Abstract
We evaluated the efficacy and safety of the combination of twice-daily fludarabine and cytarabine (BIDFA) in patients with refractory/relapsed acute myeloid leukemia (AML), high-risk myelodysplastic syndromes (MDS), and chronic myeloid leukemia in myeloid blast phase (CML-BP). One hundred seven patients were enrolled. Overall, 27 (26%) patients responded with a complete remission (CR) rate of 21% and CR without platelet recovery (CRp) of 5%. The overall 4-week mortality rate was 9%. In conclusion, BIDFA is active and safe in heavily pretreated patients with myeloid malignancies.
Background
The purpose of this study was to evaluate the efficacy and safety of the combination of twice-daily fludarabine and cytarabine (BIDFA) in patients with refractory/relapsed acute myeloid leukemia (AML), high-risk myelodysplastic syndromes (MDS), and chronic myeloid leukemia in myeloid blast phase (CML-BP).
Patients and Methods
One hundred seven patients with refractory/relapsed AML, intermediate and high-risk MDS, and CML-BP, with a performance status of 3 or less and normal organ function were treated. Patients received fludarabine 15 mg/m2 intravenously (IV) every 12 hours on days 1 to 5 and cytarabine 0.5 g/m2 IV over 2 hours every 12 hours on days 1 to 5. Gemtuzumab ozogamicin (GO) was administered at 3 mg/m2 IV on day 1 in the first 59 patients. Patients with CML-BP were allowed to receive concomitant tyrosine kinase inhibitors.
Results
Overall, 27 (26%) patients responded with a complete remission (CR) rate of 21% and CR without platelet recovery of 5%. The overall 4-week mortality rate was 9%. The CR rates for patients with relapsed AML with first CR duration greater than or equal to 12 months, relapsed AML with first CR duration less than 12 months, and refractory/relapsed AML beyond first salvage were 56%, 26%, and 11%, respectively. With a median follow-up of 7 months, the 6-month event-free survival, overall survival, and complete remission CR duration rates were 18%, 35%, and 70%, respectively.
Conclusion
BIDFA is active with an overall response rate of 26% in a heavily pretreated population. This combination is safe with a low 4-week mortality rate of 9%.
Keywords: Acute myeloid leukemia, Efficacy, Refractory, Safety
Introduction
Treatment options for patients with relapsed and refractory acute myeloid leukemia (AML) are limited. Retreatment with a high-dose cytarabine–containing regimen is considered standard for patients whose disease relapses after an initial complete remission (CR) lasting for a year or more. For patients whose disease relapses after a shorter remission or with primary refractory disease, no true standard options exist and investigational therapy is considered appropriate.
The combination of fludarabine and cytarabine was shown to be superior to cytarabine alone in patients with relapsed AML who had a relatively long first remission.1 The effectiveness of fludarabine and cytarabine may be due to the ability of fludarabine to modulate the pharmacologic behavior of cytarabine. Specifically, at intracellular concentrations of greater than or equal to 10 μM, 9-β-d-Arabinofuranosyl-2-fluoroadenine 5′-triphosphate (F-ara-ATP), the active metabolite of fludarabine, increases accumulation of arabinofuranosylcytosine triphosphate (ara-CTP) in AML cells beyond levels possible with the dose escalation of cytarabine.2 Once plasma cytarabine levels are greater than 10-μM, ara-CTP formation plateaus. Previous work indicated that F-ara-ATP accumulation in AML cells is generally 10 μM or more at fludarabine doses of 15 mg/m.2–4 Because the half-life of ara-CTP elimination is rapid (~3 hours) in myeloid blasts, this active metabolite is eliminated from circulating blasts by 24 hours. Thus it was postulated that the administration of 2 doses of fludarabine, each at 15 mg/m2, and 2 doses of cytarabine daily would result in doubling of the ara-CTP area under the curve for that day compared with that observed with fludarabine and cytarabine given once daily.5,6 This led to a phase I study in which 65 patients received fludarabine 15 mg/m2 every 12 hours followed by cytarabine 0.5 g/m2 infused over 2 hours, a dose rate that results in approximately 10-μM plasma levels of ara-C, which saturates cellular ara-CTP accumulation7; the variable of interest was the number of doses administered. Five days of administration were found to be safe (4 days in adults ≥ 65 years of age) and effective, with a CR rate of 28%.
