Abstract
Writer’s cramp is a task-specific focal hand dystonia characterized by involuntary excessive muscle contractions during writing. Although abnormal striatal dopamine receptor binding has been implicated in the pathophysiology of writer’s cramp and other primary dystonias, endogenous dopamine release during task performance has not been previously investigated in writer’s cramp. Using positron emission tomography imaging with the D2/D3 antagonist 11C-raclopride, we analysed striatal D2/D3 availability at rest and endogenous dopamine release during sequential finger tapping and speech production tasks in 15 patients with writer’s cramp and 15 matched healthy control subjects. Compared with control subjects, patients had reduced 11C-raclopride binding to D2/D3 receptors at rest in the bilateral striatum, consistent with findings in previous studies. During the tapping task, patients had decreased dopamine release in the left striatum as assessed by reduced change in 11C-raclopride binding compared with control subjects. One cluster of reduced dopamine release in the left putamen during tapping overlapped with a region of reduced 11C-raclopride binding to D2/D3 receptors at rest. During the sentence production task, patients showed increased dopamine release in the left striatum. No overlap between altered dopamine release during speech production and reduced 11C-raclopride binding to D2/D3 receptors at rest was seen. Striatal regions where D2/D3 availability at rest positively correlated with disease duration were lateral and non-overlapping with striatal regions showing reduced D2/D3 receptor availability, except for a cluster in the left nucleus accumbens, which showed a negative correlation with disease duration and overlapped with striatal regions showing reduced D2/D3 availability. Our findings suggest that patients with writer’s cramp may have divergent responses in striatal dopamine release during an asymptomatic motor task involving the dystonic hand and an unrelated asymptomatic task, sentence production. Our voxel-based results also suggest that writer’s cramp may be associated with reduced striatal dopamine release occuring in the setting of reduced D2/D3 receptor availability and raise the possibility that basal ganglia circuits associated with premotor cortices and those associated with primary motor cortex are differentially affected in primary focal dystonias.
Keywords: dystonia, dopamine, PET, raclopride, striatum
Introduction
Primary dystonia is a neurological disorder characterized primarily by involuntary muscle spasms giving rise to patterned movements and abnormal postures occurring in the absence of a brain lesion, neurodegeneration or identifiable exogenous cause (Tarsy and Simon, 2006; Albanese et al., 2011). Focal dystonias affect one body part or region and can be task-specific, affecting the fine motor control required for tasks such as writing (writer’s cramp), speaking (spasmodic dysphonia), playing a musical instrument, or running (Torres-Russotto and Perlmutter, 2008). Evidence from a number of investigations suggests that primary focal dystonias might share a common aetiology and that abnormalities in the striatal dopamine system may contribute to dystonia pathophysiology (Hallett, 2006a, 2006b; Defazio et al., 2007; Breakefield et al., 2008).
Most dopaminergic imaging studies in primary focal dystonia have focused on detecting abnormalities in striatal D2-like receptor binding. Striatal D2/D3 receptor availability has been reported to be reduced in writer’s cramp (Horstink et al., 1997; Berger et al., 2007), cervical dystonia (Naumann et al., 1998), blepharospasm (Horie et al., 2009), and in a mixed group of patients with hand and cranial dystonia (Perlmutter et al., 1997b). However, some conflicting findings on dopamine receptor binding have been reported. For example, no significant differences in striatal D2/D3 availability were detected in earlier studies in patients with cervical dystonia (Leenders et al., 1993; Hierholzer et al., 1994; Becker et al., 1997). Similarly, a recent study of a mixed group of hand and cranial dystonia patients using the more selective D2 receptor radioligand 18F-N-methyl-benperidol found no significant differences from control subjects (Karimi et al., 2011). Importantly, binding by the radioligands used in dopamine receptor imaging studies can be affected by competition with synaptic concentration of dopamine (Laruelle, 2012). Thus, discrepancies in striatal D2-like receptor availability among previous dopamine receptor imaging studies might be a consequence of abnormal tonic release of striatal dopamine when subjects were scanned at rest.
Although the number of pathological studies in primary focal dystonia is limited, most have not found evidence for abnormal cellular degeneration of nigro-striatal neurons in focal dystonia (Standaert, 2011). Nevertheless, abnormal striatal dopamine release may contribute to the disorder. In non-human primates, transient dystonia is seen following an acute reduction in striatal dopamine induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydopyriine—a neurotoxin that selectively destroys dopaminergic neurons (Perlmutter et al., 1997a; Tabbal et al., 2006). In addition, low levels of striatal dopamine have also been associated with the manifestation of dystonia in DYT5 or DOPA-responsive dystonia caused by genetic mutations interfering with dopamine production, typically without the loss of nigro-striatal neurons (Knappskog et al., 1995; Clot et al., 2009).
In post-mortem studies of generalized early-onset DYT1 dystonia caused by the TOR1A mutation, striatal dopamine has also been found to be reduced (Hornykiewicz et al., 1986; Furukawa et al., 2000; Augood et al., 2002). The reduction in striatal dopamine in these cases was not accompanied by degeneration of neurons within the substantia nigra except in one case where the individual tested positive for the TOR1A mutation, but during life was diagnosed with parkinsonism at age 53 and treated with dopamine replacement therapy (Augood et al., 2002). Further support for reduced dopamine release in primary dystonia has been suggested in mouse models of DYT1 dystonia (Balcioglu et al., 2007; Page et al., 2010; Song et al., 2012), which may be linked to altered dopamine transporter function (Torres et al., 2004; Hewett et al., 2010). Normal or increased levels of striatal dopamine, however, have also been reported in mouse models of DYT1 dystonia (Dang et al., 2005, 2006; Grundmann et al., 2007; Zhao et al., 2008), and in a mouse model of DYT11 or myoclonus-dystonia caused by mutations in the epsilon sarcoglyan gene (Zhang et al., 2012). Although these animal models of dystonia have showed some inconsistencies in how striatal dopamine is altered in dystonia, they do help support the notion that dystonia is associated with dysfunction of the striatal dopamine system.
To date, imaging investigations into presynaptic dopaminergic function in adult-onset primary focal dystonia have been restricted to assessments during a resting state. One group using 18F-fluorodopa reported increased presynaptic dopaminergic activity in the striatum of patients with idiopathic dystonia compared with control subjects (Otsuka et al., 1992), but the study included patients with generalized dystonia in addition to focal hand and cervical dystonia and was further confounded by the inclusion of patients with dystonic symptoms at rest. In another study involving patients with cervical dystonia who had reduced D2/D3 availability at rest, the presynaptic dopamine transporter was found to be normal (Naumann et al., 1998). Abnormal presynaptic dopamine release in these patients, however, could still account for the reduced D2/D3 availability despite normal levels of dopamine transporter being detected.
The aims of the present study were 2-fold: (i) examine striatal dopamine receptor availability using the PET D2/D3 antagonist radioligand 11C-raclopride (11C-RAC) during the resting state in patients with writer’s cramp who have no dystonic symptoms at rest; and (ii) examine 11C-RAC displacement induced by endogenous dopamine release during performance of a sequential finger-tapping task involving the dystonic hand and an asymptomatic sentence production task. 11C-RAC was used because it is a reversible binder of D2/D3 receptors that has been well validated in providing indirect measures of task-induced synaptic dopamine release during behavioural or pharmacological challenges in the living human brain (Laruelle, 2012).
We hypothesized that reduced D2/D3 receptor availability in writer’s cramp would be accompanied by abnormal 11C-RAC displacement during the sequential finger-tapping task that involved recruitment of similar muscle groups as those affected by writer’s cramp. Additionally, we hypothesized that compensatory mechanisms could lead to abnormal 11C-RAC displacement during performance of an asymptomatic speech production task. A finger-tapping task rather than a writing task was used to assess hand motor performance in patients with writer’s cramp to limit the potential confounding of excessive muscle spasms occurring during writing and motor and sensory feedback influences during writing on brain responses (Torres-Russotto and Perlmutter, 2008). A speech production task was examined as a putative unaffected task in patients with writer’s cramp that involved motor control pathways distinct from writing. We further hypothesized that D2/D3 receptor availability and changes in task-induced 11C-RAC displacement in writer’s cramp would relate to the clinical features of symptom severity and disease duration.
Materials and methods
Participants
We studied 15 patients with writer’s cramp and 15 age-matched healthy control subjects (Table 1). All subjects were right-handed (Oldfield, 1971), and none had any history of neurological or psychiatric disorders with the exception of dystonia in patients with writer’s cramp. Subjects were excluded if they were taking medication known to affect dopaminergic function. All patients with writer’s cramp had adult-onset dystonia (>26 years old), had no dystonia in other body regions or at rest, and had normal laryngeal anatomy and function confirmed by nasolaryngoscopy. Nine of the patients with writer’s cramp were receiving regular botulinum toxin injections, but none had received injections within the past 4 months and were fully symptomatic. Patients were excluded if they had dystonic symptoms at rest to avoid the potential confound of dystonic spasms occurring during the rest scan. A similar investigation into dopaminergic function in patients with spasmodic dysphonia was conducted at the same time as the present study, and some control subjects were shared in the two studies (Simonyan et al., 2013a). Some control subjects were also reported in a previous study by Simonyan et al. (2013b). The Institutional Review Boards of the National Institutes of Health (NIH) and the Icahn School of Medicine at Mount Sinai, as well as the NIH Radiation Safety Committee approved this study, and all participants gave their written informed consent before participation.
