Abstract
Asthma is a complex disorder of the airways that is characterized by T helper type 2 (Th2) inflammation. The pleiotrophic cytokine TSLP has emerged as an important player involved in orchestrating the inflammation seen in asthma and other atopic diseases. Early research elucidated the role of TSLP on CD4+ T cells, and recent work has revealed the impact of TSLP on multiple cell types. Furthermore, TSLP plays an important role in the sequential progression of atopic dermatitis to asthma, clarifying the key role of TSLP in the pathogenesis of asthma, a finding with therapeutic implications.
Introduction
Asthma is a disorder of the airways that affects over 300 million people worldwide and is increasing in prevalence in developed countries [1]. Asthma is characterized by airway obstruction, excessive mucus production, and airway hyperresponsiveness (AHR). In allergic asthma, inflammation of the airways is a key component resulting in airway remodeling and damage [1]. Th2 immunity and Th2 cytokines, including IL-3, IL-4, IL-5, IL-9, IL-13, and GM-CSF, have been shown to play a major role in this disease, resulting in mast cell and eosinophil differentiation and maturation, basophil recruitment, and B cell isotype switching to IgE synthesis [1]. Recently, the cytokine thymic stromal lymphopoietin (TSLP) has emerged as an important factor in the pathogenesis of asthma, with higher concentrations of TSLP found in the lungs of asthmatics, correlating with increased Th2 responses and disease severity [2,3]. Further, multiple genome-wide association studies have identified TSLP as a susceptibility locus [4,5], and TSLP polymorphisms and single-nucleotide polymorphisms in the TSLP promoter in humans are associated with a higher risk of developing asthma [6-10], further strengthening data indicating a key role for TSLP the pathogenesis of this disease.
TSLP: Sources, Inducing Factors, and Mechanism of Signaling
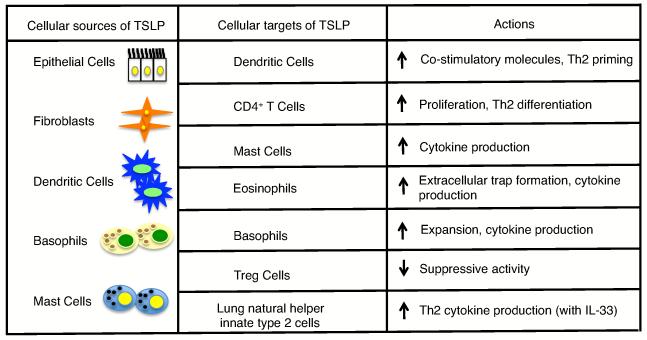
TSLP is a short-chain four α-helical bundle type I cytokine that was initially isolated from a thymic stromal cell line supernatant and was determined to be a factor that supported the maturation of B cells [11]. Initially, TSLP primarily was thought to be a survival and maturation factor for B and T cells, but it has now been shown to act on a broad range of cell types including those implicated in the development of lung inflammation and asthma, such as dendritic cells (DCs), CD4+ T cells, eosinophils, basophils, mast cells, innate type II cells (Figure 1), as well as on CD8+ T cells, B cells, natural killer T cells and smooth muscle cells [11-13]. TSLP is most highly produced by epithelial and stromal cells lining the barrier surfaces of the skin, gut, and lungs; however, it has recently also been shown to be produced by other cell types implicated in asthma, including dendritic cells, mast cells, and basophils (Figure 1), and there are reports of its expression by smooth muscle cells as well [13,14•]. Although TSLP is a member of the IL-2 family of cytokines and shares homology with IL-7, its receptor does not contain the common cytokine receptor γ cytokine chain that is common to the IL-2 family of cytokines, but instead the high affinity TSLP receptor is composed of the IL-7Rα chain paired with a TSLP-specific receptor component, TSLPR. Unlike IL-7 signaling which utilizes JAK1 and JAK3 to activate STAT proteins, upon TSLP binding to its high affinity receptor, TSLPR recruits JAK2 and IL-7Rα recruits JAK1, resulting in the activation of primarily STAT5A and STAT5B, as well as STAT1, STAT3, and other STAT proteins to a lesser extent, depending on the cell type [15•,16].
Figure 1.
