Abstract
Lactating female rats without their pups exhibit lower HPA responsiveness to stress than nonlactating females. However, responsiveness to stress is similar when lactating females are tested with their pups and the stressor involves a potential threat to the offspring. This study constitutes the first comparison of stress responsiveness in lactating and nonlactating female nonhuman primates. Subjects were 53 multiparous female free-ranging rhesus macaques. Approximately half of the females were lactating and half were nonpregnant/nonlactating. Blood samples were obtained after capture and after overnight housing in an individual cage. Lactating females were tested with their infants. Lactating females had significantly higher plasma cortisol levels than nonlactating females on both days. Having or not having an infant was also a better predictor of plasma cortisol levels among all females than their age, dominance rank, group of origin, time of day at which the sample was obtained, and time elapsed since beginning of the sampling procedure or since anesthesia. Plasma cortisol levels of lactating females were not significantly correlated with post-partum stage or with the cortisol levels of their infants. Capture, handling, and individual housing in a cage are powerful psychological stressors for free-ranging primates. We suggest that the higher plasma cortisol levels exhibited by lactating females reflect greater responsiveness to stress associated with perception of risks to infants. Hyporesponsiveness to stress may not be a general characteristic of lactation in all mammalian species, but a short-term effect of infant suckling that is most apparent with stressors unrelated to the offspring.
Keywords: Responsiveness to stress, Cortisol, Lactation, Infants, Humans, Nonhuman primates
The activity of the hypothalamic-pituitary-adrenal (HPA) axis and its responsivity to stress during lactation have been best characterized in rodents, while relatively little is known in humans and other primates. In rats, the post-partum period is characterized by elevated levels of plasma ACTH and corticosterone in basal conditions (e.g., Stern et al., 1973; Lightmann, 1992), and blunted CRF, ACTH and corticosterone responses to stress (see Lightman et al., 2001; Neumann, 2001; Heinrichs et al., 2002; Walker et al., 2004; Tu et al., 2005; for reviews). Behavioral and neuroendocrine hyporesponsiveness to stress in lactating rats has generally been demonstrated in studies in which females were exposed to physical, pharmacological, or environmental stressors in the absence of the pups (see above references). When lactating rats, however, were exposed to a psychosocial stressor (a male intruder or predator odor) in the presence of their pups their ACTH and corticosterone responses were higher and not different from those of virgin females (Deschamps et al., 2003; see also Leonhardt et al., 2007 for further evidence of high corticosterone responses to stress by lactating rats). There have been only a few studies comparing HPA responsiveness to stress in lactating and nonlactating women and the evidence is mixed. Studies by Altemus and Carter reported that lactating primiparous women (8–18 weeks after delivery) exhibited lower HPA activation than nonlactating women following treadmill exercise but not in response to psychosocial stress (Altemus et al., 1995; 2001; see also Carter and Altemus, 1997; Carter et al., 2001). A recent study by Tu et al. (2006) reported no differences in plasma cortisol elevations in response to the Trier Social Stress Test (TSST) among primiparous mothers who were breastfeeding, bottle-feeding, or nonpost-partum (with children aged 1–6 years), whereas among multiparous women, breastfeeding women exhibited reduced cortisol elevations in response to the TSST or the viewing of an emotional film evoking a threat to a child relative to bottle-feeding mothers or nonpost-partum mothers. Finally, it has been suggested that, in contrast to rats, in women lactation does not result in a general reduction of HPA activity in response to psychosocial stress; rather, infant suckling can exert a short-term suppression of the cortisol stress response (Heinrichs et al., 2001; see also Mezzacappa et al., 2003; Tilbrook et al., 2006). Research with nonhuman primates could complement human studies in this area and further assess the extent to which the findings from nonprimate mammals can be extrapolated to humans.
