Abstract
Accelerated cholesterol and lipid metabolism are the hallmarks of cancer and contribute to malignant transformation due to the obligatory requirement for cholesterol for the function of eukaryotic membranes. To build new membranes and maintain active signaling, cancer cells depend on high intensity of endogenous cholesterol biosynthesis and uptake of lipid particles. This metabolic dependency of cancer cells on cholesterol and other lipids is tightly regulated by the cholesterol homeostasis network including: 1) sterol response element binding proteins (SREBP), master transcriptional regulators of cholesterol and fatty acid pathway genes; 2) nuclear sterol receptors (liver X receptors, LXR) which coordinate growth with the availability of cholesterol; 3) lipid particle receptors such as LDL receptor providing exogenous sterols and lipids to cancer cells. In addition, activity of oncogenic receptors such as MUC1 or EGFR, accelerates sterols uptake and biosynthesis. Therefore, a general strategy of reducing the cholesterol pool in cancer cell is challenged by the highly efficient feedback loops compensating for a blockade at a single point in the cholesterol homeostatic network.
Besides the well-established structural role of cholesterol in membranes, recent studies uncovered potent biological activities of certain cholesterol metabolic precursors and its oxidized derivatives, oxysterols. The former, meiosis activating sterols, exert effects on trafficking and signaling of oncogenic epidermal growth factor receptor (EGFR). Cholesterol epoxides, the highly active products of cholesterol oxidation, are being neutralized by the distal sterol pathway enzymes, EBP and DHCR7. These recently discovered “moonlighting” activities of the cholesterol pathway genes and metabolites expand our understanding of the uniquely conserved roles these sterol molecules play in the regulation of cellular proliferation and in cancer.
Background
Synthesis of cholesterol and its intermediates
Cholesterol is a crucial component of cell membranes and its homeostasis is critical for normal cell functioning (1). Cholesterol biosynthesis is highly conserved in all the eukaryotes with a minimal difference between the end-products, human cholesterol and fungal ergosterol, arising at the level of zymosterol conversion (2). A series of elongation reactions of the non-aromatic fatty acid produces farnesyl pyrophosphate, which is converted to squalene – the first four-ring sterol precursor in the pathway (3). The pre-squalene steps of the cholesterol pathway produce isoprenoids, farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are critical for membrane anchoring of multiple signaling oncogenic proteins such as RAS (4), phosphoinositie-3-kinase (PI3K) (5) and AKT (6). Squalene epoxidase (SQLE) and lanosterol synthase (LSS) catalyze the conversion of squalene to a relatively inert sterol, lanosterol, which is highly abundant in skin appendages such as hair (from lanus, Latin, wool) (3). The subsequent steps produce a series of precursors possessing various biological activities. For instance, highly biologically active C4-methylated sterols are also known as meiosis-activating sterols (7) for their unique role in regulating the second division of meiosis in the gonads. The final product of the pathway, cholesterol, is subjected to a series of oxidative conversions in the molecule’s “tail” and the “B” ring, to produce bile acids, steroid hormones and vitamin D (8, 9). Metabolic arrest of the pre-squalene steps of cholesterol pathway during normal development is universally lethal in all eukaryotes due to the disruption of critical membrane-based signaling. Contrastingly, mutations in the distal cholesterol pathway genes are viable but produce severe developmental defects (10). Therapeutic trials of cholesterol supplementation have led to only modest improvements (11, 12), thus suggesting unique biological activities for the accumulating intermediate sterol metabolites specific for each genetic lesion.
