Abstract
Class B scavenger receptors, such as SR-BI/II or CD36, bind lipoproteins, but also mediate bacterial recognition and phagocytosis. In evaluating whether blocking receptors can prevent intracellular bacterial proliferation, phagocyte cytotoxicity and proinflammatory signaling in bacterial infection/sepsis, we found that SR-BI/II- or CD36-deficient phagocytes are characterized by a reduced intracellular bacterial survival, a lower cytokine response, and were protected from bacterial cytotoxicity in the presence of antibiotics. Mice deficient in either SR-BI/II or CD36 are protected from antibiotic-treated cecal ligation and puncture (CLP)-induced sepsis, with greatly increased peritoneal granulocytic phagocyte survival (8-fold), a drastic diminution in peritoneal bacteria counts and 50 to 70% reduction in systemic inflammation (serum levels of IL-6, TNF-α and IL-10) and organ damage relative to CLP in wild-type mice. CD36-deficient mice survival after CLP was 58% compared to 17% in control mice. When compensated for mineralocorticoid and glucocorticoid deficiency, SR-BI/II-deficient mice had almost a 50% survival rate vs. 5% in mineralo-/glucocorticoid-treated controls. Targeting SR-B receptors with L-37pA, a peptide that functions as an antagonist of SR-BI/II and CD36 receptors, also increased peritoneal granulocyte counts, reduced peritoneal bacteria and bacterium-induced cytokine secretion. In the CLP mouse sepsis model L-37pA improved survival from 6% to 27%, reduced multiple organ damage, and improved kidney function. These results demonstrate that the reduction of both SR-BI/II- and CD36-dependent bacterial invasion and inflammatory response in the presence of antibiotic treatment results in granulocyte survival, local bacterial containment, and also reduces systemic inflammation, organ damage and improve animal survival during severe infections.
Introduction
Sepsis is a combination of clinical syndromes and pathologic processes, including septic shock, disseminated intravascular coagulation, and endothelial barrier dysfunction, followed by multiorgan failure resulting from generalized infection. Sepsis, particularly bacterial, develops when a critical mass of bacteria overcomes local containment and enters the general circulation, leading to bacteremia and triggering excessive systemic inflammation, hypotension, intravascular coagulation, and endothelial dysfunction, which eventually leads to multiorgan failure and death. Despite considerable patient care advances in intensive care units, the mortality rate from sepsis is still increasing in critically ill patients (1–3). An incomplete understanding of sepsis pathophysiology has slowed the development of effective treatments that are needed to reduce the intractably high mortality rate(4). Several strategies for treating human sepsis based on targeting of various systemic pro-inflammatory mediators or systemic coagulation have failed in large multi-center clinical trials (5–7). Because the current sepsis treatment approaches remain limited to a combination of antibiotic intervention and supportive therapy, novel strategies are needed to improve sepsis therapeutics and outcomes.
Bacterial infection begins with limited local bacterial proliferation followed by a local inflammatory response and rapid granulocyte recruitment to the infected site (8–10). These initial events are critically dependent on pattern recognition receptors which mediate bacterial binding and downstream intracellular signaling in responsive cells, resulting in the secretion of cytokines and chemokines(10, 11). Highly expressed TLR, NOD, and RIG receptors on phagocytic cells make them primary sensors of infection and inflammation(11, 12). In contrast to TLRs, which mediate only bacteria-induced signaling leading to cytokine production, scavenger receptors play a dual role that includes both pathogen-induced signaling followed by cytokine secretion and bacterial phagocytosis/clearance (13–16). Recent data describes a critical role for CD36 and SR-BI/II, class B scavenger receptors which are also known as lipoprotein receptors(17–21). CD36 mediates bacterial adhesion, internalization and lysosomal sequestration (17, 22). Lysosomal sequestration of gram-positive bacteria in CD36-expressing cells facilitates lysosomal-integrated TLR2-dependent signaling and cross-talk (22). CD36 also has been reported to mediate a direct activation of Fyn/Lyn small GTP-binding proteins followed by the downstream activation of JNK, p38 and ERK1/2 kinases, independent of TLR signaling (17, 23–25). Similarly, we and others have demonstrated that SR-BI/II receptors mediate bacterial phagocytosis (21) and bacterium-induced secretion of cytokines(18, 23). Bacterial interaction with SR-BI/II can also lead to incomplete phagocytosis during which bacteria escape from the lysosomal compartment to cytosol. Lysosomal escape/incomplete phagocytosis protects bacteria from phagocytic destruction, but also shields them from systemic antibiotics and, hence, bacteria can proliferate (21). Our findings suggest that SR-B family receptors might accelerate sepsis because they promote inflammation and mediate incomplete phagocytosis. Evasion from systemic antibiotics due to cytosolic escape can allow unimpeded intracellular bacterial expansion and could potentially facilitate granulocyte cytotoxicity (17, 21) as well as systemic dissemination of bacteria. Genetic or pharmacological inhibition of SR-B receptors, therefore, might enhance containment of the local infection and reduce systemic inflammation.
We have proposed that the absence of SR-B receptors or targeting of SR-B receptors utilizing receptor ligands/antagonists can reduce downstream events following interactions between receptor(s) and entities recognized by SR-B such as pathogens, their cell components, or other proinflammatory factors/inflammogenes, including acute phase and heat-shock proteins(17, 18, 23). We and others have recently established that L-37pA, an amphipathic helical synthetic peptide, is a ligand for SR-BI/II (18, 26) and CD36 (17, 23) and a potent receptor antagonist(17). L-37pA blocks phagocytosis of bacteria and bacterium-induced cytokine secretion mediated by both SR-BI (18, 21) and human CD36(17, 23). Since L-37pA also reduces bacterium-stimulated immune cell activation, it can be potentially beneficial by limiting bacterial proliferation and systemic inflammation and reducing tissue damage in sepsis.
In this study, we analyzed the potential benefits of targeting SR-BI/II and CD36 in a mouse model of peritoneal sepsis induced by cecal ligation and puncture (CLP). The CLP model, especially when adequate antibiotic treatment and fluid resuscitation are used, replicates many of the hallmarks of severe septic shock, such as polymicrobial bacteremia, hypotension, and multiple organ failure, including acute kidney injury (27). We studied the effects of the absence (SR-BI/II- or CD36-knockout mice) and blockade (with L-37pA) of these receptors on sepsis survival, multiple organ damage, and inflammation. Our data indicate that SR-B absence or blockade increases animal survival by increasing local peritoneal granulocyte infiltration, limiting bacterial proliferation, therefore reducing tissue toxicity and systemic inflammation.
Material and methods
Animals
The National Institutes of Health (NIH) criteria for laboratory animal care were used in this study. CD36-knockout (KO) mice (C57BL/6 background) were kindly provided by Dr. Moore’s lab and grown in a colony at an NIH animal facility. SR-BI/II mice (C57BL/6 background) were obtained from Jackson Laboratories and a colony was established at NIH. To ensure the development of sepsis-induced acute kidney injury in the C57BL/6 background (27, 28), age-matched C57BL/6 (24–30 weeks old, NCI-DCT, Frederick, MD, USA) were used as controls for knockout mouse studies. For pharmacological studies, CD-1 mice (6–8 weeks old, NCI-DCT, Frederick, MD, USA) were used because they develop sepsis-acute kidney injury (AKI) at a younger age (29).
Surgery
The CLP procedure was described in detail previously (27, 28, 30–33). Briefly, the cecum at 12 mm in length was ligated and punctured through with a 21-gauge needle and gently squeezed to express 1 mm of fecal material, and then returned to the central abdominal cavity. In sham-operated animals, the cecum was just identified and replaced in the peritoneal cavity. Immediately after surgery, pre-warmed normal saline (30 ml/kg) was given intraperitoneally and subcutaneous antibiotics were administered (imipenem/cilastatin; 14 mg/kg in 1 mL/30 g mouse of normal saline at 6 h, and 7 mg/kg in 1 mL/30 g mouse of 2/3 normal saline at 18 h). Blood was collected by cardiac puncture for measurement of serum markers of organ injury and cytokine responses at 24 h after surgery. Kidneys were fixed in 10% neutral buffered formalin for histology.