Table 1.
Subject demographic data
| Patients with writer’s cramp (n = 15) | Control subjects (n = 15) | P-value | |
|---|---|---|---|
| Age (mean ± SD, years) | 52.4 ± 9.0 | 55.1 ± 9.3 | 0.42 |
| Gender | 10M:5F | 6M:9F | 0.15 |
| Handedness | Right | Right | NA |
| Disease duration (mean ± SD, years) | 14.4 ± 6.5 | − | NA |
| Disease severity (WCRS) | 13.1 ± 4.7 | − | NA |
M = male; F = female; WCRS = Writer's Cramp Rating Scale; NA = not applicable.
Patient disease duration was estimated based on patients’ report of earliest recalled symptoms of hand cramping while writing. Patient symptom severity was assessed using the Writer’s Cramp Rating Scale (Wissel et al., 1996; Kruisdijk et al., 2007). The Writer’s Cramp Rating Scale scores the manifestation of dystonia during writing involving the first three fingers, wrist, and elbow (rated 0 to 2), as well as the latency to dystonia onset (rated 1 to 2) and presence of tremor (rated 0 to 2) during writing.
Magnetic resonance imaging scanning
All participants underwent MRI scanning on a 3 T GE Signa Excite system (General Electric Medical Systems) for PET image registration and to rule out presence of gross brain abnormalities. A high-resolution anatomical MRI of each participant’s brain was obtained using a T1-weighted MPRAGE: echo time, 3 ms; inversion time, 450 ms; flip angle = 10°; matrix size = 256 × 256; field of view = 240 mm; slice thickness = 1.2 mm.
Positron emission tomography scanning
PET scanning with 11C-RAC was used to investigate striatal D2/D3 receptor binding potential at rest and estimate changes in 11C-RAC binding potential during task production as a measure of striatal dopamine release. 11C-RAC binds to D2/D3 receptors with sufficiently low binding affinity to compete with endogenous dopamine and therefore a reduction in binding potential can be used to estimate an increase in extracellular concentration associated with dopamine release in response to a variety of interventions (Seeman et al., 1989; Laruelle, 2000, 2012; Watabe et al., 2000; Garraux et al., 2007). Subjects were instructed not to drink any beverages containing caffeine or alcohol for 24 h and to fast for a minimum of 3 h before the PET scans. All PET scanning was started in the morning (before noon) to limit the effect of possible diurnal fluctuations in dopamine transmission on our findings. Head movements during PET scanning were minimized by the use of a thermoplastic mask moulded around the subject’s head.
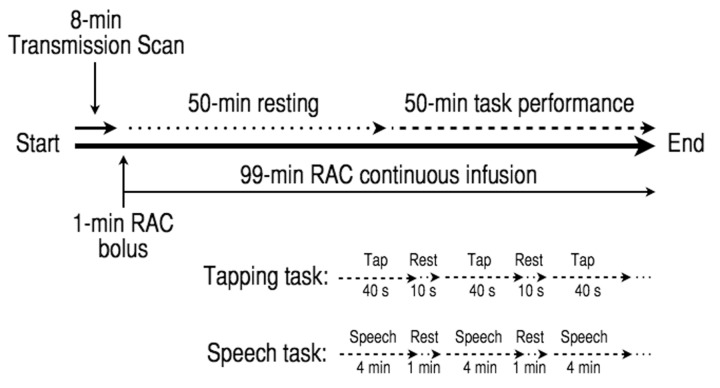
All PET data were acquired on a GE Advance tomograph (GE Medical Systems). An 8-min transmission scan using a 68Ge source was obtained before tracer injection for attenuation correction. A target dose of 20 mCi of 11C-RAC was delivered in a 20 ml syringe and administered as a 1-min bolus injection followed by a 99-min constant infusion using a computer-controlled pump (Harvard Instruments). Based on prior recommendations on how to maximize the signal-to-noise ratio (Watabe et al., 2000), a bolus-plus-infusion method was used (Fig. 1). Two 100-min 11C-RAC scans were acquired, during which subjects initially rested and then performed either sequential finger tapping or sentence production, respectively. During each 100-min dynamic 11C-RAC PET scan, 27 frames of 30 s to 5 min epochs were generated (reconstructed resolution 6 mm in all directions) according to a PET scanning timeline for assessment of task-related dopamine release as previously reported by our group (Garraux et al., 2007; Simonyan et al., 2013a, 2013b). The mean actual administered 11C-RAC doses [mean ± standard deviation (SD)] did not significantly differ between patients with writer’s cramp and control subjects for both the finger tapping (writer’s cramp: 20.07 ± 1.11 mCi, control subjects: 20.45 ± 0.30 mCi; P = 0.22) and speech production scans (writer’s cramp: 20.20 ± 0.42 mCi, control subjects: 19.63 ± 1.56 mCi; P = 0.19).
Figure 1.
Experimental design for PET scans with 11C-raclopride (RAC) using a bolus-plus-infusion design to assess D2/D3 receptor availability at rest and endogenous striatal dopamine release as measured by displacement of 11C-RAC during sequential finger tapping and speech production tasks.
Tasks
All participants underwent two 100-min 11C-RAC PET scans separated by a minimum of 1 day. Each PET scan consisted of two consecutive experimental conditions of 50-min duration each: (i) resting quietly with eyes closed without falling asleep (Baseline); and (ii) performance of a task (Task). The tasks included a five-finger sequential tapping sequence that subjects performed by alternating 40 s of continuous tapping with 10 s of rest, and a speech production that subjects performed by alternating 4 min of repeating sentences aloud (e.g. ‘Tom is in the army’) with 1 min of rest (Fig. 1). The order of the scans was pseudo-randomized by alternating the order of task acquisition with each subject enrolled.
Participants were instructed in the tapping sequence on the day of scanning and briefly practiced it at a pace of ∼2 taps/s until comfortable with the sequence. Tapping was performed by the dominant right hand at an internally paced rate and consisted of a repeating sequence starting with the thumb (I) as follows: I→II→III→IV→V→IV→III→II. Finger tapping presses were recorded using a five-finger fibre-optic button response unit (Psychology Software Tools) and PowerLab 8/30 amplifier with LabChart6 Pro software (ADInstruments). For the speech production task, subjects were instructed immediately before scanning to listen attentively to an acoustically presented sample sentence and repeat each sentence four times at the comfortable conversational speech level.
Volumetric magnetic resonance image processing
The high-resolution MPRAGE images of each participant’s brain underwent processing using FreeSurfer (v4.5.0), an automated program for calculating volumetric segmentation and cortical thickness (http://www.surfer.nmr.mgh.harvard.edu). Subcortical segmented volumes were produced based on the geometric structures of the white/grey matter interface and regions of interest based on standardized caudate, putamen, and nucleus accumbens segmentation units in Freesurfer. Following automated segmentation, all images were visually inspected to rule out segmentation processing errors. If any segmenting errors in striatal structures were found, the segmentation routine was repeated and the repeat results inspected. Despite several attempts, the anatomical MRI scan for one healthy control subject (62-year-old female) did not accurately segment the subcortical volumes and so her data were not included in the final analysis. Subcortical volumes were compared using two-sample t-tests with a significance threshold of P < 0.05.
Positron emission tomography imaging data processing
Preprocessing of 11C-RAC PET images included correction for possible head motion-induced artefacts during task performance using the registered attenuation correction method as previously described (Garraux et al., 2007; Simonyan et al., 2013a, 2013b). Emission images were first reconstructed with filtered back-projection and without attenuation correction. All emission frames were then registered with mutual information to the prime emission image and smoothed with a 6-mm full-width at half-maximum Gaussian filter using FMRIB’s Linear Image Registration Tool from the FMRIB Software library (Jenkinson et al., 2002). The transmission images were registered to the same prime emission image, and the transmission frame reconstructed with filtered back-projection in order to perform attenuation correction.
PMOD (PMOD Technologies) and analysis of functional neuroimages (AFNI; Cox, 1996) software were used to perform registrations of each subject’s 11C-RAC scans to their individual anatomical brain MRI followed by normalization of all 11C-RAC images to the Montreal Neurological Institute’s Colin N27 brain in standard Talairach space (Talairach and Tournoux, 1988). Voxel-based analyses of PET data were performed with AFNI using volumes of interest (caudate nucleus, putamen, and cerebellum) derived from cytoarchitectonic probability maps distributed with AFNI for the Colin N27 brain (Eickhoff et al., 2005). The caudate nucleus and putamen volumes were sampled across their entire rostro-caudal and dorso-ventral extents, while the cerebellar volume was restricted to grey matter defined over five consecutive slices of both hemispheres.
