Abstract
Our aim was to investigate the natural evolution of cataplexy and polysomnographic features in untreated children with narcolepsy with cataplexy. To this end, clinical, polysomnographic, and cataplexy-video assessments were performed at diagnosis (mean age of 10 ± 3 and disease duration of 1 ± 1 years) and after a median follow-up of 3 years from symptom onset (mean age of 12 ± 4 years) in 21 children with narcolepsy with cataplexy and hypocretin 1 deficiency (tested in 19 subjects). Video assessment was also performed in two control groups matched for age and sex at first evaluation and follow-up and was blindly scored for presence of hypotonic (negative) and active movements. Patients’ data at diagnosis and at follow-up were contrasted, compared with controls, and related with age and disease duration. At diagnosis children with narcolepsy with cataplexy showed an increase of sleep time during the 24 h; at follow-up sleep time and nocturnal sleep latency shortened, in the absence of other polysomnographic or clinical (including body mass index) changes. Hypotonic phenomena and selected facial movements decreased over time and, tested against disease duration and age, appeared as age-dependent. At onset, childhood narcolepsy with cataplexy is characterized by an abrupt increase of total sleep over the 24 h, generalized hypotonia and motor overactivity. With time, the picture of cataplexy evolves into classic presentation (i.e. brief muscle weakness episodes triggered by emotions), whereas total sleep time across the 24 h decreases, returning to more age-appropriate levels.
Keywords: children, narcolepsy, cataplexy, sleep, sleepiness
Introduction
Narcolepsy with cataplexy is a rare lifelong disorder characterized by excessive daytime sleepiness, cataplexy, sleep paralysis, hallucinations, and fragmented nocturnal sleep (American Academy of Sleep Medicine, 2005). Narcolepsy with cataplexy usually arises in adolescence or young adulthood (Dauvilliers et al., 2001), but the diagnosis is typically established after a long period with a mean delay across Europe from symptom onset to diagnosis of 14 years (Luca et al., 2013). Narcolepsy with cataplexy is believed to have a stable course, but longitudinal studies are scarce (Vignatelli et al., 2011). The number of childhood diagnoses has recently increased especially in some countries, probably because of higher disease awareness, promoted in Italy by media campaigns of the Italian Association of Narcoleptic patients (AIN onlus), and also related to the awareness of narcolepsy in the context of the possible association with H1N1 pandemic and vaccination (Han et al., 2011; Partinen et al., 2012).
Cataplexy is the tell-tale symptom of narcolepsy with cataplexy and is classically defined as a sudden loss of muscle tone evoked by emotions (American Academy of Sleep Medicine, 2005). In younger children closer to onset, it may co-occur with a complex movement disorder with prominent facial involvement (Serra et al., 2008), and persistent hypotonia. These abnormalities are associated with ‘active’ movements (ranging from peri-oral to frankly dyskinetic-dystonic movements even with stereotypies) that increase during emotional stimulation (Plazzi et al., 2011). The current study aimed to describe the natural evolution of this symptomatology over time, together with other clinical and polysomnographic features. The lack of drugs specifically registered for childhood narcolepsy with cataplexy and the concerns about treatment safety led some parents to delay treatment for months or years after diagnosis (Murali and Kotagal, 2006; Aran et al., 2010; Lecendreux et al., 2012); in these cases we were able to document the natural (i.e. without pharmacological treatment) disease course.
Materials and methods
Subjects
We performed a prospective study including 21 of 87 patients with narcolepsy with cataplexy under the age of 18, newly diagnosed between January 2001 and December 2012, whose parents refused pharmacological treatment after diagnosis because of concerns about possible side effects. The baseline clinical and polysomnographic features of children included in the study were similar to those of children with narcolepsy with cataplexy whose parents agreed to initiate pharmacological treatment at the time of diagnosis. Behavioural strategies (scheduled naps) were systematically suggested to all patients and families. All patients were regularly followed up at the Outpatient Clinic for Narcolepsy of the University of Bologna, and underwent a second hospitalization after at least 6 months from first evaluation and before parents decided to start treatment. The study was performed according to the Declaration of Helsinki and approved by local institutional review board; consent was obtained from parents and assent from patients.
The study included the following systematic procedures conducted at baseline (i.e. diagnosis) and at last follow-up evaluation: clinical assessment, video recordings, and hospitalization with polysomnographic recordings and blood samples for anti-streptolysin-O titres.
Clinical assessment
The same sleep medicine specialist (G.P.) systematically evaluated each patient, monitoring onset and presence of symptoms (sleepiness, cataplexy, sleep paralysis, hypnagogic hallucinations, and disturbed nocturnal sleep). Subjective sleepiness was assessed by the Epworth Sleepiness Scale modified for children (Murali and Kotagal, 2006). Anthropometric measures (height, weight and body mass index) were also collected. Body mass index percentiles were calculated according to latest Italian normative values, and patients between the 85th and 97th percentiles or over the 97th percentile were classified as overweight or obese, respectively (Cacciari et al., 2006).
Video recordings
Concurrent with each evaluation, video recordings were performed with the subject sitting and/or standing for ∼30 min. This included 5 min of baseline recording (‘without stimulation’) and up to 25 min while subjects were watching funny movies (‘under stimulation’). Videos were subsequently scored by an independent blinded observer (H.P.) for the occurrence of ‘negative’ (paroxysmal head drops and falls, persistent eyelid narrowing and tongue protrusion, persistent facial hypotonia, persistent generalized hypotonia) and ‘active’ (raising of the eyebrows, perioral and tongue movements, facial grimaces, swaying of the head or trunk, stereotyped motor behaviour, dyskinetic or dystonic movements) motor phenomena using a purpose designed score sheet. These ratings were performed separately for the ‘without stimulation’ and ‘under stimulation’ conditions on an ordinal scale ranging from 0 (absent) to 3 (continuously present). All single ‘negative’ and ‘active’ motor phenomena scores were summed to obtain composite scores separately for the ‘without stimulation’ and ‘under stimulation’ conditions. The presence or absence of two motor patterns (neck extension viewing or puppet-like movements) during the whole video recording was also evaluated. Two groups of healthy controls, matched for age and sex with patients with narcolepsy with cataplexy at baseline and at follow-up, underwent the same video recording procedures, and anthropometric data collection.