L-37pA intervention study
The amphipathic helical synthetic peptide (18, 26), L- 37pA, (an SR-B receptor antagonist) or L3D-37pA (a non-helical peptide with same sequence but having three L to D substitutions) was injected intravenously at a dose of 10 mg/kg in 0.3 ml PBS. PBS was used as another control. The intervention was repeated at 6 h after surgery. To explore the dosing window for these peptides, L- 37pA at doses of 10 and 50 mg/kg were injected at 0 and 6 h after CLP, respectively.
Survival study
The animals were observed for survival every 6–12 h after surgery. Subcutaneous antibiotics and fluid (imipenem/cilastatin 14 mg/kg in 1 ml of normal saline) were administered at 6 h after surgery then repeated (imipenem/cilastatin 7 mg/kg in 1 ml of normal saline) every 12 h for 4 days. In the case of SR-BI/II KO mice, there is a substantial glucocorticoid and mineralocorticoid deficiency (Baranova et al 2011, in press); therefore, the animals received 2 × 10−5 M dexamethasone (for glucocorticoid replacement) and 2 × 10−5 M fludrocortisone acetate (for mineralocorticoid replacement) in drinking water starting at 24 h before surgery for the duration of the experiment. One set of control mice also received the same hormone-containing drinking water. Animals with extreme morbidity were euthanized.
Renal pathology
Tissue was fixed in 10% formalin and embedded in paraffin. 4 μm sections were cut and then stained with periodic acid-Schiff (PAS) reagent. Tubular damage was defined as tubular epithelial swelling, loss of brush border, vacuolar degeneration. The degree of kidney damage was estimated as previously described (30).
Blood chemistry and cytokine measurement
Blood urea nitrogen (BUN), aspartate transaminase (AST) and alanine transaminase (ALT) were measured using an autoanalyzer (Hitachi 917, Boehringer Mannheim, Indianapolis, IN, USA). Serum creatinine was measured by high-performance liquid chromatography (HPLC) as previously described (34). Serum TNF-α, IL-6 and IL-10 were determined by ELISA (R&D Systems, Minneapolis, MN, USA).
Peritoneal cell and bacteria proliferation analyses
Mice were anesthetized at 18 h for blood drawing and organ collection. Immediately after blood collection, peritoneal lavage was performed with 5 ml of sterile PBS using an 18-gauge needle (9). The lavage fluid was collected in sterile tubes and placed on ice. Peritoneal cells were counted and, after cytospin and Giemsa staining, the percentages of monocytes and PMNs were estimated. Bacterial counts were determined by plating peritoneal lavage on blood agar at various dilutions (21). Cell and bacterial counts were then calculated and expressed per mouse. Immunohistochemical staining of the peritoneal cells was also performed, as previously described, with anti-active caspase-3 antibody (Cell Signaling Technology, Beverly, MA), a marker of apoptosis. The number of apoptotic cells was examined in ten randomly chosen 400X fields, and expressed as the percentage of caspase-3 positive cells (30).
Cell culture
HEK 293 and HeLa cells were stably transfected to express human SR-BI, SR-BII or CD36 (20, 23). The cells were plated in cell culture treated plates (Costar) and cultured in DMEM containing 10% FBS and penicillin/streptomycin.
Bone marrow cells (BMC) were isolated from normal, SR-BI/II and CD36 knockout mice (17, 21). To produce granulocytic cells, isolated BMC were plated on various culture treated plates for real-time and dynamic monitoring of cell proliferation and viability of adherent cells (ACEA) 16-well plates (ACEA Biosciences, San Diego, CA) or 8-well immunochemistry slide chambers in RPMI-1640 containing 10% FBS and 10 ng/ml of mouse granulocyte-macrophage colony stimulating factor and cultured for at least for 5 days. About 90–95% of plate-adherent BM derived cells were GR-1 positive, as revealed by anti-Gr-1 immunostaining (data not shown).
Fluorescently-labeled bacteria and lysosomes
Live E. coli and Staphylococcus aureus were labeled with Alexa Fluor 488, using a protein labeling kit (Invitrogen), following the vendor’s instructions. All bacterial uptake and phagocytosis studies were performed by using DMEM containing 2 mg/ml BSA without antibiotics. HeLa cells were incubated with bacteria at ≈50 labeled bacteria per cultured cell. Culture plates were briefly centrifuged to accelerate bacterial sedimentation and the cells were incubated at 37°C for 1 h. After three washings with PBS to remove unattached bacteria, slides were incubated with fluorescent LysoTracker Red. After a 30-min incubation with trackers, slides were washed with PBS, fixed with 4% paraformaldehyde, and sealed or viewed immediately without fixation. Visualization was achieved by using Alexa Fluor 488/594-labeled anti-mouse/rabbit IgG secondary Abs (Invitrogen). To assess subcellular localization, images were obtained with a Zeiss 510 laser scanning confocal microscope, using a krypton-argon Omnichrome laser with excitation wavelengths of 488 and 568 nm for Alexa 488 and Alexa 568 labels, respectively.
Antibiotic Protection Assay
Cultured cells were incubated in DMEM containing 10% FCS, 100 ug/ml penicillin/streptomycin and K12 E. coli (approximately 10 bacteria per cell) in culture wells. After 1 h incubation at 37°C, plates were washed with PBS and lysed in H2O for 20 min on ice. Lysates were plated on blood agar dishes at various dilutions and incubated overnight at 37° C.
Granulocyte cytotoxicity/cytokine response assays
Cells were aliquoted into ACEA 16-well plates and allowed to sediment at room temperature for 30 min. The plates were then inserted into analyzing slots of the ACEA instrument (ACEA Biosciences, San Diego, CA). Cells were cultured for 24–96 h until reaching confluency (various genetically modified HEK 293 cells) or differentiating into granulocytic cells (BMC isolated from various mice). K12 E. coli were added at a concentration of approximately 10 bacteria/cell. Cell layer resistance was analyzed for the next 48–72 h. To establish the role of CD36 and SR-BI/II in pathogen/pathogen-associated product induced inflammation, various cells were incubated with bacteria, LPS or GroEL for 24 h followed by media harvesting. Conditioned media was used to measure IL-6 and IL-8 in mouse and human cells, respectively.
Statistical analyses
All the data are expressed as mean±SE. The analysis of variance (ANOVA) with a multiple comparison correction or t-test by Sigma-Stat program (Systat Software, Point Richmond, CA, USA) was used for detecting differences between groups. A P-value of < 0.05 was accepted as statistically significant.
Results
SR-BI/II and CD36 are important for bacterial phagocytosis, cytosolic invasion, granulocyte survival and cytokine secretion in vitro
SR-BI/II (CLA-1/2) and CD36 are an important part of host defense system, mediating bacterial recognition through bacterial binding and internalization and bacteria-induced inflammation (17, 21, 22, 35). Bacterial internalization is associated with both bacterial lysosomal sequestration (phagocytosis) and lysosomal escape (incomplete phagocytosis). Given that bacterial phagocytosis is important for bacterial killing and clearance, lysosomal escape can lead to cytosolic invasion, unchecked bacterial proliferation and cytotoxicity. To analyze the potential impact of class B scavenger receptors on bacterial phagocytosis and lysosomal escape, we first assessed uptake and co-localization of fluorescently labeled bacteria (green signal) with a specific lysosomal marker, LysotrackerRed (red signal) in HeLa cells stably transfected with human SR-BI (CLA-1), SR-BII (CLA-2) or human CD36. As seen in Fig. 1, CLA-1, CLA-2 or CD36 expression greatly increases bacterial uptake when compared to HeLa cells stably transfected with empty plasmid mock controls. In HeLa cells expressing each one of the three class B scavenger receptors, both E. coli and S. aureus were substantially internalized into lysosomes (yellow signal/co-localization). At the same time, a number of bacteria remained outside of the lysosomal compartment, which may reflect lysosomal escape and cytosolic proliferation.