Changes in HPA function in the pre- and post-partum periods in nonhuman primates appear to be generally similar to those observed in humans. In humans, plasma concentrations of CRH, ACTH and cortisol rise steadily during late gestation (as a result of both maternal and placental contributions), peak during labor, and drop following parturition (e.g., McLean and Smith, 1999; Mastorakos and Ilias, 2003). Basal plasma CRH and ACTH concentrations fall to nonpregnant levels within hours following parturition, while basal cortisol begins to drop in the first few hours post-partum but may take 5 to 7 days to return to pre-pregnancy levels (e.g., Taylor et al., 1994; McLean and Smith, 1999; Mastorakos and Ilias, 2003; see Owens et al., 1987 for data on post-partum cortisol sensitivity to feedback inhibition). Similar to human research, studies of rhesus macaques and other nonhuman primates have reported a pre-partum increase in plasma CRH and plasma, fecal, or urinary cortisol, followed by a rapid post-partum decline (Bahr et al., 1998; Smith et al., 1999; Bowman et al., 2001; Bardi et al., 2003; Saltzman and Abbott, 2005). The precise time course of the resetting of the HPA axis following parturition is not known in human or nonhuman primates, and there seems to be considerable variation among individuals (Magiakou et al., 1996; Carter et al., 2001). Although pregnant baboons exhibit a blunted ACTH and cortisol response to the CRH challenge similar to that observed in rats and humans (Goland et al., 1990), no studies to date have specifically compared HPA axis responses to endocrine challenges or stress in lactating and nonlactating monkey females. Therefore, the extent to which the post-partum period is accompanied by hyporesponsiveness to stress in nonhuman primates is not known.
As the changes in HPA axis activity illustrate, the post-partum period is not only a period characterized by the onset of new activities such as lactation and maternal care, but also a period in which a female resets her neuroendocrine systems following the major alterations associated with pregnancy and parturition. An important question that neither animal nor human studies have fully addressed is whether the hyporesponsiveness to stress exhibited by lactating females is linked to the post-partum resetting of neuroendocrine systems or other early post-partum processes, or whether it is a general characteristic of lactation or maternal care that occurs above and beyond the post-partum period. Statements such as “In animal research it is generally believed that HPA axis hyporesponsiveness can be observed during the whole period of lactation” (Heinrichs et al., 2002, p. 199) seem to be entirely based on research with rats (and, for the most part, lactating rats tested without pups). In rats, however, the lactation and maternal care periods are of short duration and concentrated in the post-partum period, whereas in primates and many other mammals the vast majority of lactation and maternal care occurs outside the post-partum period. During this period of extended maternal care, which for humans and some other primates may last several years, mothers continue to be exposed to many infant-related psychosocial stressors and are vulnerable to emotional disorders such as anxiety and depression. Therefore, we need not only more studies comparing responsiveness to stress between lactating and nonlactating females in primates, but also more studies examining the HPA axis responses to stress by lactating females well beyond the early post-partum period.
In the present study we compared the plasma cortisol responses to stress of lactating and nonlactating females in free-ranging rhesus macaques. Rhesus macaques are an ideal model to address this question given our close phylogenetic relatedness and our similarities in HPA axis responses to stress. For the present study, the stressors involved capture, brief handling, and overnight individual housing of the animals in a cage. In our free-ranging population, these procedures are conducted annually for colony management purposes and biosampling, and have been shown to result in behavioral stress responses and elevations of plasma cortisol levels shortly after capture, the following day, and possibly for several weeks thereafter (Berman, 1989; Laudenslager et al., 1999). In our study, all lactating females were tested with their infants, and plasma cortisol was measured in both mothers and infants. Since the lactating females ranged from 8 days to approximately 5 months post-partum, this variation allowed us to examine whether possible differences in their stress responsiveness relative to nonlactating females varied as a function of time elapsed since parturition.
Methods
Subjects
This study was conducted with the free-ranging population of rhesus macaques on Cayo Santiago, a 15.2 ha island located 1 km off the southeastern coast of Puerto Rico. This colony was established in 1938, with free-ranging monkeys captured in India (Rawlins and Kessler, 1986). Since then, no new individuals have been introduced into the population, except through births. To maintain a stable population size, a fraction of the yearlings and two-year olds are culled each year. During the study period, the population included approximately 850 animals distributed among 6 naturally formed social groups. Monkeys on Cayo Santiago forage on vegetation and are provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders. In the Cayo Santiago population, there is a 6-month mating season beginning in March–April, followed by a 6-month birth season beginning in September-October. Some rhesus females give birth every year while others do so every other year. Colony records are updated with daily censuses of all animals. The Cayo Santiago database includes information on each animal’s date of birth and death, maternal relatedness and genealogy, and group membership, reproductive, and health history.