Hospitalization
Within a month from first and last clinical assessment, patients were hospitalized. They underwent 48 h of polysomnographic recordings (24 h for adaptation and 24 h for diagnostic purposes) before a multiple sleep latency test (MSLT). MSLT was performed carefully instructing the children to lie down and close their eyes, and a parent was allowed to stay in the laboratory room whenever requested by the patient to avoid any fear of the unfamiliar environment. Sleep studies were visually scored by a registered polysomnographic technician (S.V.) who was blinded to clinical details. Total sleep time from the second 24 h, sleep data from the second nocturnal recording, and MSLT results were collected. Patients underwent lumbar puncture for cerebrospinal hypocretin 1 assessment whenever possible, together with blood samples for human leukocyte antigen (HLA) typing at first hospitalization, and anti-streptolysin-O titres (first and follow-up hospitalizations).
Categorical and continuous data were explored by means of frequency and mean ± standard deviation. Group comparisons (between subjects) were tested using Mann-Whitney or Chi-square tests. Within subjects comparisons were assessed using Wilcoxon signed rank or Chi-square tests, and variables showing a significant change over time were tested against disease duration and age using linear regressions. Pearson correlation coefficient analysis was used to study relationships between continuous variables.
Results
Clinical and biological assessment
Individual clinical data of the patients are reported in Supplementary Table 1.
At baseline (mean 1 ± 1 years from onset of narcolepsy with cataplexy), patients with narcolepsy with cataplexy (71% males; 18 Caucasian, three Asian) were 10.0 ± 3.2 years old. They presented with the following symptoms, presence (age at onset): 100% excessive daytime sleepiness (8.9 ± 3.1 years old), 100% cataplexy (9.1 ± 3.1 years old), 19% sleep paralysis (8.3 ± 3.5 years old), 57% hypnagogic hallucinations (8.7 ± 3.3 years old), and 48% disturbed nocturnal sleep (8.9 ± 3.4 years old). Mean modified Epworth Sleepiness Scale score was 13.7 ± 3.3. Mean weight and height were 46.4 ± 22.2 kg and 1.4 ± 0.2 m, with a corresponding mean body mass index of 22.5 ± 5.1 kg/m2 (range 15.8–32.2 kg/m2). According to Italian reference values, eight (38%) patients were overweight and six (29%) were obese. Additionally, three (14%) children had precocious puberty and 13 (62%) had behavioural alterations (i.e. aggressive behaviour or irritability reported by patients). Nineteen of 21 (90.5%) patients were HLA-DQB1*06:02-positive. All 19 tested patients were hypocretin 1-deficient (all patients were under the diagnostic cut-off 110 pg/ml, and 13 of 19 below 40 pg/ml that can be considered a safe detection limit across different assays), including two HLA-DQB1*06:02-negative subjects. Two patients underwent hypocretin 1 assay twice, after 4 months and 1.5 years from symptoms onset, and after 11 months and 2 years from symptoms onset, respectively (all measurements were <40 pg/ml). Mean anti-streptolysin-O titres were 320.3 ± 250.7 IU/ml, with 14 patients (67%) being over 400 IU/ml.
At follow-up assessment, performed 3.1 ± 1.9 years from the onset of narcolepsy with cataplexy and 2.1 ± 1.7 years (range 0.5–5.2) from first observation, patients had a mean age of 12.1 ± 3.9 years. Compared to baseline, we found a non-significant increase in the occurrence of sleep paralysis (24%, age at onset of 9.2 ± 3.4 years old), hypnagogic hallucinations (67%, age at onset of 8.8 ± 3.0 years old), and disturbed nocturnal sleep (62%, age at onset of 10.0 ± 4.0 years old). Modified Epworth Sleepiness Scale was stable over time (mean of 13.5 ± 4.6, P = 0.47). Over the follow-up period, children grew up to mean weight of 50.0 ± 22.2 kg (P = 0.1), height of 1.5 ± 0.2 m (P = 0.007), and body mass index of 23.4 ± 5.1 (range 16.3–32.1, P = 0.5). According to normative data, four (20%) and six (29%) patients were overweight and obese, respectively, a slight decrease from baseline. Decreased ratio of abnormal to normal body mass index, however, did not reach the statistical significance. Mean anti-streptolysin-O titres were stable (P = 0.8) showing mean values of 375.6 ± 282.1 IU/ml at follow-up, and the decreased ratio over time of patients > 400 IU/ml (n = 8, 40%) did not reach statistical significance (P = 0.66).
Polysomnograhic data
Polysomnographic data at baseline and at follow-up assessment are reported in Table 1. Nocturnal sleep features were stable over time, apart from a significant shortening of sleep latency.
Table 1.
