Zeisberger SM, Baruscotti I, Wacker J, Feng J, Zaugg K, Dubey R, Zisch AH, Zaugg M. Stem cell-like human endothelial progenitors show enhanced colony-forming capacity after brief sevoflurane exposure: preconditioning of angiogenic cells by volatile anesthetics. Anesth Analg. 2009;109:1117–26. doi: 10.1213/ANE.0b013e3181b5a277. [DOI] [PubMed] [Google Scholar]
- 32.van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–10. doi: 10.1161/CIRCRESAHA.108.175505. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, Sato T, Seharaseyon J, Liu Y, Johns DC, O’Rourke B, Marban E. Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels. Mol Pharmacol. 1999;55:1000–5. [PubMed] [Google Scholar]
- 34.Sellitto AD, Maffit SK, Al-Dadah AS, Zhang H, Schuessler RB, Nichols CG, Lawton JS. Diazoxide maintenance of myocyte volume and contractility during stress: evidence for a non-sarcolemmal K(ATP) channel location. J Thorac Cardiovasc Surg. 2010;140:1153–9. doi: 10.1016/j.jtcvs.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