11C-RAC binding potential was determined under equilibrium conditions from the radioactivity concentration in the D2 receptor-rich target regions (C, putamen and caudate nucleus) and in a receptor-poor background region (C’, cerebellum) using the equation: binding potential = [(C − C′)] / C′ (Carson, 2000). Parametric images of 11C-RAC binding potential during baseline and task performance were created using frames 14–16 (40–50 min) and frames 20–27 (60–100 min), respectively. Task-induced dopamine release was estimated by calculating the percent change in 11C-RAC binding potential (ΔBP) during task performance: ΔBP = [(BPBaseline − BPTask) / BPBaseline] × 100% (Watabe et al., 2000).
To examine outliers in data and remove noisy data that could contribute to signal variability, we calculated ‘spikiness’ of each voxel in all individual subject 11C-RAC binding potential and 11C-RAC ΔBP images. Outliers were identified using the AFNI program ‘3dToutcount’, which was used to calculate voxelwise median absolute deviations for each individual data set. The data set was excluded as an outlier if their median lay outside ± 3.5 × median absolute deviations. Applying these methods led to one patient with writer’s cramp and one healthy control subject being removed from the resting 11C-RAC binding potential analysis. No subjects were removed as outliers from either of the task-induced 11C-RAC ΔBP analyses.
Assessment of between-group differences in 11C-RAC binding potential at rest and 11C-RAC ΔBP during task performance was performed using voxel-wise two-sample t-tests. The AFNI Monte Carlo simulation program ‘3dClustSim’ was used to estimate the probability of false positive clusters within a volume restricted to the striatum. Based on the smoothness of noise in individual subject 11C-RAC binding potential and 11C-RAC ΔBP scans estimated using the AFNI program ‘3dFWHMx’, a family-wise error (FWE)-corrected P < 0.05 was reached using a voxel-level threshold of P = 0.025 and cluster size threshold of 212 voxels for the 11C-RAC binding potential contrasts and 100 voxels for the 11C-RAC ΔBP contrasts.
Relation of positron emission tomography findings to clinical features
AFNI software was used to perform voxel-wise Spearman’s correlation analyses in order to investigate potential relationships between 11C-RAC binding potential at rest and 11C-RAC ΔBP during task performance in patients with writer’s cramp with disease duration and symptom severity. Significance level for the correlations was set at a voxel-level threshold of P < 0.025, to account for the two clinical measures evaluated, with a correlation coefficient threshold of |r| ≥ 0.6 and cluster size threshold of 100 voxels.
Region of interest analyses
Conventional region of interest analyses were performed to complement the voxel-based analyses, quantify changes in 11C-RAC binding potential at rest and 11C-RAC ΔBP during task performance in patients with writer’s cramp, and examine scatter plots of relationships observed between PET findings and clinical features. Extractions of 11C-RAC binding potential at rest and 11C-RAC ΔBP during task performance from individual subject data were performed with AFNI using the caudate nucleus and putamen volumes of interest derived from probability maps distributed with AFNI as described above (Eickhoff et al., 2005). Additionally, significant clusters identified in the voxel-based analyses at the group level were used to generate masks for extraction of 11C-RAC binding potential at rest and 11C-RAC ΔBP during task performance from individual subject data. Two-sample t-tests were used to assess significant differences between patients with writer’s cramp and control subjects, and a statistical threshold of P < 0.05 applied.
Results
Volumetric magnetic resonance imaging
No significant differences in the volumes of the putamen, caudate nucleus, or nucleus accumbens between patients with writer’s cramp and healthy control subjects were found (Table 2).
Table 2.
Striatal region volumes in patients with writer’s cramp compared with healthy control subjects
| Striatal region | Volume (mean ± SD) |
||
|---|---|---|---|
| Healthy controls (n = 14) | Patients with writer’s cramp (n = 15) | P-value | |
| Putamen | |||
| Left | 5437 ± 727 | 5500 ± 790 | 0.82 |
| Right | 5281 ± 638 | 5252 ± 720 | 0.91 |
| Caudate nucleus | |||
| Left | 3166 ± 494 | 3488 ± 577 | 0.12 |
| Right | 3255 ± 480 | 3549 ± 548 | 0.14 |
| Nucleus accumbens | |||
| Left | 618 ± 98 | 584 ± 127 | 0.43 |
| Right | 637 ± 104 | 618 ± 148 | 0.70 |
Participants and positron emission tomography scanning performance
Patients with writer’s cramp and control subjects did not significantly differ in age (P = 0.42) or gender (P = 0.15) (Table 1). The total number of taps during the tapping task scans also did not significantly differ between patients and control subjects (writer’s cramp: 4378 ± 1649, control subjects: 4151 ± 929; two-tailed t-test: P = 0.66). Tapping count data from two control subjects, however, were lost because of technical issues. Following the tapping task scanning, four patients with writer’s cramp reported experiencing a vague sense of ‘tightness’ in their hands, but all denied experiencing their typical dystonic symptoms or feeling hand muscle fatigue. None of the subjects reported experiencing dystonia-like laryngeal spasms during speech production during scanning. Furthermore, no dystonic hand posturing while tapping was observed and no speech changes consistent with laryngeal dystonia were heard during continuous monitoring of patients with writer’s cramp during task performance.
11C-raclopride binding potential at rest
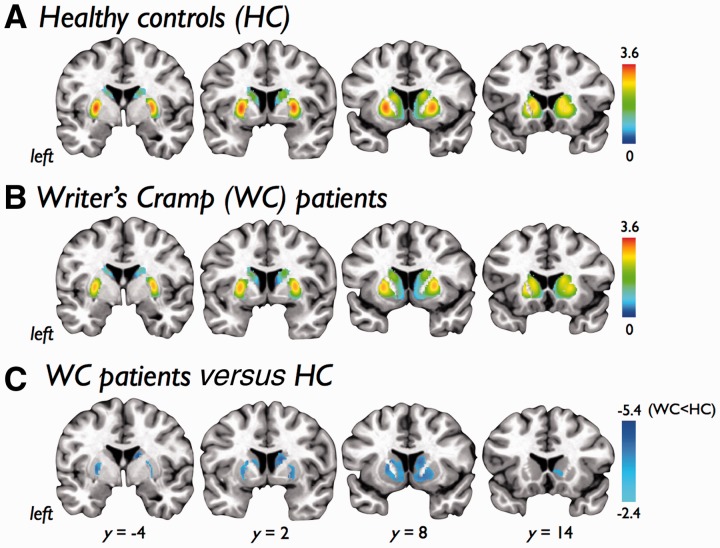
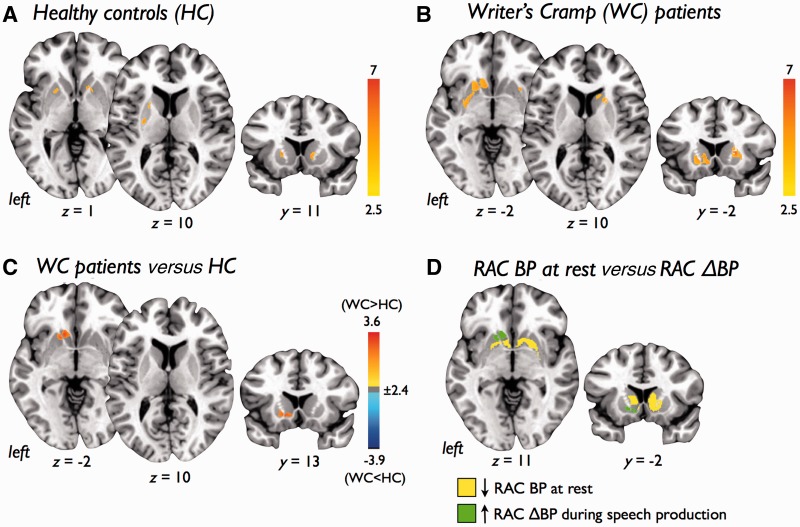
Voxel-wise analysis of 11C-RAC binding potential at rest revealed significantly reduced striatal D2/D3 availability in the bilateral striatum in patients with writer’s cramp (Fig. 2A–C and Table 3). There were no significant differences between the resting 11C-RAC binding potential scans acquired before the finger-tapping task to those scans acquired before the speech production task.
Figure 2.
Statistical parametric maps showing 11C-raclopride binding potential to striatal dopamine receptors (D2/D3) at rest in (A) healthy control subjects (HC) and (B) patients with writer’s cramp (WC). A contrast analysis (C) showed patients with writer’s cramp had a significant reduction in D2/D3 binding in the bilateral striatum. Colour bars show range of t-values displayed. Images are shown at P < 0.05, FWE corrected, on coronal slices of a standard brain in Talairach space.
Table 3.