Polysomnographic and MSLT data of patients with narcolepsy with cataplexy at baseline and follow-up hospitalizations with statistical comparison
| Baseline |
Follow-up |
Wilcoxon test | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-Value | |
| PSG nocturnal sleep latency | 5.35 | 2.86 | 2.99 | 2.59 | 0.0185 |
| PSG nocturnal total sleep time | 503.86 | 105.17 | 454.33 | 94.48 | n.s. |
| PSG nocturnal sleep efficiency | 86.92 | 11.38 | 84.64 | 10.43 | n.s. |
| PSG nocturnal arousal index | 16.69 | 4.84 | 15.94 | 4.27 | n.s. |
| PSG sleep stages shift index | 9.84 | 3.05 | 10.53 | 3.37 | n.s. |
| PSG nocturnal REM latency | 15.36 | 24.54 | 57.25 | 111.16 | n.s. |
| PSG nocturnal time spent in non-REM stage 1 (%) | 12.51 | 5.15 | 12.92 | 8.12 | n.s. |
| PSG nocturnal time spent in non-REM stage 2 (%) | 41.39 | 6.58 | 42.69 | 9.49 | n.s. |
| PSG nocturnal time spent in slow wave sleep (%) | 23.02 | 8.12 | 23.74 | 7.85 | n.s. |
| PSG nocturnal time spent in REM sleep (%) | 23.10 | 6.35 | 20.66 | 7.81 | n.s. |
| PSG nocturnal number of sleep cycles | 4.90 | 1.26 | 4.10 | 1.52 | n.s. |
| PSG Daytime total sleep time | 130.41 | 84.12 | 94.44 | 61.77 | n.s. |
| PSG 24 hours total sleep time | 630.45 | 72.72 | 551.56 | 128.77 | 0.004 |
| MSLT mean sleep latency | 4.19 | 3.15 | 3.96 | 3.27 | n.s. |
| MSLT number of sleep onset REM periods | 3.80 | 1.01 | 4.25 | 0.85 | n.s. |
n.s. = non-significant; PSG = continous polysomnography.
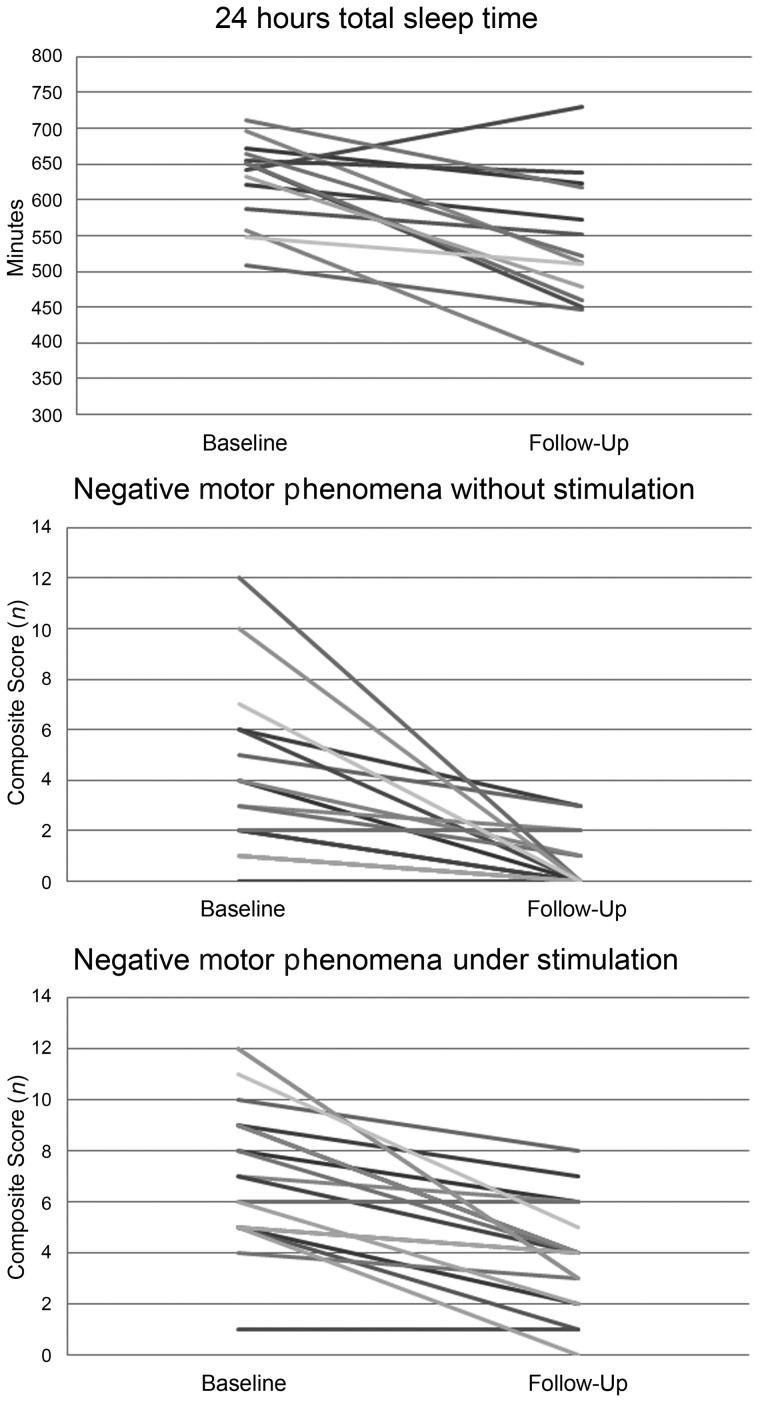
Daytime sleep propensity and the number of sleep onset REM periods at the MSLT were also stable, whereas the 24 h total sleep time decreased from first to follow-up evaluation (P = 0.004, Fig. 1) as the final result of the (non-significant) decreases of daytime and night-time sleep.
Figure 1.
Individual 24 h total sleep time, negative motor phenomena scores without and with stimulation at baseline and at follow-up evaluations.
Video recordings of cataplexy: negative and active motor phenomena
Table 2 shows anthropometric data and the single and composite scores of ‘negative’ and ‘active’ motor phenomena of patients with narcolepsy with cataplexy in parallel with healthy controls at baseline and follow-up evaluation.
Table 2.