The data presented here were collected as part of a larger study of stress and reproduction in multiparous, experienced females. Study subjects were all adult females in the population who, when the study began, were between 15 and 25 years of age (n = 53; mean age ± SE: 18.3 ± 0.3). All subjects were multiparous and belonged to 6 different groups (F=17; R=16; S=7; V=5; HH=4; KK=4). All subjects, with the exception of a few individuals, were classified as being high-, middle-, or low-ranking depending on whether their rank fell within the top, middle, or bottom third of the dominance hierarchy within their group. Dominance hierarchies were established on the basis of data on aggressive and submissive interactions collected by trained observers. At the time of capture, between January and March 2007, 25 females were lactating (“lactating group”) and had a live infant (infant age range: 8–134 days; mean ± SE = 72.8 ± 7.5 days), 3 of them had had an infant within 6 months but the infant had died for unknown reasons, 2 of them were pregnant, and 23 of them were neither pregnant nor lactating (“nonlactating group”). All females were inspected by a veterinarian at the time of sampling and found to be in general good health. Therefore, there was no hint that the nonlactating females in this study did not conceive for health problems, or that the lactating females were in poor health. Pregnancy and nonpregnancy were determined at the time of sampling through a physical inspection (palpation of the abdomen) by the veterinarian and reconfirmed retrospectively at the end of the birth season.
Procedure
Focal subjects and their infants were trapped between January and March 2007. Monkeys were captured in a feeding corral, approximately 100m2, which was provisioned daily with monkey chow. Trapping generally occurred between 8:30 and 12:00. Subjects were netted or captured by hand in the corral, immediately anesthetized with ketamine (approximately 10 mg/kg via IM injection), and transferred to a holding cage (0.62 × 0.42 × 0.62 m). The subjects were moved to a small field laboratory located in a trailer, where blood samples were collected. Blood samples following capture were collected between 9:00 and 12:10 (average time of day: 10:5 ± 12.9 min). Samples were collected, on average, 16.5 ± 0.9 min after capture (range: 7–45 min) and 14.8 ± 0.9 min after ketamine administration. All blood samples were collected from the femoral vein into heparin-treated Vacutainer tubes and placed into dry ice. After blood collection, the monkeys were placed into a standard squeeze cage for overnight housing. The following morning, the monkeys were anesthetized again and a second blood sample was collected from all adult females. This time, infants were anesthetized along with their mothers and a blood sample was collected from them as well. With one exception, only infants older than 2 months of age (n = 16) were sampled due to the very small size of younger infants.
Blood samples on the second day were collected between 7:15 and 10:40 (average time of day: 8:10 ± 12.9 min). Samples were collected, on average, 63.9 ± 7.4 min after the door of the laboratory was first opened (range: 7–207 min), and 28.3 ± 4.8 min after ketamine administration (range 0–127 min). Thus, the sample collected on the first day presumably reflects the acute stress response to capture, while the sample collected on the second day presumably reflects the cumulative stressful effects of capture and overnight housing (see Laudenslager et al., 1999, for a similar interpretation). Since some monkeys are captured more quickly or easily than others, we tested whether there was significant variation in the timing of the sampling procedures among individuals in relation to their social group. Group membership did not significantly affect any of the time variables for sample collection following capture (one-way ANOVA; time of sampling, F5,47 = 0.78, NS; time since capture, F5,47 = 0.49, NS; time since ketamine injection, F5,47 = 0.87, NS). All blood samples were centrifuged and stored at −80 until shipped to the Biomarker Assay Core Lab of the Yerkes National Primate Research Center, where they were assayed for cortisol by radioimmunoassay using a commercially available kit (Diagnostic Systems Laboratories, Webster, TX). Intra-assay coefficient of variation was 4.90% and inter-assay coefficients were 4.50% and 8.74%.
Analyses
Cortisol data were log-transformed to ensure normality of distribution and homogeneity of variance. Statistical tests included Student’s t tests for paired and unpaired samples, Pearson’s correlations, analysis of variance (ANOVA), simple and multiple regression analyses, and chi squares. All tests were two-tailed. Probabilities ≤ 0.05 were considered statistically significant.
Results
Lactating and nonlactating females did not differ significantly in their age (lactating: 17.8 ± 0.3 years; nonlactating: 19.0 ± 0.6; t = 1.59, df = 46, NS), dominance rank (lactating: high = 6; middle = 7; low = 11; nonlactating: high = 6; middle = 7; low = 4; chi square: 2.13, df = 2; NS), or any variables related to blood sampling (time of day, time since beginning of the procedure, and time since ketamine injection) on either day. Cortisol concentrations measured on Day 1 and Day 2 were not significantly correlated (r = 0.17; n = 53, NS).
