Abstract
Alzheimer’s disease is the commonest cause of dementia in the elderly, but its pathological determinants are still debated. Amyloid-β plaques and neurofibrillary tangles have been implicated either directly as disruptors of neural function, or indirectly by precipitating neuronal death and thus causing a reduction in neuronal number. Alternatively, the initial cognitive decline has been attributed to subtle intracellular events caused by amyloid-β oligomers, resulting in dementia after massive synaptic dysfunction followed by neuronal degeneration and death. To investigate whether Alzheimer’s disease is associated with changes in the absolute cell numbers of ageing brains, we used the isotropic fractionator, a novel technique designed to determine the absolute cellular composition of brain regions. We investigated whether plaques and tangles are associated with neuronal loss, or whether it is dementia that relates to changes of absolute cell composition, by comparing cell numbers in brains of patients severely demented with those of asymptomatic individuals—both groups histopathologically diagnosed as Alzheimer’s—and normal subjects with no pathological signs of the disease. We found a great reduction of neuronal numbers in the hippocampus and cerebral cortex of demented patients with Alzheimer’s disease, but not in asymptomatic subjects with Alzheimer’s disease. We concluded that neuronal loss is associated with dementia and not the presence of plaques and tangles, which may explain why subjects with histopathological features of Alzheimer’s disease can be asymptomatic; and exclude amyloid-β deposits as causes for the reduction of neuronal numbers in the brain. We found an increase of non-neuronal cell numbers in the cerebral cortex and subcortical white matter of demented patients with Alzheimer’s disease when compared with asymptomatic subjects with Alzheimer’s disease and control subjects, suggesting a reactive glial cell response in the former that may be related to the symptoms they present.
Keywords: ageing, amyloid-β, dementia, neuronal loss, isotropic fractionator
Introduction
Alzheimer’s disease is currently the commonest cause of dementia in the elderly. Since its first description (Alzheimer, 1907; Fischer, 1907; Goedert, 2009), amyloid-β neuritic plaques and neurofibrillary tangles have often been considered as the main pathological hallmarks of Alzheimer’s disease (Mattson, 2004; Nestor et al., 2004; Palop and Mucke, 2010), despite growing post-mortem evidence for amyloid-β deposition in the cognitively normal elderly (Price and Morris, 1999; Bennett et al., 2006; Negash et al., 2011). Indeed, abundant amyloid-β neuritic plaques can be found in the brains of ageing subjects with documented normal cognition, a condition defined as asymptomatic Alzheimer’s disease. It is conceivable, therefore, that amyloid-β deposition per se is not the cause of cognitive impairment in Alzheimer’s disease. In fact, intracellular transcriptional and post-transcriptional events have been recently suggested as determinants of the first signs of cognitive dysfunction (e.g. abnormal production of amyloid-β oligomers: Gong et al., 2003; Lacor et al., 2004; Ferreira and Klein, 2011; and tau hyperphosphorylation: De Felice et al., 2008; Jin et al., 2011; Flunkert et al., 2012). Plaques and tangles, then, would be late events of the pathological cascade. Considering their late appearance in the brain, amyloid-β plaques were even proposed to act as protective buffers against toxic oligomers, being a possible antioxidant defence mechanism (Lee et al., 2004; Lesné et al., 2008).
Alternatively, Alzheimer’s disease aetiopathogeny has been related to anomalous cell cycle re-entrance before neuronal death (Herrup, 2010, 2012), presumably causing a relevant reduction in the number of neurons, and the subsequent progressive cognitive impairment that ends in dementia (Whitehouse et al., 1982; Neniskyte et al., 2011). According to this view, the initial cognitive decline would be caused by subtle intracellular events, and dementia would follow due to massive cell death. Indeed, a significant neuronal loss was reported in the hippocampus of patients with Alzheimer’s disease (Simic et al., 1997, Korbo et al., 2004; Giannakopoulos et al., 2009). For the neocortex as a whole (Bundgaard et al., 2001) and cerebellum (Andersen et al., 2003, 2012), despite hypometabolism and atrophy detected by neuroimaging in demented patients (Thompson et al., 2003; Chételat et al., 2008; Thomann et al., 2008), no evidence of significant neuronal loss has been found.
To clarify these inconsistencies, we sought to use the isotropic fractionator (Herculano-Houzel and Lent, 2005) to investigate whether dementia is associated with changes in the quantitative cellular composition of ageing brains. Using this novel technique, it is possible to determine the absolute cell composition of the brain and of brain regions with considerable precision (Azevedo et al., 2009, 2013; Lent et al., 2012). Therefore, by comparing the absolute neuronal and non-neuronal cell composition of the brains of cognitively-normal elderly with those of severely demented and asymptomatic patients with Alzheimer’s disease, we were able to investigate whether neuronal loss severity is associated with plaque and tangle burden, or whether it is dementia that could be related to changes of absolute cell composition.
We found a great reduction of neurons in the hippocampus and cerebral cortex of demented patients with Alzheimer’s disease when compared with asymptomatic subjects with Alzheimer’s disease and the cognitively-normal elderly, but no significant difference between the two latter groups. In contrast, we found an increase in non-neuronal cell numbers in the cortex and subcortical white matter of the demented Alzheimer’s group when compared to asymptomatic Alzheimer’s subjects and controls. The cerebellum, however, showed no changes in its absolute cell composition in all groups.
Materials and methods
Subjects
Fourteen female brains (Table 1) were obtained from the Brazilian Brain Bank of the Aging Brain Study Group (Grinberg et al., 2007) of the University of São Paulo Medical School. Brains were removed and fixed within 24 h of death. All procedures were approved by the local and national Ethics Committees. Informed consent for removal of the brains was provided by a knowledgeable informant, who also responded to semi-structured questionnaires designed to evaluate several functional domains (Ferretti et al., 2010). Cognition was assessed using the Clinical Dementia Rating Scale (Morris, 1993) and by the Informant Questionnaire on Cognitive Decline in the Elderly–Retrospective Version (IQCODE; Jorm and Jacomb, 1989).
Table 1.
Subjects demographic data
| Age (years) | Group | Mass (g) | CDR | IQCODE | Braak | CERAD | Histopathology | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 71 | Control | 1403.2 | 0 | 3.11 | <III* | 0 | Normal | Pulmonary oedema |
| 73 | Control | 1363.9 | 0 | 3.30 | I | 0 | Normal | Myocardiosclerosis |
| 74 | Control | 1289.32 | 0 | 3.00 | II | 0 | Normal | Cardiac tamponade |
| 82 | Control | 1385.9 | 0 | 3.00 | II | 0 | Normal | Pulmonary oedema |
| 84 | Control | 1013.7 | 0 | 3.00 | <III* | 0 | Normal | Hemoperitoneum |
| 82 | Asymptomatic Alzheimer’s disease | 1338.2 | 0 | 3.00 | V | Frequent | Alzheimer’s disease | Myocardiosclerosis |
| 82 | Asymptomatic Alzheimer’s disease | 1332.0 | 0 | 3.00 | IV | Moderate | Alzheimer’s disease | Pulmonary oedema |
| 80 | Asymptomatic Alzheimer’s disease | 1209.86 | 0 | 3.00 | IV | Moderate | Alzheimer’s disease | Pulmonary oedema |
| 80 | Asymptomatic Alzheimer’s disease | 1190.32 | 0 | 3.00 | VI | Frequent | Alzheimer’s disease | Pulmonary thromboembolism |
| 74 | Demented Alzheimer’s disease | 1402.2 | 3 | 5.00 | IV | Frequent | Alzheimer’s disease | Bronchopneumonia |
| 83 | Demented Alzheimer’s disease | 1255.7 | 3 | 5.00 | III | Moderate | Alzheimer’s disease | Pulmonary oedema |
| 86 | Demented Alzheimer’s disease | 1136.84 | 3 | 5.00 | VI | Frequent | Alzheimer’s disease | Bronchopneumonia |
| 88 | Demented Alzheimer’s disease | 1280.16 | 3 | 5.00 | IV | Moderate | Alzheimer’s disease | Serofibrinous pericarditis |
| 88 | Demented Alzheimer’s disease | 1204.2 | 3 | 5.00 | VI | Moderate | Alzheimer’s disease | Pulmonary oedema |
*Argyrophilic grain disease.