Video assessment of patients with narcolepsy with cataplexy at baseline and follow-up in comparison with age and sex balanced control groups
| Controls (n = 21) |
Baseline narcolepsy with cataplexy (n = 21) |
Narcolepsy with cataplexy versus controls | Controls (n = 21) |
Follow-up narcolepsy with cataplexy (n = 21) |
Narcolepsy with cataplexy versus controls | Baseline versus follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing data | Mean | S.D | Mean | SD | P-value | Mean | SD | Mean | SD | P-value | P-Value |
| Age | 11.72 | 3.14 | 10.03 | 3.16 | n.s. | 12.30 | 3.04 | 12.11 | 3.92 | n.s. | 0.000 |
| Male sex | 62% | 71% | n.s. | 62% | 71% | n.s. | |||||
| Overweight | 14% | 38% | 0.007 | 10% | 20% | 0.039 | |||||
| Obesity | 5% | 29% | 5% | 29% | |||||||
| Overweight and obesity | 19% | 67% | 0.002 | 15% | 49% | 0.014 | |||||
| Negative motor phenomena | |||||||||||
| Head drops B | 0.00 | 0.00 | 0.57 | 0.98 | 0.004 | 0.00 | 0.00 | 0.00 | 0.00 | n.s. | 0.016 |
| Head drops T | 0.05 | 0.22 | 1.75 | 0.79 | 0.000 | 0.00 | 0.00 | 1.05 | 0.59 | 0.000 | 0.007 |
| Eyelids and tongue B | 0.00 | 0.00 | 1.29 | 0.84 | 0.000 | 0.00 | 0.00 | 0.33 | 0.66 | 0.019 | 0.001 |
| Eyelids and tongue T | 0.00 | 0.00 | 2.15 | 0.67 | 0.000 | 0.00 | 0.00 | 1.47 | 0.93 | 0.000 | 0.001 |
| Facial hypotonia B | 0.00 | 0.00 | 1.14 | 1.06 | 0.000 | 0.00 | 0.00 | 0.24 | 0.44 | 0.019 | 0.003 |
| Facial hypotonia T | 0.00 | 0.00 | 1.85 | 0.87 | 0.000 | 0.00 | 0.00 | 0.81 | 0.75 | 0.000 | 0.001 |
| Generalized hypotonia B | 0.00 | 0.00 | 0.90 | 1.09 | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | n.s. | 0.003 |
| Generalized hypotonia T | 0.00 | 0.00 | 1.45 | 0.99 | 0.000 | 0.00 | 0.00 | 0.38 | 0.50 | 0.002 | 0.000 |
| Active motor phenomena | |||||||||||
| Eyebrows raising B | 0.05 | 0.22 | 0.48 | 0.60 | 0.004 | 0.05 | 0.22 | 0.24 | 0.44 | n.s. | n.s. |
| Eyebrows raising T | 0.38 | 0.50 | 1.50 | 0.76 | 0.000 | 0.43 | 0.51 | 1.05 | 0.59 | 0.001 | 0.013 |
| Perioral and tongue B | 0.29 | 0.46 | 0.67 | 0.80 | n.s. | 0.33 | 0.48 | 0.33 | 0.58 | n.s. | n.s. |
| Perioral and tongue T | 0.76 | 0.62 | 1.70 | 0.66 | 0.000 | 0.81 | 0.60 | 1.43 | 0.81 | 0.008 | n.s. |
| Facial grimaces B | 0.14 | 0.36 | 0.29 | 0.72 | n.s. | 0.14 | 0.36 | 0.24 | 0.54 | n.s. | n.s. |
| Facial grimaces T | 0.57 | 0.51 | 1.35 | 0.74 | 0.000 | 0.62 | 0.49 | 0.95 | 0.74 | n.s. | 0.021 |
| Head/trunk swaying B | 0.00 | 0.00 | 0.52 | 0.60 | 0.000 | 0.00 | 0.00 | 0.29 | 0.46 | 0.009 | n.s. |
| Head/trunk swaying T | 0.19 | 0.40 | 1.60 | 0.68 | 0.000 | 0.14 | 0.36 | 1.14 | 0.73 | 0.000 | 0.048 |
| Stereotyped behaviour B | 0.05 | 0.22 | 0.33 | 0.58 | 0.039 | 0.05 | 0.22 | 0.48 | 0.60 | 0.004 | n.s. |
| Stereotyped behaviour T | 0.48 | 0.51 | 1.15 | 0.87 | 0.007 | 0.43 | 0.51 | 1.19 | 0.98 | 0.007 | n.s. |
| Dyskinetic dystonic B | 0.00 | 0.00 | 0.23 | 0.44 | 0.019 | 0.00 | 0.00 | 0.14 | 0.36 | n.s. | n.s. |
| Dyskinetic dystonic T | 0.09 | 0.30 | 0.85 | 0.87 | 0.000 | 0.09 | 0.30 | 0.86 | 0.79 | 0.000 | n.s. |
| Consistent motor pattern | |||||||||||
| Neck extension viewing | 0.00 | 0.00 | 0.38 | 0.50 | 0.002 | 0.00 | 0.00 | 0.29 | 0.46 | 0.009 | n.s. |
| Puppet-like movements | 0.00 | 0.00 | 0.24 | 0.44 | 0.019 | 0.00 | 0.00 | 0.09 | 0.30 | n.s. | n.s. |
| Negative and active phenomena composite scores | |||||||||||
| NEGATIVE composite B | 0.00 | 0.00 | 3.90 | 3.74 | 0.000 | 0.00 | 0.00 | 0.57 | 1.03 | 0.009 | 0.000 |
| NEGATIVE composite T | 0.05 | 0.22 | 7.20 | 2.86 | 0.000 | 0.00 | 0.00 | 3.71 | 2.12 | 0.000 | 0.000 |
| ACTIVE composite B | 0.52 | 0.68 | 2.52 | 2.86 | 0.000 | 0.57 | 0.68 | 1.71 | 2.02 | 0.037 | n.s. |
| ACTIVE composite T | 2.09 | 1.30 | 6.65 | 2.96 | 0.000 | 2.09 | 1.18 | 6.62 | 3.71 | 0.000 | n.s. |
NA = not available; B = ‘without stimulation’ condition; T = ‘under stimulation’ condition.
