Abstract
The neuroendocrine response to episodes of acute stress is crucial for survival whereas the prolonged response to chronic stress can be detrimental. Learning and memory are particularly susceptible to stress with cognitive deficits being well characterized consequences of chronic stress. Although there is good evidence that acute stress can enhance cognitive performance, the mechanism(s) for this are unclear. We find that hippocampal slices, either prepared from rats following 30 min restraint stress or directly exposed to glucocorticoids, exhibit an N-methyl-d-aspartic acid receptor-independent form of long-term potentiation. We demonstrate that the mechanism involves an NMDA receptor and PKA-dependent insertion of Ca2+-permeable AMPA receptors into synapses. These then trigger the additional NMDA receptor-independent form of LTP during high frequency stimulation.
Keywords: long-term potentiation, metaplasticity, glucocorticoids, glutamate receptor, calcium
Introduction
Chronic stress is well recognized to be an important risk factor for both depression and memory impairment (McEwen et al., 2012) and a recent study in over 11 000 individuals from the Swedish Twin Registry showed that chronic morbidity is associated with significant impairment of cognitive function (Caracciolo et al., 2013). In experimental studies, stress has been specifically associated with effects on cognition (Kim and Diamond, 2002; de Kloet et al., 2005; Roozendaal et al., 2010) and memory processing (de Quervain et al., 2009). Deleterious effects are most apparent when stress has been prolonged, whereas in contrast the acute response to a stressor is adaptive with increased attention, vigilance and improved cognitive performance (de Kloet et al., 1999; Joels et al., 2008, 2011; Sandi, 2011). These and many other studies clearly show that in response to stress the brain exhibits both structural and functional plasticity, and it is this capacity that in part provides us with an opportunity to develop novel pharmacological strategies for the treatment of a wide range of clinical conditions from dementia and depression to epilepsy and stroke.
The first stage in the pathway to translating the positive effects of the acute stress response is to establish the mechanism(s) through which the stressor enhances synaptic plasticity, and in particular how the stress hormones cortisol (in human) and corticosterone (in the rodent) alter synaptic function. Previous studies suggest that glucocorticoids rapidly modulate excitatory synaptic transmission (Karst et al., 2005), at least in part through their regulation of glutamate receptors (Groc et al., 2008; Yuen et al., 2011). As excitatory glutamate receptors are critically involved in long-term synaptic plasticity and learning and memory (Bliss and Collingridge, 1993; Neves et al., 2008), the mechanisms underlying the glucocorticoid regulation of glutamatergic synaptic plasticity might provide links between glucocorticoids and the modulation of memory processes during stress.
The α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) is a major glutamatergic receptor involved in excitatory synaptic transmission. The trafficking of AMPARs to the synapse is widely accepted to be critical in long-term synaptic plasticity (Isaac et al., 1995; Hayashi et al., 2000; Kessels and Malinow, 2009), a process thought to underlie learning and memory (Bliss and Collingridge, 1993; Lamprecht and LeDoux, 2004; Neves et al., 2008; Ho et al., 2011). Both the number and subunit composition of postsynaptic AMPARs are able to determine the activity-dependent changes responsible for long-term potentiation (LTP) (Malinow and Malenka, 2002; Bredt and Nicoll, 2003; Sheng and Hyoung Lee, 2003). In particular, LTP involves the insertion of AMPARs into the synaptic region and a concurrent increase in AMPAR-mediated transmission (Kessels and Malinow, 2009). In addition to changes in synaptic receptor number during synaptic events, changes in AMPAR subunit composition can also be a fundamental process in regulating synaptic strength (Liu and Cull-Candy, 2000; Cull-Candy et al., 2006; Liu and Zukin, 2007). Such events occur in response to stress; exposure to stressors has been shown to mediate the synaptic insertion of GluA2-lacking, Ca2+-permeable AMPARs (CP-AMPARs) (Clem and Huganir, 2010; Savtchouk and Liu, 2011). However, the mechanism through which stress modifies AMPAR composition, and the consequences of this for synaptic plasticity, are unknown.
We hypothesized that acute stress or transient exposure to glucocorticoids would enhance mechanisms underlying synaptic efficacy. In the present study, we have characterized the effects of acute stress and glucocorticoid exposure on the magnitude and induction mechanism of hippocampal LTP using ex vivo and in vitro models. We find that acute stressors elicit an N-methyl-d-aspartate receptor (NMDAR)-dependent type of metaplasticity that enhances LTP through the priming of a form of LTP that is independent of, but additive with, NMDAR-dependent LTP. This stress-induced LTP is independent of protein synthesis but is associated with activation of protein kinase A (PKA), phosphorylation of S845 of GluA1, and insertion of GluA1 subunits into the plasma membrane. Stress-induced LTP requires Ca2+ for its induction and is blocked by IEM-1460 (IEM), an inhibitor of CP-AMPARs. Thus at CA1 synapses in the hippocampus, conventional NMDAR-LTP can coexist with a distinct form of LTP that is primed by stress and involves CP-AMPARs in its induction.
Materials and methods
Animals
Four to five-week old male Wistar rats were received from Charles River. They were housed in small groups with free access to water and food. They were subjected to a 12 h light/12 h dark cycle with the light phase commencing at 8.00 am. Animals were sacrificed between 10:00 am and 11:00 am by cervical dislocation in accordance with the UK Animals Scientific Procedures Act of 1986.
Restraint stress
Rats were physically restrained in 50 ml Falcon tubes for 30 min without food or water. Control rats were housed in their usual cages under normal conditions. Animals were sacrificed immediately following restraint stress by decapitation.
Slice preparation
The brain was quickly removed and transferred to ice-cold artificial CSF containing: 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1.25 mM NH2PO4, 2 mM CaCl2, 1 mM MgSO4, and 10 mM glucose. A mid-sagittal cut was made in the brain and one hemisphere was placed back into the ice-cold artificial CSF until it was required. Transverse hippocampal slices (400 µm) were cut using a Mcllwain tissue chopper (Mickle Laboratory Engineering Co. Ltd.) and allowed to stabilize in artificial CSF for 1 h while constantly perfused in 95% O2 / 5% CO2 mixture.
Electrophysiology
A recovery period, of approximately 60 min, was allowed for the tissue to recover from the slicing procedure and for stable responses to be obtained. Extracellular field potentials were recorded in the CA1 region using glass electrodes containing NaCl (3 M). Stimulating electrodes were placed in the subiculum and CA2 (Schaffer collateral pathway). Stimuli were delivered alternately to the two electrodes (each electrode 0.016 Hz). LTP was evoked by two trains of tetanus stimuli (each 100 Hz, 1 s; repeated after a 30 s interval). The slope of the evoked field potential responses was measured and expressed relative to the normalized preconditioning baseline. Data were captured and analysed using WinLTP (www.winltp.com). Experiments in which changes in the fibre volley occurred were discarded.