Figure 1. Role of Scavenger Receptor Class B proteins in bacterial uptake and phagocytosis.

HeLa cells transfected with empty vector (Mock), CLA-1, CLA-2, or CD36 were incubated with Alexa 488-labeled K12 E. coli (upper panels) or with Staphylococcus aureus (lower panels) for 1 hour in DMEM containing 10% FCS; washed and further incubated in bacteria-free media in the presence of Lysotracker Red for 30 minutes. Live cultures were viewed using confocal microscopy to analyze phagocytosis of bacteria. Co-localization of Alexa 488-E. coli (green) and Lysotracker Red (red) is seen as yellow. The blue color shows DAPI stained nuclei. Examples of the most intense co-localization are within white circles. The white bars show 10 µm.
To assess the role of extra-lysosomal bacterial sequestration in bacterial proliferation and cytotoxicity, we analyzed intracellular bacterial counts in HeLa cells and granulocytes infected either with K12 E. coli or S. aureus in the presence of antibiotics (antibiotic protection assay). Importantly, when bacteria were incubated with HeLa cells overexpressing scavenger receptors (CLA-1, CLA-2, or CD36) or BMD granulocytes in the presence of antibiotics, the number of surviving bacteria was solely dependent on the rate of bacteria internalization and, consequently, intracellular escape from antibiotic killing and lysosomal degradation (21). In HeLa cells, the number of intracellular bacteria was greatly increased in SR-BI-, SR-BII-, or CD36-transfected HeLa cells (Fig. 2A). Granulocytes deficient in SR-BI/II and CD36 had 30% and 80% reductions in E. coli bacterial counts, respectively (Fig. 2B). When gram-positive bacteria were used, they preferentially required CD36 because CD36 KO granulocytes had 90% reduced bacterial survival (Fig. 2B). Since SR-B deficiency reduced the ability of bacteria to invade BMC-derived granulocytes while receptor overexpression increased, we analyzed whether the presence/absence of these receptors affects the survival of cells incubated with bacteria in the presence of antibiotics. As seen in Fig. 2C, HEK cells stably transfected to express either human SR-BI, SR-BII, or CD36 rapidly deteriorated when compared to mock-transfected HEK cells, which have low endogenous levels of these three receptors. In complementary experiments, scavenger class B receptor knockout granulocytes demonstrated improved viability after bacterial challenge, surviving 8 and 14 h longer with SR-BI/II- and CD36-deficient cells, respectively (Fig. 2D). These data indicate that SR-class B deficiency could be potentially beneficial in preventing sepsis and bacterial proliferation but only in the presence of antibiotics due to reduced incomplete phagocytosis, lysosomal escape, antibiotic evasion and, as a consequence, decreased bacterial cytotoxicity.
Figure 2. Role of SR-BI/II and CD36 in bacterial uptake, bacterial cytotoxicity, and pathogen-induced cytokine secretion.
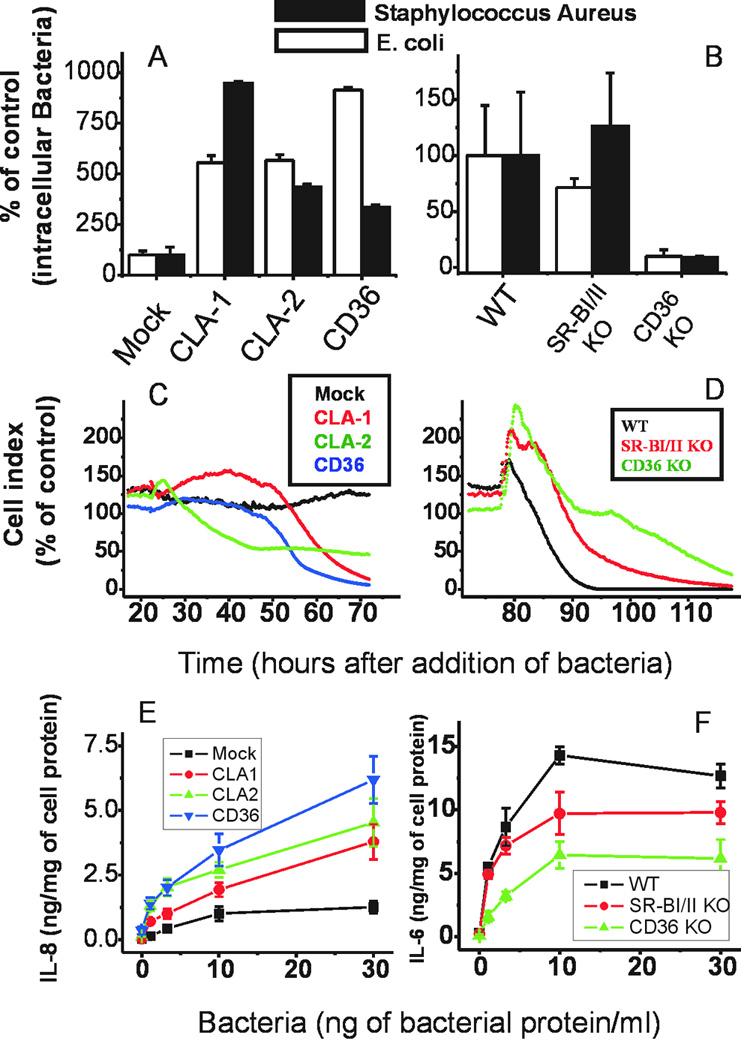
HeLa or HEK293 cells expressing CLA-1, CLA-2, CD36, or empty plasmid or bone marrow derived granulocytes isolated from control, SR-BI/II KO, and CD36 KO mice were incubated with various bacteria (K12 E. coli, white bars, A,B; Staphylococcus aureus, black bars A,B; or peritoneal polybacterial culture C–F) to analyze bacterial uptake utilizing an antibiotic protection assay in HeLa cells (panel A), granulocytes (panel B), E. coli-induced cell death utilizing ACEA in HEK cells (panel C), granulocytes (panel D); and peritoneal bacteria-induced IL-8 secretion in HEK 293 cells (panel E) and granulocytes (panel F). For panel E (IL-8 secretion) mice were subjected to CLP, peritoneal bacteria were isolated and expanded to provide a polybacterial culture, which was then added to transfected HEK 293 cells. After an 18-hour incubation of bacteria with HEK 293 cells or granulocytes, media was harvested and used for IL-6 and IL-8 ELISA evaluations. Colored codes are shown in the corresponding panels.
Another important function of bacterial recognition by SR-B is the receptor-mediated inflammatory response. Indeed, an essential hallmark of severe sepsis is the development of septic shock due to overwhelming systemic inflammation, where intervention could be beneficial. When we compared wild-type HEK 293 cells vs. HEK cells stably transfected with CLA-1 (SR-BI), CLA-2 (SR-BII), or CD36 scavenger receptors, peritoneal bacteria increased IL-8 secretion to varying degrees: CD36 > SR-BII> SR-BI > mock (Fig. 2E). BMC-derived granulocytes from SR-BI/II- and CD36-deficient mice produced 40% and 70% less IL-6, respectively (Fig. 2F). Receptors were overexpressed at relatively similar levels in HeLa cells, but in bone marrow differentiated macrophages/granulocytes (Mф) CD36 is expressed at higher levels than SR-BI and SR-BII (data not shown). This is consistent with our observations that the intracellular bacterial count (survival) was less than 30% in CD36 KO versus about 70–100% in SR-BI/II KO Mф. These data indicate that SR-B deficiency could be beneficial during severe infections due to a reduced pro-inflammatory activation of phagocytes, as well as lower intracellular bacterial survival in the presence of antibiotics.