Maintenance of high sterol levels in cancer cells
More than hundred years ago an association between lipid metabolism and tumor progression was first investigated by John Holden Webb, who suggested that cancer was due to crystallization of cholesterol from living cells (13). Since that time the involvement of lipid metabolism in tumorigenesis has been thoroughly investigated. Cholesterol composition of cellular membranes has been established as an essential metabolic requirement for cell divisions (14, 15) and it was shown that proliferating cells increase cholesterol uptake (16, 17). Cancer cells adapt to maintain high intracellular cholesterol through different mechanisms (Fig. 2) including accelerated endogenous production of cholesterol and fatty acids (18) regulated by the sterol response element binding proteins (SREBP) (19), or by reducing cholesterol efflux trough ATP-binding cassette (ABC) class A transporters such as ABCA1 reported in prostate cancer (20), or by increasing the uptake of low density lipid particles (LDL) (21). Deregulation of cholesterol homeostasis can be a powerful tool to suppress cancer growth and signaling of oncogenic receptors such as epidermal growth factor receptor (EGFR) family (16, 22, 23). Cholesterol promotes cancer signaling, in part, due to the assembly of cholesterol-rich membrane microdomains called lipid rafts (24) (Fig. 1). The overall dependency of rapidly dividing cells on lipid and sterol metabolism makes deregulation of these metabolic pathways a promising anti-cancer target. In this review, we will focus on the recent discoveries in the function of sterol metabolites and cholesterol pathway enzymes in cancer.
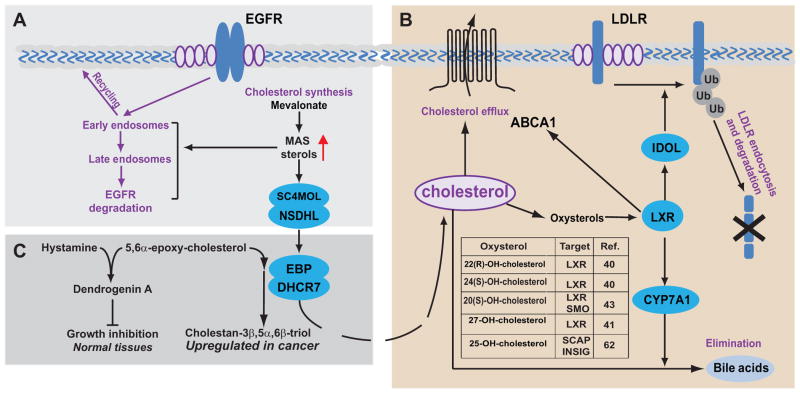
Figure 2. “Moonlighting” functions of cholesterol pathway enzymes and metabolites.
A. Depletion of the C4-demethylating enzymes (SC4MOL and NSDHL) results in accumulation of meiosis activating sterols (MAS) abundantly present in gonads. MAS influences endocytosis of surface receptors (e.g. EGFR) by promoting shuttling of the internalized EGFR to late endosomes for degradation which may be important for EGFR targeting in cancer and developmental defects in the hereditary cholesterol pathway gene deficiencies (23). B. Some endogenously generated oxysterols (e.g. 22-R-hydroxycholesterol, 25-hydroxycholesterol) bind to LXR/RXR heterodimeric nuclear receptors. LXR induces expression of ABCA1 and ABCG1 efflux pumps, and cholesterol conversion to bile acids via CYP7A1 (40, 62). LXR also promotes LDL receptor degradation via increased endocytosis and ubiquitin ligation by IDOL E3 ligase (50). C. A complex of EBP and DHCR7 known as ChEH, cholesterol epoxide hydrolase, disposes of toxic cholesterol epoxides and promotes growth of tumors cells. Contrastingly, in normal tissues, cholesterol epoxides react with histamine to produce dendrogenin A which promotes cell differentiation and suppresses cell growth (18, 26, 27).