Biotinylation and NeutrAvidin pull-down
Surface biotinylation of acute slices was performed as described previously with some modifications (Thomas-Crusells et al., 2003). Briefly, slices were initially washed twice in artificial CSF and subsequently incubated in artificial CSF containing 1 mg/ml Sulfo-NHS-SS-Biotin for 45 min at 4°C to allow for labelling of all surface membrane proteins. Biotinylated tissue was then homogenized in lysis buffer containing 25 mM Tris (pH 7.6), 150 mM NaCl, 1% Triton™ X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM NaF and a cocktail of protease inhibitors (Sigma). The lysate was centrifuged at 21 000g to remove nuclei and cellular debris. Total protein concentration was determined using the BCA assay (Pierce). A small amount of the lysate was removed for whole-cell analysis later. Subsequently, 100 µl of StreptaAvidin beads (Thermo Scientific Inc) were added to 500 µg of protein lysate and placed on a rotator at 4°C for 2 h. Samples were then washed five times in wash buffer (25 mM Tris pH 7.6, 150 nM NaCl, 0.5% Triton™ X-100); beads were pulled-down after each wash by gentle centrifugation. Bound proteins were eluted in 2× SDS reducing buffer and gently heated at 60°C for 30 min. The resulting supernatant was transferred to new tubes and heated at 90°C for 5 min before gel loading.
Western blot and data analysis
Proteins suspended in Laemmli buffer were separated using 10% SDS-PAGE. Subsequently, the proteins were transferred onto PVDF membrane (Bio-Rad) and incubated with the relevant primary antibodies. The following polyclonal antibody was used: anti-panCadherin (1/1000) from Cell Signalling. Monoclonal antibodies used include: anti-β-actin (1/10 000) from Abcam; anti-phospho GluR1 S845 (1/2,000) from Millipore; anti-GluR1 (1/250 dilution) from Santa Cruz; and anti-GluR2 (1/1000) from Chemicon. Membranes were then incubated in either rabbit or mouse IgG antibodies (1/5000 dilution, Millipore) conjugated to horseradish peroxidise and immunoblotted using the ECL detection system (Thermo Scientific Inc.). Optical densities of immune reactive bands were measured using ImageJ software (NIH) and statistical analysis conducted with SigmaPlot (Systat Software, Inc., USA). The statistical significance of the data was analysed by Students t-test and a probability level of P < 0.05 was considered statistically significant.
Results
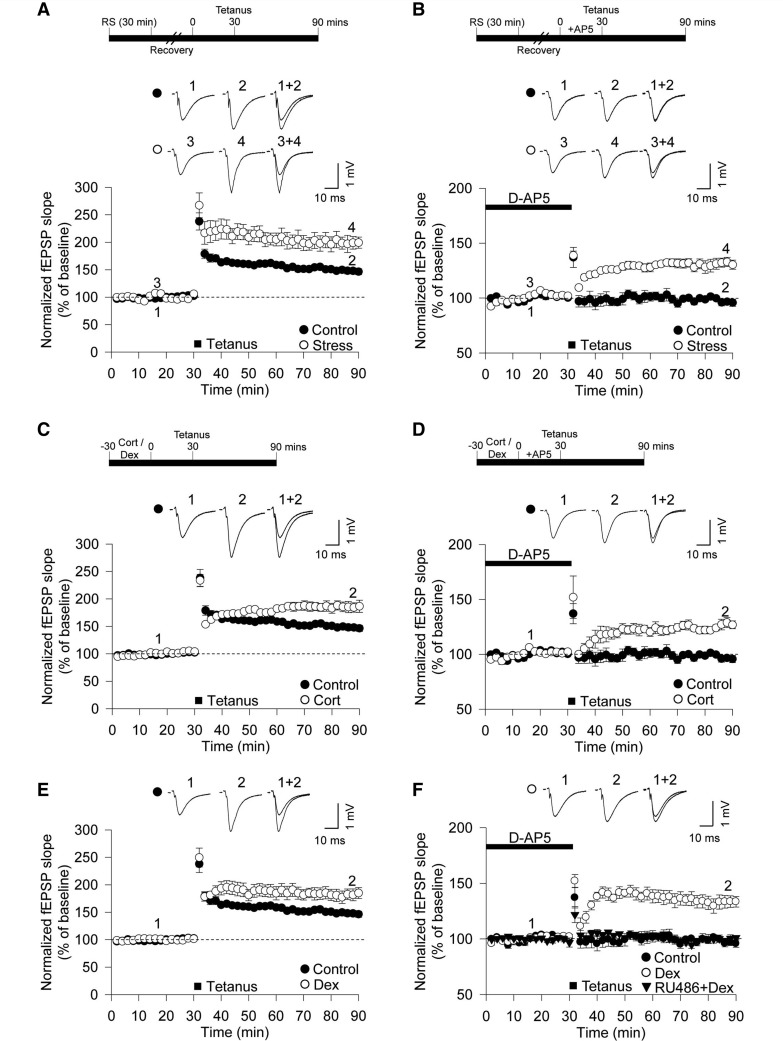
To investigate the effects of acute stress on synaptic function, we examined hippocampal synaptic plasticity in slices taken from animals that had undergone brief restraint stress for 30 min immediately before being sacrificed. The magnitude of LTP in field excitatory postsynaptic potentials was significantly greater in stressed animals compared with control animals (stress: 199 ± 11% of baseline, n = 6, open circle; interleaved controls: 150 ± 5%, n = 7, closed circle, P < 0.01, Fig. 1A). LTP induced by a tetanus (high-frequency stimulation, two trains of 100 Hz, 100 pulses) relies on the synaptic activation of NMDARs in the hippocampus (Collingridge et al., 1983). However, the enhanced LTP induced by stress (stress-induced LTP) was independent of NMDAR activation, as the NMDAR antagonist D-AP5 completely blocked LTP in control slices (97 ± 3%, n = 6, closed circle, P < 0.01, Fig. 1B), but not in slices from stressed animals (132 ± 4%, n = 6, open circle; Fig. 1B). Thus, acute stress enhances LTP through promoting an NMDAR-independent form of synaptic plasticity.
Figure 1.
Brief restraint stress and glucocorticoid treatment facilitates LTP through an NMDA receptor-independent mechanism. (A) Delivery of high frequency stimulation (two trains of 100 Hz, 100 pulses) induced LTP in the CA1 of the hippocampus. Exposure to 30 min restraint stress (RS) increased the level of LTP (open circle, n = 6) compared with non-stressed animals (closed circle; n = 7). (B) Incubation with the NMDAR antagonist D-AP5 (50 µM), during high-frequency stimulation, completely abolished LTP in control rat slices (closed circle; n = 6) but not after 30 min restraint stress (open circle, n = 6). (C) Preincubation of slices with 200 nM corticosterone (Cort) facilitated LTP induction (n = 6). (D) D-AP5 (50 µM) failed to block LTP in slices incubated with corticosterone (n = 6). (E) Facilitation of LTP induction was observed after treatment with 200 nM dexamethasone (Dex; n = 6). (F) D-AP5 (50 µM) failed to abolish LTP in slices treated with dexamethasone (n = 6), but pretreatment with RU486 (500 nM) abolished the dexamethasone-mediated facilitation of LTP (n = 6). Error bars indicate standard error of the mean (SEM). fEPSP = field excitatory postsynaptic potential.
To determine whether the effects of stress on LTP were mediated by glucocorticoids, we performed experiments using corticosterone (200 nM) or the synthetic glucocorticoid receptor agonist dexamethasone (200 nM) applied for 30 min before the tetanus and continued until the end of the experiment (Fig. 1C and E). These treatments produced effects strikingly similar to acute stress (Fig. 1A and B). Both corticosterone and dexamethasone enhanced LTP (corticosterone: 185 ± 10%, n = 6, Fig. 1C; dexamethasone: 182 ± 10%, n = 6, Fig. 1E), and D-AP5 failed to eliminate LTP in corticosterone- and dexamethasone-treated slices (corticosterone: 129 ± 5%, n = 6, Fig. 1D; dexamethasone: 133 ± 6%, n = 6, Fig. 1F). Pretreatment with the glucocorticoid receptor antagonist RU486 (500 nM) completely abolished NMDAR-independent LTP, induced by dexamethasone treatment (99 ± 4%, n = 6, Fig. 1F). In contrast, it had no effect on the induction of NMDAR-dependent LTP when applied alone (data not shown). Taken together, these results suggest that brief exposure to stress, acting through glucocorticoid receptors, enhances the magnitude of LTP in the hippocampus by priming an NMDAR-independent form of LTP.