SR-BI/II and CD36 are critically important in CLP sepsis
Despite reduced proinflammatory activation of phagocytes, the effect of SR-B receptor family deficiency in vivo was surprisingly opposite to what was expected. Without antibiotic treatment, intravenous bacterial inoculation of CD36-deficient mice revealed reduced gram positive bacterial clearance, resulting in severe bacteremia during first several hours (35). CLP-induced sepsis was also more severe in SR-BI/II-deficient than in normal mice (36–39).
These data point to an important role of these receptors in bacterial phagocytosis which is clearly deficient in SR-BI/II and CD36 KO mice. To assess the role of SR- class B receptors in sepsis and the potential for pharmacological intervention, we used antibiotic treatment as a part of the CLP model, as well to compensate for phagocytic deficiency in these mice. SR-BI/II KO mice are also characterized by defective lipoprotein metabolism causing adrenal insufficiency and impaired production of both adrenal steroid hormones (36–39). Corticosterone replacement was only partially effective at improving survival and reducing inflammation in SR-BI/II KO mice challenged with LPS or stressed by starvation (36, 39). Moreover corticosterone replacement alone was insufficient to improve SR-BI/II KO mice survival in CLP-induced sepsis(37). We have also found that aldosterone elevation, in response to CLP, LPS, or bacterial chaperonin 60, was also reduced in SR-BI/II KO mice (Baranova et al 2011, in press). Since fluid and antibiotic treatments represent first-line interventions in septic patients (6, 7), we reanalyzed the role of SR-BI/II and CD36 deficiency in the development of CLP-induced sepsis by utilizing fluid and antibacterial treatment in both SR-BI/II and CD36 KO mice with simultaneous correction of adrenal deficiency in SR-BI/II KO mice with combined glucocorticoid (GC) and mineralocorticoid (MC) replacement. The relative adrenal deficiency of SR-BI/II may mimic the situation with some older human septic patients who have compromised adrenal cholesterol reserve. Because CD36 KO mice have only minor impairment of platelet activation (40) but normal adrenal function these mice were not treated with GC/MC.
Granulocyte migration to the peritoneal cavity is an important host defense mechanism during infectious peritonitis. Higher peritoneal phagocyte counts are associated with reduced peritoneal bacterial accumulation and positively correlated with survival rate in CLP-septic mice. Since cultured SR-B KO granulocytes were protected from bacterial toxicity (Fig. 2D), we first analyzed peritoneal lavage in mice at 18 h after the CLP procedure. When compared to normal mice, the total number of peritoneal cells increased 3-fold in both SR-BI/II and CD36 KO animals (Fig. 3A). Both KO mice strains had no statistically significant changes in peritoneal monocyte/macrophage cell counts (Fig. 3C), but demonstrated greatly increased number of granulocytes/PMN (Fig. 3B). The 4–6 fold increase (p<0.05) in granulocyte/PMN accumulation was similar to data demonstrating neutrophil accumulation and improved bacterial containment in TLR9 deficient mice (10). Increased PMN accumulation inversely correlated with drastic reduction of peritoneal bacteria in both SR-BI/II and CD36 KO mice relative to CLP-treated C57BL/6 control (Fig. 3D). Representative cytospin slides (Fig. 3E) show that 70–80% of peritoneal cells in SR-BI/II- and CD36-deficient mice after CLP are mostly granulocytes/PMN, with a small number of monocyte/macrophage cells. Cytospin preparations of SR-BI/II and CD36 KO lavage cells had fewer intracellular and extracellular bacteria in peritoneal lavage samples (Fig. 3E, right). Without CLP, both wild-type and KO mice demonstrated a similar cellular composition of peritoneal cells (Fig. 3E, left panels).
Figure 3. Effect of SR-BI/II and CD36 deficiency on peritoneal fluid composition.

Peritoneal lavage was collected to count the total number of peritoneal cells (A), granulocytes/PMN (B), monocyte/macrophage cells (C) and bacteria (D) per mouse (n = 6–15) 24 h after CLP. Panel E represents typical cytospin slides stained for cell composition analyses.
These data demonstrate that SR-B deficient granulocyte/PMN cells were protected in CLP-sepsis treated with antibiotics. Since SR-B deficiency caused a 20–50 fold reduction in both intracellular and extracellular bacteria in peritoneal lavage, PMN protection likely resulted from reduced cellular toxicity and necrosis than attenuated apoptosis. The number of apoptotic cells was unchanged in peritoneal lavage cells from septic CD36 mice and was slightly increased in SR-BI/II KO mice (Supplemental Fig. 1); however more definitive experiments would be needed to eliminate this possibility. In agreement with earlier data (37), when animals were not treated with antibiotics, most SR-B KO animals did not survive 18 hrs after infection. Surviving animals demonstrated a 2–3-fold higher peritoneal bacteria count compared to CLP-treated controls (data not shown).
CLP development and progression were further analyzed by assessing systemic inflammation, organ damage, and animal survival. As seen in Fig. 4–6, sepsis in both SR-BI/II and CD36 KO was less severe than in control mice. SR-BI/II KO animals received dexamethasone (a synthetic glucocorticoid) and fludrocortisone (a glucocorticoid with predominantly mineralocorticoid activity) replacement therapy. The level of systemic inflammation, as determined by plasma IL-6 and TNF-alpha levels, was reduced by up to two-thirds in both SR-BI/II and CD36 KO animals when compared to C57BL/6 controls (Fig. 4). Total plasma cholesterol and triacylglycerol were not changed in all three mice after CLP procedure (Fig. 5 A, B). Organ damage/dysfunction was reduced and characterized by the lower levels of bilirubin, LDH, AST, ALT, and amylase (Fig. 5 D, E–H). The level of serum creatinine was half that of control animals indicating better maintained kidney function (Fig 5C). The better maintained kidney function was further substantiated by kidney histology analysis (Fig. 5I, Supplemental Fig. 2). These data indicate that SR-B deficiency is beneficial in sepsis under conditions when animals are treated with fluids (to compensate for systemic hypotension), antibiotics (to compensate for diminished phagocytosis) and mineralocorticoids/glucocorticoids (to compensate for adrenal insufficiency).
Figure 4. Systemic inflammation reduced in septic scavenger receptor-deficient mice (CD-36 and SR-BI/II deficiency).
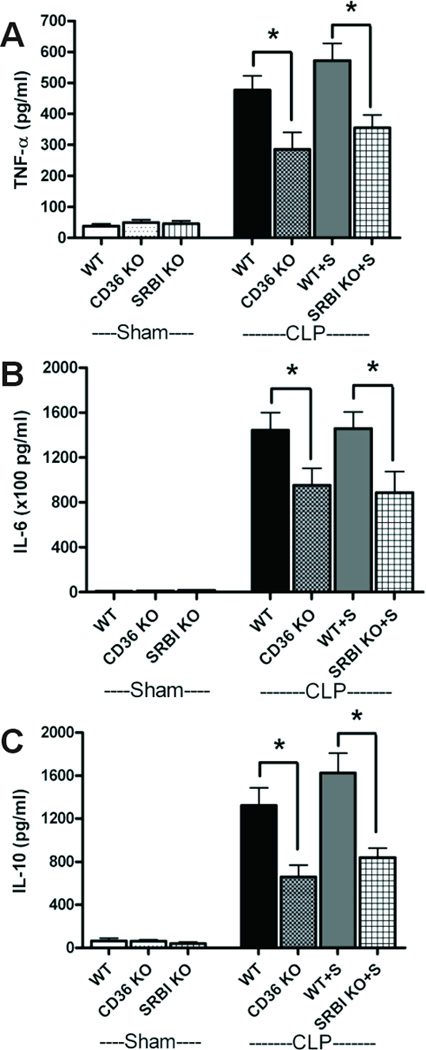
Serum pro-inflammatory cytokines (TNF-α, IL-6) (A, B) and the anti-inflammatory cytokine (IL-10) (C) were determined at 24 h after surgery in sham (n =4), WT CLP (n=7), CD36-deficient mice CLP (n=9), WT CLP with steroid supplement (n=8) and SR-BI/II-deficient mice CLP with steroid supplement (n=8). * P<0.05
Figure 6. Scavenger receptor deficiency reduces mortality from sepsis.