Clusters with significant decreases in 11C-raclopride binding at rest in patients with writer’s cramp compared with controls
| Striatal Region | Cluster size (voxels) | Talairach coordinates |
Peak t-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right caudate/ putamen | 2686 | 14 | −8 | 18 | −5.42 |
| Left caudate/ putamen | 1958 | −15 | 6 | 11 | −4.31 |
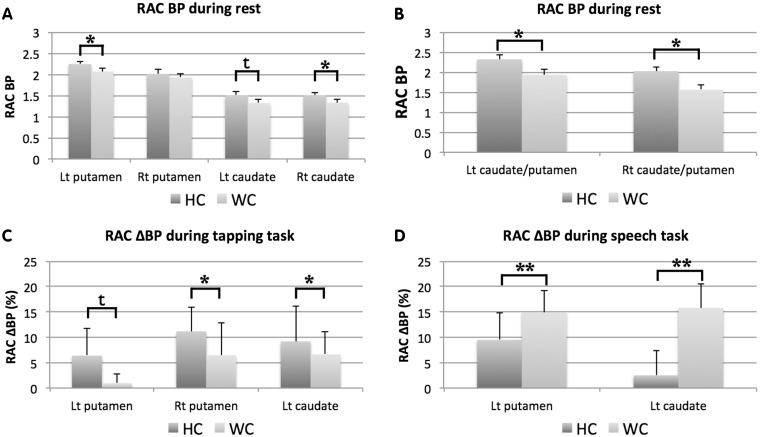
Using whole striatal volumes, 11C-RAC binding potential at rest in patients with writer's cramp was significantly reduced in the left putamen (writer’s cramp: 2.07 ± 0.07, control subjects: 2.25 ± 0.07; P < 0.05) and right caudate nucleus (writer’s cramp: 1.33 ± 0.07, control subjects: 1.51 ± 0.06; P < 0.05). Patients with writer’s cramp also showed a trend toward reduced 11C-RAC binding potential in the left caudate nucleus (writer’s cramp: 1.32 ± 0.08, control subjects: 1.51 ± 0.09; P < 0.06; Fig. 5A). Using regions of interest generated from significant clusters identified in the voxel-wise analysis, significantly reduced 11C-RAC binding potential was observed in the left striatum (writer’s cramp: 1.94 ± 0.55, control subjects: 2.32 ± 0.44; P < 0.05) and right striatum (writer’s cramp: 1.56 ± 0.48, control subjects: 2.03 ± 0.35; P < 0.05) of patients with writer’s cramp during rest (Fig. 5B). There were no significant differences between the resting 11C-RAC binding potential scans acquired before the finger-tapping task to those scans acquired before the speech production task (P > 0.05).
Figure 5.
Differences in 11C-raclopride binding potential (RAC BP) to striatal dopamine receptors (D2/D3) during rest in healthy control subjects (HC) and patients with writer’s cramp (WC) generated using (A) atlas-defined whole putamen and caudate volumes of interest and (B) clusters defined by those striatal regions found to be significantly different between healthy control subjects and writer’s cramp in the voxel-based analyses. Also shown are bar graphs showing dopamine release as measured by displacement of 11C-raclopride (11C-RAC ΔBP) during (C) right-hand finger tapping and (D) speech production tasks in healthy control subjects and patients with writer’s cramp using clusters within the striatum found to be significantly different between healthy control subjects and writer’s cramp in the voxel-based analyses. Significant differences were evaluated using t-test comparisons (*P < 0.05; **P < 0.005; t: P < 0.1). Error bars reflect standard error of the mean. Lt = left; Rt = right.
11C-raclopride Δbinding potential during task performance
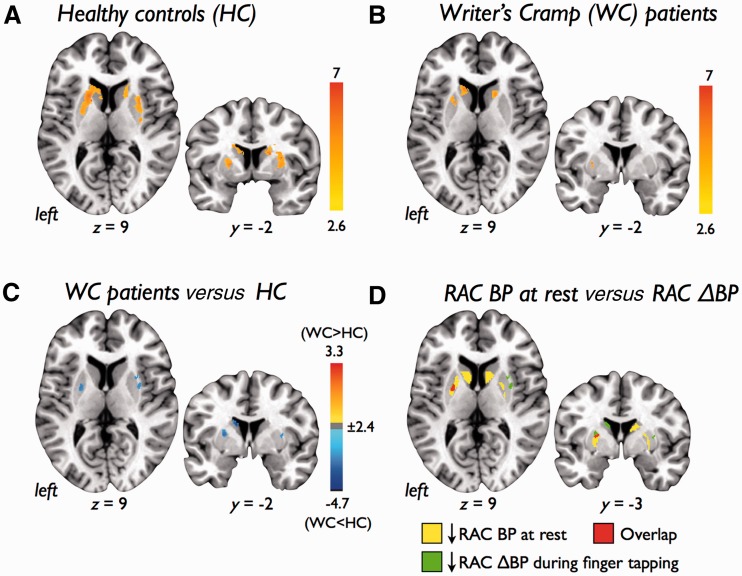
Voxel-wise analysis of 11C-RAC ΔBP revealed significant increases in the displacement of 11C-RAC throughout the bilateral striatum during finger tapping in control subjects and patients with writer’s cramp (Fig. 3A–B and Table 4). Contrasting patients with writer’s cramp with control subjects, however, revealed reduced 11C-RAC displacement (decreased 11C-RAC ΔBP) in the left caudate nucleus and bilateral putamen in patients (Fig. 3C). Post hoc analysis revealed one cluster where decreased 11C-RAC ΔBP during finger tapping overlapped with reduced 11C-RAC binding potential at rest in the left putamen (Fig. 3D).
Figure 3.
Statistical parametric maps showing endogenous dopamine release as measured by displacement of 11C-raclopride (RAC ΔBP) during a right-hand finger tapping task in (A) healthy control subjects (HC), (B) patients with writer’s cramp (WC), and (C) patients with writer’s cramp compared with healthy control subjects. (D) Left striatal regions showing decreased 11C-RAC ΔBP during the tapping task (green) and striatal regions found to have reduced D2/D3 receptor availability at rest (yellow) showed one cluster of overlap in the left putamen (red). Colour bars show range of t-values displayed. Images are shown at P < 0.05, FWE corrected, on axial and coronal slices of a standard brain in Talairach space.
Table 4.
Significant clusters of task-induced 11C-raclopride displacement (RAC ΔBP) in patients with writer’s cramp compared with control subjects
| Task | Striatal region | Cluster size (voxels) | Talairach coordinates |
Peak t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Finger tapping | Healthy control subjects | |||||
| Left putamen/caudate | 3709 | −19 | 11 | 5 | 9.64 | |
| Right caudate/putamen | 2773 | 19 | −2 | 18 | 9.67 | |
| Patients with writer’s cramp | ||||||
| Left caudate | 722 | −11 | 14 | 12 | 6.38 | |
| Left putamen | 382 | −16 | 10 | −2 | 6.00 | |
| Right caudate | 149 | 16 | 11 | 9 | 4.43 | |
| Left putamen | 144 | −26 | −2 | 7 | 5.65 | |
| Patients with writer’s cramp versus control subjects | ||||||
| Left caudate | 232 | −11 | −2 | 19 | −4.74 | |
| Right putamen | 209 | 31 | 9 | 5 | −3.66 | |
| Left putamen | 121 | −23 | −2 | 10 | −3.43 | |
| Speech production | Healthy control subjects | |||||
| Left putamen | 239 | −17 | 11 | 5 | 4.65 | |
| Right caudate | 128 | 12 | 12 | 2 | 3.96 | |
| Left putamen | 100 | −25 | −8 | 10 | 3.82 | |
| Patients with writer’s cramp | ||||||
| Right putamen/caudate | 862 | 22 | 11 | 6 | 6.02 | |
| Left putamen | 763 | −26 | −8 | −2 | 4.10 | |
| Left caudate | 490 | −6 | 12 | −3 | 4.41 | |
| Patients with writer’s cramp versus control subjects | ||||||
| Left putamen | 194 | −11 | 15 | −3 | 3.57 | |
| Left caudate | 100 | −14 | 12 | −3 | 3.14 | |
Significantly increased 11C-RAC displacement during speech production was seen in the control subjects in the bilateral putamen and right caudate nucleus, and in the bilateral striatum in patients with writer’s cramp (Fig. 4A–B and Table 3). During asymptomatic speech production, the patients with writer’s cramp compared to control subjects showed greater 11C-RAC displacement (increased 11C-RAC ΔBP) in the left caudate nucleus and left putamen, as well as a cluster of reduced 11C-RAC displacement (decreased 11C-RAC ΔBP) in the posterior left putamen (Fig. 4C). Post hoc analysis showed there was no overlap between striatal regions of altered 11C-RAC ΔBP during speech production and reduced 11C-RAC binding potential at rest (Fig. 4D).
Figure 4.
Statistical parametric maps showing endogenous dopamine release as measured by displacement of 11C-raclopride (RAC ΔBP) during a speech production task in (A) healthy control subjects (HC) and (B) patients with writer’s cramp (WC), and (C) patients with writer’s cramp compared with healthy control subjects. Colour bars show range of t-values displayed. (D) Left striatal regions showing decreased 11C-RAC ΔBP during the speech production task (green) were non-overlapping with striatal regions found to have reduced D2/D3 receptor availability at rest (yellow). Images are shown P < 0.05, FWE corrected, on axial and coronal slices of a standard brain in Talairach space.