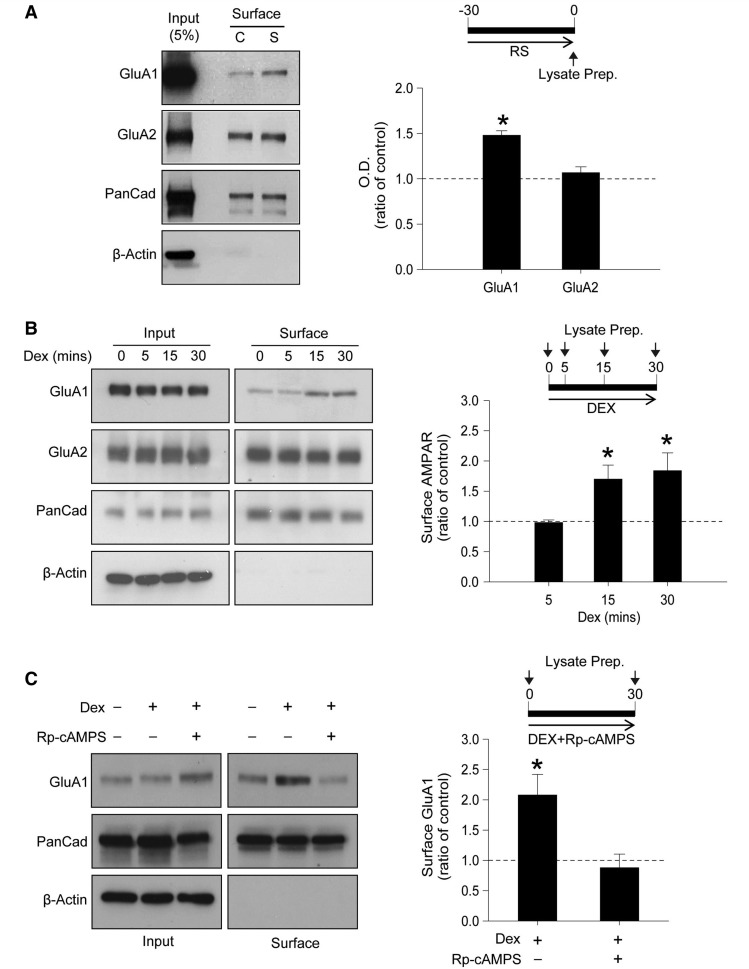
It is widely accepted that AMPARs mediate the major component of fast excitatory synaptic transmission (Derkach et al., 2007; Kessels and Malinow, 2009) and that GluA2 lacking, homomeric GluA1 AMPARs can have an important role in the generation of LTP under some (Plant et al., 2006; Wiltgen et al., 2010; Tayler et al., 2011), but not all (Adesnik and Nicoll, 2007), conditions. We therefore wondered whether the modulation of excitatory synaptic transmission by acute stress and glucocorticoid exposure, manifested in the enhancement of LTP, might be associated with changes in the synaptic expression of AMPARs and their subunit composition. Using a surface biotinylation assay, we found that there was a significant increase in surface expression of GluA1 in hippocampal slices prepared from stressed animals compared with controls (P < 0.01, n = 4, Fig. 2A). However, no difference was observed in GluA2 surface expression between the groups (P > 0.05, n = 4, Fig. 2A).
Figure 2.
Increased surface expression of GluA1 following acute stress and dexamethasone treatment requires PKA activation. (A) Biotinylation assay using hippocampal slices from rats exposed to 30 min restraint stress. GluA1 surface expression is increased in the stressed animals (S) compared with non-stressed, control animals (C) (n = 4). (B) In vitro experiments showing increased surface expression of GluA1 receptors after 15 and 30 min dexamethasone (Dex, 200 nM) treatment. No change in GluA2 expression was observed (n = 3). (C) Pretreatment with 100 µM Rp-cAMPS completely abolished the increase in GluA1 surface expression following dexamethasone treatment (n = 3). Error bars indicate SEM. *P < 0.05. PanCad = PanCadherin.
Similar results were obtained in slices treated acutely with dexamethasone (Fig. 2B). In these experiments we performed surface biotinylation experiments at various times after adding dexamethasone to establish how rapidly the effect occurred. We observed no significant difference in AMPAR receptor expression after 5 min (P > 0.05, n = 3) but GluA1 surface expression was selectively increased at 15 and 30 min (P < 0.05, n = 3 for both time-points, Fig. 2B). Given that there was no change in the expression of the GluA2 subunit, these results suggest that acute exposure to dexamethasone may increase the surface expression of homomeric GluA1-containing AMPARs. The surface expression of GluA1 AMPARs is known to be regulated by a PKA-dependent signalling mechanism (Lee et al., 2003; Lu and Roche, 2011). Therefore, we tested whether pretreatment with the PKA inhibitor Rp-cAMPS would affect the dexamethasone-mediated enhancement of GluA1 surface expression. Pretreating slices with Rp-cAMPS (100 μM) abolished the effect of dexamethasone (P < 0.05, n = 3, Fig. 2C), implicating PKA in this glucocorticoid-mediated regulation of AMPAR surface expression.
Acute stress and glucocorticoids increase S845 phosphorylation of GluA1
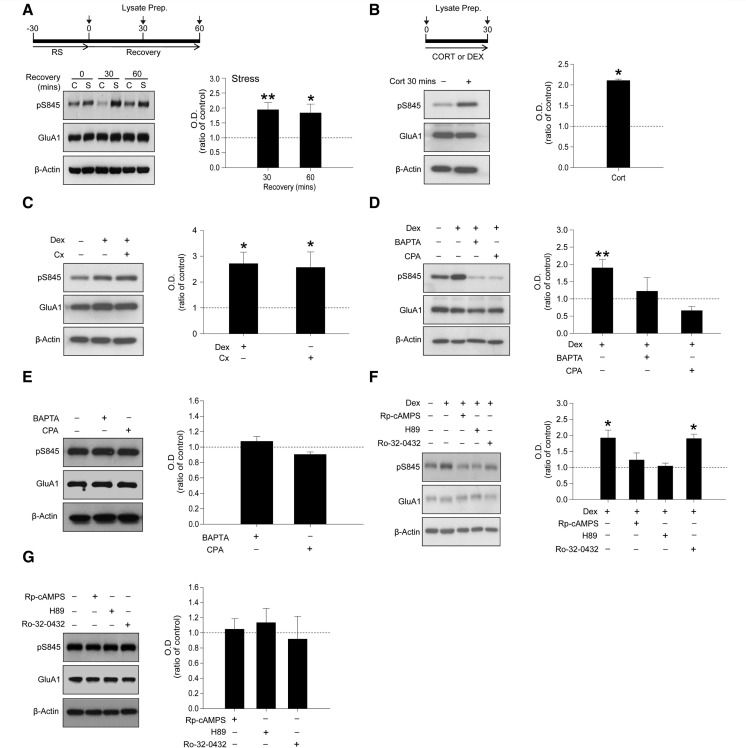
The PKA-mediated phosphorylation of the serine 845 (pS845) residue of the GluA1 subunit correlates with the surface expression of homomeric GluA1 AMPARs on the plasma membrane (Mammen et al., 1997; Lee et al., 2003; Oh et al., 2006). Western blot analysis of ex vivo samples indicated that stress induced a significant increase in pS845 that persisted for at least 60 min after slice preparation (P < 0.05, n = 5, Fig. 3A). Consistent with the ex vivo experiments, incubation for 30 min with either corticosterone (P < 0.05, n = 3, Fig. 3B) or dexamethasone (P < 0.05, n = 5, Fig. 3C) induced comparable increases in pS845. As glucocorticoid receptor activation was found to be important in the induction of stress-induced LTP (Fig. 1F), we also determined whether the glucocorticoid receptor was an important mediator in pS845 following dexamethasone treatment. The increase in pS845 following dexamethasone treatment (P < 0.05, n = 4, Supplementary Fig. 1A) was abolished by pretreatment with RU486 (500 nM), indicating an important role for glucocorticoid receptor in this acute stress model.