Gemtuzumab ozogamicin (GO) is a humanized monoclonal antibody directed against the CD33 epitope linked to calicheamicin. In phase II pivotal studies that enrolled a total of 277 patients, GO induced an overall response rate (ORR) [including CR and CR complete remission without platelet recovery (CRp)]) of 26%.8 The ORR was 35% in patients who had a first CR longer than 1 year and 11% among patients with a first CR shorter than 3 months. The Medical Research Council (MRC) 15 trial has addressed the issue of adding GO to chemotherapy for the treatment of newly diagnosed AML in patients younger than 60 years of age.9 Although the addition of GO did not impact the ORR or overall survival (OS), a predefined analysis by cytogenetic risk groups showed a significant survival benefit for patients with favorable-risk disease and a positive trend for those with intermediate risk. The dose of GO explored in the study was lower than the dose approved by the Food and Drug Administration (FDA) for use as a single agent, 3 mg/m2 intravenously (IV). The use of a lower dose was expected to reduce toxicities associated with combining GO with chemotherapy.
Based on the preceding facts, we conducted a phase II study assessing the efficacy and safety of this combination of twice-daily fludarabine and cytarabine plus or minus GO in patients with refractory/relapsed AML, high-risk myelodysplastic syndrome (MDS), and chronic myeloid leukemia in blast phase (CML-BP).
Patients and Methods
Patient Eligibility
Patients with a diagnosis of AML, high-risk MDS (≥10% blasts), or CML-BP whose disease relapsed or was refractory to frontline and/or salvage therapy were eligible. Informed consent was obtained according to institutional guidelines. Other eligibility criteria included (1) age 12 years or older, (2) performance status (PS) 0 to 3 (Eastern Cooperative Oncology Group scale), and (3) adequate renal (creatinine level 3 mg/dL or less) and hepatic (bilirubin level 3 mg/dL or less) function. Approval for the study was obtained from the Institutional Review Board (IRB) of the University of Texas MD Anderson Cancer Center. All patients signed an informed consent approved by the IRB; the study was conducted in accordance with the Declaration of Helsinki.
Treatment Regimen
The treatment included 5 days of fludarabine at 15 mg/m2 given IV over 15 to 30 minutes, every 12 hours followed 4 hours later by cytarabine at 0.5 g/m2 given IV over 2 hours every 12 hours. The treatment was given over 4 days in patients 65 years or older and over 3 days in patients with a PS of 3. GO 3 mg/m2 (up to 5 mg) was given over 2 hours IV for 1 dose on day 1 for the first 59 patients enrolled only. The drug was withdrawn from the market and therefore 48 patients received fludarabine and cytarabine only. Patients not achieving CR after 1 course could receive a second induction course if the treating physician determined this to be in the patient’s best interest. Patients achieving CR could receive up to 6 cycles of consolidation with fludarabine and cytarabine as during induction except that fludarabine and cytarabine were given for 4 days (3 days for patients ≥ 65 years and 2 days for patients with a PS of 3). Consolidation cycles were repeated every 4 to 6 weeks, depending on the recovery of neutrophil and platelet counts and toxicity. Patients with CML-BP were to receive concomitant tyrosine kinase inhibitor therapy.
Response Criteria and Definitions
CR was defined by the presence of 5% blasts or less in the bone marrow (BM), with greater than 1 × 109/L neutrophils and greater than 100 × 109/L platelets in the peripheral blood. A partial response was as for CR, but with persistence of 6% to 25% marrow blasts. A hematologic improvement had similar criteria as for CR, but without recovery of the platelet counts to 100 × 109/L or greater. This is also called marrow CR with incomplete platelet recovery, or CRp.
A partial response in MDS required improvement in at least 2 parameters: reduction of marrow blasts by 50% or more and to less than 10%, an increase of platelet counts by 100% and to greater than 100 × 109/L, and an increase of granulocyte count by 100% and to greater than 0.5 × 109/L.