At first evaluation, patients with narcolepsy with cataplexy had higher scores for all ‘negative’ and ‘active’ scores compared with controls, except peri-oral and tongue movements and facial grimaces in the ‘without stimulation’ condition. None of the patients displayed myoclonic jerks. Patients with narcolepsy with cataplexy were also more likely to have the two motor patterns we assessed (‘neck extension viewing’ and ‘puppet-like’ movements) compared with controls. All composite scores of ‘negative’ and ‘active’ movements were higher in patients than controls.
At follow-up evaluation, patients with narcolepsy with cataplexy had higher scores of all ‘negative’ motor phenomena than control subjects, except head drops and generalized hypotonia in the ‘without stimulation’ condition, meaning that this sort of spontaneous cataplexy disappears along the disease course. Narcolepsy with cataplexy patients displayed higher scores for all ‘active’ phenomena in the ‘under stimulation’ condition, except facial grimaces, together with higher scores of head and trunk swaying and stereotypies in the ‘without stimulation’ condition than healthy controls. In two patients, a myoclonic jaw sagging in the ‘under stimulation’ condition was also observed. Patients with narcolepsy with cataplexy also had more frequent motor pattern neck extension viewing than controls. All composite scores of ‘negative’ and ‘active’ movements were higher in patients than controls.
The within subjects comparison of patients with narcolepsy with cataplexy patients at baseline versus follow-up evaluation disclosed a significant decrease of all single and composite ‘negative’ scores (Fig. 1). Moreover, 2 of 21 (9.5%) and 15 of 21 (71.4%) did not show any hypotonic phenomenon (i.e. negative composite score = 0) in the ‘without stimulation’ condition at first and follow-up assessments, respectively. Conversely, only three single active motor phenomena occurring ‘under stimulation’ (eyebrows raising, facial grimaces, and head and trunk swaying) significantly decreased over time leading to a non-significant reduction of the active composite scores.
Relation between age, disease duration and evolving picture of childhood narcolepsy with cataplexy
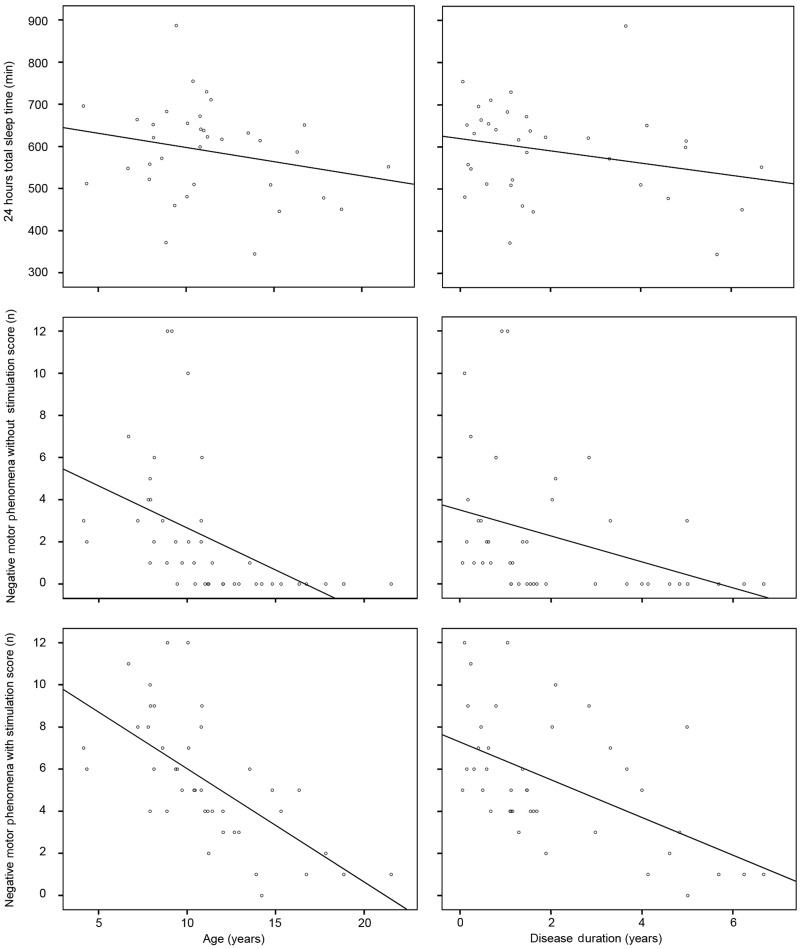
We further tested disease duration and age as predictors of 24 h total sleep time and of the composite scores of negative motor phenomena in the without stimulation and under stimulation conditions, using linear regressions (Fig. 2).
Figure 2.
Scattered graphs of age (left column) and disease duration (right column) versus 24 h total sleep time (top row), negative motor phenomena score ‘without stimulation’ (middle row) and ‘under stimulation’ (bottom row).
Age (beta = −0.237, P = 0.171) and disease duration (beta = −0.255, P = 0.139) were not significant predictors of 24 h total sleep time.