Figure 3.
Glucocorticoids enhance the phosphorylation of GluA1 via a non-genomic mechanism requiring increased Ca2+ mobilization. (A) Animals were exposed to 30 min restraint stress. Hippocampal slices were homogenized immediately following stress or allowed to recover for either 30 or 60 min. Phosphorylation levels of serine 845 (pS845) of the GluA1 sub-unit of AMPARs were increased in the stressed animals (S) compared to non-stressed control animals (C) (n = 5). (B) Preincubation with corticosterone (Cort, 200 nM) increased pS845 of GluA1 compared to control brain slices (n = 3). (C) Pretreatment with cycloheximide (cx; 100 µM) had no effect on pS845 levels following dexamethasone (Dex) treatment (n = 5). (D) Both BAPTA-AM (100 μM) and cyclopiazonic acid (CPA, 50 μM) preincubation prevented the increased phosphorylation of the GluA1 subunits induced by dexamethasone treatment (n = 4). (E) Treatment with BAPTA-AM and cyclopiazonic acid alone had no effect on pS845 (n = 4). (F) Pretreatment with PKA inhibitors Rp-cAMPS (100 µM) or H89 (10 µM) abolished dexamethasone-mediated increases in pS845 levels, whereas the protein kinase C inhibitor Ro-32-0432 (10 µM) had no effect (n = 4). Error bars indicate SEM. *P < 0.05; **P < 0.01. (G) Treatment with Rp-cAMPS, H89 or Ro-32-0432 alone had no effect on pS845 levels (n = 4).
Many well-characterized effects of stress involve alterations in gene transcription and translation (Bain et al., 2007; Heitzer et al., 2007) although fast, non-genomic actions of glucocorticoids are also documented (Losel and Wehling, 2003; Dallman, 2005). We examined whether the pS845 required de novo protein synthesis by using the translation inhibitor cycloheximide. Pretreatment for 30 min with 100 µM cycloheximide had no effect on the ability of dexamethasone (30 min) to increase the phosphorylation of S845 (P > 0.05, n = 5, Fig. 3C), suggesting that a non-genomic mechanism is involved in this action.
As Ca2+ is important for most, but not all, forms of synaptic plasticity (Esteban et al., 2003), we next tested whether glucocorticoid-mediated pS845 of GluA1 requires Ca2+. To test this, we modified levels of Ca2+ using the membrane-permeable Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM). Dexamethasone application alone significantly increased pS845 under control conditions but not following 30 min pretreatment with BAPTA-AM, (dexamethasone versus dexamethasone + BAPTA: P < 0.05, n = 4, Fig. 3D). A primary source of intracellular Ca2+ involved in certain forms of LTP is Ca2+ released from intracellular stores (Rose and Konnerth, 2001). To investigate its possible involvement in the effects of dexamethasone we used the sarcoplasmic Ca2+-ATPase inhibitor cyclopiazonic acid, as a means of depleting intracellular stores of Ca2+. Pretreatment for 30 min with 50 μM cyclopiazonic acid completely abolished the effects of dexamethasone treatment on pS845 (dexamethasone versus dexamethasone +cyclopiazonic acid: P < 0.05, n = 4, Fig. 3D). Moreover, BAPTA-AM and cyclopiazonic acid treatment alone had no significant effect on pS845 (P > 0.05 for all treatments, n = 4, Fig. 3E). These data suggest that intracellular Ca2+ mobilization is important for glucocorticoid-induced S845 phosphorylation.
Given the role of PKA in the dexamethasone-induced increase in surface GluA1 (Fig. 2) and in the phosphorylation of S845 (Banke et al., 2000), it seemed likely that this kinase mediates the increased pS845 in response to glucocorticoids. Consistent with this, preincubation for 30 min with the PKA inhibitors Rp-cAMPS (100 µM) or H89 (10 µM), prevented the effects of dexamethasone on pS845 (P < 0.05 for all treatments, n = 4, Fig. 3F). In contrast, the protein kinase C inhibitor tested, Ro-32-0432 (10 µM) was without effect (P > 0.05, n = 4, Fig. 3F). Furthermore, treatment with PKA and the protein kinase C inhibitor alone produced no significant change in pS845 (P > 0.05 for all treatments, n = 4, Fig. 3G). Collectively, these results indicate that acute stress, via the mobilization of intracellular Ca2+, induces PKA activation and leads to the insertion of GluA1, but not GluA2, subunits into the plasma membrane.
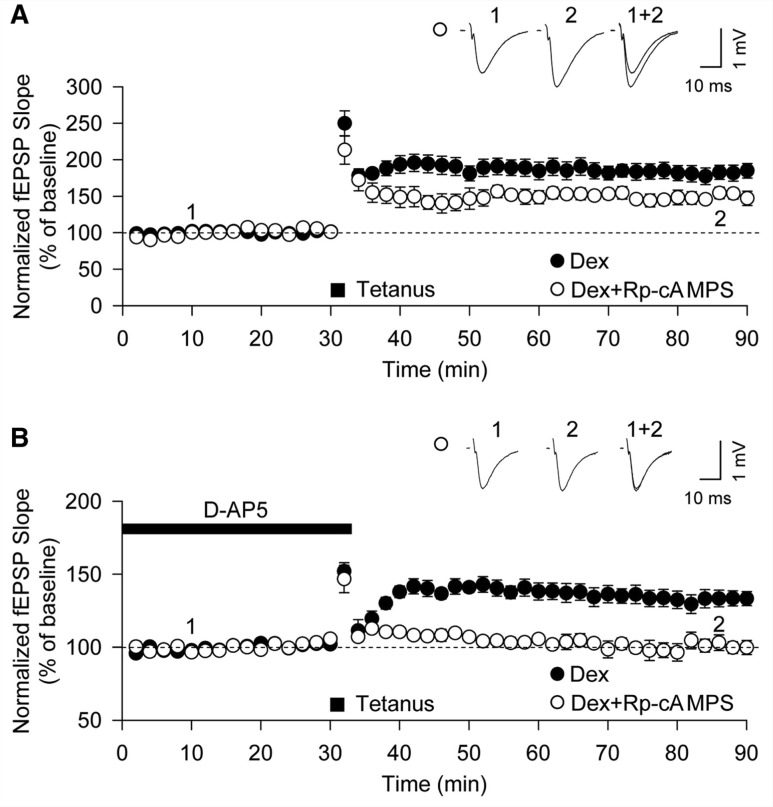
The PKA-dependent actions of dexamethasone upon pS845 and surface expression of GluA1 subunits may be causally related to the generation of stress-induced LTP or may be an epiphenomenon. To distinguish between these possibilities, we examined whether Rp-cAMPS affected the dexamethasone-induced enhancement of LTP. Preincubation with Rp-cAMPS resulted in the inhibition of enhanced LTP in the presence of dexamethasone (from 182 ± 10%, n = 6 to 155 ± 9%, n = 5; P < 0.05, Fig. 4A), a value not significantly different to that seen in control slices. Furthermore, Rp-cAMPS eliminated stress-induced LTP induced in the presence of D-AP5 following treatment with dexamethasone (101 ± 4%, n = 5, Fig. 4B). These findings are most readily explained by a process whereby the activation of glucocorticoid receptors increase GluA1-AMPAR surface expression through PKA signalling, and this permits the induction of an NMDAR-independent LTP that is additive to NMDAR-dependent LTP.