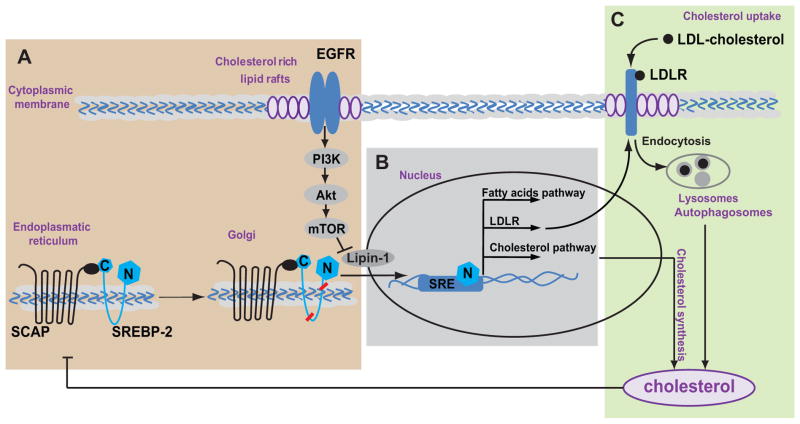
Figure 1. Cholesterol homeostasis regulation.
A. Activated EGFR and mTOR signaling promotes SREBP-SCAP complex to move to the Golgi where it undergoes cleavage to form an active nuclear SREBP fragment (N, blue diamonds). B. In the nucleus, the nuclear fragment of SREBP (N, blue diamonds) binds to the sterol regulatory element DNA sequences (56) and induces expression of fatty acid and cholesterol pathway genes and the LDL receptor (LDLR). C. Uptake of exogenous LDL cholesterol is via its binding to LDL receptor. LDLR internalization and degradation in lysosomes and autophagosomes releases free cholesterol (34). Under the conditions of excess, cholesterol acts as a negative regulator by binding to SCAP and blocking SREBP processing.
The endocytic matrix
As a major component of lipid rafts, cholesterol is the main critical component of lipid rafts activating multiple membrane-bound receptor signaling pathways (1, 24) (Fig. 1). Lipid rafts regulate endocytosis and catalytic activities of surface receptors, including signaling duration, receptor recycling and receptor stability (1). Any essential biological process involving non-diffusible protein complexes is dependent on highly coordinated budding and fusion of the cellular membranes, thus forming the so-called “endocytic matrix” within the cell (25). The function of the EGFR in the context of this “matrix” has been thoroughly investigated. Endocytosis is one of the major mechanisms of attenuation of EGFR (and other receptor tyrosine kinases) signaling as it permits the removal of receptor from the cell surface and its delivery to the sites of inactivating endoplasmic reticulum-based phosphatases (26, 27). Sigismund et al. revealed that EGFR signaling might be regulated by two different ways of internalization. They found that low EGF cell stimulation causes clathrin-mediated endocytosis (CME) predominantly, which promotes mostly recycling of the EGFR to the cell surface and this, in turn, allows for prolonged CME-dependent signaling. Stimulation with high EGF level activates non-clathrin endocytosis (NCE) that sends a sizeable pool of receptors to degradation thereby protecting the cell from overstimulation. Detailed kinetic analysis of the EGFR endocytosis and signaling showed marked dependency of NCE on the membrane cholesterol contents (28).
Participation of cholesterol metabolites in endocytic trafficking of signaling molecules has been discovered in our recent studies (23, 29): depletion of the C4-demethylating enzymes (SC4MOL and NSDHL) markedly sensitizes cancer cell lines and tumor xenografts to EGFR-targeting drugs via accelerated shuttling of the internalized EGFR endosomes towards late endosomes and lysosomes for degradation. The analysis of the highly evolutionary conserved (30, 31) interactions for these sterol pathway genes demonstrated significant enrichment for multiple components of the vesicular trafficking apparatus in the cell (23). Furthermore, genetic deficiency of NSDHL in heterozygous bare patches (Bpa1H/+) mice (32) consistently revealed a direct effect on EGFR signaling in keratinocytes. The skin areas expressing the mutant X-linked Bpa1H allele (NSDHL-null areas) showed a marked downregulation of the EGFR signaling coincident with reduced proliferation (23).
The regulation of receptor endocytosis via cholesterol homeostasis has been shown for multiple receptors, and thus may represent a general mechanism: e.g. melanocortin-4 receptor (MC4R) endocytosis to maintain MC4R responsiveness to its agonist α-MSH is highly sensitive to depletion of membrane cholesterol (33).