Age (beta = −0.460, P = 0.002; and beta = −0.461, P < 0.0005) and disease duration (beta = −0.360, P = 0.019; and beta = −0.552, P < 0.0005) were inversely correlated with negative motor phenomena score in the ‘without stimulation’ and ‘under stimulation’ conditions when tested separately. At multivariate analyses, age maintained a significant influence (beta = −0.387, P = 0.039; and beta = −0.512, P = 0.002), whereas disease duration lost significance (beta = −0.117, P = 0.523; and beta = −0.232, P = 0.135) in both conditions.
Relation between motor phenomena and biochemical evidence of streptococcal infection
Supplementary Table 2 shows the scores of negative and active motor phenomena of patients with narcolepsy with cataplexy with and without elevated anti-streptolysin-O titres (>400 IU/ml) at baseline and follow-up evaluations.
At baseline, there were no statistically significant differences in negative scores, but only significantly higher scores of single active phenomena (facial grimaces under stimulation, and stereotyped motor behaviour in both without stimulation and under stimulation conditions). The comparison of composite scores did not reach statistical significance, however, active composite scores were significantly correlated with anti-streptolysin-O titres in both the ‘without stimulation’ (Pearson coefficient = 0.525; P = 0.017) and ‘under stimulation’ (Pearson coefficient = 0.530; P = 0.020) conditions.
At follow-up evaluation, the eight patients with elevated anti-streptolysin-O titres did not differ from the others, and there were no significant relationships between composite scores and anti-streptolysin-O titres.
Discussion
We report for the first time that, in untreated patients, the picture of childhood cataplexy, characterized by a complex movement disorder with persistent hypotonia and prominent facial involvement, gradually turns into the classically reported adult phenotype. In the latter, cataplexy is only characterized by paroxysmal episodes of loss of antigravitary muscle tone exclusively evoked by emotions with normal interictal neurological examination. We also found high 24 h total sleep time close to onset that subsequently shortened over time. The study suggests that acute removal of hypocretin 1 signalling induces a true hypersomnia associated with generalized decrease of muscle tone that subsequently evolves over time.
Monitoring disease evolution in 21 drug naive cases over a mean follow-up period of 2 years (3 years from disease onset), we found that: (i) subjective and objective (MSLT) daytime sleepiness, and increased body mass index abruptly appeared at narcolepsy with cataplexy onset and remained stable over time; (ii) sleep amounts across the 24 h peaked close to onset and then shortened together with nocturnal sleep latency; (iii) cataplexy severity, as reflected by both hypotonic motor phenomena and ‘active’ movements (mainly the facial movements under stimulation), decreased over time; (iv) hypotonic features of cataplexy were age dependent; and (v) biochemical evidence of streptococcal infection correlated with motor overactivity only close to disease onset.
Therefore narcolepsy with cataplexy is not a stable phenotype in children, but displays a characteristic evolution with an abrupt onset followed by an improvement over time. Parents of children with narcolepsy with cataplexy usually report a sudden change in sleep–wake habits at first that can often be timed to a particular day. This consists of a voluntary anticipation of bedtime with increased time spent in bed, occurrence of disturbing awakenings and dreaming, and prolonged daytime naps (sometimes lasting hours) with difficulty awakening as in our case series and in a previous clinical observation (Kotagal et al., 1990). Behavioural changes such as irritability also appear (Guilleminault and Pelayo, 1998; Partinen et al., 2012). Hypotonia with prominent facial involvement (mouth opening and tongue protrusion), a global floppy aspect, and gait disturbance with falls to the ground can occur without apparent relation to emotions (Serra et al., 2008; Plazzi et al., 2011). Over the next few weeks children most often gain weight, either searching for sweet foods with increased appetite or without changing eating habits (Poli et al., 2013). Misdiagnoses at this time are frequent because the phenotype is different from adult narcolepsy with cataplexy characterized by brief, refreshing sleep attacks, and episodes of weakness evoked by strong emotions.
As suggested by a previous study in childhood NC (Plazzi et al., 2011), where hypotonic phenomena inversely correlated with age and time from narcolepsy with cataplexy onset, hypotonia symptoms gradually decreased over time leading to the disappearance of ‘spontaneous’ cataplexy, namely head drops and falls without recognizable emotional stimulation (e.g. while walking or eating), and the appearance of classical cataplexy. Accordingly, in our series the ‘adult’ cataplexy phenotype (i.e. no evidence of hypotonia without emotional stimulation) was present in 9.5% and 71.4% of children at first and follow-up evaluation, respectively. What could explain the evolution of cataplexy over time? At first, muscle atonia could reflect the widespread effect of the acute failure of hypocretinergic transmission that facilitates motor neuron activity in the brainstem and spinal cord (Yamuy et al., 2004; Mascaro et al., 2009; Schreyer et al., 2009). Compensatory mechanisms then likely occur within the CNS, despite stable low hypocretin 1 levels, as shown here in some patients. The re-emergence of transient episodes of atonia only during emotions could reflect the engagement of yet unknown compensatory mechanisms during emotional processing. Accordingly, functional studies in patients with narcolepsy with cataplexy showed altered emotional processing by cortical-subcortical structures influenced by disease duration (Schwartz et al., 2008; Ponz et al., 2010). Similarly to our childhood cases, spontaneous joint buckling with falls followed by clinical amelioration over 2 years has been recently described in an equine model of familial narcolepsy with cataplexy (Ludvikova et al., 2012).