Figure 4.
Stress-induced LTP requires PKA activation. (A) Enhancement of LTP by dexamethasone (Dex) treatment (closed circle; n = 6) was attenuated by pretreatment with 100 µM Rp-cAMPS (open circle; n = 5). (B) Rp-cAMPS (100 µM) blocked stress-induced LTP induced in the presence of D-AP5 (open circle; n = 5). fEPSP = field excitatory postsynaptic potential.
Stress-induced long-term potentiation is triggered by Ca2+-permeable AMPA receptors
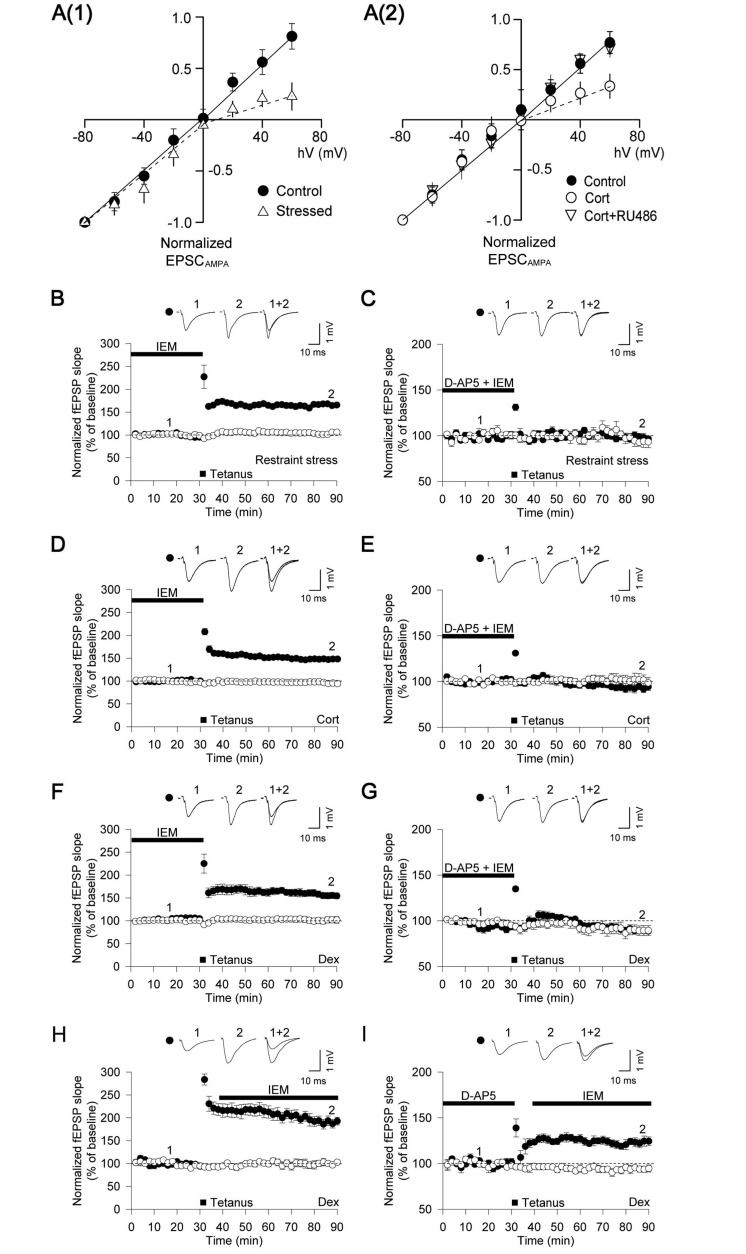
CP-AMPARs have been shown to mediate the induction of an NMDAR-independent form of LTP at CA1 in the hippocampus of mice lacking GluA2 AMPARs (Asrar et al., 2009). As we had observed that stimulation of glucocorticoid receptors results in an increase in surface GluA1, it seemed plausible that stress-induced LTP is also triggered via CP-AMPARs. To confirm this hypothesis we examined the synaptic current-voltage (I-V) relationship of excitatory postsynaptic currents (EPSC)AMPA. As expected, the I-V relationship was linear in control animals. In contrast, the restraint stress rats exhibited an inwardly rectifying I-V curve [P < 0.01, n = 7, Fig. 5A(i)]. A similarly rectified I-V curve was observed in slices treated with corticosterone [P < 0.01, n = 7, Fig. 5A(ii)]. This effect could be blocked with pretreatment with RU486 [n = 7, Fig. 5A(ii)]. This inwardly rectified I-V relationship is suggestive of an increase in the synaptic expression of CP-AMPARs. To further clarify the role of CP-AMPARs in stress-induced LTP we tested whether the CP-AMPAR inhibitor IEM (100 µM) (Buldakova et al., 2007; Asrar et al., 2009) affected LTP in ex vivo hippocampal slices prepared from stressed rats. The level of LTP observed after application of IEM (168 ± 4%, n = 6, Fig. 5B) was less than that obtained from untreated slices from stressed rats (199 ± 11%, n = 6; P < 0.01, Fig. 1A) but similar to that observed in control rats (150 ± 5%, n = 7; P > 0.05, Fig. 1A), suggesting an inhibition of the stress-induced LTP component. Consistent with this, IEM completely prevented the induction of NMDAR-independent LTP in slices from stressed rats (99 ± 3%, n = 6, Fig. 5C). We next performed experiments using acute slices treated with either corticosterone or dexamethasone. Consistent with the ex vivo experiments from stressed rats, facilitation of LTP by corticosterone or dexamethasone was prevented by IEM (corticosterone: 148 ± 3%, n = 6, Fig. 5D; dexamethasone: 155 ± 7%, n = 6, Fig. 5F). Furthermore, IEM completely blocked LTP, induced by glucocorticoid receptor stimulation in the presence of D-AP5 (corticosterone: 95 ± 4%, n = 6, Fig. 5E; dexamethasone: 91 ± 2%, n = 6, Fig. 5G).
Figure 5.