CDR = Clinical Dementia Rating scale; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease.
The control group comprised five elderly females with no cognitive impairment, who died of non-neurological causes. Five demented elderly female brains with histopathological diagnosis of Alzheimer’s disease were included in the demented-Alzheimer’s disease group. Four non-demented female brains, but with histopathological features of Alzheimer’s disease were included in the asymptomatic Alzheimer’s disease group (Table 1).
Brain handling, fixation and dissection
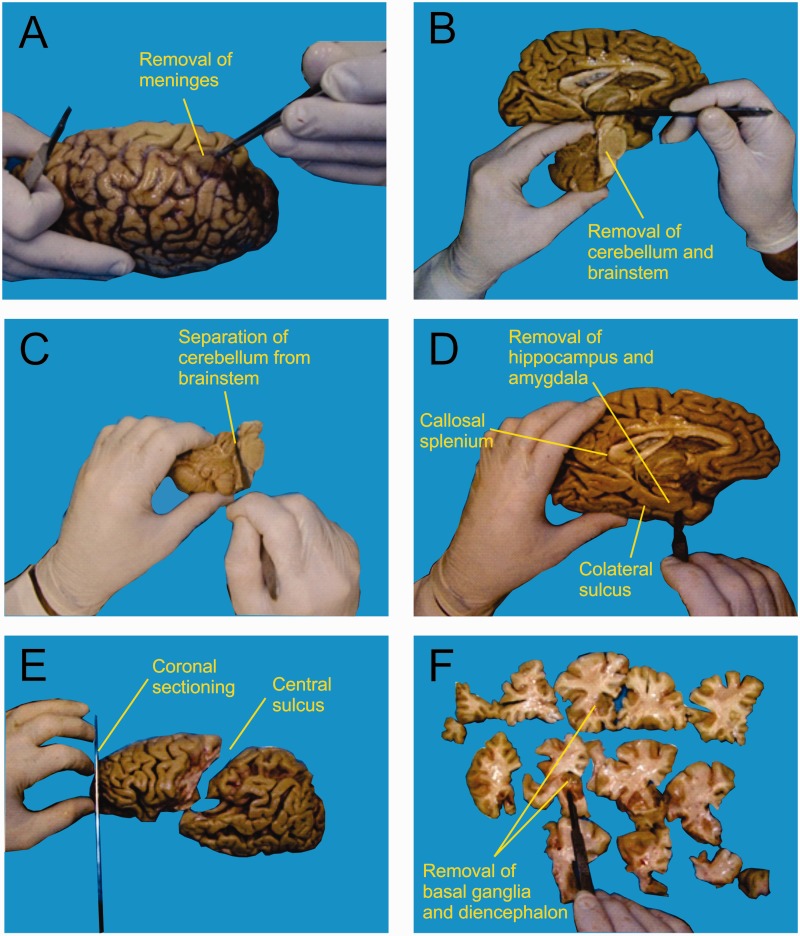
Because no significant differences were found in mass or in neuronal, non-neuronal and total cell numbers between right and left hemispheres by Azevedo et al. (2009), each brain was split on the midsagittal plane, and the left hemisphere immersed in 2% paraformaldehyde at 4°C, whereas samples of right hippocampus, amygdala, cortical areas, brainstem and cerebellum were collected for histopathological analysis, according to the Brazilian Brain Bank protocol (Grinberg et al., 2007). After removing the meninges and blood vessels (Fig. 1A), the left hemisphere was segmented into seven regions of interest: cerebellum, hippocampal formation with amygdala (hereafter termed ‘hippocampal formation’), frontal lobe grey and white matters, grey matter and white matter from other lobes, and remaining regions. The left cerebellar hemisphere was cut through the cerebellar peduncles, allowing its separation from the brainstem (Fig. 1B and C). The hippocampal formation was separated from the hemisphere by sectioning along the collateral sulcus up to the caudal-most coronal level of the callosal splenium (Fig. 1D). The frontal lobe was separated from the rest of the brain by a section made along the central sulcus (Fig. 1E), and the whole hemisphere was cut coronally into slices 1-cm thick (Fig. 1E and F). Next, the cerebral cortex and the subcortical white matter were separated from the remaining regions (basal ganglia, diencephalon, mesencephalon, pons and medulla oblongata) by cutting along the dorsolateral surface of the striatum, diencephalon and mesencephalon in each section (Fig. 1G). Finally, the coronal slices had the cortical grey matter dissected away from the underlying white matter by careful shaving around the gyri with a scalpel until grey and white matters were completely separated (not shown in Fig. 1).
Figure 1.
After removing the meninges and blood vessels (A), the left brainstem and cerebellum were separated from the brain (B), and the cerebellar peduncles were cut to isolate the cerebellum (C). The hippocampal formation was removed out from the hemisphere by sectioning along the collateral sulcus until the caudalmost coronal level of the callosal splenium (D). The frontal lobe was then separated from the rest of the brain by a section made along the central sulcus (E), and cut coronally into slices of ∼1 cm thickness (E and F). Finally (F), the cerebral cortex was separated from the remaining regions (basal ganglia, diencephalon, mesencephalon, pons and medulla oblongata).
The seven regions of interest were frozen in phosphate-buffered saline (pH 7.4) at −20°C and subjected, separately, to the isotropic fractionator (Herculano-Houzel and Lent, 2005). Each region was weighed and then cut into smaller pieces to be homogenized by an automatic machine composed of tissue grinders operated by servomotors under control of the experimenters (Azevedo et al., 2013).
Histopathological analysis and diagnostic criteria
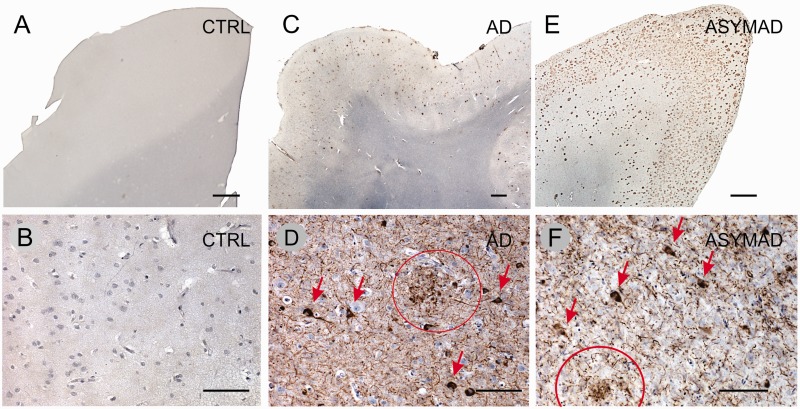
Tissue blocks from the right hemisphere were embedded in paraffin, cut and submitted to routine staining and immunohistochemistry (Fig. 2) for diagnostic purposes, according to a previously published protocol (Grinberg et al., 2007). In brief, immunohistochemistry was performed using antibodies against amyloid-β (4G8, 1/5000, Signet Laboratories), phospho-tau (PHF-1, 1/1000, provided by Peter Davies, New York), and α-synuclein (EQV-1, 1/10 000, provided by Kenji Ueda, Tokyo, Japan). If frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) was suspected, immunostaining for TDP-43 (1/500, ProteinTech) was performed, according to the brain bank protocol (Grinberg et al., 2007). Staining was performed on 8-µm thick sections cut from paraffin blocks and mounted on glass slides. For all antibodies, immunoperoxidase was employed using an avidin-biotin complex detection system (Vectastain® ABC kit; Vector Laboratories) with 3,3’diaminobenzidine as the chromogen. Slides were pretreated for antigen retrieval by immersion in citrate pH 6.0 in a steamer at 95°C for 45 min. The sections were incubated overnight at 4°C with the primary antibodies and for 1 h at room temperature with species-specific biotinylated secondary antibodies.
Figure 2.