In CML-BP, a complete hematologic response (CHR) was similar to CR. A partial hematologic response was as for CHR but with persistence of a few immature peripheral cells (<5% myelocytes and metamyelocytes) or with persistent but 50% or more reduced splenomegaly or thrombocytosis. A return to second chronic phase required disappearance of accelerated or blastic phase features.
Statistical Considerations
Survival was calculated from the date of start of therapy until death. Remission duration was calculated from the date of initial response until disease recurrence. Relapse was defined by recurrence of greater than 5% blasts in a BM aspirate unrelated to recovery or by the presence of extramedullary disease. Event-free survival (EFS) was calculated from the beginning of treatment until an event including relapse, death during induction, or death in CR. Survival and remission duration were plotted by the method of Kaplan and Meier. Toxicity was graded according to the National Cancer Institute’s NCI Common Toxicity Criteria, version 3.0.
Results
Study Group
A total of 107 patients were treated. Their characteristics are shown in Table 1. The median age was 62 years (range, 19–85 years). Ninety-three patients had AML, 5 patients had high-risk MDS, and 9 patients had CML-BP. Of the 107 patients, 52 were in first salvage: first CR duration of less than 12 months in 43 patients and more than 12 months in 9 patients. Karyotypic studies showed a diploid karyotype in 36 patients and unfavorable chromosomal abnormalities involving chromosomes 5 and 7 in 22 patients. Sixty-five (61%) patients had disease in which previous intensive chemotherapy failed, whereas 42 (39%) patients had disease in which targeted and hypomethylating agents failed.
Table 1.
Patient Characteristics (N = 107)
| Characteristic | N (%)/Median [Range] |
|---|---|
| Age (y) | 62 [19–85] |
| PS ≥ 1 | 94 (88) |
| Hemoglobin, g/dL | 9.5 [2–15.2] |
| White Blood Cell Count × 109/L | 4.8 [0.4–129] |
| Platelets × 109/L | 33 [5–607] |
| Percentage of Blasts (Peripheral Blood) | 32 [0–96] |
| Percentage of Blasts (Bone Marrow) | 46 [6–96] |
| Creatinine Level, mg/dL | 0.9 [0.4–2.8] |
| Bilirubin, mg/dL | 0.4 [0.2–2.2] |
| Disease | |
| AML | 93 (87) |
| MDS | 5 (5) |
| CML-BP | 9 (8) |
| Disease Subtype | |
| S1, CRD1 ≥ 12 mo | 9 (9) |
| S1, CRD1 < 12 mo | 43 (40) |
| ≥ S2 | 55 (51) |
| Cytogenetic Profile | |
| Diploid | 36 (33) |
| −5/−7 | 22 (21) |
| T(9;22) | 9 (9) |
| Miscellaneous | 40 (37) |
| Gemtuzumab Ozogamicin | 59 (55) |
| Previous Intensive Chemotherapy | 65 (61) |
Abbreviations: AML = acute myeloid leukemia; CML-BP = chronic myeloid leukemia in blast phase; CRD1 = first complete remission duration; MDS = myelodysplastic syndrome; PS = performance status; S1 = salvage 1; S2 = salvage 2.
Response
A total of 123 cycles was administered, with a median number of 1 cycle (range, 1–7 cycles). Overall, 22 patients (21%) achieved CR and 5 (5%) patients achieved CRp, for an ORR of 26% (Table 2). Four patients received reinduction therapy, 2 of whom achieved CR. Responses by disease category and by subset analysis are shown in Table 3. In AML, 21 (23%) of 93 patients achieved CR and 4 had CRp (4%) for an ORR of (27%). In CML-BP, 2 (22%) of 9 patients had objective responses (1 CR, 1 CRp). None of the 5 patients with MDS responded. Response rates were higher in patients with longer first CR durations; the ORRs were 56%, 30%, and 16% for patients with first salvage and first CR duration of more than 12 months, first salvage and first CR duration less than 12 months, and with second salvage and beyond, respectively. These results compare favorably with the expected response rates in matched cohorts of patients treated at MD Anderson, where the ORRs were 50%, 11%, and 7%, respectively. Response rates were similar whether patients received intensive chemotherapy and had it fail or had only received targeted and hypomethylating therapies and them fail. Response rates were higher among patients who received additional GO. The ORRs were 30% and 19%, respectively; the CR rates were 27% and 13%, respectively.