In previous observations, we have reported that childhood cataplexy is not characterized solely by peculiar hypotonic features, but also includes motor overactivity compared with control subjects. Some of these hyperkinetic features, such as choreic movements, were reminiscent of brain paediatric autoimmune post-streptococcal diseases and correlated with anti-streptolysin-O titres (Plazzi et al., 2011). These hyperkinetic movements should not be confused with the myoclonic features of cataplexy that have been documented in polygraphic studies in adults (Rubboli et al., 2000; Vetrugno et al., 2010), subjectively reported by patients (Overeem et al., 2011), and observed in our case series only at follow-up. The similarities between PANDAS (Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections), Sydenham’s Chorea and childhood narcolepsy phenotypes include a partial remission over time and an association with biochemical evidence of past streptococcal infection (Dale, 2005; Aran et al., 2009). The non-significant decrease of anti-streptolysin-O over time that may be influenced by insufficient follow-up time given the evidence of higher anti-streptolysin-O in patients closer to narcolepsy with cataplexy onset (Aran et al., 2009), and by the limited number of patients in our series and by the relatively frequent occurrence of streptococcal infections in the paediatric population. Conversely, the enhancement of these active motor phenomena during emotional stimulation suggests a relation with genuine cataplexy and thus a distinct pathophysiological role of hypocretinergic interactions.
The pathogenesis of sporadic narcolepsy with cataplexy is likely autoimmune with hypocretin neurons being a target (Kornum et al., 2011a), and possibly interacting with other neurotransmission systems during development (Sundvik et al., 2011). Narcolepsy with cataplexy is strongly associated with HLA–DQB1*06:02 (Mignot et al., 1994), an effect also modulated by the presence of other HLA subtypes (Mignot et al., 2001; Hor et al., 2010), and by polymorphisms in the T cell receptor alpha (Hallmayer et al., 2009; Hor et al., 2010), P2YR11 receptor and other loci (Kornum et al., 2011b; Faraco et al., 2013). Clinical and biochemical evidence temporally links the onset of narcolepsy with cataplexy symptoms with an activation of the immune system by infection, either bacterial such as streptococcal (Aran et al., 2009), or viral such as H1N1 flu or vaccination (Han et al., 2011; Partinen et al., 2012), or with an autoimmune response proved by production of self-targeted antibodies (Cvetkovic-Lopes et al., 2010; Kawashima et al., 2010). We speculate that the clinical evolution of childhood narcolepsy with cataplexy resembles the course of autoimmune disorders that show a partial remission after an abrupt acute onset phase (Wingerchuk et al., 1999; von Herrath et al., 2007). Further studies of the narcolepsy with cataplexy course are warranted to disentangle whether adaptive compensatory mechanisms or a partial spontaneous remission drive alone, or in combination, our clinical observations.
Our main finding of extended sleep time across the 24 h close to onset fits well with past clinical observations of prolonged, unrefreshing naps in children with narcolepsy with cataplexy (Kotagal et al., 1990), and with our clinical impression from parents’ reports. Although adult patients with narcolepsy with cataplexy show an amount of sleep over the 24 h comparable to Tafti et al. (1992), or higher than Broughton et al. (1988), healthy control subjects, children with narcolepsy with cataplexy displayed an acutely increased need to sleep in the 24 h at disease onset that subsequently stabilized at lower levels. While acknowledging a potential influence of age, we emphasize the absence of prospective studies on sleep across the 24 h, and that the life-span explored here was not large enough to impact on the MSLT, as documented in patients with narcolepsy with cataplexy of different age groups (Dauvilliers et al., 2004). Despite the absence of normative MSLT data in children, in our case series MSLT proved to be a useful and reliable diagnostic tool, with all patients at both evaluations having two or more sleep onset REM periods, but one and two patients showing a mean sleep latency > 8 min at first and follow-up evaluations, respectively, as expected (Vendrame et al., 2008).
Follow-up clinical assessments revealed a non-significant increase in the report of first occurring sleep paralysis, hypnagogic hallucinations, and disturbed nocturnal sleep together with body mass index shifting to lower percentiles. Acknowledging that distinguishing hypnagogic hallucinations from dream experiences can be difficult in children (Guilleminault and Pelayo, 1998), we cannot exclude that, over time, the full-blown narcolepsy with cataplexy symptom tetrad may develop (Dauvilliers et al., 2004; Ohayon et al., 2005). High obesity prevalence has been reported in both adults and children with narcolepsy with cataplexy in cross-sectional studies (Poli et al., 2009, 2013). Body mass index abruptly peaked at onset (Poli et al., 2013), and subsequently decreased without reaching normality in our series, thus the weight of patients with narcolepsy with cataplexy seems to stabilize above population values.
Our study also has limitations. We used video recordings without any additional neurophysiological measurement, thus preventing us from providing measurement of a definite electromyographic pattern and the precise muscles involved in the cataplectic attack. However, the prominent facial involvement in younger patients fits well with polygraphic cataplexy characterizations that suggested a grossly rostro-caudal progression of muscle atonia in adults (Rubboli et al., 2000; Vetrugno et al., 2010) confirmed by the self-reported highest involvement of the facial district (Overeem et al., 2011). Moreover, our semi-standardized setting with a single recording lasting ∼30 min does not allow us to comment on the temporal evolution of the described motor phenomena (e.g. progressive increase or habituation), but in our opinion was appropriate to describe cataplexy features in the absence of any available standardized test.
The abrupt onset of childhood narcolepsy with cataplexy, the acute movement disorder, the sudden weight gain and the hypersomnolence represent a unique and vanishing clinical framework that partially remits over time. On one hand, this can explain the diagnostic challenges and the diagnostic delay (namely, patients are more easily diagnosed once narcolepsy with cataplexy turns to the classical picture); on the other hand, the changes of symptoms can reassure parents about the prognosis of these early onset cases, but also claims for caution in interpreting results of uncontrolled therapeutical studies.
Supplementary Material
Acknowledgements
We are indebted to all the patients and families participating to this study, most notably the Italian Association of Narcolepsy (AIN onlus) patients. Without their contributions, this study would not have been possible. We also thank the reviewers for their useful suggestions.
Glossary
Abbreviation
- MSLT
multiple sleep latency test
Supplementary material
Supplementary material is available at Brain online.