Region of interest analysis of 11C-RAC ΔBP using the whole striatum volumes revealed no significant differences in 11C-RAC displacement in either putamen or caudate nucleus during finger tapping or speech production between patients with writer’s cramp and control subjects. A trend toward increased 11C-RAC displacement, however, was seen in the right caudate nucleus (writer’s cramp: 11.3 ± 20.8%, control subjects: 0.57 ± 19.0%; P = 0.06) during speech production. Using regions of interest generated from significant clusters identified in the voxel-wise analysis, significant decreases in 11C-RAC displacement were observed in the right putamen (writer’s cramp: 6.5 ± 6.4%, control subjects: 9.7 ± 4.6%; P < 0.05) and left caudate nucleus (writer’s cramp: 6.6 ± 4.4%, control subjects: 9.3 ± 6.4%; P < 0.05), with a trend toward decreased 11C-RAC displacement observed in the left putamen (writer’s cramp: 1.0 ± 1.8%, control subjects: 6.1 ± 5.1%; P < 0.1), during finger tapping (Fig. 5C). Significant increases in 11C-RAC displacement were observed in the left putamen (writer’s cramp: 14.9 ± 4.1%, control subjects: 8.3 ± 5.0%; P < 0.005) and left caudate nucleus (writer’s cramp: 15.8 ± 4.8%, control subjects: 1.3 ± 4.8%; P < 0.005) during speech production (Fig. 5D).
Correlations between positron emission tomography findings and clinical measures
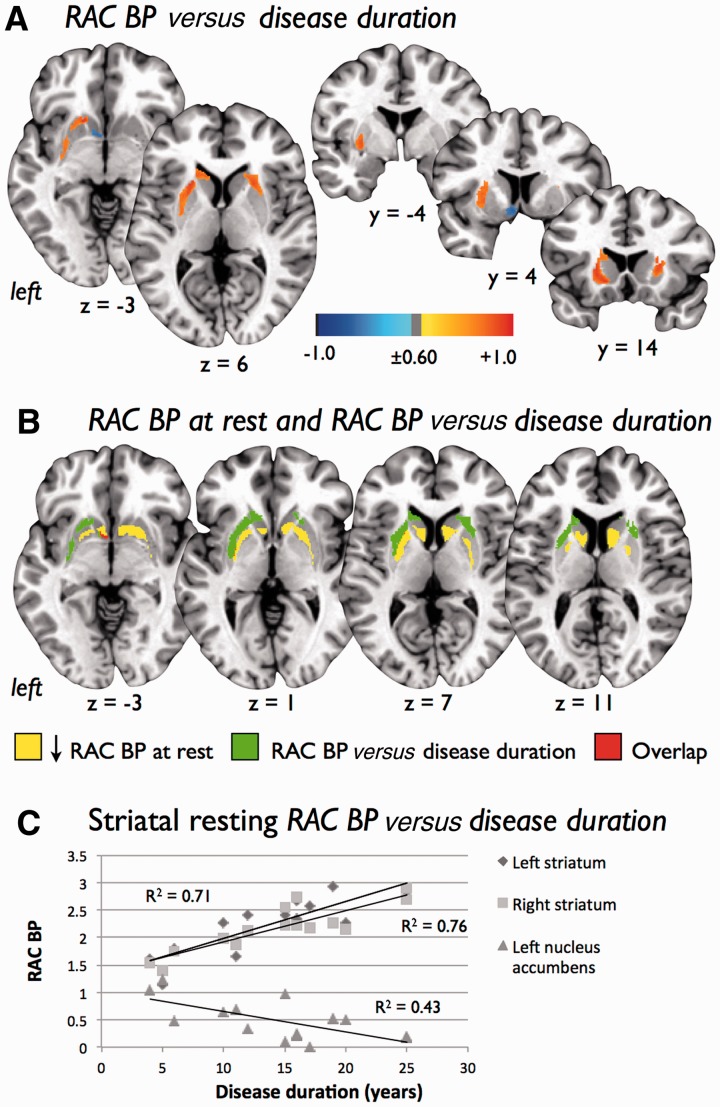
In patients with writer’s cramp, 11C-RAC binding potential at rest was positively correlated (r ≥ 0.60, voxel P < 0.025, cluster size ≥ 100 voxels) with disease duration with a greater cluster size seen in the left striatum compared with the right striatum (Fig. 6A and Table 5). A region consisting of a negative correlation between 11C-RAC binding potential at rest and disease duration (r ≤ −0.60, voxel P < 0.025, cluster size ≥ 100 voxels) was also found in the left nucleus accumbens. The striatal regions showing increasing 11C-RAC binding with writer’s cramp disease duration were lateral to and non-overlapping with those regions identified as having reduced D2/D3 binding potential at rest with the exception of a cluster of a overlap in the left nucleus accumbens (Fig. 6B). Using individual patient writer’s cramp PET data extracted with regions of interest generated from significant clusters identified in the voxel-wise correlation analysis, scatter plots supported the presence of positive correlations between 11C-RAC binding potential at rest and disease duration in the left striatum (r = 0.85, P < 0.001) and right striatum (r = 0.87, P < 0.001), as well as the negative correlation between 11C-RAC binding potential at rest and disease duration in the left nucleus accumbens (r = -0.66, P < 0.01; Fig. 6C).
Figure 6.
(A) Voxel-wise correlation analysis between D2/D3 receptor availability at rest in patients with writer’s cramp (WC) as measured by 11C-raclopride binding potential (RAC BP) and disease duration. Images are shown at a voxel threshold of P ≤ 0.025 (r = ± 0.60) with a cluster size threshold = 100, on axial and coronal slices of a standard brain in Talairach space. Colour bar shows range of r-values displayed. (B) Striatal regions showing a significant positive correlation between D2/D3 receptor availability at rest and disease duration (green) were non-overlapping and lateral to striatal regions found to have significantly reduced D2/D3 receptor availability at rest (yellow) compared to control subjects. One region of overlap (red) between these images was found in the left nucleus accumbens. (C) Scatterplots of disease duration and 11C-RAC binding potential to striatal dopamine D2/D3 receptors during rest generated using bilateral striatal and left nucleus accumbens clusters found to be significantly different between healthy control subjects and writer’s cramp in the voxel-based analyses.
Table 5.
Significant clusters from voxel-wise correlation analysis between 11C-raclopride binding at rest and disease duration in patients with writer’s cramp
| Striatal region | Cluster size (voxels) | Talairach coordinates |
Peak r-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left caudate/putamen | 2770 | −14 | 15 | −2 | 0.95 |
| Right caudate/putamen | 814 | 24 | 13 | 8 | 0.89 |
| Left nucleus accumbens | 102 | −3 | 3 | −6 | −0.75 |
No significant correlations were found between 11C-RAC binding potential at rest and symptom severity as measured by the Writer’s Cramp Rating Scale. Additionally, no significant correlations were found between 11C-RAC ΔBP during the finger tapping and speech production tasks and disease duration or symptom severity.
Discussion
This study was designed to assess binding of the D2/D3 receptor antagonist 11C-RAC at rest and displacement of 11C-RAC during performance of two tasks in patients with writer’s cramp. Using a voxel-based image analysis approach, we found decreased 11C-RAC displacement in the left striatum and right putamen in patients with writer’s cramp during a right-handed finger-tapping task that was asymptomatic, but used the same effectors that are involved in dystonia. We also found increased 11C-RAC displacement in the left striatum, as well as a region of decreased 11C-RAC displacement in the left putamen, in the same patients with writer’s cramp during an asymptomatic speech production task. Furthermore, 11C-RAC binding at rest showed a significant relationship with disease duration. These novel findings provide direct evidence that suggests adult-onset primary focal dystonia is associated with abnormal dopaminergic neurotransmission.
Reduced 11C-raclopride binding to striatal D2/D3 receptors during rest
During the resting state, 11C-RAC binding to D2/D3 receptors in the striatum of patients with writer’s cramp was reduced. This finding is consistent with the majority of prior D2-like dopamine receptor imaging studies in primary focal dystonias (Horstink et al., 1997; Perlmutter et al., 1997b; Naumann et al., 1998; Berger et al., 2007; Horie et al., 2009). Localized changes in expression of D2/D3 receptors might help explain some of the inconsistency in previously reported D2-like receptor imaging studies that applied region of interest methods and averaged across striatal structures.
As our patient cohort was a carefully selected group of patients with task-specific focal hand dystonia who did not experience any dystonic symptoms at rest, the finding of reduced 11C-RAC binding is unlikely to have been confounded by dystonic movements occurring during rest. This finding, therefore, could potentially be explained by abnormal D2-like receptor expression in writer’s cramp or possibly by a defect in receptor turnover or trafficking (Seeman et al., 1989; Volkow et al., 1994; Beaulieu and Gainetdinov, 2011). The presence of increased tonic striatal dopamine release, however, could also lead to the finding of reduced D2/D3 availability in the absence of any receptor deficit.