Histopathological features of some cases, taken from sections through the inferior temporal cortex. Upper row (A, C and E), immunostaining for amyloid-β, and lower row (B, D and F), immunostaining for phospho-Tau (see ‘Materials and methods’ section for details). (A and B) Control (CTRL; 82 years, Clinical Dementia Rating Scale = 0, Braak II; see Table 1), showing no signs of pathology. (C and D) Demented patient with Alzheimer’s disease (AD, 74 years, Clinical Dementia Rating Scale = 3, Braak IV), with an abundant number of neuritic plaques (in brown). (E and F) Asymptomatic subject with Alzheimer’s disease (ASYMAD, 82 years, Clinical Dementia Rating Scale = 0, Braak V), showing an advanced pattern of Alzheimer’s pathology. Neurofibrillary tangles are indicated with arrows, and neuritic plaques indicated with circles in D and F. Scale bars: A, C and E = 1 mm; B, D and F = 100 µm.
For Alzheimer’s disease, amyloid-β neuritic plaques (Fig. 2) were assessed following the neuropathological guidelines of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD: Mirra et al., 1991), receiving semi-quantitative descriptors: none (0), scarce, moderate or frequent (Table 1). Neurofibrillary tangles (Fig. 2) were assigned a score (0–VI) according to the Braak and Braak (1991) staging system.
The control subjects had no history of cognitive and/or behavioural deficits, cerebrovascular disease, or alcohol/drug abuse. On neuropathological evaluation, the brains showed no neuritic plaques (Fig. 2). Accordingly, the CERAD score was 0 for these subjects. Neurofibrillary changes were confined to transentorhinal and entorhinal cortex, as well as hippocampus; thus, their Braak neurofibrillary tangles scores ranged between 0 and II (Fig. 2 and Table 1). One control case had mild argyrophilic grain disease and therefore the Braak staging could not be reliably assessed in the hippocampus. However, in this case there were no tangles outside the hippocampus, assuring that the Braak stage was <III.
The asymptomatic Alzheimer’s subjects had no history of behavioural deficits, cerebrovascular disease, or alcohol/drug abuse, and were cognitively intact. On neuropathological evaluation, however, their brains showed moderate or great quantities of neuritic amyloid-β plaques, and the neurofibrillary tangles Braak scores were at least IV (Fig. 2 and Table 1), indicating that the changes extended beyond the limbic areas.
The demented patients with Alzheimer’s disease were individuals who had received a clinical diagnosis of dementia (Clinical Dementia Rating Scale = 3), and whose neuropathological brain examination showed at least a moderate number of neuritic amyloid-β plaques, and neurofibrillary tangles Braak scores ≥III, indicating that changes extended outside the hippocampal formation. The cases did not show any other potential causes of cognitive decline (Fig. 2 and Table 1).
Chemomechanical dissociation, nuclei staining, immunocytochemistry and counting procedures
The isotropic fractionator was described previously by Herculano-Houzel and Lent (2005), applied to the human brain by Azevedo et al. (2009), and automated by Azevedo et al. (2013). Briefly, after the neural tissue is properly fixated, small fragments are collected and placed into the mortars of glass tissue grinders. A saline-detergent solution (40 mM sodium citrate; 1% Triton™ X-100) was added, and through careful and constant translation and rotation movements of a tightly coupled pestle, the tissue was disrupted chemomechanically by the combination of the dissociative effects of the detergent solution with the turbulent flow produced inside the grinder. The cell membranes are broken, releasing the intact nuclei into the fluid. The result was an isotropic suspension containing all cell nuclei from the fractioned region.
For the purpose of staining the nuclei of all cells, the fluorescent DNA marker 4’-6-diamino-2-phenylindole dihydrochloride (DAPI) was added to the suspension. Intense agitation was then performed to disperse the nuclei and achieve isotropy. Aliquots from the isotropic suspension were collected, deposited into a haemocytometer (Neubauer chamber) and imaged using a fluorescence microscope (Zeiss Axioplan). The average nuclei density was determined by counting the number of nuclei within sectors of the coverslipped haemocytometer (1 mm2 area; 0.1 mm depth) for four aliquots. The total number of cells originally present in the analysed region is then obtained by multiplying density by the total volume of the suspension. To identify the fraction of neuronal nuclei among the total number of DAPI-stained nuclei, another aliquot of the isotropic suspension was collected and selectively immunolabelled with mouse primary antibody against neuronal nuclear protein (NeuN, Chemicon; MAB 377B clone A60 against murine cells; 1:200 in PBS, overnight incubation at room temperature). Anti-NeuN antibody recognizes the majority of neuronal subtypes in a variety of vertebrate species, with the exception of cerebellar Purkinje cells, mitral cells of the olfactory bulb, inferior olivary nuclei of brainstem, dentate neurons of cerebellar deep nuclei, retinal photoreceptors, and nigral neurons in some rodents (Mullen et al., 1992; Wolf et al., 1996; Sarnat et al., 1998; Kumar and Buckmaster, 2007). The quantitative contribution of these cells as compared with the total brain numbers, however, is negligible (Herculano-Houzel and Lent, 2005; Herculano-Houzel et al., 2006). Then, the nuclei were washed in saline and incubated at room temperature for at least 2 h with the secondary antibody (Alexa Fluor® 555 anti-mouse goat IgG, Molecular Probes; 1:200 in PBS) and normal goat serum (1:10). By counting the number of NeuN-labelled nuclei among at least 500 DAPI-stained nuclei, the percentage of neurons present in the sample was determined and thus the total number of neurons in the analysed region was estimated. The non-neuronal cells were quantified as the difference between the total number of cells and the total number of neurons. Photomicrographs were taken for routine documentation using a Zeiss Axioplan fluorescence microscope. For all illustrations, contrast and brightness of the pictures were adjusted using Corel Draw X3.
Statistical analyses
The statistical analyses were performed by use of the Graph Prism 5.0 software. Analysis of variance (one-way ANOVA) and Tukey’s multiple comparison post hoc tests were used to compare results from these groups. The means are shown in Supplementary Tables 1–10, and means with standard deviations (SD) are represented in Figs 3-6.
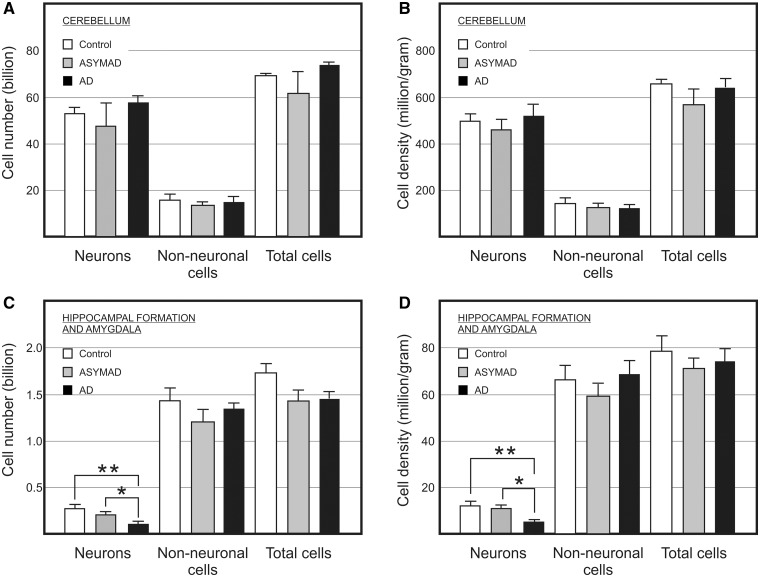
Figure 3.
Absolute bilateral cell number (A and C) and density (B and D) of cerebellar (A and B) and hippocampal formation (C and D) neuronal, non-neuronal, and total cells in control, asymptomatic Alzheimer’s disease (ASYMAD) and demented Alzheimer’s disease (AD) groups. Each bar represents mean and standard deviation. Significant differences are indicated by *P < 0.05, **P < 0.01.