A repeated measures ANOVA comparing plasma cortisol concentrations on Day 1 and Day 2 in lactating and nonlactating females revealed significant main effects of day, F (1, 45) = 66.14, p < 0.0001 and female reproductive condition, F (1,45) = 11.71, p = 0.001, but no significant interaction between these variables, F (1,45) = 0.60, NS. Cortisol concentrations were higher on Day 2 than on Day 1, and higher for lactating than for nonlactating females across the two days (Fig. 1). Fig. 1 also shows the average cortisol concentrations of pregnant females (n = 2) and of females whose infants died (n = 3), even though these individuals were too few to be included in the statistical analyses. Fig. 1 suggests that the cortisol concentrations of pregnant females were similar to (Day 1) or lower (Day 2) than those of lactating females with infants, whereas those of females whose infants had died were comparable to those of nonlactating females.
Figure 1.
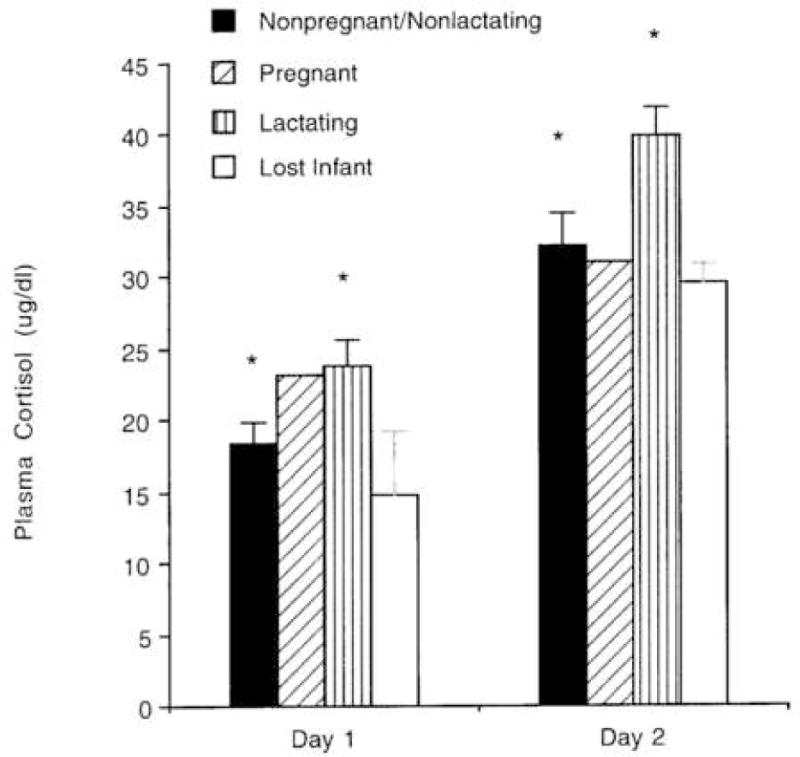
Mean (+SEM) concentrations of plasma cortisol on Day 1 and Day 2 in females who were neither pregnant nor lactating (n = 23), pregnant females (n = 2), lactating females (n = 25), and females who had given birth but their infant had died within 6 months prior to this study (n = 3). Pregnant females and females who lost their infants were not included in the statistical analysis. Cortisol levels were significantly higher on Day 2 than on Day 1, and in lactating than in nonlactating/nonpregnant females. * P ≤ 0.0001.
A multiple regression analysis using reproductive condition (i.e., with or without infant), female age, group of origin, dominance rank, and the sampling time variables as potential predictors of variation in plasma cortisol on Day 2 was statistically significant, F(7, 38) = 3.01, r = 0.64; p = 0.01, and identified reproductive condition as the best predictor of cortisol concentrations (partial F = 3.94; p = 0.05). A similar analysis conducted for Day 1 approached overall significance, F(7,39) = 2.01, r = 0.55; p = 0.08, but again identified reproductive condition as the best predictor of cortisol concentrations (partial F = 4.78; p = 0.03). Plasma cortisol concentrations measured on Day 2 showed a negative relationship with age (r = 0.23; n = 52; p = 0.09; Fig. 2a) and dominance rank (one-way factorial ANOVA: F2,43 = 2.15; p = 0.13; Fig. 2b) but neither effect was statistically significant.