Sources and targets (direct and indirect) of TSLP in asthma/lung inflammation, including the actions of TSLP on these targets.
Interestingly, TSLP can be induced by a variety of factors including the proinflammatory cytokines IL-1 and TNF-α in combination with other Th2 cytokines, commensals in the gut, bacterial and viral infections, Toll-like receptor or Nod2 signaling, and allergens [17]. NF-κB has been shown to be an important mechanism of TSLP activation, and there are NF-κB binding sites in the promoters of both the mouse and human TSLP gene [11]. In the context of lung responses TSLP has been shown to be induced by proteases that react with PAR-2 (including those found in common airborne fungal allergens) and by dsRNA produced by infections with viruses, such as Respiratory syncytial virus (RSV) and rhinovirus infection [1,11,18]. Further, recent studies have highlighted the fact that lung epithelial cells from asthmatics respond more strongly to dsRNA and produce more TSLP than cells from non-asthmatic patients [19,20]. This may help to explain why asthmatics tend to be more sensitive to lung viral infections than non-asthmatics [21]. In addition, uric acid is released in mouse lungs upon primary challenge with a common allergen, house dust mites, and is also found in the bronchoalveolar lavage fluid of asthmatic patients upon allergen challenge, resulting in an increase in TSLP and other Th2 cytokines in the lung. Thus, uric acid can also increase TSLP expression [22].
Recent studies have focused on not only understanding how TSLP influences CD4+ T cell Th2 immunity and disease progression but also have examined the broader role of TSLP in asthma pathogenesis by studying the action of TSLP on other cell types, including regulatory T cells and innate immune cells. Overall, these studies have elucidated the mechanism of TSLP action on asthma development and progression, by examining the interplay of multiple cell types. Moreover, they have provided a better understanding of the role of TSLP in the sequential progression of atopic diseases, a process termed the atopic march.
TSLP and Asthma
Early studies clearly established the importance of TSLP and Th2 cytokines in the development of asthma and other atopic diseases, such as the chronic inflammatory skin disease atopic dermatitis (AD). TSLP is highly expressed in human AD lesions [23], and overexpression of TSLP in mouse skin results in a spontaneous atopic dermatitis-like disease [24], whereas overexpression of TSLP in the lung, results in the development of severe airway inflammation and AHR [25]. Initially TSLP was shown to play a major role in pro-allergic responses by acting on dendritic cells (DCs). TSLP results in DC maturation and increased costimulatory expression, including upregulation of OX-40 ligand, thus allowing these DC to prime naïve CD4+ T cells to differentiate into proinflammatory Th2 cells expressing IL-4, IL-5, IL-13, and TNF-α[23,26]. The initial dogma was that TSLP worked indirectly on CD4+ T cells through TSLP-induced DC maturation and subsequent T cell priming; however, subsequently the direct actions of TSLP on CD4+ T cells were appreciated. Using the well-established ovalbumin (OVA) model of inhaled antigen-induced allergic inflammation of the lung, it has been shown that mice lacking TSLPR expression, or mice given a neutralizing Fc-TSLPR fusion protein, fail to develop allergic inflammation, in contrast to wildtype (TSLPR sufficient) mice [25,27]. Additionally, transfer of wildtype CD4 T cells into these TSLPR-deficient mice re-establishes OVA-induced inflammation, emphasizing the importance of the direct actions of TSLP on CD4+ T cells [27]. Recently, however, the direct action of TSLP on DCs has re-emerged as even more intriguing in light of a recent report showing that DCs themselves can produce TSLP upon TLR stimulation, suggesting that TSLP may be able to act in an autocrine manner on DCs to further amplify the Th2 response [14•].
Recent studies have increased our understanding of the broad impact of TSLP, highlighting the role of TSLP on multiple cell types including regulatory T (Treg) cells, innate immune cells such as basophils, eosinophils, mast cells, and the newly identified innate type 2 cells [11,28]. TSLP has been shown to induce natural Treg cell development in the thymus, likely in a manner redundant with IL-7 [29,30]. However, less is understood about the effect of TSLP on antigen-induced pulmonary Treg cell development and function. The depletion of Treg cells during the sensitization phase of allergen-induced lung inflammation results in an exacerbation of inflammation, indicating that Treg cells may decrease asthma pathogenesis [31]. Recent studies have clarified the interplay of TSLP and Treg cells, illustrating that TSLP from the BAL of asthmatic patients can suppress the ability of healthy control pulmonary Tregs to make IL-10 and exert their suppressive activity [32]. Moreover, even low amounts of TSLP can suppress the development of in vivo allergen-specific Treg cells in mouse lungs and in in vitro human CD4 T cell cultures [32,33]. Thus, TSLP may suppress the development of tolerance to allergens in the lungs, while enhancing pro-inflammatory Th2 responses.