Stress and glucocorticoids facilitate LTP through a mechanism involving Ca2+-permeable AMPARs. (A1) Acute restraint stress induces an inwardly rectified I-V relationship of AMPAR current (EPSCAMPA) (control, n = 9; stressed, n = 7). Spermine (100 µM) was included in the filling solution and EPSCAMPA was isolated by applying the NMDAR antagonists D-AP5 (50 µM) and MK801 (10 µM). (A2) Corticosterone (Cort) treatment induces an inwardly rectified I-V relationship of EPSCAMPA. This is attenuated by pretreatment with RU486 (500 nM) in acute slices (control, n = 7; corticosterone, n = 7; corticosterone + RU486, n = 7). (B–I) Filled symbol indicates tetanus delivered input and open symbol indicates control input. (B) The Ca2+-permeable AMPAR antagonist IEM-1460 (IEM: 100 µM) attenuated facilitation of LTP recorded following restraint stress (n = 6). (C) IEM abolished stress-induced LTP (tetanus delivered in presence of D-AP5), induced by restraint stress (n = 6). (D) IEM attenuated LTP recorded following corticosterone treatment (n = 6). (E) IEM abolished stress-induced LTP (tetanus delivered in presence of D-AP5), induced by corticosterone treatment (n = 6). (F) IEM attenuated LTP recorded after dexamethasone treatment (n = 6). (G) IEM abolished stress-induced LTP (tetanus delivered in presence of D-AP5), induced by dexamethasone treatment (n = 6). (H) IEM had no effect on pre-established LTP induced by dexamethasone treatment (n = 7). (I) IEM had no effect on the expression of stress-induced LTP (tetanus delivered in presence of D-AP5), enabled by dexamethasone treatment (n = 7). EPSP = excitatory postsynaptic potential; fEPSP = field excitatory postsynaptic potential.
These data indicate that CP-AMPAR are involved in the induction of an NMDAR-independent form of LTP. In some (Plant et al., 2006) but not all (Adesnik and Nicoll, 2007) circumstances, the insertion of CP-AMPARs may also contribute to the early expression of LTP. To determine whether this is the case for stress-induced LTP, we applied IEM to slices after high-frequency stimulation. IEM had no effect on the dexamethasone-enhanced LTP under either control conditions (186 ± 10%, n = 7, Fig. 5H), or in the presence of D-AP5 (125 ± 6%, n = 7, Fig. 5I). As the magnitude of stress-induced LTP was not affected by IEM post-tetanus, we concluded that CP-AMPARs are specifically involved during the induction of stress-induced LTP. These results therefore provide a physiological role for CP-AMPARs in the induction of a form of LTP in response to acute stress.
Stress-induced long-term potentiation is primed by the activation of NMDA receptors
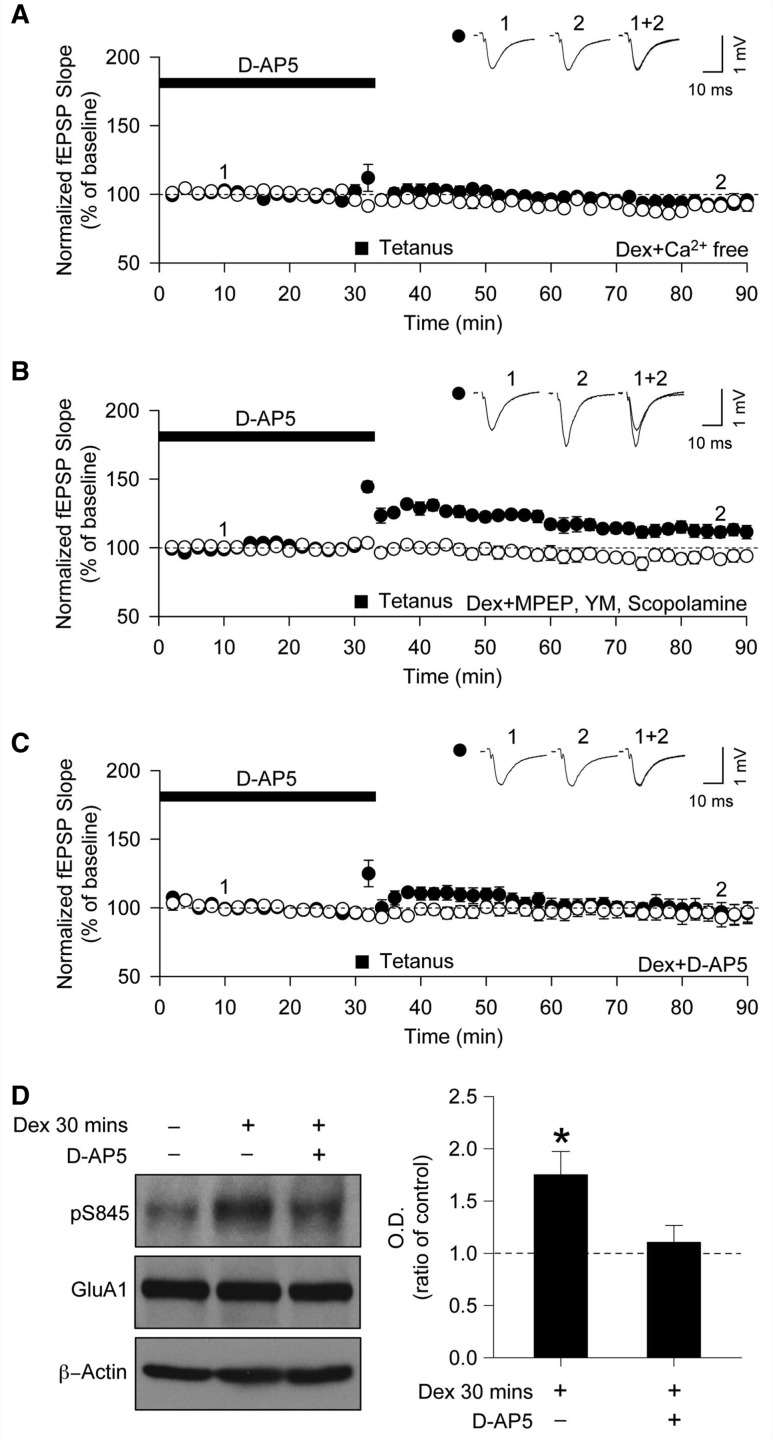
A key question concerns how the stimulation of glucocorticoid receptors leads to the state where a tetanus can induce an NMDAR-independent form of LTP; how do glucocorticoids recruit CP-AMPARs to prime the synapses for stress-induced LTP? The finding that the phosphorylation of pS845 requires Ca2+ suggests that the priming mechanism likely involves a change in intracellular Ca2+. To test for this, we used a Ca2+-free artificial CSF buffer during the dexamethasone treatment period and then reintroduced Ca2+ to test for the presence of NMDAR-independent LTP. Stress-induced LTP was absent in dexamethasone-treated slices that had been incubated in Ca2+-free artificial CSF (96 ± 3%, n = 6, Fig. 6A). This suggests that Ca2+ is required for the priming mechanism by which glucocorticoids recruit CP-AMPARs to enable stress-induced LTP.
Figure 6.
Ca2+-influx through NMDARs is required for dexamethasone to generate stress-induced LTP. (A–C) Filled symbol indicates tetanus delivered input and open symbol indicates control input. (A) Incubation with a Ca2+-free medium during dexamethasone (Dex) treatment prevented stress-induced LTP (n = 6). (B) Incubation with MPEP, YM-298198 and scopolamine during dexamethasone treatment did not affect the generation of stress-induced LTP (n = 6). (C) Incubation with D-AP5 during dexamethasone treatment prevented stress-induced LTP (n = 6). (D) Incubation with D-AP5 abolished dexamethasone-mediated enhancement of pS845 (n = 4). fEPSP = field excitatory postsynaptic potential. *P < 0.05.