Figure 2.
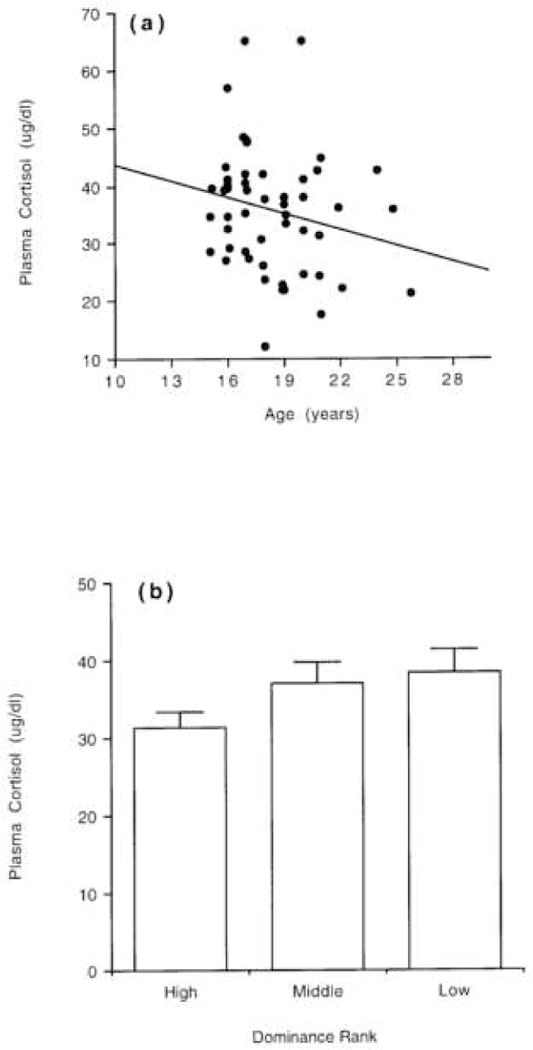
(a). Correlation between female age and plasma cortisol levels on Day 2 across all adult females. The correlation is not statistically significant (see also Downs et al. 2007). (b). Mean (+SEM) concentrations of plasma cortisol on Day 2 in high-ranking, middle-ranking, and low-ranking adult females regardless of reproductive condition. The difference among the three groups is not significant.
Post-partum period stage was not a significant predictor of plasma cortisol concentrations among lactating females with infants. Specifically, there was no significant correlation between post-partum days and cortisol concentrations measured on Day 1 (r = 0.26; n = 25; NS) or Day 2 (r = 0.09; n = 25; NS). Furthermore, the increment in plasma cortisol concentrations observed on Day 2 (difference between Day 2 and Day 1) was not significantly predicted by post-partum period stage (r = 0.08; n = 25; NS).
Plasma cortisol concentrations of infants (3.1 ± 0.1; n = 16) were significantly lower than those of adult females (3.5 ± 0.0; n = 52; t = 3.25; df = 66; p = 0.001). There were no significant correlations between infant cortisol levels and infant age (r = 0.17; n = 16; NS) and between infant cortisol and maternal cortisol (r = 0.28; n = 16; NS).
Discussion
Capture and separation from conspecifics can be significant sources of stress for group-living nonhuman primates, especially for free-ranging individuals. The free-ranging rhesus macaques on Cayo Santiago are habituated to the presence of human observers but are captured and handled only once a year, in conjunction with the annual trapping procedures. Behavioral and physiological data from previous studies have suggested that these trapping procedures are indeed stressful to the monkeys (Berman, 1989; Laudenslager et al., 1999). HPA responses to trapping on Cayo Santiago have only been reported by one previous study. Laudenslager et al. (1999) measured plasma cortisol levels shortly after trapping and the following morning, after the monkeys had been individually housed in a cage. They found that plasma cortisol concentrations on the second day were significantly higher than those on the first day. They interpreted both cortisol measures as stress responses and argued that the cortisol concentrations on the second day reflected the cumulative stress of trapping and individual housing. We used procedures virtually identical to those used by Laudenslager et al. (1999) and found a similar increase in plasma cortisol concentrations on the second day. We concur with Laudenslager et al.’s interpretation that both cortisol measures reflect stress responses rather than baseline values, and that stress was likely to be higher on the second day. In addition, in both our study and the study by Laudenslager et al. (1999), cortisol levels may have been higher on the second day because the sample was obtained earlier in the morning (in our study, 2–3 hours earlier), when plasma cortisol is higher.