Table 2.
Overall Response (N = 107)
| Parameter | N (%) |
|---|---|
| OR | 27 (26) |
| CR | 22 (21) |
| CRp | 5 (5) |
Abbreviations: CR = complete remission; CRp = complete remission without platelet recovery; OR = objective response.
Table 3.
Response by Disease and Subset Groups
| Variable | n | Percentage | ||
|---|---|---|---|---|
| OR | CR | CRP | ||
| Status | ||||
| S1, CRD1 ≥ 12 mo | 9 | 56 | 56 | 0 |
| S1, CRD1 < 12 mo | 43 | 30 | 25 | 5 |
| ≥ S2 | 55 | 16 | 11 | 5 |
| Disease | ||||
| AML | 93 | 27 | 23 | 4 |
| MDS | 5 | 0 | 0 | 0 |
| CML-BP | 9 | 22 | 11 | 11 |
| GO | ||||
| Yes | 59 | 30 | 27 | 3 |
| No | 48 | 19 | 13 | 6 |
Abbreviations: AML = acute myeloid leukemia; CML-BP = chronic myeloid leukemia in myeloid blast phase; CR = complete remission; CRD1 = first complete remission duration; CRp = complete remission without platelet recovery; GO = gemtuzumab ozogamicin; MDS = myelodysplastic syndrome; OR = objective response; S1 = salvage 1; S2 = salvage 2.
Outcome
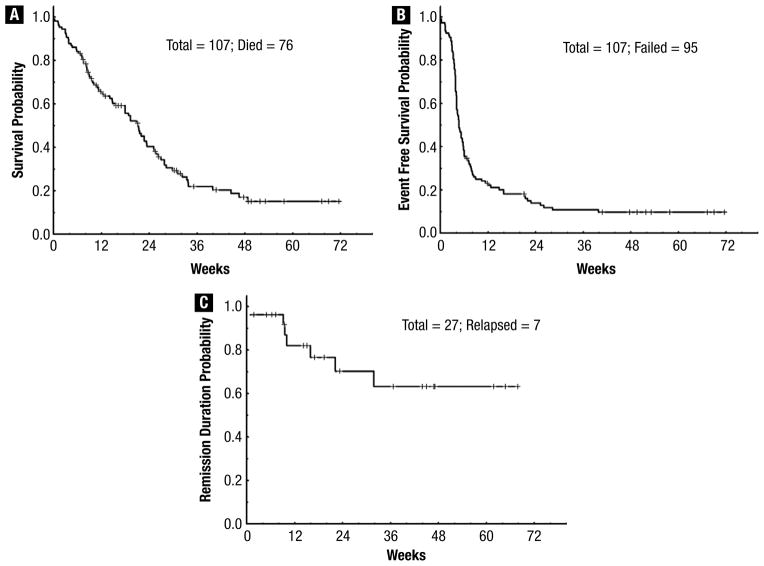
With a median follow-up of 26 weeks (range, 7–72 weeks), 31 patients (29%) remained alive. The 24-week survival rate was 38% (Figure 1A). Figure 1, B and C shows the EFS and CR duration for responders (n = 27); the median OS and EFS were 32 weeks (range, 1–72 weeks) and 5 weeks (range, 1–72 weeks), respectively. The median CR duration has not been reached so far. Patients in first salvage with a first CR duration of more than 12 months had a better outcome, with a median OS and EFS of 41, 23, and 12 weeks (P = .004) (Figure 2A), and 28, 6, and 4 weeks (P < .001) (Figure 2B), compared with patients in first salvage with a first duration of less than 12 months and patients receiving treatment for second salvage and beyond. Although there was no difference in OS and EFS between patients who did and those who did not receive GO, those who received GO had better CR duration; the median CR duration has not been reached in patients who received GO compared with 15 weeks in those who did not (P = .038). Finally, no difference in outcome was observed in patients who had previously received intensive chemotherapy or targeted and hypomethylating agents only.