References
- American Academy of Sleep Medicine. ICSD-2- International classification of sleep disorders: diagnostic and coding manual. 2nd edn. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–83. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol. 1988;70:473–81. doi: 10.1016/0013-4694(88)90145-9. [DOI] [PubMed] [Google Scholar]
- Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J Endocrinol Invest. 2006;29:581–93. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47:785–91. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Gosselin A, Paquet J, Touchon J, Billiard M, Montplaisir J. Effect of age on MSLT results in patients with narcolepsy-cataplexy. Neurology. 2004;62:46–50. doi: 10.1212/01.wnl.0000101725.34089.1e. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Montplaisir J, Molinari N, Carlander B, Ondze B, Besset A, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology. 2001;57:2029–33. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- Faraco J, Lin L, Kornum BR, Kenny EE, Trynka G, Einen M, et al. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. PLoS Genet. 2013;9:e1003270. doi: 10.1371/journal.pgen.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Pelayo R. Narcolepsy in prepubertal children. Ann Neurol. 1998;43:135–42. doi: 10.1002/ana.410430125. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- Hor H, Kutalik Z, Dauvilliers Y, Valsesia A, Lammers GJ, Donjacour CE, et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–9. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Lin L, Tanaka S, Jennum P, Knudsen S, Nevsimalova S, et al. Anti-Tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep. 2010;33:869–74. doi: 10.1093/sleep/33.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornum BR, Faraco J, Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol. 2011a;21:897–903. doi: 10.1016/j.conb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kornum BR, Kawashima M, Faraco J, Lin L, Rico TJ, Hesselson S, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011b;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagal S, Hartse KM, Walsh JK. Characteristics of narcolepsy in preteenaged children. Pediatrics. 1990;85:205–9. [PubMed] [Google Scholar]
- Lecendreux M, Poli F, Oudiette D, Benazzouz F, Donjacour CE, Franceschini C, et al. Tolerance and efficacy of sodium oxybate in childhood narcolepsy with cataplexy: a retrospective study. Sleep. 2012;35:709–11. doi: 10.5665/sleep.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca G, Haba-Rubio J, Dauvilliers Y, Lammers GJ, Overeem S, Donjacour CE, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22:482–95. doi: 10.1111/jsr.12044. [DOI] [PubMed] [Google Scholar]
- Ludvikova E, Nishino S, Sakai N, Jahn P. Familial narcolepsy in the Lipizzaner horse: a report of three fillies born to the same sire. Vet Q. 2012;32:99–102. doi: 10.1080/01652176.2012.714089. [DOI] [PubMed] [Google Scholar]
- Mascaro MB, Prosdócimi FC, Bittencourt JC, Elias CF. Forebrain projections to brainstem nuclei involved in the control of mandibular movements in rats. Eur J Oral Sci. 2009;117:676–84. doi: 10.1111/j.1600-0722.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin X, Arrigoni J, Macaubas C, Olive F, Hallmayer J, et al. DQB1*0602 and DQA1*0102 (DQ1) are better markers than DR2 for narcolepsy in Caucasian and black Americans. Sleep. 1994;17(Suppl 8):S60–7. doi: 10.1093/sleep/17.suppl_8.s60. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali H, Kotagal S. Off-label treatment of severe childhood narcolepsy-cataplexy with sodium oxybate. Sleep. 2006;29:1025–9. doi: 10.1093/sleep/29.8.1025. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12:12–18. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzi G, Pizza F, Palaia V, Franceschini C, Poli F, Moghadam KK, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134:3480–92. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli F, Plazzi G, Di Dalmazi G, Ribichini D, Vicennati V, Pizza F, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli F, Pizza F, Mignot E, Ferri R, Pagotto U, Taheri S, et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep. 2013;36:175–81. doi: 10.5665/sleep.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponz A, Khatami R, Poryazova R, Werth E, Boesiger P, Bassetti CL, et al. Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann Neurol. 2010;67:190–200. doi: 10.1002/ana.21825. [DOI] [PubMed] [Google Scholar]
- Rubboli G, d'Orsi G, Zaniboni A, Gardella E, Zamagni M, Rizzi R, et al. A video-polygraphic analysis of the cataplectic attack. Clin Neurophysiol. 2000;111 (Suppl 2):S120–8. doi: 10.1016/s1388-2457(00)00412-0. [DOI] [PubMed] [Google Scholar]
- Schreyer S, Büttner-Ennever JA, Tang X, Mustari MJ, Horn AK. Orexin-A inputs onto visuomotor cell groups in the monkey brainstem. Neuroscience. 2009;164:629–40. doi: 10.1016/j.neuroscience.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Ponz A, Poryazova R, Werth E, Boesiger P, Khatami R, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–22. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:858–65. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- Sundvik M, Kudo H, Toivonen P, Rozov S, Chen YC, Panula P. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011;25:4338–47. doi: 10.1096/fj.11-188268. [DOI] [PubMed] [Google Scholar]
- Tafti M, Villemin E, Carlander B, Besset A, Billiard M. Sleep in human narcolepsy revisited with special reference to prior wakefulness duration. Sleep. 1992;15:344–51. doi: 10.1093/sleep/15.4.344. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Havaligi N, Matadeen-Ali C, Adams R, Kothare SV. Narcolepsy in children: a single-center clinical experience. Pediatr Neurol. 2008;38:314–20. doi: 10.1016/j.pediatrneurol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, D'Angelo R, Moghadam KK, Vandi S, Franceschini C, Mignot E, et al. Behavioural and neurophysiological correlates of human cataplexy: a video-polygraphic study. Clin Neurophysiol. 2010;121:153–62. doi: 10.1016/j.clinph.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Vignatelli L, Plazzi G, Peschechera F, Delaj L, D'Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12:19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–94. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–45. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.