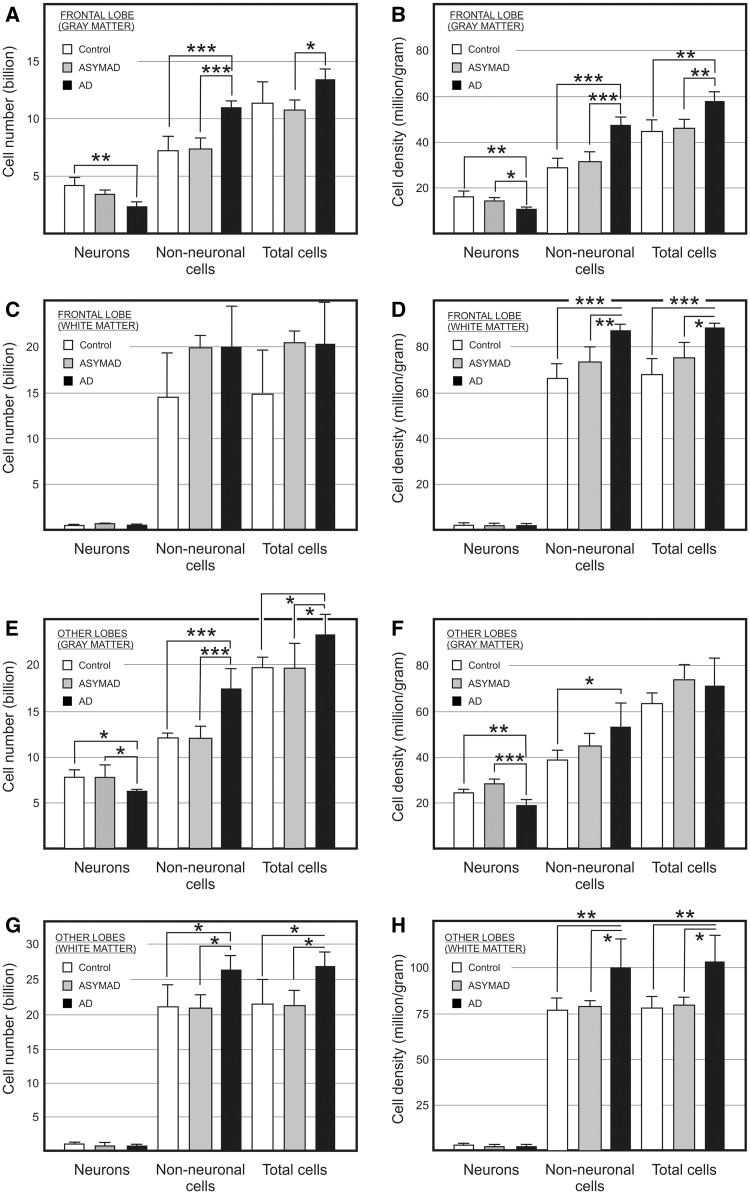
Figure 4.
Absolute bilateral cell number (A, C, E, and G) and density (B, D, F, and H) for neuronal, non-neuronal, and total cells in the grey (A and B) and white (C and D) matter of the frontal lobe (A–D) and of the other lobes as a whole (E–H) from control, asymptomatic Alzheimer’s disease (ASYMAD) and demented Alzheimer’s disease (AD) groups. Each bar represents the mean and standard deviation. Significant differences are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 5.
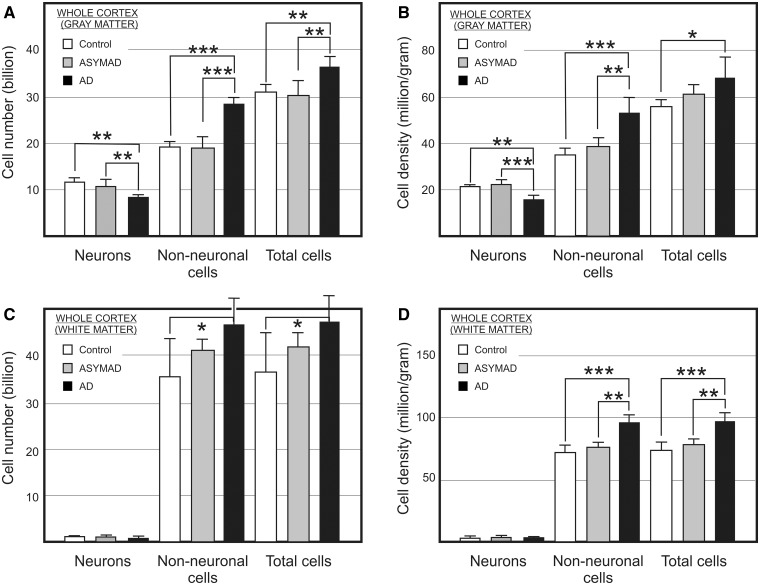
Absolute bilateral cell number (A and C) and density (B and D) for neuronal, non-neuronal, and total cells in the grey (A and B) and white (C and D) matter of the entire cerebral cortex from control, asymptomatic Alzheimer’s disease (ASYMAD) and demented Alzheimer’s disease (AD) groups. Each bar represents the mean and standard deviation. Significant differences are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 6.
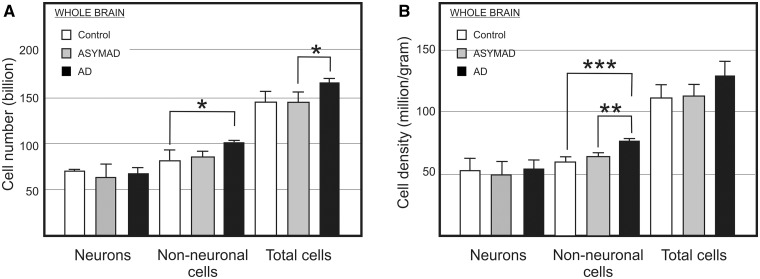
Absolute bilateral cell number (A) and density (B) for neuronal, non-neuronal, and total cells in the whole brain from control, asymptomatic Alzheimer’s disease (ASYMAD) and demented Alzheimer’s disease (AD) groups. Each bar represents the mean and standard deviation. Significant differences are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
We compared the absolute cell number and density between control subjects, asymptomatic individuals with Alzheimer’s disease, and severely demented patients with Alzheimer’s disease. Subjects’ demographic data are shown in Table 1. Numbers shown below refer to both hemispheres together, after multiplying the counts obtained for the left hemispheres by 2, as explained in the ‘Materials and methods’ section.
Cerebellum
Although representing only 8.1–9.1% of total brain mass, the cerebellum contains 77.5% to 79.8% of all brain neurons in elderly females. ANOVA and multiple comparison post hoc test, however, showed no significant differences in cerebellar mass, absolute cell number or density among groups (Fig. 3A and B; Supplementary Table 1). The non-neuronal/neuronal ratio and the fractional distribution of neurons were similar among groups.
These results suggest that this brain region seems not to be affected by Alzheimer’s disease, at least regarding absolute cellular composition and cell density.
Hippocampal formation and amygdala
Using one-way ANOVA to compare absolute neuronal number in the hippocampal formation (Fig. 3C), significant differences between groups were found. Tukey’s test indicated that only the demented patients with Alzheimer’s disease group (110 million) differed statistically from control subjects (270 million), which was not the case for the asymptomatic-Alzheimer’s disease group (220 million). Neuronal loss in demented patients with Alzheimer’s disease results in more than half the number neurons than in control subjects. For the absolute number of non-neuronal cells, ANOVA revealed no significant difference among groups (Fig. 3C and Supplementary Table 2). To verify whether the difference in neuronal number was due to variability of brain mass or by uncertainties in dissection, we focused on cell density. ANOVA and multiple comparison tests showed a significant reduction in neuronal density in demented patients with Alzheimer’s disease when compared with the control and asymptomatic Alzheimer’s disease groups, but not when asymptomatic-subjects with Alzheimer’s disease and control subjects were compared (Fig. 3D).
On the other hand, ANOVA did not reveal any significant difference in non-neuronal cell density among groups (Fig. 3D). The non-neuronal/neuronal ratio was higher in the demented patients with Alzheimer’s disease group when compared with controls and asymptomatic patients with Alzheimer’s disease. Inversely, the fractional distribution of neurons was lower in the demented Alzheimer’s disease group (Supplementary Table 2). These differences represent a great reduction of >50% in absolute neuronal number and in neuronal density of demented patients with Alzheimer’s disease compared with control subjects and asymptomatic patients with Alzheimer’s disease.
Frontal lobe
Grey matter
ANOVA comparing the three groups showed significant differences in absolute neuronal composition of the frontal grey matter. However, using a multiple comparison test, the only significant difference was found between controls (4.17 billion) and demented patients with Alzheimer’s disease (2.46 billion) (Fig. 4A and Supplementary Table 3). We analysed the neuronal density in addition to absolute numbers. ANOVA showed significant differences in neuronal density of frontal grey matter among all groups. However, when Tukey’s test was used, no significant difference in neuronal density was found between control and asymptomatic Alzheimer’s disease groups (Fig. 4B and Supplementary Table 3).