We compared the plasma cortisol responses to capture and individual housing, as well as the increment in plasma cortisol from Day 1 to Day 2, between lactating females with infants and females who were neither pregnant nor lactating. This analysis represents the first direct comparison of cortisol responses to stress in lactating and nonlactating females in nonhuman primates. We found no evidence of hyporesponsiveness to stress among lactating females. Rather, plasma cortisol levels were significantly higher in lactating than in nonlactating females, both shortly after capture and the morning after. The influence of reproductive condition on plasma cortisol levels was confirmed by a multiple regression analysis showing that being lactating or nonlactating was a better predictor of plasma cortisol levels than female age, dominance rank, group of origin, and the sampling time variables (time of day, time since beginning of the procedures, and time since anesthesia).
Our study constitutes one of the few attempts to measure acute responses to stress in free-ranging rhesus macaques. One of the limitations of our study, however, is the lack of a plasma cortisol baseline measure with which to compare the cortisol levels measured following stress (see also Laudenslager et al., 1999). Although the difference between plasma cortisol levels measured on the second and on the first day may provide information on an individual’s increment in circulating plasma cortisol following stress, this difference cannot be equated to a difference between stress and basal cortisol levels for two reasons: 1) plasma cortisol on Day 1 is not a baseline measure but is itself an acute stress measure, and 2) the stress-related increase in cortisol on Day 2 is confounded by circadian variation in cortisol because the second blood sample was obtained earlier in the morning than the first sample.
Since we do not have a true plasma cortisol baseline measure for our subjects, a possible interpretation of our findings is that the greater cortisol levels observed in lactating females do not reflect greater responsiveness to stress relative to nonlactating females, but rather higher baseline levels of plasma cortisol associated with lactation. In this view, stress responsiveness could be similar in lactating and nonlactating females, or even lower in the lactating females. Although this explanation cannot be ruled out entirely, it is unlikely to be correct because, in both nonhuman primates and humans, post-partum plasma cortisol levels return to pre-pregnancy baselines within a few days after parturition (see Introduction for references). Moreover, Altemus et al. (1995) found no significant differences in basal levels of ACTH and cortisol between lactating and nonlactating women, and Tu et al. (2006) found no significant differences in basal plasma cortisol levels between breastfeeding or bottle-feeding mothers and mothers outside the post-partum period (with children aged 1–6 years old). The lactating females in our study were tested between 8 and 134 days following parturition, i.e. at a time in which their basal plasma cortisol levels should have been comparable to pre-pregnancy levels. The rapid return to pre-pregnancy levels of basal HPA activity following parturition in human and nonhuman primates marks an important difference with rats, in which there is chronic elevation of basal HPA activity throughout lactation (e.g., Lightman et al., 2001). Increased basal secretion of corticosterone seen in lactation, however, is not viewed as the primary cause of the hyporesponsiveness to stress by lactating rats (Lightman et al., 2001).
Since it is unlikely that the higher cortisol levels of lactating females reflected higher baselines, it is possible that they reflected greater responsiveness to stress in mothers with infants. In rhesus macaques, infants from 0 to 6 months of age are highly vulnerable to predators and conspecifics and entirely dependent on their mothers for protection (e.g., Maestripieri, 1993a; 1995). Concerns over risk to their infants may explain why lactating females exhibited higher cortisol responses to trapping and individual housing and why these responses did not vary significantly across the 6 lactation months. This explanation would be consistent with the observation that females who had given birth but whose infants had died prior to the study had low plasma cortisol levels comparable to those of nonlactating females.