Altered 11C-raclopride displacement during task performance
11C-RAC displacement was significantly decreased in the left caudate nucleus and bilateral putamen during right-handed finger-tapping task in patients with writer’s cramp compared to control subjects. As motor performance was not significantly different between patients and control subjects, and since patients did not experience dystonic spasms during tapping, motor performance cannot explain the difference found between the groups. One of the two significant clusters of decreased 11C-RAC displacement within the left putamen identified during tapping overlapped with a striatal region associated with reduced D2/D3 availability. As such, the focal manifestation of dystonia in these patients may be related to deficient task-induced dopamine release occurring in the setting of reduced D2/D3 receptor availability. In a similar investigation in patients with spasmodic dysphonia, we found decreased 11C-RAC displacement during a symptomatic speech production task (Simonyan et al., 2013a). Together these findings help support the presence of abnormal task-dependent (phasic) dopaminergic neurotransmission in adult-onset primary focal dystonia. These congruous findings are in line with previous animal and human studies where reduced striatal dopamine release has been associated with the manifestation of dystonia (Knappskog et al., 1995; Perlmutter et al., 1997a; Tabbal et al., 2006; Balcioglu et al., 2007; Clot et al., 2009; Hewett et al., 2010; Page et al., 2010; Song et al., 2012).
During performance of the asymptomatic speech production task, 11C-RAC displacement was significantly increased in patients with writer’s cramp in the left striatum. In our comparable 11C-RAC study of patients with spasmodic dysphonia (Simonyan et al., 2013a), increased 11C-RAC displacement was observed during asymptomatic sequential finger tapping. These findings suggest that an increase in phasic striatal dopamine release may occur during the performance of asymptomatic tasks in patients with focal dystonia. These increases might reflect a compensatory response to reduced striatal D2/D3 receptor availability; however, we found no areas where reduced D2/D3 availability at rest overlapped with the significantly increased or decreased 11C-RAC displacement during speech production arguing against this possible explanation. Another possibility is that patients with writer’s cramp have subclinical alterations of the dopaminergic system involved in speech production. To test this hypothesis, future investigations focused on identifying speech initiation and production disturbances in patients with writer’s cramp will be needed.
Correlations of 11C-raclopride findings with clinical measures
Our voxel-based correlation analysis identified positive relations between D2/D3 availability at rest as measured by 11C-RAC binding potential and disease duration in writer’s cramp throughout the lateral aspects of the left striatum and to a lesser degree in the right striatum. Our analysis also revealed a small region of negative correlation between D2/D3 availability at rest and disease duration in writer’s cramp within the left nucleus accumbens. This region of negative correlation overlapped with an area of reduced D2/D3 availability found in writer’s cramp compared to control subjects. Interestingly, however, the lateral striatal regions showing increasing D2/D3 availability with disease duration were non-overlapping with the medial distribution of reduced D2/D3 availability found in writer’s cramp compared with control subjects.
Non-human primate neuroanatomical tracing studies (Takada et al., 1998; Nambu et al., 2002, Kelly and Strick, 2004; Miyachi et al., 2006; Haber and Calzavara, 2009; Nambu, 2011) and human imaging studies (Maillard et al., 2000; Gerardin et al., 2003; Bingel et al., 2004) have helped elucidate the organization of the striatum and other basal ganglia structures, which is thought to reflect somatotopically organized cortical inputs to the striatum and outputs back to the cortex. Disorganization of the striatal somatotopic organization has been reported both in mouse models of dystonia (Chiken et al., 2008) as well as in humans (Vitek et al., 1999; Delmaire et al., 2005). A medio-lateral organization for the striatum has also been described with medial regions receiving somatotopic inputs from the supplementary motor area and lateral regions receiving inputs primarily from the primary motor cortex (Nambu, 2011). Thus, the reduced D2/D3 availability at rest in the medial striatum may indicate that basal ganglia circuits involving premotor cortices and those involving the primary motor cortex are differentially affected in writer’s cramp. Although this hypothesis is supported by a voxel-based morphometry study that found patients with cervical dystonia had decreased grey matter density in the supplementary motor area accompanied by increased grey matter density in primary motor cortex (Draganski et al., 2003), reported changes in premotor cortices in patients with focal hand dystonia have been more varied (Garraux et al., 2004; Delmaire et al., 2007; Egger et al., 2007; Granert et al., 2010). Still, premotor dysfunction in writer’s cramp has been well supported in these mophometry studies and in a large number of electrophysiological (Yazawa et al., 1999; Zeuner et al., 2009) and functional imaging studies (Odergren et al., 1998; Ibanez et al., 1999; Oga et al., 2002; Hu et al., 2006; Castrop et al., 2012). Abnormal dopaminergic function specifically affecting the medial striatal regions that are connected to premotor cortices, and thereby associated with the generation, planning and selection of movements (Chouinard and Paus, 2006; Cohen et al., 2010; Da Cunha et al., 2012), might underlie the task-specific nature of the focal dystonia in writer’s cramp. Future multimodal imaging investigations, such as those combining functional MRI and PET techniques, however, will be needed to try and link the molecular changes and larger scale physiological changes identified in writer’s cramp.
The one region where decreased striatal D2/D3 availability at rest in patients with writer’s cramp overlapped with reducing D2/D3 availability over disease duration was the left nucleus accumbens. This finding suggests that the nigro-striatal and mesolimbic dopamine pathways could be affected differently in writer’s cramp. Given the prevailing findings of previous investigations, along with the widespread striatal dopaminergic dysfunction we found in patients with writer’s cramp and patients with spasmodic dysphonia (Simonyan et al., 2013a), dysfunction within the nigro-striatal dopaminergic system in primary dystonia is well supported. The role that mesolimbic dopamine pathways stemming from the ventral tegmental area might play in primary dystonia is not known. In a hamster model of idiopathic primary generalized dystonia, however, D2 binding was decreased in the dorsomedial striatum and increased in nucleus accumbens (Nobrega et al., 1996). In the same hamster model, significant decreases in dopamine transporter binding were also found in the nucleus accumbens and ventral tegmental area of those animals expressing severe dystonia (Nobrega et al., 1999). In a post-mortem examination of two patients with early-onset generalized dystonia, reduced concentrations of dopamine were detected in the nucleus accumbens (Hornykiewicz et al., 1986). In a more recent voxel-based morphometry study of a group of patients with focal hand dystonia and cervical dystonia, the volume of the nucleus accumbens was found to be increased (Egger et al., 2007). Together these findings suggest that alterations within the mesolimbic dopamine pathway could be present in primary dystonia. While involvement of the mesolimbic pathway might help explain the increased prevalence of depression and anxiety in the disorder (Kuyper et al., 2011; Stamelou et al., 2012), its role and contribution to symptoms in primary dystonia remain to be determined.
Limitations
One potential limitation in our study is that patients with writer’s cramp were not scanned during the dystonia-inducing task of writing. The use of the finger-tapping task, however, allowed for a more accurate matching of motor behaviour between patients and control subjects and helped avoid the confounding effect of excessive muscle spasms occurring during writing on our findings. Additionally, the fact that no patient with writer’s cramp experienced dystonic spasms during the tapping task helped substantiate the isolated and task-specific nature of focal dystonia in these patients. Still, future studies on dopamine release during dystonic-inducing tasks could help better define the relationship between dopaminergic neurotransmission dysfunction and manifestation of symptoms in focal hand dystonia. Likewise, PET investigations using task-free pharmacologically-induced dopamine release or radioligands with presynaptic targets could help better characterize dopaminergic dysfunction in primary dystonia (Cummings et al., 2011).
Given the limited spatial resolution of PET, it is possible partial volume effects may have affected some of our findings. As no volumetric differences were detected in the striatum of our patients with writer’s cramp compared to healthy control subjects, however, morphometric alterations, such as those that have been previously reported in a variety forms primary dystonia (Neychev et al., 2011; Zoons et al., 2011), are unlikely to have contributed significantly to our findings. Another limitation is that PET, in contrast to microdialysis, provides indirect measures of dopamine release. 11C-RAC, however, has been previously shown to have specific affinity for D2/D3 receptors and behave as predicted by the occupancy (competitive) model such that the magnitude of changes in 11C-RAC displacement corresponds directly to the amount of change in synaptic dopamine concentration (Seeman et al., 1989; Laruelle, 2000, 2012). Still, dopamine release is thought to likely exert a non-competitive action on 11C-RAC binding through mechanisms such as dopamine-induced internalization of striatal D2 receptors (Ginovart, 2005). Non-competitive actions of dopamine on 11C-RAC binding, however, have largely been described under amphetamine-induced dopamine release conditions in which a massive release is triggered. It is not known to what extent similar mechanisms occur under more physiological task-induced dopamine release conditions. Furthermore, non-competitive mechanisms like internalization of D2 receptors are implicated in late stage responses to dopamine surges (Laruelle, 2012), and so likely play a limited role during the relatively brief 50 min of task performance used in our study. Regardless of the extent non-competitive mechanisms contribute to our 11C-RAC displacement findings, endogenous dopamine release is the trigger that leads to changes in 11C-RAC binding in both the competitive and non-competitive models proposed to date. Still, if writer’s cramp is associated with dysregulation of D2 receptor trafficking as has been described in some genetic forms of primary dystonia (Torres et al., 2004; Esapa et al., 2007), our results may be an underestimate or exaggeration of the amount of striatal dopamine release depending on whether there is reduced or increased internalization of D2 receptors, respectively.