Figure 1.
(A) Overall Survival for the Entire Population. (B) Event-Free Survival for the Entire Population. (C) Complete Response Duration Among the 27 Responders
Figure 2.
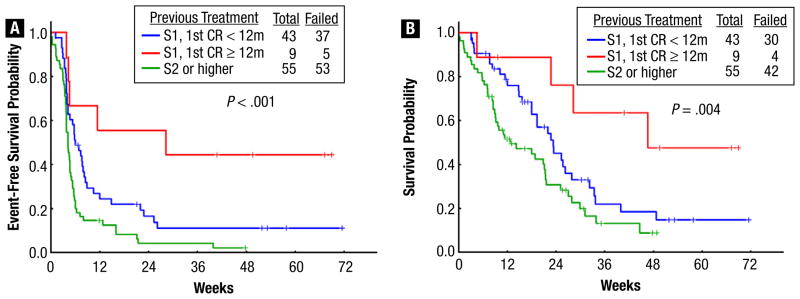
(A) Event-Free Survival by Salvage Number and First Remission Duration for the Entire Population. (B) Overall Survival by Salvage Number and First Remission Duration for the Entire Population
Prognostic Factors for Response and Outcome
We assessed the association of pretreatment characteristics with response, OS, and EFS. In the univariate analysis (Table 4A), patients with abnormal karyotype and in second salvage therapy and beyond had a lower response rate. Second salvage therapy and beyond, abnormal karyotype, increasing percentage of peripheral blood blasts, and increase in the white blood cell count were associated with a lower rate of 6-month EFS. These factors, in addition to poor performance status, anemia, and an increase in percentage of bone marrow blasts, were associated with a lower rate of 6-month OS.
Table 4A.
Univariate Analysis of Prognostic Factors for Response and Survival
| Variable | Parameter | Total N (%) |
Response | % at 6 Months | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | P | EFS | P | OS | P | |||
| Age (y) | < 60 | 45 (42) | 14 (31) | .26 | 19 | .065 | 46 | .08 |
| ≥ 60 | 62 (58) | 13 (21) | 9 | 29 | ||||
| Performance Status | 0 | 13 (12) | 4 (31) | .73 | 31 | .076 | 73 | .01 |
| ≥ 1 | 94 (88) | 23 (24) | 10 | 32 | ||||
| Disease | AML | 93 (87) | 25 (27) | .39 | 15 | .290 | 38 | .84 |
| MDS | 5 (5) | 0 (0) | 0 | 60 | ||||
| CML-BP | 9 (8) | 2 (22) | 0 | 25 | ||||
| Disease Subtype | S1, CRD1 ≥ 12 mo | 9 (8) | 5 (56) | .03 | 56 | .001 | 76 | < .001 |
| S1, CRD1 < 12 mo | 43 (40) | 13 (30) | 14 | 39 | ||||
| ≥ S2 | 55 (51) | 9 (16) | 4 | 28 | ||||
| Cytogenetic Profile | Diploid | 36 (34) | 15 (42) | .03 | 26 | .000 | 67 | < .001 |
| −5/−7 | 22 (21) | 5 (23) | 11 | 16 | ||||
| T(9;22) | 9 (8) | 2 (22) | 0 | 40 | ||||
| Miscellaneous | 40 (37) | 5 (13) | 5 | 24 | ||||
| Gemtuzumab Ozogamicin | Yes | 59 (55) | 18 (31) | .18 | 17 | .129 | 36 | .72 |
| No | 48 (45) | 9 (19) | 8 | 41 | ||||
| BM BL, % | ≤ 45 | 49 (49) | 11 (22) | .64 | 14 | .430 | 46 | .05 |
| > 45 | 50 (51) | 14 (28) | 12 | 33 | ||||
| PB BL, % | ≤ 32 | 46 (50) | 11 (24) | .43 | 16 | .010 | 53 | < .001 |
| > 32 | 46 (50) | 7 (15) | 0 | 20 | ||||
| HGB, g/dL | < 10.0 | 70 (65) | 17 (24) | .82 | 11 | .100 | 33 | .05 |
| ≥ 10.0 | 37 (35) | 10 (27) | 17 | 49 | ||||
| WBC × 109/L | < 4.9 | 54 (51) | 18 (33) | .07 | 23 | < .001 | 45 | < .001 |
| ≥ 4.9 | 53 (50) | 9 (17) | 4 | 31 | ||||
| PLT × 109/L | < 33 | 53 (50) | 16 (30) | .27 | 13 | .800 | 36 | .43 |
| ≥ 33 | 54 (51) | 11 (20) | 13 | 39 | ||||
| Creatinine, mg/dL | < 1.0 | 64 (60) | 16 (25) | 1.00 | 19 | .150 | 44 | .10 |
| ≥ 1.0 | 43 (40) | 11 (26) | 7 | 28 | ||||
| Bilirubin, mg/dL | < 0.5 | 58 (54) | 14 (24) | .83 | 13 | .400 | 43 | .75 |
| ≥ 0.5 | 49 (46) | 13 (27) | 13 | 34 | ||||
| Previous Intensive Therapy | Chemotherapy | 65 (61) | 15 (23) | .65 | 14 | .689 | 40 | .73 |
| Targeted therapy | 42 (39) | 12 (29) | 11 | 31 | ||||