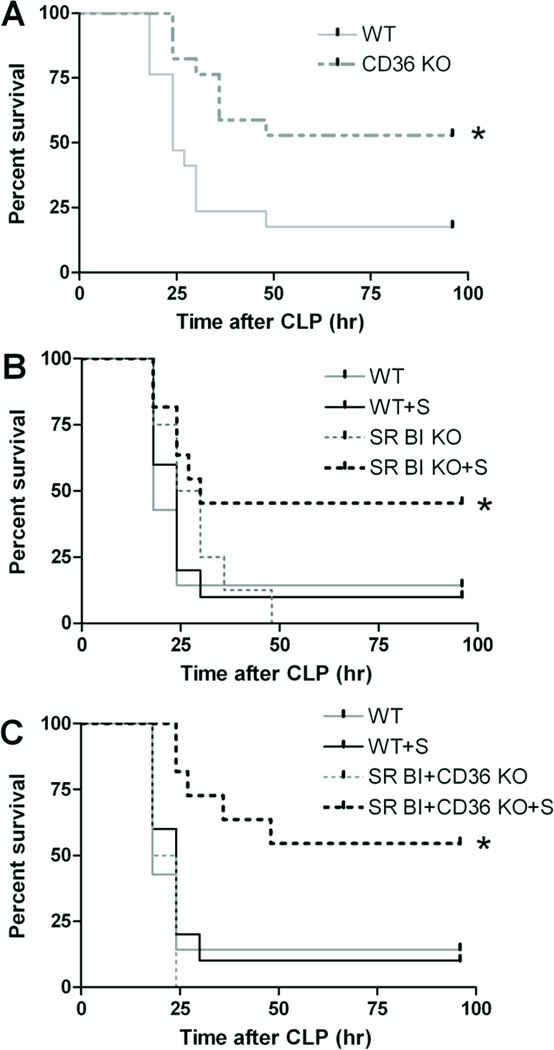
Survival analysis of age- and background-matched WT (n = 17) vs. CD36 KO (n = 17) mice (A); WT (n = 7), WT with GC/MC supplementation (n= 10), SR-BI/II KO (n = 8), and SR-BI/II KO with GC/MC supplementation (n = 9) mice (B); WT (n = 7), WT with GC/MC supplementation (n= 10), CD36- SR-BI/II DKO (n = 4), and CD36- SR-BI/II DKO with GC/MC supplementation (n = 11) mice (C).
Figure 5. Sepsis severity is attenuated in scavenger receptor-deficient mice (CD-36 and SR-BI/II deficiency).

Serum hyperlipidemia (Cholesterol, A; Triglyceride, B), kidney injury (Scr, C; BUN, D), LDH (E), liver damage (ALT, F; AST, G), pancreatic injury (amylase, H), and semiquantitative kidney injury score (I) at 24 h after CLP surgery in sham (n =4–5/ group), WT (n=8), CD36-deficient (n=19), WT with steroid supplement (n = 4) and SR-BI/II-deficient mice with steroid supplement (n = 9). * P<0.05.
Survival at 96 h after CLP (Fig. 6) was improved in both CD36 KO (Fig. 6A) and steroid hormone-compensated SR-BI/II (Fig. 6B) mice (55 and 48%, respectively) when compared to control C57BL/6 mice with (5%) or without steroid hormone (17%). Because CD36 KO mice do not develop adrenal deficiency, neither they nor their C57BL/6 controls were treated with adrenal hormones. In the absence of steroids, SR-BI/II KO did not survive CLP sepsis (Fig. 6B). When we analyzed SR-BI/II / CD36 double KO, mice, we have found that these mice do not survive without steroids, but when GC/MC deficiency was compensated by steroid supplementation, the double KO mice had a survival rate similar to CD36 KO, although slightly attenuated.
L-37pA treatment increases peritoneal granulocyte accumulation and reduces bacterial proliferation in CLP septic mice
Amphipathic helical peptides (AHP) can reduce inflammatory activity in vitro (41), as well as systemic inflammation and lethality in endotoxin-induced septic shock in mice (42), and CLP-induced sepsis in rats (43). The effect of AHP was suggested to be a result of LPS binding to AHP, leading to LPS neutralization (41). We and others have also demonstrated that AHP, such as L-37pA, are strong antagonists/ligands for both CD36 and SR-BI/II (17, 18) and can block bacterial uptake and inflammatory activity in vitro (17, 18, 21). Because SR-B receptor deficiency was associated with reduced bacterial cytotoxicity, systemic inflammation and decrease in mortality, we investigated whether L-37pA can improve peritoneal PMN cell survival, reduce peritoneal bacteria and attenuate CLP-induced sepsis in mice (27, 29). To study these pharmacological effects of L-37pA, 6–8 week old CD-1 mice, which develop acute kidney injury due to CLP-induced sepsis at a much earlier time than C57BL/6 mice, were used. Intravenous administration of L-37pA (10 mg/kg) immediately and 6 h after CLP surgery attenuated sepsis severity in 6–8 week-old CD-1 mice. All mice were additionally treated with fluid and antibiotics to simulate the central strategy for treating critically ill septic patients (4). As negative controls, mice received either PBS or the non-helical L3D-L37pA peptide, which has three L- to D-amino acid substitutions (18) and does not bind to SR-BI/II and CD36.
Peritoneal lavage from PBS IV injected septic mice at 18 h contained 7.6 × 106 cells/mice, mostly PMN and monocyte/macrophage cells with a number of internalized bacteria (Fig. 7A). Although the total number of cells was only slightly higher than the 5 × 106 cells seen in non-septic (sham control) mice, there was a 10-fold increase of peritoneal granulocytes (Fig. 7B), similar to previously reported results (9, 10). In L-37pA-treated mice there was further 2-fold increase in peritoneal cell count, which was predominantly associated with a 2.5-fold increase in granulocyte/PMN cell count when compared to PBS-treated mice (Fig. 7B). A number of monocyte/macrophage cells remained similar to PBS-treated septic controls (Fig. 7C). Despite the absence of statistically significant overall changes of peritoneal bacteria in L-37pA-treated versus PBS-treated mice, about 25% of L-37pA-treated mice demonstrated a 95% reduction of bacteria in peritoneal fluids (Fig. 7D). These data indicate that L-37pA increased the number of granulocytes accumulating in the peritoneum similar to the effect of SR-B deficiency seen in Fig. 3.
Figure 7. Effect of L-37pA on peritoneal fluid composition.