Although the order of the 100-min PET scans were counterbalanced in terms of which task was performed in the second half of the scan, and all scanning procedures and their timing kept consistent across patients and control subjects to limit factors that might alter 11C-RAC binding, we did not include a separate control group that performed no task during the second half of the scan. If 11C-RAC binding differences exist between patients with dystonia and control subjects during the second 50 min compared to the initial baseline 50 min (e.g. from differing levels of or altered competition with cold raclopride), it might affect measures of 11C-RAC displacement during the performance of the tasks. As such, future investigations assessing differences in task-induced dopamine release could potentially benefit from adding a non-task performing control group to test whether 11C-RAC binding differs at rest between the first and second half of the scan in patients with writer’s cramp. Another limitation to our study is the lack of a counterbalanced design in terms of the order of the task and rest conditions within a scanning session. Unfortunately, counterbalanced task designs are not able to be implemented using 11C-RAC and the bolus-plus-infusion method because pharmacological challenges and performance of tasks induce changes in dopamine receptor binding that persist for over an hour and would therefore lead to a contamination of the resting state if it followed the task (Laruelle et al., 1997; Carson, 2000; Houston et al., 2004). The bolus-plus-infusion method, however, does have a number of advantages over bolus-only studies including the ability to maintain 11C-RAC in equilibrium while both control and task data are collected as well as allowing for 11C-RAC displacement to be measured during a single scanning session thereby limiting the effects of changes in cerebral blood flow and other physiological processes on obtained measures during different scanning sessions (Watabe et al., 2000; Slifstein and Laruelle, 2001).
Lastly, while the 11C-RAC scanning procedures used here have been shown to maximize signal-to-noise ratio for detection of synaptic dopamine release (Watabe et al., 2000), a limitation to the imaging method used is its inability to distinguish primary from secondary changes or between cause and effect (Zoons et al., 2011). Thus, the striatal dopaminergic dysfunction detected could stem from distinct yet linked pathophysiological processes such as inhibitory deficits, maladaptive neuroplasticity, and defective sensorimotor function (Hallett, 2006a, 2011; Breakefield et al., 2008; Peterson et al., 2010; Neychev et al., 2011).
Conclusion
In conclusion, our findings suggest that reduced striatal D2/D3 receptor availability at rest as measured by decreased 11C-RAC binding potential in writer’s cramp is accompanied by disturbances in task-induced endogenous dopamine release as measured by a change in 11C-RAC binding. During asymptomatic sequential finger tapping involving the affected right hand, dopamine release was reduced in the left striatum in patients with writer’s cramp. In contrast, during asymptomatic speech production, dopamine release was increased in the left striatum. The manifestation of a task-specific primary focal dystonia in a susceptible individual may therefore stem from reduced task-induced dopaminergic neurotransmission occurring in the setting of reduced dopamine receptor availability. The positive correlation between disease duration and D2/D3 receptor availability in the lateral striatum raises the possibility that there may be a localized compensatory response or that the pathophysiological process in writer’s cramp selectively involves circuits associated with the medial striatum.
Acknowledgements
We would like to thank Sandra B. Martin, MS, and Pamela R. Kearney, MD, for subject’s clinical evaluation. We also wish to thank Richard Reynolds, MS, for help with imaging data analysis and the staff at the PET Department of the NIH Clinical Centre for assistance with radiopharmaceutical production and PET data acquisition.
Glossary
Abbreviation
- AFNI
analysis of functional neuroimages
- BP
binding potential
- FWE
family-wise error
- PET
positron emission tomography
- RAC
11C-raclopride
- ROI
region of interest
- SD
spasmodic dysphonia
- WC
writer’s cramp
- WCRS
writer’s cramp rating scale
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders, NIH [DC009629 to K.S.]; and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH.
References
- Albanese A, Asmus F, Bhatia K, Elia A, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- Augood S, Hollingsworth Z, Albers D, Yang L, Leung J, Muller B, et al. Dopamine transmission in DYT1 dystonia: a biochemical and autoradiographical study. Neurology. 2002;59:445–8. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Kim M, Sharma N, Cha J, Breakefield X, Standaert D. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem. 2007;102:783–8. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu J, Gainetdinov R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Becker G, Naumann M, Scheubeck M, Hofmann E, Deimling M, Lindner A, et al. Comparison of transcranial sonography, magnetic resonance imaging, and single photon emission computed tomography findings in idiopathic spasmodic torticollis. Mov Disord. 1997;12:79–88. doi: 10.1002/mds.870120114. [DOI] [PubMed] [Google Scholar]
- Berger H, van der Werf S, Horstink C, Cools A, Oyen W, Horstink M. Writer's cramp: restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism Relat Disord. 2007;13:170–3. doi: 10.1016/j.parkreldis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Bingel U, Gläscher J, Weiller C, Büchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex. 2004;14:1340–5. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- Breakefield X, Blood A, Li Y, Hallett M, Hanson P, Standaert D. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Carson R. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27:657–60. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Castrop F, Dresel C, Hennenlotter A, Zimmer C, Haslinger B. Basal ganglia-premotor dysfunction during movement imagination in writer's cramp. Mov Disord. 2012;27:1432–9. doi: 10.1002/mds.24944. [DOI] [PubMed] [Google Scholar]
- Chiken S, Shashidharan P, Nambu A. Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J Neurosci. 2008;28:13967–77. doi: 10.1523/JNEUROSCI.3834-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard P, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–52. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Clot F, Grabli D, Cazeneuve C, Roze E, Castelnau P, Chabrol B, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with Dopa-responsive dystonia. Brain. 2009;132(Pt 7):1753–63. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmutter S, Prut Y. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol. 2010;20:696–703. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cummings J, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain. 2011;134(Pt 11):3146–66. doi: 10.1093/brain/awr177. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Gomez-A A, Blaha C. The role of the basal ganglia in motivated behavior. Rev Neurosci. 2012;23:747–67. doi: 10.1515/revneuro-2012-0063. [DOI] [PubMed] [Google Scholar]
- Dang M, Yokoi F, McNaught K, Jengelley T, Jackson T, Li J, et al. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol. 2005;196:452–63. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Dang M, Yokoi F, Pence M, Li Y. Motor deficits and hyperactivity in Dyt1 knockdown mice. Neurosci Res. 2006;56:470–4. doi: 10.1016/j.neures.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–93. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Krainik A, Tézenas du Montcel S, Gerardin E, Meunier S, Mangin J, et al. Disorganized somatotopy in the putamen of patients with focal hand dystonia. Neurology. 2005;64:1391–6. doi: 10.1212/01.WNL.0000158424.01299.76. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton J, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. 2007;69:376–80. doi: 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–31. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Egger K, Mueller J, Schocke M, Brenneis C, Rinnerthaler M, Seppi K, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. 2007;22:1538–42. doi: 10.1002/mds.21619. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Esapa C, Waite A, Locke M, Benson M, Kraus M, McIlhinney R, et al. SGCE missense mutations that cause myoclonus-dystonia syndrome impair epsilon-sarcoglycan trafficking to the plasma membrane: modulation by ubiquitination and torsinA. Hum Mol Genet. 2007;16:327–42. doi: 10.1093/hmg/ddl472. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Hornykiewicz O, Fahn S, Kish S. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology. 2000;54:1193–5. doi: 10.1212/wnl.54.5.1193. [DOI] [PubMed] [Google Scholar]
- Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–9. doi: 10.1002/ana.20113. [DOI] [PubMed] [Google Scholar]
- Garraux G, Peigneux P, Carson RE, Hallett M. Task-related interaction between basal ganglia and cortical dopamine release. J Neurosci. 2007;27:14434–41. doi: 10.1523/JNEUROSCI.1595-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin E, Lehéricy S, Pochon J, Tézenas du Montcel S, Mangin J, Poupon F, et al. Foot, hand, face and eye representation in the human striatum. Cereb Cortex. 2003;13:162–9. doi: 10.1093/cercor/13.2.162. [DOI] [PubMed] [Google Scholar]
- Ginovart N. Imaging the dopamine system with in vivo [11C]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol. 2005;7:45–52. doi: 10.1007/s11307-005-0932-0. [DOI] [PubMed] [Google Scholar]
- Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, et al. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage. 2010;54:32–41. doi: 10.1016/j.neuroimage.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hübener J, Teismann P, Hauser T, et al. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Haber S, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Pathophysiology of dystonia. J Neural Transm Suppl. 2006a:485–8. doi: 10.1007/978-3-211-45295-0_72. [DOI] [PubMed] [Google Scholar]