In addition to its effects on adaptive immune cells and dendritic cells, TSLP can act on multiple innate immune cells that play a role in Th2 immunity and asthma pathogenesis [11]. Asthma is characterized by increased innate immune cells responses, as the prototypical Th2-type cytokines influence basophil recruitment (GM-CSF and IL-3), eosinophil maturation, and survival (GM-CSF, IL-3 and IL-5), and mast cell differentiation and maturation (IL-3, IL-9 and IL-13) [1]. In the last few years, it has also been shown that TSLP can promote basophil responses [34], and that during an allergen-induced Th2 response, basophils can function as antigen-presenting cells [34-36]. Furthermore, TSLP can act directly on bone marrow progenitors to induce basophil hematopoiesis in an IL-3 independent manner [37•]. Therefore, TSLP plays an important role in basophil responses and may aid in Th2 responses by recruiting basophils that can act as APCs upon allergen challenge; however, these effects have yet to be determined in the context of asthma. In addition, TSLP can act on eosinophil and mast cells to increase their Th2-type cytokine production [38,39]. Recent data indicate that TSLP may also enhance the formation of bactericidal extracellular traps consisting of cytotoxic granule proteins and DNA, termed eosinophil extracellular traps, however, the function of these traps in asthma is not yet well understood [40]. Together, these data show that TSLP can affect innate immune cells, which may play a role in amplifying Th2 inflammation.
In addition to adaptive and traditional innate immune cell responses, the newly characterized innate type 2 cells have been implicated in playing a role in Th2-like response in lung inflammation [28]. In the last few years, multiple non-T non-B cells have been identified in mice that participate in allergic conditions and have been generally characterized as innate type 2 cells. There appear to be several human counterparts to these cells, so these cells may play a role in human allergic diseases as well [28]. Although relatively little is known about the role of these cells in asthma, it is clear that one innate type 2 cell population, the nuocyte, is present during allergic lung inflammation and can induce airway hyper-responsiveness [41]; however, it is not yet known if TSLP plays a role in this process. Furthermore, another innate lymphocyte population called lung natural helper cells (LNH), can respond to IL-33 in combination with TSLP, IL-2, or IL-7 to produce IL-5 and IL-13, and LNH play a crucial role in protease allergen-induced inflammation [42•]. These data indicate that innate type 2 cells play a role in lung inflammation and that TSLP may be involved; therefore, it is important to determine the role of TSLP on these cells and their role in asthma.