Ca2+ can be elevated in neurons through various pathways, including the activation of G-coupled receptors. The predominant Gq/11-coupled receptors in CA1 neurons are mGlu1, mGlu5 and m1AChR, all of whose activation can induce release Ca2+ from intracellular stores. We found, however, that a cocktail of inhibitors for these receptors (2-methyl-6-(phenylethynyl)pyridine hydrochloride) (MPEP 1 µM; mGluR5 antagonist), desmethyl-YM-298198 (YM; 500 nM; mGluR1 antagonist) and scopolamine (20 µM; mAChR antagonist) could not prevent stress-induced LTP (115 ± 4%, n = 6, Fig. 6B). The synaptic activation of postsynaptic NMDARs also regulates Ca2+ mobilization (Alford et al., 1993; Yuste et al., 2000). We therefore tested whether Ca2+ flux through NMDARs is involved in the priming of stress-induced LTP. Interestingly, LTP was completely blocked by D-AP5 in slices pretreated with dexamethasone plus D-AP5 (96 ± 7%, n = 6, Fig. 6C). Consistent with these data, we found that 30 min dexamethasone treatment caused an increase in pS845 (P < 0.05, n = 4, Fig. 6D), which was prevented by pretreatment with D-AP5. These results suggest that the induction of stress-induced LTP requires the glucocorticoid-induced activation of NMDARs and subsequent Ca2+ influx.
Discussion
Alterations in synaptic function after either acute stress or emotional arousal can facilitate cognition (Cahill et al., 1994; Shors and Servatius, 1995; Shors and Mathew, 1998; Blank et al., 2002; Weiss et al., 2005; Joels et al., 2006; Bangasser and Shors, 2007).This enhanced cognitive performance after exposure to a stressor serves an important survival strategy, and understanding the underlying mechanism could provide insights into new translational strategies for the treatment of memory impairment in man. The first step is to ascertain the underlying mechanism, and we now describe a novel mechanism by which acute stress can enhance synaptic plasticity, a major process involved in learning and memory (Bliss and Collingridge, 1993). We find that stimulation of glucocorticoid receptors leads to an NMDAR-dependent form of metaplasticity that involves the PKA-dependent insertion of GluA1-containing CP-AMPARs into synapses. These newly inserted AMPARs can then induce a form of LTP that is entirely independent of NMDARs but that is additive to conventional NMDAR-dependent LTP. In this way, acute stress is able to enhance the magnitude of LTP at hippocampal synapses (Supplementary Fig. 1B).
Previous studies have shown that glucocorticoids are able to rapidly modify glutamatergic function. For example, it has been shown that corticosterone induces the mobilization of GluA1 and GluA2 to the plasma membrane and can facilitate a chemical form of LTP observed in dissociated hippocampal neurons (Groc et al., 2008; Conboy and Sandi, 2009). The effect is complex, with time dependent actions through muscarinic receptors and glucocorticoid receptors (Groc et al., 2008; Yuen et al., 2011). In prefrontal cortex neurons, acute stress and corticosterone have been shown to enhance synaptic transmission, through facilitated AMPAR and NMDAR function (Yuen et al., 2009, 2011; Lee et al., 2012). More broadly, acute stress or exposure to glucocorticoids can cause a rapid increase in glutamate release (Moghaddam, 1993; Abraham et al., 1996; Venero and Borrell, 1999; Reznikov et al., 2007). Although these changes in excitatory synaptic transmission have been suggested to regulate synaptic plasticity, no underlying rapid non-genomic mechanism has been revealed. Our study has shown a role for the selective insertion of CP-AMPARs that enables the induction of an NMDAR-independent form of LTP.
AMPARs that lack the GluA2 subunit, such as homomeric GluA1 receptors, have a higher Ca2+ permeability and single channel conductance than GluA2 containing AMPARs (Lomeli et al., 1994; Swanson et al., 1997). These properties have been shown to be important for the induction and/or expression of various forms of synaptic plasticity in the CNS (Mahanty and Sah, 1998; Liu and Cull-Candy, 2000; Lei and McBain, 2002; Cull-Candy et al., 2006; Liu and Zukin, 2007; Shepherd, 2012). For example, it has been shown that in transgenic mice lacking GluA2 that CP-AMPARs can mediate a form of NMDAR-independent LTP (Jia et al., 1996). In addition, it has been shown that CP-AMPARs can be transiently inserted following the induction of NMDAR-LTP, where they trigger the subsequent expression of neuronal plasticity (Plant et al., 2006; but see also Adesnik and Nicoll, 2007). We found that in stress-induced LTP, CP-AMPARs are required for the induction, rather than the expression, of LTP. Thus, IEM was able to fully block the induction of NMDAR-independent LTP as observed either in isolation from or in addition to NMDAR-LTP. However, IEM had no effect on either baseline transmission or the expression of LTP when applied shortly after its induction. Further work will be required to establish the precise mechanism of stress-induced LTP expression and how this is initiated through the transient activation of CP-AMPARs. One possible explanation is that stress results in the insertion of CP-AMPARs into the plasma membrane at extra-synaptic sites. During high-frequency stimulation these could become activated, possibly by the ‘spill-over’ of l-glutamate (Yang et al., 2005; Okubo et al., 2010), triggering the synaptic insertion of GluA2-containing AMPARs.
Although NMDARs are not required for the induction of stress-induced LTP, their activation is essential for the metaplasticity that primes CA1 synapses for stress-induced LTP. Thus, inhibition of NMDARs during the application of dexamethasone completely prevented the subsequent ability to induce NMDAR-independent LTP. This leads to the question regarding the mechanism underlying this important form of metaplasticity. It is known that glucocorticoids can rapidly enhance NMDAR activation and subsequently increase intracellular Ca2+ levels in the CA1 region of the hippocampus (Takahashi et al., 2002; Sato et al., 2004; Xiao et al., 2010). Our observation that this form of metaplasticity is prevented by BAPTA is consistent with these observations. The synaptic activation of NMDARs is known to result in the release of Ca2+ from intracellular stores (Alford et al., 1993) and we have found that release from stores is required for the metaplasticity. This priming is specific to NMDAR activation since inhibition of G-protein coupled receptors, which can also modulate the release of Ca2+ from intracellular stores, did not affect stress-induced LTP. We also found that PKA is required for the priming of stress-induced LTP, and that its activation is associated with pS845 and the increased surface expression of GluA1 subunits. The most plausible mechanism therefore is that Ca2+ associated with NMDAR stimulation activates a Ca2+-sensitive adenylyl cyclase (Pierre et al., 2009), which leads to PKA-mediated phosphorylation of S845 to trigger the AMPAR trafficking that underlies the priming effect. Consistent with this model, NMDAR triggered activation of PKA (Roberson and Sweatt, 1996) has been shown to drive the synaptic expression of GluA1-containing AMPARs (Esteban et al., 2003). This mechanism does not require de novo protein synthesis and accordingly, we found that the ability of dexamethasone to prime stress-induced LTP was unaffected by treatment with cycloheximide. However, the possibility remains that stress could have a secondary action to upregulate the gene expression of components of this pathway to achieve a longer lasting effect. In this regard, it is interesting to note that PKA has also been shown to play a role in a late, protein synthesis-dependent phase of LTP (Frey et al., 1993; Huang and Kandel, 1994; Abel et al., 1997; Nayak et al., 1998).