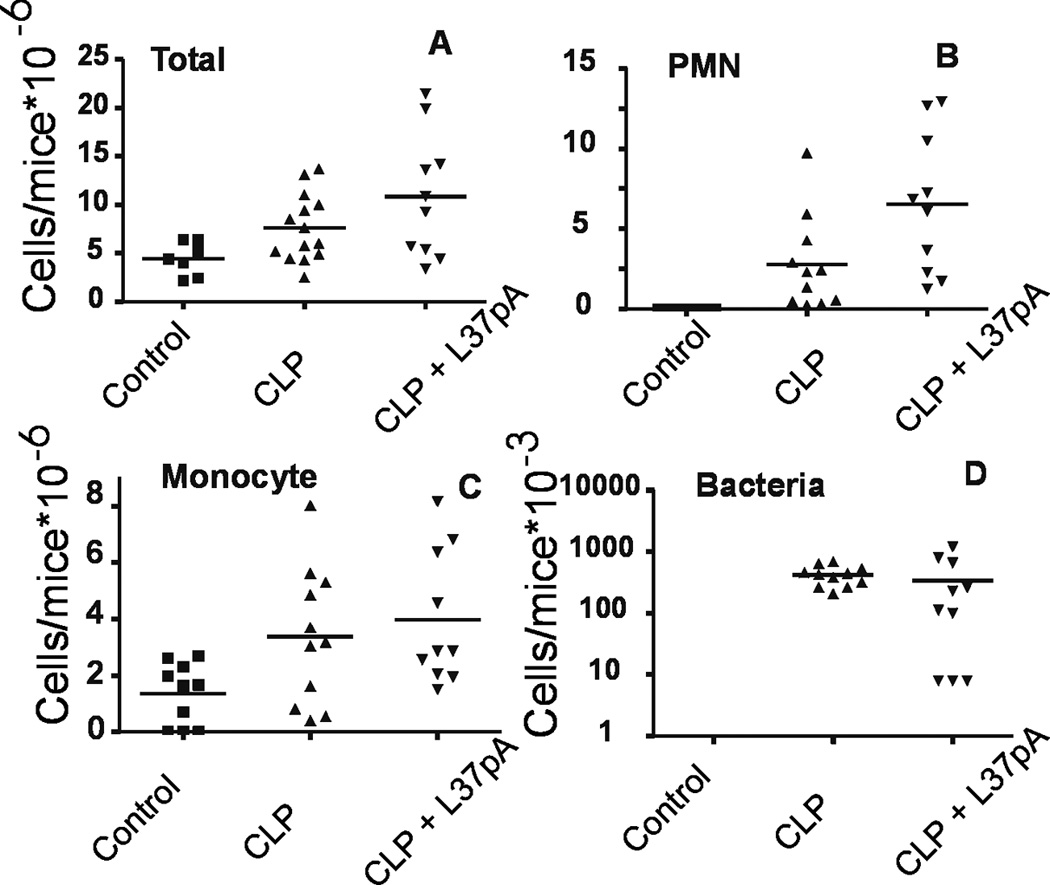
Peritoneal lavage was collected to count the number of total peritoneal cells (A), granulocytes/PMN (B), monocyte/macrophage cells (C) and bacteria (D) in 7–15 animals undergoing CLP at 24 h.
L-37pA inhibits systemic inflammation, reduces organ damage and improves survival in murine CLP-induced sepsis
In L-37pA-treated mice, IL-6, IL-10 and TNF-alpha levels were significantly reduced (Fig. 8) when compared with PBS and L3D-37pA-injected animals. Lower pro- and anti-inflammatory cytokine levels indicate improved control of infection in L-37pA-treated animals. The survival rate at 96 h after CLP increased 4-fold from 6% in L3D-37pA or PBS-treated mice to 27% in L-37pA-treated animals (Fig. 9A). A lower dose of L-37pA (10 mg/kg) did not affect plasma cholesterol and triacylglycerol levels whereas a higher dose of 50 mg/ml, led to hypertriglyceridemia and slight hypercholesterolemia (Fig. 9B–C). These effects of L-37pA are similar to the effects of D-4F peptide, which has also been demonstrated to increase rat survival during sepsis by two-fold (43). The increased animal survival with L-37pA treatment was associated with significantly reduced CLP-induced multi-organ damage (improved serum creatinine, BUN, AST, ALT, amylase, LDH) (Fig. 9, D–I) and improved kidney histology (Fig. 9J, Supplemental Fig. 3). These data indicate that the final stages of sepsis such as decreased organ function and increased organ damage were significantly attenuated in mice treated with L-37pA.
Figure 8. L-37pA inhibits inflammatory cytokine response in murine sepsis model.
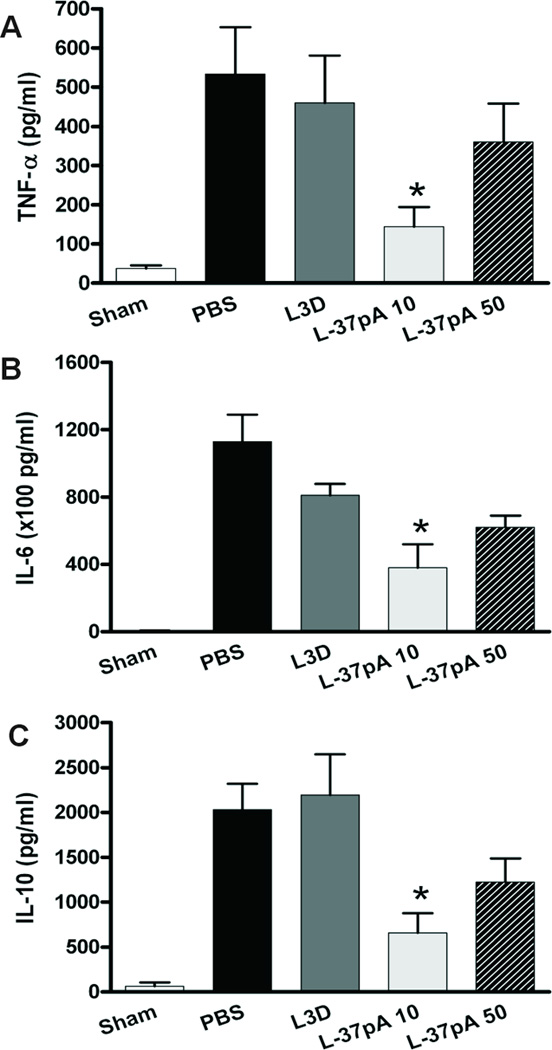
Serum inflammation-associated cytokines, TNF-α (A), IL-6 (B) and IL-10 (C) were tested at 24 h after surgery in sham (n =4), PBS control (n=6), L3D-37pA control peptide (n=7) and L-37pA (n=7) 10 mg/kg at 0 and 6 h after CLP (* P<0.05 vs. PBS control).
Figure 9. L-37pA improves survival and attenuates organ injury in murine CLP sepsis model with antibiotic treatment.

Survival analysis of PBS control (n = 15), L3D-37pA control (n = 18), and L-37pA (n = 18) treated mice (A). Serum lipids (Cholesterol, B; Triglyceride, C), kidney injury (Scr, D; BUN, E), liver damage (ALT, F; AST, G), LDH (H), pancreatic injury (amylase, I), and semiquantitative kidney injury score (J) at 24 h after CLP surgery in sham (n =4), PBS control (n=6), L3D-37pA control peptide (L3D: n=7), L-37pA 10 mg/kg (n=7) and L-37pA 50 mg/kg (n=7) administered at 0 and 6 h after CLP (* P<0.05 vs. PBS control).
Discussion
Modeling sepsis remains challenging in small experimental animals. Recent reviews indicate that CLP-induced sepsis best duplicates the septic peritonitis often seen in older human patients (44, 45). CLP mimics many features of human sepsis including hemodynamic changes, activation of pro- and anti-inflammatory cytokines, systemic inflammation, disseminated intravascular coagulation, multiple organ failure, systemic immune-depression, and death. Also very importantly, the use of outbred 6–9 week-old CD-1 or older 4–6 month-old C57BL/6 mice allows stably reproducible sepsis-induced acute kidney injury commonly observed in human sepsis. The CLP model was used throughout our study to investigate role of SR-BI/II and CD36 in C57BL/6 mice or the pharmacological effects of L37pA using much younger and much less expensive CD-1 mice.