Membrane cholesterol concentration also regulates the extracellular LDL cholesterol uptake via binding with the LDL receptor (LDLR) and its following internalization. The “classical” way of LDLR internalization is based on clathrin-dependent endocytosis. Acidification of early endocytic vesicles liberates LDL from the receptor and allows the cargo to be delivered to lysosomes where the lipoprotein particle is degraded and cholesterol is salvaged for cellular use (34) (Fig. 1). The endocytosis of LDLR is tightly regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9), a serine endoproteinase which binds to the extracellular domain of LDLR and induces its internalization (35). Endocytosis of LDLR via the clathrin-mediated pathway is accelerated in cells with low intracellular sterol level. The uptake of LDL cholesterol is highly responsive to even small changes of cholesterol concentration in the ER membranes since deprivation of cholesterol leads to SREBP cleavage-activating protein (SCAP)-mediated induction of the SREBP transcription factors activation (36), which in turn regulates LDLR transcription (37).
Conversely, increased sterol concentrations activate the sterol-sensing nuclear receptors LXRα and LXRβ (Fig. 2). Activated LXRs, as heterodimers with retinoid X receptors (RXR), generally reduce intracellular cholesterol through the expression of cholesterol efflux proteins such as ATP-binding cassette (ABC) transporters ABCA1 and ABCG1, ADP-ribosylation factor-like 7 (ARL7) and apolipoprotein E (38). LXR also reduce uptake of the LDL cholesterol via the transcription of an E3 ubiquitin ligase, inducible degrader of LDLR (IDOL) (39). IDOL promotes internalization and degradation of LDLR in a clathrin-independent manner (34). An LXRα binding site in the proximal promoter region of the rat 7α-hydroxylase CYP7A also promotes the removal of cholesterol by increasing its conversion to bile acids (40). Under physiological conditions, oxysterols such as 22(R)-hydroxycholesterol, 24(S),25-epoxycholesterol and 24(S)-hydroxycholesterol, strongly induce LXRα and LXRβ transcriptional targets (40), but not in response to other sterols (lanosterol, desmosterol, steroid hormone precursors, testosterone, progesterone or bile acids) (41).
“Moonlighting” functions of cholesterol pathway
While cholesterol composition of the cellular membranes has been the focus of research, recent data highlight the unique biological properties of the sterol pathway metabolites. Among the signaling pathways that are critically dependent on the intact cholesterol biosynthesis, sonic hedgehog is arguably the best studied (42). Oncoprotein Smoothened (Smo), one of the principal signaling effectors of hedgehog signaling, contains a so-called sterol sensing domain capable of binding sterols and other lipid molecules. While the structural details of the sterol sensing domain interaction with individual sterol species remain unclear, certain naturally occurring products of cholesterol oxidation, oxysterols such as 20(S)-hydroxycholesterol, directly bind and activate SMO causing SMO translocation to the primary cilium and interaction with GLI (43).
Another example of sterol pathway metabolites involvement in cancer is meiosis activating sterols (MAS). MAS are the cholesterol pathway intermediaries downstream of lanosterol, and they play a unique physiological role to activate meiotic resumption in mouse oocytes in vitro (7). FF-MAS (4,4-dimethyl-5a-cholesta-8, 14, 24-triene-3b-ol) was extracted from human preovulatory follicular fluid and T-MAS (4,4-dimethyl-5a-cholest-8,24-diene-3b-ol) from the bull testicular tissue (44). In addition, MAS have been identified as natural ligands for liver X receptors (LXR) alpha and beta (41). We have recently reported that sterol C4-methyl oxidase-like (SC4MOL) and NADP-dependent steroid dehydrogenase-like (NSDHL), catalyzing oxidative demethylation of the MAS cholesterol precursors at C4 position, also regulate the endocytic traffic of EGFR in normal and cancer cells (23).