Thus, a significant reduction of ∼41% in the absolute number of neurons was found in the demented Alzheimer’s disease group when compared with control subjects. Similarly, a significant reduction of 34% neuronal density in frontal grey matter was found in the demented Alzheimer’s disease group when compared with control subjects, and 26% when compared to the asymptomatic patients with Alzheimer’s disease group. The fractional distribution of neurons in the demented patients with Alzheimer’s disease was lower than in the other groups, such as the proportion of all neurons found in the whole brain.
ANOVA and multiple comparison test showed a significant increase of 47% in absolute non-neuronal cell number in demented patients with Alzheimer’s disease (10.97 billion) when compared with control subjects (7.48 billion), and 49% when compared with asymptomatic patients with Alzheimer’s disease (7.34 billion), but no significant difference was found when control and asymptomatic Alzheimer’s disease groups were compared. Correcting for mass effects, ANOVA and post hoc test showed a significant increase of 64% in non-neuronal cell density in demented Alzheimer’s disease group (47.26 million/g) when compared with controls (28.79 million/g), and 52% when compared with asymptomatic subjects with Alzheimer’s disease (30.98 million/g). However, no significant difference was found when asymptomatic patients with Alzheimer’s disease and the control group were compared (Fig. 4A).
The non-neuronal/neuronal ratio was higher in demented-patients with Alzheimer’s disease when compared with the other two groups. However, ANOVA showed no significant differences of frontal lobe grey matter mass among groups (Supplementary Table 3).
White matter
ANOVA showed no significant statistical difference of absolute cell composition of the frontal lobe white matter between controls, asymptomatic patients with Alzheimer’s disease and the demented Alzheimer’s disease group (Fig. 4C). However, post hoc tests showed a significant increase of 32% in non-neuronal cell density in demented patients with Alzheimer’s disease (86.68 million/g) when compared with controls (65.66 million/g), and 19% when compared with asymptomatic subjects with Alzheimer’s disease (72.61 million/g). However, no significant difference was found when control subjects and asymptomatic patients with Alzheimer’s disease were compared (Fig. 4D and Supplementary Table 4). The non-neuronal/neuronal ratio was higher in demented-patients with Alzheimer’s disease.
Other lobes
Grey matter
ANOVA and multiple comparison test found significant differences in the absolute neuronal composition between control subjects (7.62 billion) and demented patients with Alzheimer’s disease (5.91 billion), and between asymptomatic subjects with Alzheimer’s disease (7.69 billion) and the demented Alzheimer’s disease group, but not between control subjects and asymptomatic patients with Alzheimer’s disease (Fig. 4E). Thus, a significant reduction of ∼22% in the absolute number of neurons of the grey matter was found in the demented patients with Alzheimer’s disease when compared with control subjects, and about the same proportion when compared with the asymptomatic subjects with Alzheimer’s disease. Using ANOVA and Tukey’s test, a significant reduction of 24% in neuronal density was found in demented patients with Alzheimer’s disease when compared with controls, and almost 36% when compared with asymptomatic subjects with Alzheimer’s disease (Fig. 4F). Thus, the proportion of neurons was lower in Alzheimer’s disease when compared with the other two groups (Supplementary Table 5).
When ANOVA and multiple comparison test of absolute non-neuronal cell number was performed among all groups, a significant increase of 45% in the demented patients with Alzheimer’s disease (17.35 billion) was found when compared with the control group (11.93 billion), and the same proportion when compared with the asymptomatic subjects with Alzheimer’s disease (11.85 billion), but was not significant when control and asymptomatic Alzheimer’s disease groups were compared. ANOVA and multiple comparison test found a significant increase of 42% in non-neuronal cell density in demented patients with Alzheimer’s disease (54.59 million/g) when compared with controls (38.47 million/g), but not when compared to asymptomatic subjects with Alzheimer’s disease (44.58 million/g), and no significant difference was found when asymptomatic subjects with Alzheimer’s subjects and controls were compared (Fig. 4B and Supplementary Table 5).
The non-neuronal/neuronal ratio was higher in demented-Alzheimer’s disease when compared to the other groups.
White matter
ANOVA found significant differences in the absolute non-neuronal cell number among all groups. Multiple comparison test analysis showed a significant increase of 26% in absolute non-neuronal cells in demented patients with Alzheimer’s disease (26.4 billion) when compared with control subjects (20.93 billion), and the same proportion when compared with asymptomatic patients with Alzheimer’s disease (20.92 billion). However, no significant difference was found when control and asymptomatic Alzheimer’s disease groups were compared (Fig. 4G). ANOVA showed a significant difference in non-neuronal cell density of the white matter among control, asymptomatic subjects with Alzheimer’s disease and demented patients with Alzheimer’s disease (Fig. 4H). Tukey’s post hoc multiple comparison test showed a significant increase of 30% and 28% in the demented patients with Alzheimer’s disease (99.92 million/g) when compared with control subjects (76.71 million/g) and to asymptomatic subjects with Alzheimer’s disease (78.17 million/g), respectively. However, no significant difference was found between control and asymptomatic Alzheimer’s disease groups.
The non-neuronal/neuronal ratio was higher in demented patients with Alzheimer’s disease when compared with the other groups (Supplementary Table 6).
Cerebral cortex
The cerebral cortex as a whole (here comprising cortical grey and subcortical white matter) in cognitively normal elderly females, was found to represent ∼82% of brain mass. Neurons therein amount to 12.7 billion (19%), and non-neuronal cells reach 54.9 billion (81%).
Grey matter
ANOVA of the absolute number of neurons showed significant differences among groups (Fig. 5A and Supplementary Table 7). Using Tukey’s multiple test, a significant reduction of 29% was found in the demented-patients with Alzheimer’s disease (8.38 billion) when compared with controls (11.8 billion), and 25% when compared with the asymptomatic subjects with Alzheimer’s disease (11.12 billion), but no difference when asymptomatic Alzheimer’s disease and control groups were compared.
ANOVA also showed significant differences of absolute non-neuronal cell number in the cerebral cortex, among all groups. Using the multiple comparison post hoc test, a significant increase of ∼46% was revealed in demented patients with Alzheimer’s disease (28.32 billion) when compared with control subjects (19.4 billion), and a similar increase when compared with asymptomatic patients with Alzheimer’s disease (19.2 billion). However, no significant difference was found when control and asymptomatic subjects with Alzheimer’s disease were compared. For non-neuronal cell density, one-way ANOVA showed a significant difference among groups (Fig. 5B). When compared with control subjects (34.04 million/g), a significant increase of 51% was found in the demented patients with Alzheimer’s disease (51.49 million/g) and of 35% when compared with the asymptomatic subjects with Alzheimer’s subjects (38.19 million/g). However, no significant difference was found between asymptomatic Alzheimer’s disease and control groups.
The non-neuronal/neuronal ratio was higher in demented patients with Alzheimer’s disease when compared with the other groups (Supplementary Table 7). Inversely, the whole cerebral cortex of demented patients with Alzheimer’s disease contains a lower proportion of all neurons in the brain (Supplementary Tables 7 and 10). ANOVA showed no significant difference of cortical mass among groups.
Correcting for mass, ANOVA revealed a significant difference of neuronal density in the cerebral cortex among groups (Fig. 5B). Post hoc analysis showed a significant reduction of 26% in demented patients with Alzheimer’s disease (15.24 million/g) when compared with control subjects (20.7 million/g) and ∼31% when compared with the asymptomatic subjects with Alzheimer’s disease (22.12 million/g), but no difference when asymptomatic Alzheimer’s disease and control groups were compared (Supplementary Table 7).
White matter
ANOVA showed no significant difference in the absolute neuronal composition and neuronal density of total subcortical white matter between control, asymptomatic subjects with Alzheimer’s disease, and demented patients with Alzheimer’s disease. The proportion of neurons was low and similar in all groups (Fig. 5C and D).