In anthropomorphic primates, females are biologically predisposed to be in almost continuous contact with their infants and to breastfeed them on demand. Furthermore, some of the major stressors to primate lactating females are psychosocial and infant-related (e.g., Maestripieri 1999). Therefore, although hyporesponsiveness to stress exhibited by lactating females without their offspring may be adaptive to territorial animals such as rodents, in which mothers park their offspring in a nest and defend the boundaries of their territory from intruders (e.g., Fleming and Luebke, 1981; Maestripieri & D’Amato, 1991; Maestripieri et al., 1991; Neumann, 2001), the possible adaptive significance of a similar trait in primates would be unclear. Evidence of high physiological responsiveness to stress in lactating primate females tested with their infants is consistent with behavioral studies of rhesus macaque mothers, which suggest that motherhood (in some individuals more than others) is associated with high reactivity to anxiety-eliciting situations, particularly those involving risk to their infants (Maestripieri, 1993a; 1995). Moreover, in both human and nonhuman primates, maternal arousal and anxiety (in moderate levels) play an important role in activating the maternal attachment system and eliciting maternal protectiveness (Maestripieri, 1993b; Fleming et al., 1987; 1997; see Dix, 1991, for the important role of emotions as elicitors of parenting behavior in humans). Therefore, it is possible that higher reactivity to environmental challenges, particularly when these challenges occur in the presence of offspring and offspring are still dependent on their mothers for protection, could be adaptive to primate mothers. Clearly, more studies of human and nonhuman primates investigating stress responsiveness by lactating females under different circumstances are needed before any firm conclusions can be drawn.
Lactation and parental care in humans are associated with a number of physical, physiological, and psychosocial stressors resulting from the demands of infant care, sleep deprivation, changes in lifestyle, and in social relationships with a partner or other individuals. Chronic activation of stress-sensitive physiological systems following the birth of a child may render a mother vulnerable to emotional and mood disorders including depression, anxiety, and panic attacks (e.g., Buckwalter et al., 1999). Both chronic stress and maternal emotional disturbances can affect parenting behavior, and this in turn, may have long-term consequences for child development. Therefore, understanding the functioning of the neuroendocrine systems underlying emotion regulation and responsiveness to stress during lactation has important implications for mental health and child development. Given the similarities in the processes of lactation and in the relation between emotions and parenting behavior in human and nonhuman primates, studies of emotion regulation and responsiveness to stress in nonhuman primate mothers can make a significant contribution to our understanding of both adaptive and maladaptive biological and behavioral processes associated with motherhood.
Acknowledgments
We are grateful to the staff of the Caribbean Primate Research Center for their assistance with the animal trapping and sampling procedures. We also thank Mark Wilson, Susie Lackey, and the staff of the Biomarker Assay Core Lab of the Yerkes National Primate Research Center for running the hormonal assays. This research was supported by a grant from the Brain Research Foundation to D.M., NIH-NCRR grant CM-5-P40RR003640 to the Caribbean Primate Research Center, and NIH-NCRR grant RR-00165 to the Yerkes National Primate Research Center. The Yerkes Center is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalamic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80:2954–2958. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Bahr NI, Pryce CR, Dobeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol Behav. 1998;64:429–437. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum cortisol levels and mother-infant interactions in Japanese macaques. Am J Phys Anthropol. 2003;120:298–304. doi: 10.1002/ajpa.10150. [DOI] [PubMed] [Google Scholar]
- Berman CM. Trapping activities and mother-infant relationships on Cayo Santiago: A cautionary tale. Puerto Rican Health Science Journal. 1989;8:73–78. [PubMed] [Google Scholar]
- Bowman ME, Lopata A, Jaffe RB, Golos TG, Wickings J, Smith R. Corticotropin-releasing hormone-binding protein in primates. Am J Primatol. 2001;53:123–130. doi: 10.1002/1098-2345(200103)53:3<123::AID-AJP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Buckwalter JG, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, Goodwin TM. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology. 1999;24:69–84. doi: 10.1016/s0306-4530(98)00044-4. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann NY Acad Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Progr Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups’ presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. Neuroendocrinology. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Dix T. The affective organization of parenting: Adaptive and maladaptive processes. Psych Bull. 1991;110:3–25. doi: 10.1037/0033-2909.110.1.3. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: Emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Anderson V. Hormonal and attitudinal correlates of maternal behavior during the early postpartum period in first-time mothers. J Reprod Inf Psychol. 