One step below SC4MOL and NSDHL, emopamyl binding protein (EBP) in a complex with dihydrocholesterol-7 reductase (DHCR7) catalyzes isomerization of the double-bond between C7 and C8 in the second cholesterol ring (45). Besides binding of different structural classes of ligands such as ring B oxysterols, there appears to be a new “moonlighting” function for this protein complex. As it turns out, this complex also mediates a previously enigmatic activity of cholesterol epoxide hydrolase (46). Furthermore, the biological activities of the cholesterol epoxides regulated by EBP/DHCR7 extend far beyond the canonical cholesterol. A naturally occurring steroidal alkaloid, 5alpha-Hydroxy-6beta-[2-(1H-imidazol-4-yl)ethylamino]cholestan-3beta-ol, otherwise known as dendrogenin A, is produced in normal, but not in cancer cells, via an enzymatic conjugation of 5,6alpha-epoxy-cholesterol and histamine (47, 48). This sterol conjugate has been shown to suppress cancer cell growth and to induce differentiation in vitro in various tumor cell lines of different types of cancers (48). It also inhibited tumor growth in melanoma xenografts studies in vivo and prolonged animal survival: 40% survivors at day 50 compared with none in the control (47). The activity of another sterol synthesizing enzyme, DHCR24, has been implicated in regulation of KRAS-induced senescence via deregulation of P53 (49, 50).
Clinical-Translational Advances
Opportunities for targeting cholesterol homeostasis in cancer
Dependency of cholesterol reveals a range of therapeutic opportunities to target cholesterol homeostasis in cancer.
SREBPs and LXR are considered as potent targets, since coordinated activities of these proteins control cellular proliferation (51). Effective and orally bioavailable synthetic LXR agonists such as GW3965 may offer an opportunity to antagonize the G1 to S cell cycle progression in cancer cells via the active form of Rb and deregulation of cyclin-dependent kinases as well as induction of the negative regulator p15 (52). Anti-proliferative effect of the LXR agonist T0901317 was demonstrated to suppress β-catenin transcriptional activity by direct interaction in colon cancer HCT116 cell line in vitro (53). Activation of LXR in vivo in the azoxymethane/dextran and ApcMin/+ animal models of colon cancer resulted in blocking cells at the G1 phase of the cell cycle and activating an apoptosis program, that led to the 70% reduction in tumor number and approximately 40% in tumor size (54).
Another possibility to target cholesterol homeostasis is based on the ability of cancer cells to develop mechanisms to accumulate cholesterol. For example, prostate cancer cells showed high expression of HMG-CoA reductase (HMGCR) but failed to express the major cholesterol exporter ABCA1 (55). Statins block the de novo synthesis of cholesterol at its rate-limiting step (16, 56). The efficacy of this approach however is limited by toxicities arising from the impairment of mitochondrial metabolism and of small GTPases (e.g. Rho and Ras) dependent on isoprenoids for membrane anchoring (56). Moreover, cancer cells can bypass the effects of statins by unrestrained cholesterol importation via the LDLR pathway (16) and increased expression of the pathway genes via the SREBPs (57).
To overcome such redundancy of cholesterol regulation, blocking multiple compensatory targets may potentially yield synthetic lethal interactions (29). In cancer cells, cholesterol uptake and endogenous production are stimulated by the oncogenic signaling. For example, glioblastoma multiforme, an aggressive form of brain tumor, a constitutively active mutated EGFR-variant III promotes LDLR expression and tumor growth (16). Such a dependency relationship between the EGFR pathway and the lipid biosynthesis is regulated via transcriptional activity of SREBP1 (16).