On the other hand, although one-way ANOVA showed a significant difference of absolute non-neuronal cell numbers among groups, multiple comparison post hoc analysis revealed a significant increase of almost 27% in the demented Alzheimer’s disease group (44.97 billion) when compared with control subjects (35.51 billion), but neither when compared with asymptomatic patients with Alzheimer’s disease (40.88 billion), nor when control and asymptomatic Alzheimer’s disease groups were compared. Furthermore, we found a significant increase of non-neuronal cell density of ∼31% in the demented Alzheimer’s disease group (94 million/g) when compared with control subjects (71.75 million/g), and 25% when compared with asymptomatic subjects with Alzheimer’s disease (75.36 million/g), but no difference was found when control and asymptomatic Alzheimer’s disease groups were compared.
The non-neuronal/neuronal ratio was higher in demented patients with Alzheimer’s disease when compared with the other groups (Supplementary Tables 8 and 10).
Remaining regions
The remaining regions comprise the basal nuclei, diencephalon and brainstem considered together for counting purposes.
ANOVA and Tukey’s post hoc test indicated a significant reduction of 45% in the absolute neuronal number of the remaining regions in demented patients with Alzheimer’s disease (330 million) when compared with controls (600 million), and 47% when compared with asymptomatic subjects with Alzheimer’s disease (620 million). Further, a significant reduction of 45% of neuronal density was found in the demented Alzheimer’s disease group (3.3 million/g) when compared with control subjects (6 million/g), and 49% when compared with asymptomatic subjects with Alzheimer’s disease (6.5 million/g). However, there was no significant difference between control and asymptomatic Alzheimer’s disease groups (Supplementary Table 9). Remaining regions from demented patients with Alzheimer’s disease contain a lower proportion of all neurons in the brain when compared to the other groups.
On the other hand, neither ANOVA nor Tukey’s test showed any significant difference of absolute non-neuronal cell composition and non-neuronal cell density between these groups.
The non-neuronal/neuronal ratio was higher in demented patients with Alzheimer’s disease when compared with the other groups (Supplementary Table 9).
Whole brain
One-way ANOVA showed no significant difference of absolute neuronal composition and neuronal density in the whole brain between controls (67.31 billion/52.13 million/g), asymptomatic patients with Alzheimer’s disease (63.16 billion/49.83 million/g) and demented patients with Alzheimer’s disease (67.82 billion/53.66 million/g), and the same was found when using the multiple comparison test (Fig. 6A and B; Supplementary Table 10). In contrast, ANOVA and the post hoc test indicated a significant increase of 23% in absolute non-neuronal cells in demented patients with Alzheimer’s disease (96.8 billion) when compared with control subjects (78.6 billion), but not when compared with asymptomatic Alzheimer’s disease subjects (81.9 billion), or when asymptomatic Alzheimer’s disease subjects and control groups were compared (Fig. 6A).
Further, ANOVA and post hoc analysis indicated a significant increase of 26% in non-neuronal cell density in the demented patients with Alzheimer’s disease (76.6 million/g) when compared with the controls (60.86 million/g), and ∼18% when compared with asymptomatic Alzheimer’s disease subjects (64.6 million/g) (Fig. 6B and Supplementary Table 10).
Discussion
The classical hypothesis that Alzheimer’s dementia would be caused by neuronal loss because of amyloid-β plaques and neurofibrillary tangles has weakened, as these pathological deposits were found in autopsied brains of cognitively-normal elderly. Alternative explanations have been proposed, such as a synaptic attack by amyloid-β oligomers (Gong et al., 2003; Lacor et al., 2004; Vieira et al., 2007; Ferreira and Klein, 2011; Sebollela et al., 2012), and tau hyperphosphorylation (De Felice et al., 2008; Jin et al., 2011; Flunkert et al., 2012), but the causal relation of amyloid-β with cell cycle re-entrance (Herrup, 2010, 2012) and neuronal death (Neniskyte et al., 2011) has heated the debate again. We here report a quantitative investigation of the relation between neuronal loss and Alzheimer’s disease in different brain regions of severely demented and asymptomatic patients with Alzheimer’s disease.
We found a robust, significant reduction of neurons in the hippocampus, cerebral cortex and subcortical regions (except the cerebellum), and a concomitant increase of non-neuronal cell number in cortical grey and subcortical white matter of demented-patients with Alzheimer’s disease as compared to asymptomatic-Alzheimer’s subjects. We conclude that it is dementia that correlates with neuronal loss and glial increase, not Alzheimer’s disease itself when defined by histopathological features. It will be important for future work to confirm our data with larger samples, and to relate the quantitative data and ascertain the histopathological diagnosis with determined biochemical levels of amyloid-β.
Neuronal loss in Alzheimer’s disease brains correlates with dementia, not with plaques and tangles
Because neurons in the human cortex seem not to be significantly replaced after birth (Rakic, 1985; Bhardwaj et al., 2006), it is conceivable that neuronal loss can lead to irreversible cognitive changes. Nevertheless, a large wealth of data has associated cognitive decline with progressive cortical mass atrophy, not necessarily linked to cell death. Mouton et al. (1998) implicate synapse degeneration, whereas Thompson et al. (2003) report neurofibrillary tangle accumulation. However, some studies have suggested that distribution and severity of tangle accumulation closely follows the progress of neuronal loss (Gomez-Isla et al., 1996, 1997; Morrison and Hof, 1997, 2007). Furthermore, others have attributed atrophy to a set of events besides neuronal loss, such as cell shrinkage, reduced dendritic extent, and synaptic loss (DeKosky and Scheff, 1990; Uylings and de Brabander, 2002).
We here report a significant reduction of neurons in the demented Alzheimer’s disease group in both frontal and posterior cortical regions when compared with asymptomatic subjects with Alzheimer’s disease. These results indicate that cognitive impairment correlates with lower neuronal numbers in the cerebral cortex, but not to amyloid-β and neurofibrillary tangles, as brains from asymptomatic subjects with Alzheimer’s disease did not show any difference compared with control subjects. It has been proposed that the Alzheimer’s disease syndrome includes cognitive impairment of higher-order functions, and that a pronounced, widespread loss of cortical neurons takes place in these cases (Gomez-Isla et al., 1997; Morrison and Hof, 1997, 2007; Neniskyte et al., 2011); our quantitative data support this idea.
Similar results were obtained in the hippocampus. Classical studies have proposed that neuronal loss therein is a morphological correlate of memory impairment in Alzheimer’s disease (Ball, 1977; Hyman et al., 1984; Mani et al., 1986). Indeed, in demented patients with Alzheimer’s disease, the hippocampus is severely affected (Braak and Braak, 1991), and the consistency of hippocampal histopathology has led to a description of Alzheimer’s disease as a ‘hippocampal dementia’ (Ball et al., 1985). Our results are in agreement with these histopathological findings, and support previous stereological (Kril et al., 2004; West et al., 2004) and neuroimaging studies (Apostolova et al., 2010; Stoub et al., 2010).
Besides cerebral cortex and hippocampus involvement in Alzheimer’s disease, some pathology studies have suggested that Alzheimer’s syndrome actually begins in the brainstem and disseminates throughout the brain (Yamamoto and Hirano, 1985; Hardy et al., 1986; German et al., 1987; Grinberg et al., 2009; Simic et al., 2009; Braak et al., 2011; Grinberg et al., 2011). These authors argue that the current clinical criteria for Alzheimer’s disease diagnosis are focused on cognitive deficits produced by dysfunction of hippocampal and high order neocortical areas, whereas other behavioural and psychological symptoms of dementia such as disturbances in mood, emotion, wake-sleep cycle, confusion and depression, may reflect brainstem involvement, more specifically of serotonergic nuclei, in the pathogenesis of Alzheimer’s disease (Mann and Yates, 1983; Aletrino et al., 1992; Michelsen et al., 2008).
In addition to brainstem involvement, pathological (Whitehouse et al., 1981; Braak and Braak, 1990) and neuroimaging studies (Barber et al., 2002; Bruen et al., 2008; De Jong et al., 2008) have found a correlation in the degree of cognitive decline with volume reduction of basal ganglia and thalami in demented patients. These changes were correlated linearly with impaired cognitive performance, and strongly suggest that, besides cortical and hippocampal atrophy, deep grey matter structures in Alzheimer’s disease suffer atrophy as well, and that degenerative processes in the putamen and thalamus may contribute to the cognitive decline seen in Alzheimer’s disease.