TSLP and the Atopic March
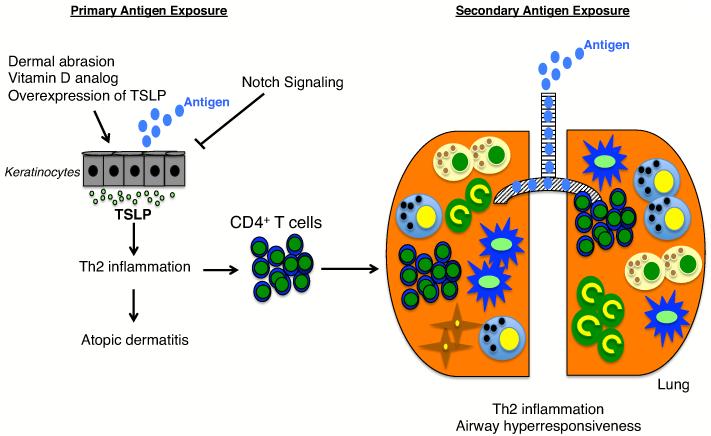
Persons with one atopic disease have an increased probability of acquiring other atopic diseases, and often this progression follows a sequential path from atopic dermatitis to asthma or rhinitis, with approximately half or two-thirds of all AD patients progressing to develop asthma or allergic rhinitis, respectively [43,44]. TSLP has been shown to be involved in both AD and asthma, and recent studies have focused on understanding the role of TSLP in the atopic march. Previous studies have shown that overexpression of TSLP in the skin, achieved either by topical application of a vitamin D analog (MC903) or by targeting Notch signaling in the skin in genetically altered mice, coupled to antigen sensitization results in airway hyper-responsiveness upon allergen challenge in the lung[18,45]. These data suggest that TSLP may play a role in the atopic march (see Figure 2). In these earlier studies the methods used resulted in high systemic levels of TSLP, which are not normally present in patients with atopic dermatitis, but after intradermal injection of TSLP and OVA antigen, which resulted in AD-like symptoms but no detectable systemic TSLP, intranasal challenge with OVA induced lung inflammation and AHR, confirming a role for TSLP in the atopic march [46•]. Memory CD4+ T cells derived from the skin sensitization phase were the key mediators of this TSLP-induced response [46•], consistent with the key role previously shown for CD4+ T cells in the development of OVA-induced allergic lung inflammation [27]and the identification of functional TSLP receptors on mouse and human CD4+ T cells [47]. Interestingly, allergic asthma was blocked by oral antigen exposure as long as the oral exposure preceded skin sensitization. These data indicate that allergic asthma can result even without TSLP being expressed in the lung or systemically, and that once sensitization to the antigen occurs in the presence of TSLP, oral tolerance to the antigen can no longer be achieved [46•]. Whereas previous studies commonly used OVA as an allergen, a recent study has proven that TSLP plays a role in atopic march to a common natural aeroallergen, the house dust mite [48]. A more physiological model of atopic dermatitis, tape-stripping, was also used to recreate the reduced barrier function of the skin caused by dermal abrasion, which is often seen with AD patients upon scratching. Tape-stripping combined with OVA antigen administration on the skin resulted in increased TSLP production by keratinocytes and these mice developed asthma-like symptoms upon intranasal challenge with OVA [49]. Finally, when IL-13 was overexpressed in the skin of mice to induce AD, upon allergen sensitization and challenge, these animals develop asthma-like manifestations characterized by increased mucus production, Th2 responses, and AHR via a TSLP dependent mechanism [50]. Together, these data show that TSLP plays an integral role in the atopic march.
Figure 2.
The role of TSLP in the progression of atopic dermatitis to asthma. TSLP is induced in keratinocytes by dermal abrasion (tape-stripping), application of a vitamin D analog, inhibition of Notch signaling, or by TSLP overexpression. Primary exposure of antigen to the skin results in Th2 inflammation, antigen-specific CD4+ T cell activation and atopic dermatitis. Upon secondary exposure to antigen in the lung (right side of panel), CD4+ T cells previously primed in the skin induce Th2 inflammation, resulting in airway hyperresponsiveness. Other inflammatory cells, including DCs, basophils, mast cells, and fibroblasts are shown (see symbol scheme shown in Figure 1).