Another mTOR-dependent mechanism of lipid biosynthesis activation has been discovered recently involving lipin-1, a conserved negative regulator of nuclear translocation of SREBP1 (58). Lipin-1 is highly homologous to the C.elegans LPIN-1, and has been shown to regulate nuclear envelope and nuclear shape (58). Torin-1, an mTOR kinase inhibitor, has been effective in preventing lipin-1 phosphorylation by mTOR and SREBP1 nuclear translocation. Thus, mTOR kinase inhibitors may be effective in suppressing mTOR pathway-induced sterol and lipid biosynthesis in cancer. Finally, direct inhibitors of SREBP have been developed. One such compound, betulin, a natural pentacyclic triterpene, specifically inhibits SREBP proteolysis by facilitating the interaction of the SREBP cleavage activating protein (SCAP) and a negative SREBP regulator, INSIG. As the result, the cholesterol biosynthesis is inactivated both in vitro and in vivo (59).
Conclusions
In summary, high dependency of cancer cells on accelerated biogenesis and uptake of lipids and cholesterol lends itself as a therapeutic opportunity for metabolic targeting of cancer growth. Most previous attempts to target cholesterol metabolism in cancer have utilized HMGCR inhibitors, statins, or farnesyl transferase inhibitors acting at the level of non-aromatic steps of the cholesterol pathway. Multiple redundancies and feedback mechanisms highly abundant in the mammalian metabolic systems (60), often preclude successful pathway targeting at a single gene level. Instead, combinations of signaling inhibitors of the EGFR and/or mTOR pathways with a growing number of inhibitors of various regulatory molecules in the cholesterol homeostasis may prove to be the winning anti-cancer strategy. Emerging data on unprecedented biological activities of certain sterol metabolites (23, 47), and opportunities to activate LXRs (16), or direct inhibition of SREBPs (59, 61) may be fruitful strategies to win over cancer by cholesterol starvation.
Acknowledgments
Grant Support: This work was supported by NIH core grant CA-06927, by the Pew Charitable Fund, and by a generous gift from Mrs. Concetta Greenberg to Fox Chase Cancer Center. Some of the authors were supported by Tobacco Settlement funding from the State of Pennsylvania, National Institute of Health b’K22 CA160725, R21 CA164205 and a career development award from Genentech (IA).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicology and applied pharmacology. 2012;259:311–9. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids research. 2012;40:D109–14. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acimovic J, Rozman D. Steroidal triterpenes of cholesterol synthesis. Molecules. 2013;18:4002–17. doi: 10.3390/molecules18044002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janosi L, Li Z, Hancock JF, Gorfe AA. Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8097–102. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Molecular and cellular biology. 1996;16:4117–27. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis-Sobreiro M, Roue G, Moros A, Gajate C, de la Iglesia-Vicente J, Colomer D, et al. Lipid raft-mediated Akt signaling as a therapeutic target in mantle cell lymphoma. Blood cancer journal. 2013;3:e118. doi: 10.1038/bcj.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byskov AG, Andersen CY, Nordholm L, Thogersen H, Xia GL, Wassmann O, et al. Chemical-Structure of Sterols That Activate Oocyte Meiosis. Nature. 1995;374:559–62. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]
- 8.Javitt NB. Bile acid synthesis from cholesterol: regulatory and auxiliary pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1994;8:1308–11. doi: 10.1096/fasebj.8.15.8001744. [DOI] [PubMed] [Google Scholar]
- 9.Miller WL. Steroid hormone synthesis in mitochondria. Molecular and cellular endocrinology. 2013 doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. Journal of lipid research. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. American journal of medical genetics Part A. 2006;140:1511–8. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- 12.Tierney E, Conley SK, Goodwin H, Porter FD. Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith-Lemli-Opitz syndrome. American journal of medical genetics Part A. 2010;152A:91–5. doi: 10.1002/ajmg.a.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb J. CANCER, ITS NATURE AND ITS TREATMENT. The Lancet. 1901;158:976–8. [Google Scholar]