Although we investigated the cellular composition of the brainstem together with basal ganglia (remaining regions), our results may give support to the evidence described above. We found a significant reduction of neurons in the remaining regions of the demented patients with Alzheimer’s disease when compared with asymptomatic patients with Alzheimer’s disease. These findings strengthen the idea that plaques and tangles are not the main cause for the clinical presentation of these patients, and that the memory deficit is not the only symptom seen in Alzheimer’s disease, but one among other dysfunctions.
Although the cerebellum is traditionally considered a neural region in charge of posture, balance, motor coordination and learning (Marr, 1969; Thach, 1998; Wegiel et al., 1999; Boyden et al., 2004; Morton and Bastian, 2004), recent studies implicate it in non-motor functions, such as cognitive, behavioural and affective processing (Ito, 1989; Leiner et al., 1991; Bower, 1997; Timmann and Daum, 2007; Schmahmann, 2010). Nevertheless, we failed to find any significant difference in the absolute cellular composition and density among asymptomatic Alzheimer’s disease and demented Alzheimer’s disease subjects compared with controls, suggesting that the cerebellum is not quantitatively vulnerable to Alzheimer’s disease.
Widespread increase of non-neuronal cell number also correlates with dementia, not with plaques and tangles
It has been proposed that reduction of white matter volume may be associated with weakening of cognitive functions because of a fall in propagation speed of electrical impulses (Marner et al., 2003; Burns et al., 2005; Jørgensen et al., 2008). Indeed, reduction of white matter volume has been observed in demented patients with Alzheimer’s disease by use of both in vivo MRI and post-mortem stereological techniques. Atrophy of the corpus callosum has been recognized as a well-known feature of Alzheimer’s disease (Capizzano et al., 2003; Chaim et al., 2007), and the same for the fornix, cingulum bundle, perforant path and temporal white matter (Hyman et al., 1986; Villain et al., 2008).
Despite these changes, few studies have elucidated the impact of Alzheimer’s disease on glial cell numbers. We approached this issue by quantifying the total number of non-neuronal cells in Alzheimer’s disease. Although the isotropic fractionator does not discern glial cells from other non-neuronal cells in the brain for lack of specific nuclear markers, it is possible to infer the number of glial cells using morphological criteria to count their nuclei.
We showed an increase of glial cell number in demented patients with Alzheimer’s disease as compared with cognitively normal and asymptomatic subjects with Alzheimer’s disease in both white and grey matters of the cerebral cortex, more pronounced in the frontal lobe than elsewhere, in contrast to another report (Pelvig et al., 2003). These results can be correlated with the frontal impairment seen in demented patients, and therefore this would be because of glial cell increase, together with neuronal reduction.
There is cellular and histological evidence in support of this interpretation. Using post-mortem MRI and neuropathological approaches, Polvikoski et al. (2010) found a correlation between frontal white matter signal increase and tangles in demented patients with Alzheimer’s disease. It could be, thus, that axonal degeneration would follow neuronal atrophy in these cases. Other authors (Sjöbeck and Englund, 2003; Sjöbeck et al., 2005) have reported that astrocyte number and reactivity, and astrocyte/oligodendrocyte ratio, are significantly greater in demented patients with Alzheimer’s disease, whereas oligodendrocyte counts are significantly lower. According to Sjöbeck and Englund (2003), astrocyte/oligodendrocyte ratio is positively correlated with severity of white matter disease. As these represent the majority of glial cell types in the brain (Pelvig et al., 2008), functional, morphological (Rodríguez et al., 2009; Zhao et al., 2011), and cell number changes in Alzheimer’s disease (Sjöbeck and Englund, 2003; Sjöbeck et al., 2005) could give support to our interpretation.
On the other hand, although reactive astrocytes and activated microglial cells are commonly associated with dense-core amyloid plaques, thus suggesting that amyloid-β triggers gliosis (Itagaki et al., 1989; Pike et al., 1995; Vehmas et al., 2003), we failed to find any significant increase of glial cell numbers anywhere in the cortex of asymptomatic subjects with Alzheimer’s disease.
Conclusion
Despite the relatively small sample, our findings show a robust correlation between dementia and neuronal loss in hippocampus, cortex and subcortical regions, and reinstate that the presence of amyloid-β plaques and neurofibrillary tangles per se is not the main feature responsible for the change in neuronal composition, or for dementia of patients with Alzheimer’s disease. These results emphasize the need to orient future research to look for a direct link between the subtle initial disruption of cell function, resulting in cell cycle re-entrance and apoptosis, as suggested by Herrup (2010, 2012). In summary, our quantitative results may mean, at least partially, that the asymptomatic subjects with histopathological features of Alzheimer’s disease do not present with dementia because they lack both neuronal loss and glial increase in their brains.
Funding
This work was supported by grants provided to R.L. by the Brazilian Council for Science and Technology Development (CNPq), the Rio de Janeiro Foundation for the Support of Science (FAPERJ), and the Brazilian Ministry of Science, Technology and Innovation (Program of National Institutes of Science and Technology, MCTI-INCTs). C.H.A.M. and A.V.O.P. received CAPES PhD fellowships during the course of this work. L.T.G. was funded by NIH grant R01AG040311-01 and the São Paulo Foundation for the Support of Science (FAPESP). R.E.P.L. and R.D.R. were funded by FAPESP. The Brain Bank of the Brazilian Aging Brain Study Group was funded by LIM-22 FMUSP, Hospital Israelita Albert Einstein, FAPESP and CAPES.
Supplementary material
Supplementary material is available at Brain online.
References
- Aletrino MA, Vogels OJ, Van Domburg PH, Ten Donkelaar HJ. Cell loss in the nucleus raphes dorsalis in Alzheimer’s disease. Neurobiol Aging. 1992;13:461–8. doi: 10.1016/0197-4580(92)90073-7. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Uber einen eigenartige Erkranung der Himrinde. Allgem Zeit Psychiatr Psychisch-Gerichtisch Med. 1907;64:146–8. [Google Scholar]
- Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging. 2012;33:11–20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–88. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FAC, Andrade-Moraes CH, Curado MR, Oliveira-Pinto AV, Guimarães DM, Szczupak D, et al. Automatic isotropic fractionation for quantitative cell analysis of large brains and brain regions. J Neurosci Meth. 2013;212:72–8. doi: 10.1016/j.jneumeth.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LR, Grinberg LT, Farfel JM, Ferretti R, Leite R, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–41. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Ball MJ. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia: a quantitative study. Acta Neuropathol. 1977;37:111–8. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Ball MJ, Fisman M, Hachinski V, Blume W, Fox A, Kral VA, et al. A new definition of Alzheimer’s disease: a hippocampal dementia. Lancet. 1985;325:14–16. doi: 10.1016/s0140-6736(85)90965-1. [DOI] [PubMed] [Google Scholar]
- Barber R, McKeith I, Ballard C, O’Brien J. Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. J Neurol Neurosurg Psychiatry. 2002;72:406–7. doi: 10.1136/jnnp.72.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12546–68. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bower JM. Control of sensory data acquisition. Int Rev Neurobiol. 1997;41:489–513. doi: 10.1016/s0074-7742(08)60367-0. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol. 1990;49:215–24. [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455–63. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Bundgaard MJ, Regeur L, Gundersen HJG, Pakkenberg B. Size of neocortical neurons in control subjects and Alzheimer’s disease. J Anat. 2001;198:481–9. doi: 10.1046/j.1469-7580.2001.19840481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–6. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Ación L, Bekinschtein T, Furman M, Gomila H, Martínez A, et al. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. Neurol Neurosurg Psychiatry. 2003;75:822–7. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaim TM, Duran FL, Uchida RR, Périco CA, De Castro CC, Busatto GF. Volumetric reduction of the corpus callosum in Alzheimer’s disease in vivo as assessed with voxel-based morphometry. Psychiatry Res. 2007;154:59–68. doi: 10.1016/j.pscychresns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;9:1334–47. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong LW, Van der Hiele K, Veer IM, Houwing JJ, Westendorp RGJ, Bollen ELEM, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain. 2008;131:3277–85. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Ferreira ST, Klein WL. The Aβ oligomer hypothesis for synaptic failure and memory loss in Alzheimer's disease. Neurobiol Learn Mem. 2011;96:529–43. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti REL, Damin A, Brucki SMD, Morillo LS, Balbino ES, Lima MCA, et al. Postmortem diagnosis of dementia by informant interview. Dement Neuropsychol. 2010;4:138–44. doi: 10.1590/S1980-57642010DN40200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer O. Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmassige Veranderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiatr Neurol. 1907;22:361–72. [Google Scholar]