Conclusion
TSLP is a pleiotropic cytokine that is expressed at barrier surfaces, such as the lung, and plays an important role in allergic inflammation and asthma. Recent studies have increased our understanding of what stimuli induce TSLP production and have helped elucidate how TSLP contributes to the development and progression of atopic diseases, such as asthma. Through its actions on both adaptive and innate immune cells, TSLP promotes and amplifies Th2 immunity, which can help shift the immune response to antigens/allergens away from tolerance towards that of a proinflammatory response. Furthermore, TSLP in the skin can drive atopic dermatitis upon allergen exposure and can promote asthma-like characteristics in mice upon inhaled (intranasal) allergen challenge, even without systemic levels of TSLP. These data highlight the important role of TSLP in atopic diseases and the atopic march. The recent appreciation of the importance of TSLP and other epithelial-derived cytokines, such as IL-33 and IL-25, in Th2 type responses have expanded the field by highlighting not only the role of adaptive immune cells, but also the contribution of stromal/epithelial cells and innate immune cells to Th2 responses in atopic diseases. Moreover, it appears that some of these epithelial-derived cytokines can cooperate and act on multiple cell types, as it is known that IL-25 can enhance the Th2 memory cell response initiated by TSLP-primed DCs, and that IL-33 and TSLP can act cooperatively on a subset of innate type 2 cells, lung natural helper (LNH) cells [42,51]. Thus, a better understanding of how these cytokines interact to help orchestrate allergic diseases is important. In addition, whereas innate type 2 cells have been shown to play an important role in Th2 responses to helminth infections, it is unknown how these cells contribute to asthma and other atopic diseases and whether TSLP may play a role, an area that warrants more research. Lastly, the role of microbes and viruses in lung inflammation and asthma is now better appreciated. Initially the lung was thought to be a relatively sterile environment, but over 2,000 bacterial genomes are found per square centimeter and there is evidence that there are differences in the microbial colonies of the airways of asthmatic patients compared to non-asthmatic patients [52]. This along with recent evidence that antibiotic treatment can exacerbate Th2 lung inflammation to house dust mite challenge [53••], indicate that commensal bacteria may play an important role in the lungs. In addition to bacterial stimuli, TSLP can also be induced by viral infection, such as rhinovirus infection, and rhinovirus infection early in life has been associated with increased risk of asthma later in life [1]. Elucidating whether there is a link between TSLP, rhinovirus, and asthma risk is important to better understand the initiation of asthma. Overall, while there are still many questions to answer, TSLP has proven to play an important role in the development of atopic diseases and in the atopic march, and provides a rational target for the therapy of allergic diseases and asthma.
Acknowledgements
We thank Dr. Rosanne Spolski, NHLBI, for critical comments. Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: W.J.L. is an inventor on patents related to TSLP.
References
- 1.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 2.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 3.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 4.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB, Baurley JW, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse north american populations. Nat Genet. 2011;43(9):887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, Fujita K, Miyatake A, Enomoto T, Miyagawa T, Adachi M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43(9):893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, Miyagawa T, Doi S, Kameda M, Fujita K, Miyatake A, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44(6):787–793. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuko H, Sakamoto T, Kaneko Y, Iijima H, Naito T, Noguchi E, Hirota T, Tamari M, Hizawa N. Lower FEV1 in non-COPD, nonasthmatic subjects: Association with smoking, annual decline in FEV1, total IgE levels, and TSLP genotypes. Int J Chron Obstruct Pulmon Dis. 2011;6:181–189. doi: 10.2147/COPD.S16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu M, Rogers L, Cheng Q, Shao Y, Fernandez-Beros ME, Hirschhorn JN, Lyon HN, Gajdos ZK, Vedantam S, Gregersen P, Seldin MF, et al. Genetic variants of TSLP and asthma in an admixed urban population. PLoS One. 2011;6(9):e25099. doi: 10.1371/journal.pone.0025099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunninghake GM, Soto-Quiros ME, Avila L, Kim HP, Lasky-Su J, Rafaels N, Ruczinski I, Beaty TH, Mathias RA, Barnes KC, Wilk JB, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. 2010;65(12):1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, Zamar D, Bosse Y, Kozyrskyj AL, James A, Laprise C, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H, Ziegler SF. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91(6):877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181(11):7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redhu NS, Gounni AS. Function and mechanisms of TLSP/TSLPR complex in asthma and COPD. Clin Exp Allergy. 2012;42(7):994–1005. doi: 10.1111/j.1365-2222.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 14•.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187(3):1207–1211. doi: 10.4049/jimmunol.1100355. •This is the first paper to show that both mouse and human dendritic cells are a source of TSLP and can produce TSLP upon Toll-like receptor signaling. TSLP is shown to be produced by dendritic cells in the house dust mite model of murine lung inflammation.
- 15•.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107(45):19455–19460. doi: 10.1073/pnas.1008271107. •This study demonstrates that counter to prior reports, TSLP signals via the JAK-STAT pathway, activating JAK1 and JAK2 to mediate STAT5 activation, providing the first example of a cytokine to mediate STAT5 activation via this combination of JAKs.