- 14.Martinez-Botas J, Suarez Y, Ferruelo AJ, Gomez-Coronado D, Lasuncion MA. Cholesterol starvation decreases p34(cdc2) kinase activity and arrests the cell cycle at G2. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1359–70. doi: 10.1096/fasebj.13.11.1359. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Botas J, Ferruelo AJ, Suarez Y, Fernandez C, Gomez-Coronado D, Lasuncion MA. Dose-dependent effects of lovastatin on cell cycle progression. Distinct requirement of cholesterol and non-sterol mevalonate derivatives. Biochimica et biophysica acta. 2001;1532:185–94. doi: 10.1016/s1388-1981(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 16.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer discovery. 2011;1:442–56. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundelin JP, Stahlman M, Lundqvist A, Levin M, Parini P, Johansson ME, et al. Increased expression of the very low-density lipoprotein receptor mediates lipid accumulation in clear-cell renal cell carcinoma. PloS one. 2012;7:e48694. doi: 10.1371/journal.pone.0048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5837–41. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Molecular cancer research : MCR. 2012;10:133–42. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, et al. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer research. 2013;73:1211–8. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudling MJ, Angelin B, Peterson CO, Collins VP. Low density lipoprotein receptor activity in human intracranial tumors and its relation to the cholesterol requirement. Cancer research. 1990;50:483–7. [PubMed] [Google Scholar]
- 22.Huang J, Das SK, Jha P, Al Zoughbi W, Schauer S, Claudel T, et al. The PPARalpha agonist fenofibrate suppresses B-cell lymphoma in mice by modulating lipid metabolism. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbalip.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukhanova A, Gorin A, Serebriiskii IG, Gabitova L, Zheng H, Restifo D, et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer discovery. 2013;3:96–111. doi: 10.1158/2159-8290.CD-12-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer research. 2002;62:2227–31. [PubMed] [Google Scholar]
- 25.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 26.Zwaenepoel K, Goris J, Erneux C, Parker PJ, Janssens V. Protein phosphatase 2A PR130/B”alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:538–47. doi: 10.1096/fj.09-140228. [DOI] [PubMed] [Google Scholar]
- 27.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 28.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Developmental cell. 2008;15:209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Astsaturov I, Ratushny V, Sukhanova A, Einarson MB, Bagnyukova T, Zhou Y, et al. Synthetic lethal screen of an EGFR-centered network to improve targeted therapies. Science signaling. 2010;3:ra67. doi: 10.1126/scisignal.2001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinci G, Xia X, Veitia RA. Preservation of genes involved in sterol metabolism in cholesterol auxotrophs: facts and hypotheses. PloS one. 2008;3:e2883. doi: 10.1371/journal.pone.0002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo C, Valachovic M, Randall SK, Nickels JT, Bard M. Protein-protein interactions among C-4 demethylation enzymes involved in yeast sterol biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9739–44. doi: 10.1073/pnas.112202799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XY, Dangel AW, Kelley RI, Zhao W, Denny P, Botcherby M, et al. The gene mutated in bare patches and striated mice encodes a novel 3beta-hydroxysteroid dehydrogenase. Nature genetics. 1999;22:182–7. doi: 10.1038/9700. [DOI] [PubMed] [Google Scholar]
- 33.McDaniel FK, Molden BM, Mohammad S, Baldini G, McPike L, Narducci P, et al. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to alpha-melanocyte-stimulating hormone (alpha-MSH) The Journal of biological chemistry. 2012;287:21873–90. doi: 10.1074/jbc.M112.346890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorrentino V, Nelson JK, Maspero E, Marques AR, Scheer L, Polo S, et al. The LXR-IDOL axis defines a clathrin, caveolae, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation. Journal of lipid research. 2013 doi: 10.1194/jlr.M037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbikay M, Mayne J, Chretien M. PCSK9, an enzyme turned escort protein: hepatic and extra hepatic functions. Journal of diabetes. 2013 doi: 10.1111/1753-0407.12064. [DOI] [PubMed] [Google Scholar]
- 36.Nohturfft A, Yabe D, Goldstein JL, Brown MS, Espenshade PJ. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102:315–23. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–97. [PubMed] [Google Scholar]