Peritoneal granulocyte/PMN mobilization is a primary host defense mechanism to prevent bacterial expansion and potential systemic bacterial dissemination (10, 46, 47). Increased or decreased granulocyte/PMN migration reversibly correlates with the rate of peritoneal bacterial proliferation and severity of CLP-induced sepsis (9, 10). Because both systemic inflammation and multiorgan damage were reduced in L-37pA-treated mice and SR-B deficient animals, we hypothesize that the effects observed in this study could be due to improved peritoneal granulocyte survival and subsequent local bacterial containment. We suggest that SR-B family targeting in combination with systemic antibiotic therapy, could be beneficial because of reduced cytosolic bacterial proliferation and a subsequent decrease in peritoneal phagocyte damage and death (21).
These data also establish an important role of class B scavenger receptors in the development of local and systemic innate immune responses during severe infection (sepsis). In Fig. 10, we propose a model of sepsis that integrates the data from this study as well as previous observations made by us and others on SR-B receptor family-dependent endocytosis (17, 21, 35), bacterial lysosomal escape (21), indirect, internalization-dependent TLR receptor cross-talk (22, 48) and direct pathogen-induced SR-B family-mediated cytokine production (17). According to this model, SR-B family receptors are both complementary and redundant to various bacterial receptors such as the Fc receptor, scavenger receptors of various classes (13–16), and other pathogen-recognizing structures such as TLR, NOD and RIG (11, 12). Initial stages of bacterial recognition are mediated to a significant extent by TLR receptors. Upon interaction with surface TLR2/4 receptors bacteria induce downstream activation of various nuclear factors, which induce production of various cytokines. TLR receptors do not mediate bacterial internalization, and this function is mostly fulfilled by other receptors including scavenger receptors of the class B family. After receptor-mediated bacterial engulfment and endocytosis, lysosomally degraded bacterial products, can interact with endosomally/lysosomally sequestered TLR7 and TLR9, thus amplifying TLR function. In contrast to the classical phagocytic Fc receptor, B class scavenger receptors also mediate signaling in both a TLR-dependent and independent manner. Thus, it has been demonstrated that pathogen interaction with CD36 (17, 40, 49) directly induces JNK-dependent signaling, leading to a direct stimulation of cytokine secretion in stably transfected HEK293 cells. Similarly, SR-BI/II mediated bacteria- and LPS-inducible signaling can lead to cytokine production in SR-BI/II expressing cells (manuscript in preparation). Because of the redundancy among pathogen/pattern recognizing receptors (PRR), phagocytic cells deficient in a single receptor may demonstrate limited or no reduction in pathogen-associated downstream events including endocytosis and nuclear factor activation. In some cell types with a unique combination of expressed PRR receptors, the absence of one can even lead to increased pathogen recognition through other receptors and unexpectedly stronger proinflammatory signaling. For example, SR-BI/II-deficient macrophages had a modestly increased, relative to normal cells, cytokine production in response to LPS (37). We have also seen such effects for peritoneal macrophages but not for BMC-derived granulocytes, where SR-BI/II and CD36 deficiency resulted in a 50–60% reduction of cytokine secretion (Fig. 9). In addition to gene activation that can lead to cytokine production, pathogens induce downstream signaling to activate endocytic processes. After the initial bacterial binding/adhesion, SR-B receptors mediate bacterial internalization/lysosomal sequestration (21); however, SR-BI can lead to incomplete phagocytosis, allowing bacteria to escape from the lysosomes into the cytosol (21) where they can evade systemic antibiotics. In this protected niche, bacteria can proliferate and activate intracellular TLRs and other pathogen receptors with resultant gene activation and inflammatory reactions (22, 35). Blocking such lysosomal escape could be beneficial under conditions when non-internalized/extracellular bacterial proliferation is blocked by antibiotic treatment. Blocking such receptors without antibiotic intervention is detrimental because both SR-BI/II and CD36 KO mice were more sensitive to sepsis and bacterial infection in the absence of antibiotics (37). Based on such a hypothesis we propose that blocking SR-B class receptors either by L-37pA or using genetically manipulated mice defective in receptor expression improves outcomes of antibiotic/fluid-treated animals with severe infections/sepsis due to better containment of bacteria by polymorphonuclear/granulocytic phagocytes. Extensive investigation will be required to determine how this mechanism interacts with many other mediators/pathways in the complex pathophysiology of sepsis (8, 50).
Figure 10. Proposed roles of SR-BI/II and CD36 in bacterial intracellular proliferation and subsequent pro-inflammatory signaling in phagocytic cells.
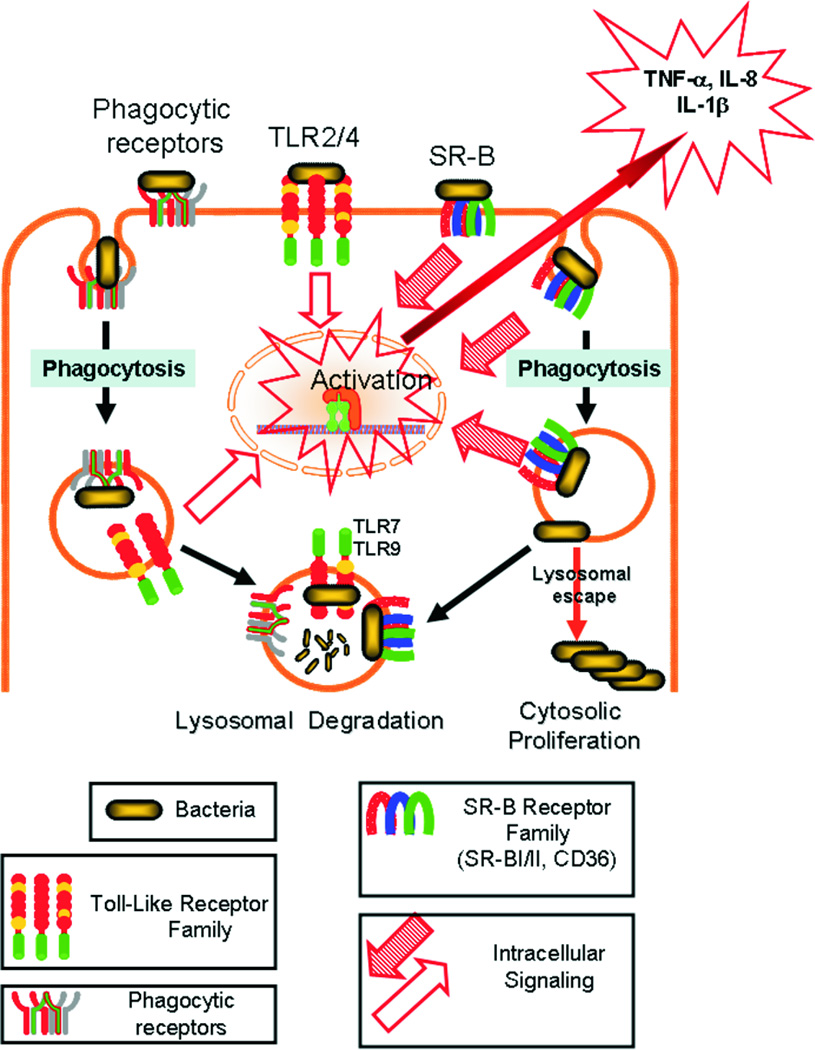
TLR, SR-A and -B classes were identified as pathogen recognizing receptors, mediating their signaling via various mechanisms. Cell surface TLR2/4 does not require pathogen internalization to initiate downstream signaling from the plasma membrane, inducing cytokine production. In contrast, TLR7/9 is localized in an endocytic compartment, where signaling requires additional mechanisms for pathogen endocytosis to trigger intracellular pathogen recognition. SR-A/B receptors combine functions of pathogen-induced signaling and pathogen-triggered phagocytosis. Due to their unique attributes SR-A/B mediate signaling upon pathogen recognition and facilitate TLR7/9-mediated gene activation, transporting bacteria (or its degradation products) to TLR7/9-expressing cell compartments. In addition, scavenger receptors mediate bacterial clearance and killing. Under conditions of incomplete phagocytosis or lysosomal bacterial escape, SR-A/B can act as pathogenic receptors that contribute to bacterial intracellular proliferation and growth.