- 16.Lu N, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206(10):2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takai T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61(1):3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 18.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7(5):e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uller L, Leino M, Bedke N, Sammut D, Green B, Lau L, Howarth PH, Holgate ST, Davies DE. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010;65(7):626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 20.Brandelius A, Yudina Y, Calven J, Bjermer L, Andersson M, Persson C, Uller L. dsRNA-induced expression of thymic stromal lymphopoietin (TSLP) in asthmatic epithelial cells is inhibited by a small airway relaxant. Pulm Pharmacol Ther. 2011;24(1):59–66. doi: 10.1016/j.pupt.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125(6):1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34(4):527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 24.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202(4):541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6(10):1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202(6):829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hams E, Fallon PG. Innate type 2 cells and asthma. Curr Opin Pharmacol. 2012;12(4):503–509. doi: 10.1016/j.coph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Lee JY, Lim YM, Park MJ, Min SY, Cho ML, Sung YC, Park SH, Kim HY, Cho YG. Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4(+)CD8(-)CD25(-) naive cells in a dendritic cell-independent manner. Immunol Cell Biol. 2008;86(2):206–213. doi: 10.1038/sj.icb.7100127. [DOI] [PubMed] [Google Scholar]
- 30.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112(8):3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baru AM, Hartl A, Lahl K, Krishnaswamy JK, Fehrenbach H, Yildirim AO, Garn H, Renz H, Behrens GM, Sparwasser T. Selective depletion of Foxp3+ Treg during sensitization phase aggravates experimental allergic airway inflammation. Eur J Immunol. 2010;40(8):2259–2266. doi: 10.1002/eji.200939972. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: Association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin Immunol. 2010;6(1):4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei L, Zhang Y, Yao W, Kaplan MH, Zhou B. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186(4):2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(h)2 cytokine-dependent immunity. Nat Immunol. 2009;10(7):697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(h)2-IgE responses in vivo via IL-4 production and presentation of peptide-mhc class ii complexes to CD+ T cells. Nat Immunol. 2009;10(7):706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 36.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10(7):713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–233. doi: 10.1038/nature10329. •This work illustrates that TSLP plays an important role in the generation of basophils by an IL-3-independent mechanism. The basophils elicted by TSLP are functionally distinct from IL-3 elicited basophils, and they promote Th2 inflammation.
- 38.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43(3):305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 40.Morshed M, Yousefi S, Stockle C, Simon HU, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. 2012;67(9):1127–1137. doi: 10.1111/j.1398-9995.2012.02868.x. [DOI] [PubMed] [Google Scholar]
- 41.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129(1):191–198. doi: 10.1016/j.jaci.2011.09.041. e191-194. [DOI] [PubMed] [Google Scholar]
- 42•.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. •This study shows that lung natural helper cells (LNH) play an important role in protease-allergen induced airway inflammation. Furthermore, TSLP can act on LNH, as TSLP combined with IL-33 results in enhanced Th2 cyokine production by these cells.
- 43.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 44.Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. J Allergy Clin Immunol. 2000;106(5 Suppl):S258–263. doi: 10.1067/mai.2000.110159. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, Chambon P. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci U S A. 2009;106(5):1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, Comeau MR, Marshak-Rothstein A, Ziegler SF. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5(3):342–351. doi: 10.1038/mi.2012.14. • This study expands earlier studies (ref. 45) that demostrated an important role of TSLP in the atopic march, showing that expression of low levels of TSLP in the skin that are not detectable systemically can result in lung inflammation upon antigen challenge in the lung. CD4+ T cells were known to be critical for TSLP-mediated lung inflamation (ref. 27), and this study shows the importance of memory CD4+ T cells from the skin for lung inflammation and airway hyperresponsiveness.
- 47.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: Direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178(11):6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 48.Jiang H, Hener P, Li J, Li M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy. 2012;67(8):1078–1082. doi: 10.1111/j.1398-9995.2012.02857.x. [DOI] [PubMed] [Google Scholar]
- 49.Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.239. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Z, Oh MH, Yu J, Liu YJ, Zheng T. The role of TSLP in IL-13-induced atopic march. Sci Rep. 2011;1(23) doi: 10.1038/srep00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, et al. Il-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204(8):1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, Larosa DF, Renner ED, Orange JS, Bushman FD, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–546. doi: 10.1038/nm.2657. • The authors demonstrate that antibiotic treatment can exhacerbate Th2 lung inflammation to house dust mice challenge, thus, commensal bacteria may play an important role in regulating allergen-induced lung inflammation.