There is strong evidence to support the notion that acute stress can facilitate memory through enhanced synaptic plasticity (Conrad et al., 1999; Blank et al., 2002; Hui et al., 2004; Nijholt et al., 2004; Yuen et al., 2011), though the mechanism underlying this process is unknown. To establish how this mechanism contributes to cognition is a major undertaking, given how much time has been devoted to understanding how NMDAR-dependent LTP is involved in learning and memory (Morris et al., 1986; Martin et al., 2000; Neves et al., 2008); a topic for which aspects of the relationship still remain controversial (Bannerman et al., 2012). It is interesting to note, however, that in animals engineered to lack GluA2, the total level of LTP and the proportion that is dependent on NMDARs is similar to those observed in this present study after acute stress (Jia et al., 1996). Studies have shown that NMDAR-dependent and independent forms of LTP mediate different behaviours (Wiltgen et al., 2010). It has also been proposed that NMDAR-independent LTP might explain the resistance of hippocampal-dependent learning to NMDAR antagonism observed in water maze tasks (Morris et al., 1986; Bannerman et al., 1995, 2012) and other behaviours (Abel et al., 1997) under certain conditions, most notably in the ‘upstairs/downstairs’ water maze experiments (Morris et al., 1986; Bannerman et al., 1995). However, no physiological context has previously been found where this occurs in normal animals. Therefore, our findings that acute stress readily induces an NMDAR-independent form of LTP may be especially pertinent in this context (Wiltgen et al., 2010).
Therefore, it is plausible that the priming of synaptic plasticity observed in this study may be associated with periods of heightened cognition. As modifications in synaptic function are thought to be fundamental in the efficient formation of memory (Alford et al., 1993; Blank et al., 2002; Pierre et al., 2009), we propose that glucocorticoids play a role in fine-tuning synaptic function and regulating the memory trace through the expression of CP-AMPARs.
Supplementary Material
Acknowledgements
The study was conceived and designed by K.C. Electrophysiological studies were conducted by J.J., E.H., G.B.M., D.H.K., P.R., L.H., T.K., B.C.K. and D.J.W. Molecular and biochemical assays were conducted by G.W., T.P., G.S., G.H.S., E.W. and H.S. The manuscript was written by G.W., D.J.W., P.R., S.L.L., G.L.C. and K.C.
Glossary
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester)
- LTP
long-term potentiation
- NMDAR
N-methyl-d-aspartic acid receptor
- PKA
protein kinase A
Funding
K.C., S.L.L. and G.L.C. were supported by BBSRC. G.W. was supported by BBSRC PhD studentship. G.L.C. was supported by WCU Programme (Korea). K.C. and G.L.C. were supported by UK Wellcome Trust-Medical Research Council Neurodegenerative Disease Initiative Programme. D.K. was supported by Korea-UK Alzheimer’s disease Research Consortium Programme (the Korean Ministry of Health and Welfare). K.K. and G.H.S. were supported by Frontier Programme in Neuroscience (Korea). J.J., T.P. and B.C.K. were supported by Chonnam National University Hospital Frontier Lab Programme for Translational Neuroscience. K.C. and D.J.W. were supported by Department for Business, Innovation and Skill GFP program (UK). K.C. was supported by the Wolfson Research Merit Award and Royal Society, London.
Supplementary material
Supplementary material is available at Brain online.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–26. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abraham I, Juhasz G, Kekesi KA, Kovacs KJ. Effect of intrahippocampal dexamethasone on the levels of amino acid transmitters and neuronal excitability. Brain Res. 1996;733:56–63. doi: 10.1016/0006-8993(96)00538-0. [DOI] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol. 1993;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrar S, Zhou Z, Ren W, Jia Z. Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoS One. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol. 2007;69:201–20. doi: 10.1146/annurev.physiol.69.031905.160308. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10:1401–3. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–6. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Bus T, Taylor A, Sanderson DJ, Schwarz I, Jensen V, et al. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012;15:1153–9. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–94. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–79. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Buldakova SL, Kim KK, Tikhonov DB, Magazanik LG. Selective blockade of Ca2+ permeable AMPA receptors in CA1 area of rat hippocampus. Neuroscience. 2007;144:88–99. doi: 10.1016/j.neuroscience.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Caracciolo B, Gatz M, Xu W, Marengoni A, Pedersen NL, Fratiglioni L. Relationship of subjective cognitive impairment and cognitive impairment no dementia to chronic disease and multimorbidity in a nation-wide twin study. J Alzheimers Dis. 2013;36:275–84. doi: 10.3233/JAD-122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-Permeable AMPA Receptor Dynamics Mediate Fear Memory Erasure. Science. 2010;330:1108–12. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2009;35:674–85. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–97. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–8. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–6. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–70. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–4. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–70. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Rev Endocr Metab Disord. 2007;8:321–30. doi: 10.1007/s11154-007-9059-8. [DOI] [PubMed] [Google Scholar]
- Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334:623–8. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–34. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–56. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–8. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Joels M, Krugers H, Karst H. Stress-induced changes in hippocampal function. Prog Brain Res. 2008;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15:280–8. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–7. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–43. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee JB, Wei J, Liu W, Cheng J, Feng J, Yan Z. Histone deacetylase 6 gates the synaptic action of acute stress in prefrontal cortex. J Physiol. 2012;590:1535–46. doi: 10.1113/jphysiol.2011.224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–33. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–34. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–8. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–13. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol. 2011;22:473–9. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–7. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–33. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–7. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–6. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature. 1998;394:680–3. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–83. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–8. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, et al. Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci USA. 2010;107:6526–31. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8:321–35. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–4. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–14. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271:30436–41. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–46. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–22. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci. 2011;34:165–76. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sato S, Osanai H, Monma T, Harada T, Hirano A, Saito M, et al. Acute effect of corticosterone on N-methyl-D-aspartate receptor-mediated Ca2+ elevation in mouse hippocampal slices. Biochem Biophys Res Commun. 2004;321:510–3. doi: 10.1016/j.bbrc.2004.06.168. [DOI] [PubMed] [Google Scholar]
- Savtchouk I, Liu SJ. Remodeling of synaptic AMPA receptor subtype alters the probability and pattern of action potential firing. J Neurosci. 2011;31:501–11. doi: 10.1523/JNEUROSCI.2608-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–34. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Shepherd JD. Memory, plasticity and sleep—a role for calcium permeable AMPA receptors? Front Mol Neurosci. 2012;5:49. doi: 10.3389/fnmol.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. Stress-induced sensitization and facilitated learning require NMDA receptor activation. Neuroreport. 1995;6:677–80. doi: 10.1097/00001756-199503000-00023. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn Mem. 1998;5:220–30. [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, Hattori TA, Yasumatsu N, Kawato S. Corticosterone acutely prolonged N-methyl-d-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem. 2002;83:1441–51. doi: 10.1046/j.1471-4159.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Lowry E, Tanaka K, Levy B, Reijmers L, Mayford M, et al. Characterization of NMDAR-Independent Learning in the Hippocampus. Front Behav Neurosci. 2011;5:28. doi: 10.3389/fnbeh.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–73. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learn Mem. 2005;12:138–43. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, et al. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One. 2010;5:e12818. doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Feng C, Chen Y. Glucocorticoid rapidly enhances NMDA-evoked neurotoxicity by attenuating the NR2A-containing NMDA receptor-mediated ERK1/2 activation. Mol Endocrinol. 2010;24:497–510. doi: 10.1210/me.2009-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–93. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci. 2000;3:653–9. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.