Abbreviations: AML = acute myeloid leukemia; BL = blast; BM = bone marrow; CML-BP = chronic myeloid leukemia in blast phase; CRD = complete remission duration; HGB = hemoglobin; MDS = myelodysplastic syndrome; PLT = platelet; S = salvage; WBC = white blood cell
A multivariate analysis (Table 4B) identified an abnormal karyotype as the only independent adverse prognostic factor for response. Abnormal karyotype, second salvage and beyond, older age, and an increase in percentage of peripheral blood blasts were independently associated with worse EFS. Abnormal karyotype increase in percentage of peripheral blood blasts and renal failure were independently associated with a significantly worse OS.
Table 4B.
Multivariate Analysis of Prognostic Factors for Response and Survival
| Characteristic | Response | EFS | OS | |||
|---|---|---|---|---|---|---|
| Odds Ratio | P | HR | P | HR | P | |
| Abnormal Cytogenetic Profile | 0.28 | .01 | 2.22 | .002 | 2.75 | .001 |
| ≥ S2 | NS | 1.99 | .004 | NS | ||
| Older Age | NS | 1.02 | .01 | NS | ||
| Increasing PB Blast Percentage | NS | 1.01 | .05 | 1.01 | .002 | |
| Increasing Creatinine | NS | NS | 1.87 | .03 | ||
Abbreviations: EFS = event-free survival; HR = hazard ratio; NS = not significant; OR = objective response; OS = overall survival; PB = peripheral blood; S2 = salvage 2.
Toxicity
The regimen was reasonably well tolerated, with most side effects being grade 1 and grade 2 (Table 5). The 4-week mortality rate was 9%; these rates were 0%, 12%, and 10% for patients with first salvage and first CR duration of more than 12 months, first salvage and first CR duration less than 12 months, and with second salvage and beyond, respectively. The most common toxicities were gastrointestinal, including nausea, vomiting, diarrhea, and mucositis. Transient liver dysfunction and skin rashes were less frequently observed. Grade 3/4 liver dysfunction was uncommon, and no venoocclusive disorders were observed.
Table 5.
Nonhematologic Side Effects (n = 107)
| Adverse Event | All Grades (%) | Grade 3–4 (%) |
|---|---|---|
| Nausea | 27 | 0 |
| Mucositis | 14 | 0 |
| Diarrhea | 16 | 0 |
| Rash | 12 | 2 |
| Vomiting | 10 | 0 |
| Hepatic | 13 | 1 |
| Neurologic | 2 | 1 |
| Renal | 2 | 0 |
| Hand-Foot Syndrome | 2 | 0 |
Discussion
This study evaluated the efficacy and safety of a combination therapy consisting of twice-daily fludarabine and cytarabine plus or minus GO in patients with pretreated refractory/relapsed AML. This combination was found to be effective. Overall, 22 (21%) of the 107 patients treated achieved CR and 5 (5%) patients achieved CRp, for an objective response rate of 26%. In addition to these encouraging response rates, the toxicity profile of this combination was noteworthy. Side effects were tolerable and mostly reversible. The 4-week mortality rate was 9%. Myelosuppression-associated complications were similar to those seen in other salvage therapies in leukemia.10–12 Of importance is the activity of this regimen given twice daily at doses that were not associated with severe extramedullary side effects, particularly neurotoxicity.