- 38.El Roz A, Bard JM, Huvelin JM, Nazih H. The anti-proliferative and pro-apoptotic effects of the trans9, trans11 conjugated linoleic acid isomer on MCF-7 breast cancer cells are associated with LXR activation. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:265–72. doi: 10.1016/j.plefa.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Scotti E, Calamai M, Goulbourne CN, Zhang L, Hong C, Lin RR, et al. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Molecular and cellular biology. 2013;33:1503–14. doi: 10.1128/MCB.01716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. The Journal of biological chemistry. 1997;272:3137–40. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 41.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 42.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nature genetics. 2003;33:508–13. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 43.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nature chemical biology. 2012;8:211–20. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byskov AG, Andersen CY, Leonardsen L, Baltsen M. Meiosis activating sterols (MAS) and fertility in mammals and man. The Journal of experimental zoology. 1999;285:237–42. [PubMed] [Google Scholar]
- 45.Herman GE, Kelley RI, Pureza V, Smith D, Kopacz K, Pitt J, et al. Characterization of mutations in 22 females with X-linked dominant chondrodysplasia punctata (Happle syndrome) Genet Med. 2002;4:434–8. doi: 10.1097/00125817-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 46.de Medina P, Paillasse MR, Segala G, Poirot M, Silvente-Poirot S. Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc Natl Acad Sci U S A. 2010;107:13520–5. doi: 10.1073/pnas.1002922107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Medina P, Paillasse MR, Segala G, Voisin M, Mhamdi L, Dalenc F, et al. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nature communications. 2013;4:1840. doi: 10.1038/ncomms2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Medina P, Paillasse MR, Payre B, Silvente-Poirot S, Poirot M. Synthesis of new alkylaminooxysterols with potent cell differentiating activities: identification of leads for the treatment of cancer and neurodegenerative diseases. Journal of medicinal chemistry. 2009;52:7765–77. doi: 10.1021/jm901063e. [DOI] [PubMed] [Google Scholar]
- 49.Salem NE, Saito M, Kasama Y, Ozawa M, Kawabata T, Harada S, et al. Genomic polymorphisms in 3beta-hydroxysterol Delta24-reductase promoter sequences. Microbiol Immunol. 2013;57:179–84. doi: 10.1111/1348-0421.12025. [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature. 2004;432:640–5. doi: 10.1038/nature03173. [DOI] [PubMed] [Google Scholar]
- 51.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vedin LL, Gustafsson JA, Steffensen KR. The oxysterol receptors LXRalpha and LXRbeta suppress proliferation in the colon. Molecular carcinogenesis. 2012 doi: 10.1002/mc.21924. [DOI] [PubMed] [Google Scholar]
- 53.Uno S, Endo K, Jeong Y, Kawana K, Miyachi H, Hashimoto Y, et al. Suppression of beta-catenin signaling by liver X receptor ligands. Biochemical pharmacology. 2009;77:186–95. doi: 10.1016/j.bcp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, Martelli N, et al. Liver x receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497–507. e13. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Murtola TJ, Syvala H, Pennanen P, Blauer M, Solakivi T, Ylikomi T, et al. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PloS one. 2012;7:e39445. doi: 10.1371/journal.pone.0039445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campia I, Lussiana C, Pescarmona G, Ghigo D, Bosia A, Riganti C. Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. British journal of pharmacology. 2009;158:1777–86. doi: 10.1111/j.1476-5381.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, et al. Dysregulation of the mevalonate pathway promotes transformation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15051–6. doi: 10.1073/pnas.0910258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–20. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell metabolism. 2011;13:44–56. doi: 10.1016/j.cmet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–52. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamisuki S, Mao Q, Abu-Elheiga L, Gu Z, Kugimiya A, Kwon Y, et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chemistry & biology. 2009;16:882–92. doi: 10.1016/j.chembiol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6511–8. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]