1987;5:193–205. [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Goland RS, Stark RI, Wardlaw SL. Response to corticotropin-releasing hormone during pregnancy in the baboon. J Clin Endocrinol Metab. 1990;70:925–929. doi: 10.1210/jcem-70-4-925. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Neumann I, Ehlert U. Lactation and stress: protective effects of breast-feeding in humans. Stress. 2002;5:195–203. doi: 10.1080/1025389021000010530. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Rasmussen KL, Berman CM, Lilly AA, Shelton SE, Kalin NH, Suomi SJ. A preliminary description of responses of free-ranging rhesus monkeys to brief capture experiences: behavior, endocrine, immune, and health relationships. Brain Behav Imm. 1999;13:124–137. doi: 10.1006/brbi.1998.0548. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Matthews SG, Meaney MJ, Walker CD. Psychological stressors as a model of maternal adversity: Diurnal modulation of corticosterone responses and changes in maternal behavior. Horm Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lightmann SL. Alterations in hypothalamic-pituitary-adrenal responsiveness during lactation. Ann NY Acad Sci. 1992;652:340–346. doi: 10.1111/j.1749-6632.1992.tb34365.x. [DOI] [PubMed] [Google Scholar]
- Lightmann SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamic-pituitary-adrenal axis. Progr Brain Res. 2001;133:111–130. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993a;95:19–31. [Google Scholar]
- Maestripieri D. Maternal anxiety in rhesus macaques Macaca mulatta. II. Emotional bases of individual differences in mothering style. Ethology. 1993b;95:32–42. [Google Scholar]
- Maestripieri D. Assessment of danger to themselves and their infants by rhesus macaque (Macaca mulatta) mothers. J Comp Psychol. 1995;109:416–420. doi: 10.1037/0735-7036.109.4.416. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. The biology of human parenting: Insights from nonhuman primates. Neurosci Biobehav Rev. 1999;23:411–422. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Badiani A, Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci. 1991;105:663–668. doi: 10.1037//0735-7044.105.5.663. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, D’Amato FR. Anxiety and maternal aggression in house mice (Mus domesticus): A look at interindividual variability. J Comp Psychol. 1991;105:295–301. doi: 10.1037/0735-7036.105.3.295. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Rabin D, Bubbert B, Gold PW, Chrousos GP. Hypothalamic CRH suppression during the postpartum period: implications for the increase of psychiatric manifestations in this period. J Clin Endocrinol Metab. 1996;81:1912–1917. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab. 1999;10:174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Tu AY, Myers MM. Lactation and weaning effects on physiological and behavioral response to stressors. Physiol Behav. 2003;78:1–9. doi: 10.1016/s0031-9384(02)00889-2. [DOI] [PubMed] [Google Scholar]
- Neumann I. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Progr Brain Res. 2001;133:143–151. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- Owens PC, Smith R, Brinsmead MW, Hall C, Rowley M, Hurt D, Lovelock M, Chan EC, Lewin T. Postnatal disappearance of the pregnancy-associated reduced sensitivity of plasma cortisol to feedback inhibition. Life Sci. 1987;41:1745–1750. doi: 10.1016/0024-3205(87)90603-5. [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ, editors. The Cayo Santiago Macaques. History, Behavior, and Biology. Albany, NY: SUNY Press; 1986. [Google Scholar]
- Saltzman W, Abbott DH. Diminished maternal responsiveness during pregnancy in multiparous female common marmosets. Horm Behav. 2005;47:151–163. doi: 10.1016/j.yhbeh.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Smith R, Wickings EJ, Bowman JME, Belleoud M, Dubreuil G, Davies JJ, Madsen A. Corticotropin-releasing hormone in chimpanzee and gorilla pregnancies. J Clin Endocrinol Metab. 1999;84:2820–2825. doi: 10.1210/jcem.84.8.5906. [DOI] [PubMed] [Google Scholar]
- Stern JM, Goldman L, Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12:179–181. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Taylor A, Littlewood J, Adams D, Dore’ C, Glover V. Serum cortisol levels are related to moods of elation and dysphoria in new mothers. Psychiatr Res. 1994;54:241–247. doi: 10.1016/0165-1781(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Ibbott MD, Clarke IJ. Activation of the hypothalamic-pituitary-adrenal axis by isolation and restraint stress during lactation in ewes: effect of the presence of the lamb and suckling. J Clin Endocrinol Metab. 2006;147:3501–3509. doi: 10.1210/en.2005-1632. [DOI] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, Walker CD. Measuring stress responses in postpartum mothers: perspectives from studies in human and animal populations. Stress. 2005;8:19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, Walker CD. Multiparity reveals the blunting effect of breastfeeding on physiological reactivity to psychological stress. J Neuroendocrinol. 2006;18:494–503. doi: 10.1111/j.1365-2826.2006.01441.x. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, Lupien S, Gallo-Payet N, Richard D. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J Psychiatr Neurosci. 2004;29:364–382. [PMC free article] [PubMed] [Google Scholar]