In the presence of antibiotic and fluid treatment, L-37pA reduced both systemic inflammation and tissue damage in CLP mice. L-37pA also improved mouse survival and profoundly increased neutrophil accumulation in the peritoneal cavity. Furthermore, an increased peritoneal neutrophil accumulation and a reduction in peritoneal bacterial proliferation were found in both CD36 and SR-BI/II KO mice. Both types of animals demonstrated improved organ function, reduced systemic inflammation, and increased animal survival only when adrenal deficiency was appropriately compensated with both mineralocorticoids and glucocorticoids. In summary, our findings indicate that the absence or blockade of SR class B receptor family proteins can reduce both inflammation and tissue toxicity by diminishing bacteria-mediated damage to peritoneal phagocytes (mainly granulocytes). This enhances local granulocyte accumulation and bacterial containment, and also reduces systemic inflammation and organ damage leading to improved animal survival in sepsis.
Supplementary Material
Footnotes
This research was supported by the NIH Intramural Research Programs including NIDDK/CC/NHLBI/NIAID
References
- 1.Angus DC. Caring for the critically ill patient: challenges and opportunities. JAMA. 2007;298:456–458. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl. J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Sessler CN, Perry JC, Varney KL. Management of severe sepsis and septic shock. Curr.Opin. Crit. Care. 2004;10:354–363. doi: 10.1097/01.ccx.0000139363.76068.7b. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Tomayko JF, Angus DC, Opal S, Cupo MA, McDermott S, Ducher A, Calandra T, Cohen J. Efficacy and safety of a phospholipid emulsion (GR270773) in Gram-negative severe sepsis: results of a phase II multicenter, randomized, placebo-controlled, dose-finding clinical trial. Crit. Care Med. 2009;37:2929–2938. doi: 10.1097/CCM.0b013e3181b0266c. [DOI] [PubMed] [Google Scholar]
- 6.Russell JA. Management of sepsis. New Engl. J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 7.Schultz MJ, van der Poll T. Modulation of innate immune responses in the treatment of sepsis and pneumonia. Curr. Drug Tar. Inflam. Allergy. 2004;3:11–17. doi: 10.2174/1568010043483962. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. The New Engl. J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 9.Knapp S, Matt U, Leitinger N, van der Poll T. Oxidized phospholipids inhibit phagocytosis and impair outcome in gram-negative sepsis in vivo. J Immunol. 2007;178:993–1001. doi: 10.4049/jimmunol.178.2.993. [DOI] [PubMed] [Google Scholar]
- 10.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Tolllike receptor 9 inhibition reduces mortality in polymicrobial sepsis. J. Exp. Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of Toll-like receptors in the development of sepsis. Shock. 2008;29:315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 12.Opitz B, Eitel J, Meixenberger K, Suttorp N. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. Thromb. Haem. 2009;102:1103–1109. doi: 10.1160/TH09-05-0323. [DOI] [PubMed] [Google Scholar]
- 13.Amiel E, Acker JL, Collins RM, Berwin B. Uncoupling scavenger receptor A-mediated phagocytosis of bacteria from endotoxic shock resistance. Infec. Imm. 2009;77:4567–4573. doi: 10.1128/IAI.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl. Acad. Sci. USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiser L, De Winther MP, Makepeace K, Hollinshead M, Coull P, Plested J, Kodama T, Moxon ER, Gordon S. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 2002;70:5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiser L, Makepeace K, Pluddemann A, Savino S, Wright JC, Pizza M, Rappuoli R, Moxon ER, Gordon S. Identification of Neisseria meningitidis nonlipopolysaccharide ligands for class A macrophage scavenger receptor by using a novel assay. Infect. Immun. 2006;74:5191–5199. doi: 10.1128/IAI.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranova IN, Kurlander R, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, Patterson AP, Eggerman TL. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008;181:7147–7156. doi: 10.4049/jimmunol.181.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocharov AV, Baranova IN, Vishnyakova TG, Remaley AT, Csako G, Thomas F, Patterson AP, Eggerman TL. Targeting of scavenger receptor class B type I by synthetic amphipathic alpha-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. J. Biol. Chem. 2004;279:36072–36082. doi: 10.1074/jbc.M314264200. [DOI] [PubMed] [Google Scholar]
- 19.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 20.Vishnyakova TG, Bocharov AV, Baranova IN, Chen Z, Remaley AT, Csako G, Eggerman TL, Patterson AP. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 2003;278:22771–22780. doi: 10.1074/jbc.M211032200. [DOI] [PubMed] [Google Scholar]
- 21.Vishnyakova TG, Kurlander R, Bocharov AV, Baranova IN, Chen Z, Abu- Asab MS, Tsokos M, Malide D, Basso F, Remaley A, Csako G, Eggerman TL, Patterson AP. CLA-1 and its splicing variant CLA-2 mediate bacterial adhesion and cytosolic bacterial invasion in mammalian cells. Proc. Natl. Acad. Sci. USA. 2006;103:16888–16893. doi: 10.1073/pnas.0602126103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baranova IN, Vishnyakova TG, Bocharov AV, Kurlander R, Chen Z, Kimelman ML, Remaley AT, Csako G, Thomas F, Eggerman TL, Patterson AP. Serum amyloid A binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J. Biol. Chem. 2005;280:8031–8040. doi: 10.1074/jbc.M405009200. [DOI] [PubMed] [Google Scholar]
- 24.Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 25.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metabolism. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams DL, de La Llera-Moya M, Thuahnai ST, Lund-Katz S, Connelly MA, Azhar S, Anantharamaiah GM, Phillips MC. Binding and cross-linking studies show that scavenger receptor BI interacts with multiple sites in apolipoprotein A-I and identify the class A amphipathic alpha-helix as a recognition motif. J. Biol. Chem. 2000;275:18897–18904. doi: 10.1074/jbc.M002411200. [DOI] [PubMed] [Google Scholar]
- 27.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney 29 and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832–836. doi: 10.1038/sj.ki.5000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 29.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am. J. Physiol. Renal Physiol. 2008;295:F1825–F1835. doi: 10.1152/ajprenal.90442.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)- dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PS, Star RA. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 2008;294:F1050–F1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am. J. Physiol. Renal Physiol. 2004;286:F1116–F1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 35.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36- mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Inves. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Song Z, Li M, Wu Q, Wang D, Feng H, Bernard P, Daugherty A, Huang B, Li XA. Scavenger Receptor BI Protects against Septic Death through Its Role in Modulating Inflammatory Response. J. Biol. Chem. 2009;284:19826–19834. doi: 10.1074/jbc.M109.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand RB, Kruijt JK, Van Eck M, Van Berkel TJ. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J. Lipid Res. 2008;49:738–745. doi: 10.1194/jlr.M700475-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Hoekstra M, Ye D, Hildebrand RB, Zhao Y, Lammers B, Stitzinger M, Kuiper J, Van Berkel TJ, Van Eck M. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J. Lipid Res. 2009;50:1039–1046. doi: 10.1194/jlr.M800410-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ. Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 42.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, Chatham J, Anantharamaiah GM, White CR. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H866–H873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit. Care Med. 2009;37:1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alves-Filho JC, Benjamim C, Tavares-Murta BM, Cunha FQ. Failure of neutrophil migration toward infectious focus in severe sepsis: a critical event for the outcome of this syndrome. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl 1):223–226. doi: 10.1590/s0074-02762005000900038. [DOI] [PubMed] [Google Scholar]
- 47.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 48.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 49.Baranova IN, Bocharov AV, Vishnyakova TG, Kurlander R, Chen Z, Fu D, Arias IM, Csako G, Patterson AP, Eggerman TL. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 2010;285:8492–8506. doi: 10.1074/jbc.M109.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