- Hallett M. Pathophysiology of writer's cramp. Hum Mov Sci. 2006b;25:454–63. doi: 10.1016/j.humov.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–84. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J, Johanson P, Sharma N, Standaert D, Balcioglu A. Function of dopamine transporter is compromised in DYT1 transgenic animal model in vivo. J Neurochem. 2010;113:228–35. doi: 10.1111/j.1471-4159.2010.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J, Cordes M, Schelosky L, Richter W, Keske U, Venz S, et al. Dopamine D2 receptor imaging with iodine-123-iodobenzamide SPECT in idiopathic rotational torticollis. J Nuclear Med. 1994;35:1921–7. [PubMed] [Google Scholar]
- Horie C, Suzuki Y, Kiyosawa M, Mochizuki M, Wakakura M, Oda K, et al. Decreased dopamine D receptor binding in essential blepharospasm. Acta Neurol Scand. 2009;119:49–54. doi: 10.1111/j.1600-0404.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish S, Becker L, Farley I, Shannak K. Brain neurotransmitters in dystonia musculorum deformans. N Engl J Med. 1986;315:347–53. doi: 10.1056/NEJM198608073150602. [DOI] [PubMed] [Google Scholar]
- Horstink CA, Praamstra P, Horstink M, Berger HJC, Booij J, VanRoyen EA. Low striatal D2 receptor binding as assessed by I-123 IBZM SPECT in patients with writer's cramp. J Neurol Neurosurg Psychiatry. 1997;62:672–3. doi: 10.1136/jnnp.62.6.672-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston G, Hume S, Hirani E, Goggi J, Grasby P. Temporal characterisation of amphetamine-induced dopamine release assessed with [11C]raclopride in anaesthetised rodents. Synapse. 2004;51:206–12. doi: 10.1002/syn.10296. [DOI] [PubMed] [Google Scholar]
- Hu X, Wang L, Liu H, Zhang S. Functional magnetic resonance imaging study of writer's cramp. Chin Med J (Engl) 2006;119:1263–71. [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer's cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Karimi M, Moerlein SM, Videen TO, Luedtke RR, Taylor M, Mach RH, et al. Decreased striatal dopamine receptor binding in primary focal dystonia: A D2 or D3 defect? Mov Disord. 2011;26:100–6. doi: 10.1002/mds.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Strick P. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–59. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Knappskog P, Flatmark T, Mallet J, Lüdecke B, Bartholomé K. Recessively inherited L-DOPA-responsive dystonia caused by a point mutation (Q381K) in the tyrosine hydroxylase gene. Hum Mol Genet. 1995;4:1209–12. doi: 10.1093/hmg/4.7.1209. [DOI] [PubMed] [Google Scholar]
- Kruisdijk JJ, Koelman JH, Ongerboer de Visser BW, de Haan RJ, Speelman JD. Botulinum toxin for writer's cramp: a randomised, placebo-controlled trial and 1-year follow-up. J Neurol Neurosurg Psychiatry. 2007;78:264–70. doi: 10.1136/jnnp.2005.083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM. Nonmotor manifestations of dystonia: a systematic review. Mov Disord. 2011;26:1206–17. doi: 10.1002/mds.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Measuring dopamine synaptic transmission with molecular imaging and pharmacological challenges: the state of the art. In: Gründer G, editor. Molecular imaging in the clinical neurosciences: neuromethods. New York, NY USA: Humana Press; 2012. pp. 163–203. [Google Scholar]
- Laruelle M, Iyer R, al-Tikriti M, Zea-Ponce Y, Malison R, Zoghbi S, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leenders K, Hartvig P, Forsgren L, Holmgren G, Almay B, Eckernas SA, et al. Striatal [11C]-N-methyl-spiperone binding in patients with focal dystonia (torticollis) using positron emission tomography. J Neural Transm Park Dis Dement Sect. 1993;5:79–87. doi: 10.1007/BF02251198. [DOI] [PubMed] [Google Scholar]
- Maillard L, Ishii K, Bushara K, Waldvogel D, Schulman A, Hallett M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology. 2000;55:377–83. doi: 10.1212/wnl.55.3.377. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Lu X, Imanishi M, Sawada K, Nambu A, Takada M. Somatotopically arranged inputs from putamen and subthalamic nucleus to primary motor cortex. Neurosci Res. 2006;56:300–8. doi: 10.1016/j.neures.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Nambu A. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 2011;5:1–9. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Kaneda K, Tokuno H, Takada M. Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol. 2002;88:1830–42. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- Naumann M, Pirker W, Reiners K, Lange KW, Becker G, Brucke T. Imaging the pre- and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: a SPECT study using I-123 epidepride and I-123 beta-CIT. Mov Disord. 1998;13:319–23. doi: 10.1002/mds.870130219. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega J, Gernert M, Löscher W, Raymond R, Belej T, Richter A. Tyrosine hydroxylase immunoreactivity and [3H]WIN 35,428 binding to the dopamine transporter in a hamster model of idiopathic paroxysmal dystonia. Neuroscience. 1999;92:211–17. doi: 10.1016/s0306-4522(98)00753-2. [DOI] [PubMed] [Google Scholar]
- Nobrega J, Richter A, Tozman N, Jiwa D, Löscher W. Quantitative autoradiography reveals regionally selective changes in dopamine D1 and D2 receptor binding in the genetically dystonic hamster. Neuroscience. 1996;71:927–37. doi: 10.1016/0306-4522(95)00511-0. [DOI] [PubMed] [Google Scholar]
- Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer's cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer's cramp: an fMRI study. Brain. 2002;125:895–903. doi: 10.1093/brain/awf083. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness - Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Ichiya Y, Shima F, Kuwabara Y, Sasaki M, Fukumura T, et al. Increased striatal 18F-dopa uptake and normal glucose metabolism in idiopathic dystonia syndrome. J Neurol Sci. 1992;111:195–9. doi: 10.1016/0022-510x(92)90068-v. [DOI] [PubMed] [Google Scholar]
- Page M, Bao L, Andre P, Pelta-Heller J, Sluzas E, Gonzalez-Alegre P, et al. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (DeltaE) TorsinA in transgenic mice. Neurobiol Dis. 2010;39:318–26. doi: 10.1016/j.nbd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter J, Tempel L, Black K, Parkinson D, Todd R. MPTP induces dystonia and parkinsonism. Clues to the pathophysiology of dystonia. Neurology. 1997a;49:1432–8. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGeeMinnich L, Jankovic J, et al. Decreased F-18 spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997b;17:843–50. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D, Sejnowski T, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37:558–73. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Guan H, Niznik H. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: implications for positron emission tomography of the human brain. Synapse. 1989;3:96–7. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 2013a;33:14705–14. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Herscovitch P, Horwitz B. Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: A combined PET, fMRI and DTI study. Neuroimage. 2013b;70:21–32. doi: 10.1016/j.neuroimage.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Song C, Fan X, Exeter C, Hess E, Jinnah H. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiol Dis. 2012;48:66–78. doi: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamelou M, Edwards M, Hallett M, Bhatia K. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135(Pt 6):1668–81. doi: 10.1093/brain/awr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert D. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–51. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbal S, Mink J, Antenor J, Carl J, Moerlein S, Perlmutter J. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neuroscience. 2006;141:1281–7. doi: 10.1016/j.neuroscience.2006.04.072. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–28. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–29. doi: 10.1056/NEJMra055549. [DOI] [PubMed] [Google Scholar]
- Torres G, Sweeney A, Beaulieu J, Shashidharan P, Caron M. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci USA. 2004;101:15650–5. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Russotto D, Perlmutter JS. Task-specific dystonias: a review. Year Neurol. 2008;2008:179–99. doi: 10.1196/annals.1444.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek J, Chockkan V, Zhang J, Kaneoke Y, Evatt M, DeLong M, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–62. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of C-11 raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–30. [PubMed] [Google Scholar]
- Wissel J, Kabus C, Wenzel R, Klepsch S, Schwarz U, Nebe A, et al. Botulinum toxin in writer's cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry. 1996;61:172–5. doi: 10.1136/jnnp.61.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kaji R, Terada K, Nagamine T, Toma K, et al. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain. 1999;122(Pt 7):1357–66. doi: 10.1093/brain/122.7.1357. [DOI] [PubMed] [Google Scholar]
- Zeuner K, Peller M, Knutzen A, Groppa S, Holler I, Kopper F, et al. Slow pre-movement cortical potentials do not reflect individual response to therapy in writer's cramp. Clin Neurophysiol. 2009;120:1213–19. doi: 10.1016/j.clinph.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yokoi F, Parsons D, Standaert D, Li Y. Alteration of striatal dopaminergic neurotransmission in a mouse model of DYT11 myoclonus-dystonia. PLoS One. 2012;7:e33669. doi: 10.1371/journal.pone.0033669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M, LeDoux M. Abnormal motor function and dopamine neurotransmission in DYT1 DeltaGAG transgenic mice. Exp Neurol. 2008;210:719–30. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoons E, Booij J, Nederveen A, Dijk J, Tijssen M. Structural, functional and molecular imaging of the brain in primary focal dystonia–a review. Neuroimage. 2011;56:1011–20. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]