This combination was effective across all patient subgroups, whether they had primary refractory disease or not. The objective response rates were 56%, 30%, and 16% in patients with first salvage and first CR duration of more than 12 months, first salvage and first CR duration less than 12 months, and with second salvage and beyond, respectively. These results compare favorably with the expected response rates in matched-cohort patients treated at our institution, where the objective response rates were 50%, 11%, and 7%, respectively.10–12 This is particularly true among patients in first relapse after a first remission duration of less than a year in which the combination of twice-daily fludarabine and cytarabine plus or minus GO induced an objective response rate of 30% compared with an expected rate of 11% in historical controls. The achievement of a long first remission remains a major determinant for outcome as previously reported, thus reflecting a chemosensitivity to cytarabine.5,10 The median OS and EFS in first salvage and first CR duration of more than 12 months were 28 and 41 weeks, respectively, compared with 6 and 23 weeks, respectively, in patients receiving first salvage after a first remission duration shorter than 12 months. Furthermore, the fludarabine, cytarabine, and granulocyte colony-stimulating factor combination used in the treatment of core binding factor newly diagnosed AML induced an objective response rate of more than 90%, resulting in improved EFS, therefore reflecting significant antileukemia activity in at least a subset of patients with AML.13 In contrast, older patients with abnormal karyotype and refractory disease beyond their second salvage therapy had an independent worse outcome, requiring additional options.
Price and colleagues recently reported CR rates of 24% and 38%, respectively, with 2 different salvage regimens: MEC (mitoxantrone, etoposide, cytarabine) and CLAG (cladribine, high-dose cytarabine, granulocyte colony-stimulating hormone) in patients with relapsed/refractory AML.14 Although our combination induced overall CR rates similar to those in the MEC regimen and lower rates compared with the CLAG regimen, more than 50% of our patients were heavily pretreated and their disease had failed more than 2 previous regimens. In patients receiving first salvage, results were comparable.
The addition of GO at a dose of 3 g/m2 on day 1 did significantly improve the response rate and the CR duration with no additional side effects. This highlights the potential role of this drug in AML. This is in line with the results of the phase II trial of a combination of GO, fludarabine, cytarabine, and the multi-drug resistance (MDR) modifier, cyclosporine (MFAC) conducted in 32 patients with primary resistant or relapsed AML, in which the objective response rate was 34%.15 In the MRC15 trial, the addition of GO (3 mg/m2) to the induction and consolidation phases did not impact the overall response or survival; however a predefined analysis by cytogenetic risk groups showed a significant survival benefit.9 In a similar study, conducted by the Southwest Oncology Group, in which the addition of GO (6 mg/m2) did not improve the CR and EFS rates, an analysis of the outcomes by the pretreatment cytogenetic profile identified a significant benefit for the favorable-risk patients who received GO.16
Through the ability of fludarabine to modulate the pharmacologic behavior of cytarabine, the combination of fludarabine and cytarabine was shown be superior to cytarabine alone, particularly in patients with relapsed AML with a relatively long first remission.12 New generations of nucleoside analogues, designed with a higher potency and less toxicity than first-generation purine analogues, proved effective at inducing remission in patients with newly diagnosed and relapsed/refractory AML. Clofarabine induced an objective response rate of 48% in a phase II study in patients with relapsed and refractory AML, MDS, CML-BP, and acute lymphocytic leukemia.17 When combined with low-dose cytarabine in patients older than 60 years of age with newly diagnosed AML, response rates with clofarabine have been reported at 68% and maintained for 14 months.18 Sapacitabine, another nucleoside analogue, causes irreparable single-strand breaking, which leads to cell cycle arrest at the G2 phase. Initial investigations in elderly patients with newly diagnosed or relapsed AML showed sapacitabine was effective, with the highest CR reported in patients assigned to 400 mg twice daily administered 3 days per week.19 Trials assessing the combination of new nucleoside analogues with cytarabine or other new agents such as hypomethylating agents or histone deacetylase inhibitors are ongoing and may offer additional benefit.20
The combination regimen was well tolerated, with low early mortality. The major toxicities were gastrointestinal, including nausea and vomiting, diarrhea, and mucositis. Transient liver test abnormalities were also seen. The addition of a lower dose of GO did not increase the hepatic toxicities and no venoocclusive disease occurred. This is in line with what was observed in the MRC15 trial, in which the dose explored was lower than the dose approved by the FDA for use as a single agent—3 mg/m2 IV.9 Finally, myelosuppression-associated complications were similar to those seen in other salvage programs in leukemia.