- Flunkert S, Hierzer M, Löffler T, Rabl R, Neddens J, Duller S, et al. Elevated levels of soluble total and hyperphosphorylated Tau result in early behavioral deficits and distinct changes in brain pathology in a new Tau transgenic mouse model. Neurodegener Dis. 2012 doi: 10.1159/000338152. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- German DC, White CL, III, Sparkman DR. Alzheimer’s disease: neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience. 1987;21:305–12. doi: 10.1016/0306-4522(87)90123-0. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Kovari E, Gold G, von Gunten A, Hof PR, Bouras C. Pathological substrates of cognitive decline in Alzheimer’s disease. Front Neurol Neurosci. 2009;24:20–9. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- Goedert M. Oskar Fischer and the study of dementia. Brain. 2009;132:1102–1111. doi: 10.1093/brain/awn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, Jr, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, et al. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci. 2003;18:10417–22. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg L, Ferreti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, et al. Brain bank of Brazilian aging brain study group: a milestone reached and more than 1600 collected brains. Cell Tissue Bank. 2007;8:151–62. doi: 10.1007/s10561-006-9022-z. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, et al. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;4:406–16. doi: 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rueb U, Heinsen H. Brainstem: neglected locus in neurodegenerative diseases. Front Neurol. 2011;2:42. doi: 10.3389/fneur.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Mann DM, Wester P WinblAlzheimer’s disease B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer’s disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–21. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103:12138–43. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. The involvement of cell cycle events in the pathogenesis of Alzheimer's disease. Alzheimers Res Ther. 2010;2:13. doi: 10.1186/alzrt37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. The contributions of unscheduled neuronal cell cycle events to the death of neurons in Alzheimer's disease. Front Biosci. 2012;4:2101–9. doi: 10.2741/527. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol. 1986;20:472–81. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–82. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011;108:5819–24. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen AMB, Marner L, Pakkenberg B. No change in total length of white matter fibers in Alzheimer’s disease. J Neurosci. 2008;157:878–83. doi: 10.1016/j.neuroscience.2008.06.075. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Korbo L, Amrein I, Lipp H-P, Wolfer D, Regeur L, Oster S, et al. No evidence for loss of hippocampal neurons in non-Alzheimer dementia patients. Acta Neurol Scand. 2004;109:132–39. doi: 10.1034/j.1600-0404.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Hodges J, Halliday G. Relationship between hippocampal volume and CA1 neuron loss in brains of humans with and without Alzheimer’s disease. Neurosci Lett. 2004;361:9–12. doi: 10.1016/j.neulet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Neuron-specific nuclear antigen NeuN is not detectable in gerbil substantia nigra pars reticulata. Brain Res. 2007;1142:54–60. doi: 10.1016/j.brainres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusbeci OY, Bas O, Gocmen-Mas N, Karabekir HS, Yucel A, Ertekin T, et al. Evaluation of cerebellar asymmetry in Alzheimer’s disease: a stereological study. Dement Geriatr Cogn Disord. 2009;28:1–5. doi: 10.1159/000228544. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer’s-related amyloid β-oligomers. J Neurosci. 2004;45:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Zhu X, Takeda A, Perry G, Smith MA. Senile plaques and amyloid-β as protective Alzheimer’s diseaseaptions to Alzheimer’s disease. Ann NY Acad Sci. 2004;1019:1–4. doi: 10.1196/annals.1297.001. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–28. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Lent R, Azevedo FAC, Andrade-Moraes CH, Oliveira-Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci. 2012;35:1–9. doi: 10.1111/j.1460-9568.2011.07923.x. [DOI] [PubMed] [Google Scholar]
- Lesné S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neurosci. 2008;151:745–9. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani RB, Lohr JB, Jeste DV. Hippocampal pyramidal cells and aging in the human: a quantitative study of neuronal loss in sectors CA1 to CA4. Exp Neurol. 1986;94:29–40. doi: 10.1016/0014-4886(86)90269-4. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO. Serotonin nerve cells in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1983;46:96. doi: 10.1136/jnnp.46.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–64. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris JC. The CDR: current version and scoring rules. Neurology. 1993;43:2412–3. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–19. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol. 2004;92:2497–509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Martin LJ, Calhoun ME, Dal Forno G, Price DL. Cognitive decline strongly correlates with cortical atrophy in Alzheimer’s dementia. Neurobiol Aging. 1998;5:371–7. doi: 10.1016/s0197-4580(98)00080-3. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–11. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory and Aging Project. Curr Alzheimer Res. 2011;4:336–40. doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neniskyte U, Neher JJ, Brown GC. Neuronal death induced by nanomolar amyloid-β is mediated by primary phagocytosis of neurons by microglia. J Biol Chem. 2011;286:39904–13. doi: 10.1074/jbc.M111.267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer’s disease. Nat Med. 2004;10(Suppl):S34–41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Rev Neurosci. 2010;7:812–18. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeurb L, Osterc S, Pakkenberg B. Neocortical glial cell numbers in Alzheimer’s disease: a stereological study. Dement Geriatr Cogn Disord. 2003;16:212–19. doi: 10.1159/000072805. [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–62. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer’s disease. Exp Neurol. 1995;132:172–9. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Polvikoski TM, van Straaten ECW, Barkhof F, Sulkava R, Aronen HJ, Niinisto L, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–8. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–6. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009;16:378–85. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in the early human fetal nervous system. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in corgnition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–60. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Sebollela A, Freitas-Correa L, Oliveira FF, Paula-Lima AC, Saraiva LM, Martins SM, et al. Amyloid-β oligomers induce differential gene expression in Alzheimer’s diseaseult human brain slices. J Biol Chem. 2012;287:5021–32. doi: 10.1074/jbc.M111.298471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379:482–94. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Simic G, Stanić G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR. Annotation - Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol. 2009;35:532–54. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöbeck M, Englund E. Glial levels determine severity of white matter disease in Alzheimer’s disease: a neuropathological study of glial changes. Neuropathol Appl Neurobiol. 2003;29:159–69. doi: 10.1046/j.1365-2990.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Sjöbeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer’s disease: a neuropathological study. Int J Geriatr Psychiatry. 2005;20:919–26. doi: 10.1002/gps.1384. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, de Toledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: relation to memory function. Neurobiol Aging. 2010;31:1089–98. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem. 1998;70:177–88. doi: 10.1006/nlme.1998.3846. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Schläfera C, Seidl U, Santos VD, Essig M, Schröder J. The cerebellum in mild cognitive impairment and Alzheimer’s disease—a structural MRI study. J Psychiatr Res. 2008;14:1198–202. doi: 10.1016/j.jpsychires.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;3:159–62. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002;49:268–76. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;2:321–31. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Vieira MNN, Forny-Germano L, Saraiva LM, Sebollela A, Martinez AMB, Houzel JC, et al. Soluble oligomers from a non-disease related protein mimic Aβ-induced tau hyperphosphorylation and neurodegeneration. J Neurochem. 2007;103:736–48. doi: 10.1111/j.1471-4159.2007.04809.x. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, ViAlzheimer’s diseaseer F, de la Sayette V, Mézenge F, Landeau B, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci. 2008;28:6174–81. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM, Dziewiatkowski J, Badmajew E, Tarnawski M, Reisberg B, et al. Cerebellar atrophy in Alzheimer’s disease—clinicopathological correlations. Brain Res. 1999;818:41–50. doi: 10.1016/s0006-8993(98)01279-7. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer’s disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–9. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Shcmidt-Kastner PK, Pietsch T, Wiestler OD, et al. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–71. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hirano A. Nucleus raphe dorsalis in Alzheimer’s disease: neurofibrillary tangles and loss of large neurons. Ann Neurol. 1985;17:3–7. doi: 10.1002/ana.410170608. [DOI] [PubMed] [Google Scholar]
- Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. doi: 10.1186/1742-2094-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.