In summary, the combination of twice-daily fludarabine and cytarabine appears to be active, with an ORR of 26% in a heavily pretreated population. This combination appears to be safe as well, with a low of 4-week mortality rate of 9%.
Clinical Practice Points.
Treatment options for patients with relapsed and refractory AML are limited.
BIDFA is active, with an objective response rate of 26% in a heavily pretreated AML population. This combination is safe with a low of 4-week mortality rate of 9%.
Trials assessing the combination of new nucleoside analogues with cytarabine or other new agents such as hypomethylating agents or histone deacetylase inhibitors are ongoing and may offer additional benefit.
Acknowledgments
This work was supported in part by grant CA100632 (leukemia SPORE) from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estey EH. How I treat older patients with AML. Blood. 2000;96:1670–3. [PubMed] [Google Scholar]
- 2.Gandhi V, Estey E, Keating MJ, et al. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11:116–24. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi V, Estey E, Du M, et al. Modulation of the cellular metabolism of cytarabine and fludarabine by granulocyte-colony-stimulating factor during therapy of acute myelogenous leukemia. Clin Cancer Res. 1995;1:169–78. [PubMed] [Google Scholar]
- 4.Gandhi V, Estey E, Du M, et al. Minimum dose of fludarabine for the maximal modulation of 1-beta-D-arabinofuranosylcytosine triphosphate in human leukemia blasts during therapy. Clin Cancer Res. 1997;3:1539–45. [PubMed] [Google Scholar]
- 5.Estey E, Plunkett W, Dixon D, et al. Variables predicting response to high dose cytosine arabinoside therapy in patients with refractory acute leukemia. Leucemia. 1987;1:580–3. [PubMed] [Google Scholar]
- 6.Estey EH, Keating MJ, McCredie KB, et al. Cellular ara-CTP pharmacokinetics, response, and karyotype in newly diagnosed acute myelogenous leukemia. Leucemia. 1990;4:95–9. [PubMed] [Google Scholar]
- 7.Estey E. Treatment of relapsed or refractory acute myelogenous leukemia. Leucemia. 2000;14:476–9. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 8.Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–52. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–77. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 10.Giles F, O’Brien S, Cortes J, et al. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–54. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- 11.Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116:5818–23. doi: 10.1182/blood-2010-07-296392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estey E, Plunkett W, Gandhi V, et al. Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma. 1993;9:343–50. doi: 10.3109/10428199309148532. [DOI] [PubMed] [Google Scholar]
- 13.Borthakur G, Kantarjian H, Wang X, et al. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer. 2008;113:3181–5. doi: 10.1002/cncr.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price SL, Lancet JE, George TJ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: is one better? Efficacy comparison between CLAG and MEC regimens. Leuk Res. 2011;35:301–4. doi: 10.1016/j.leukres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Tsimberidou A, Cortes J, Thomas D, et al. Gemtuzumab ozogamicin, fludarabine, cytarabine and cyclosporine combination regimen in patients with CD33+ primary resistant or relapsed acute myeloid leukemia. Leuk Res. 2003;27:893–7. doi: 10.1016/s0145-2126(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 16.Petersdorf S, Kopecky K, Stuart RK, et al. Preliminary results of Southwest Oncology Group Study S0106: An international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:abstract 790. [Google Scholar]
- 17.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–86. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 18.Faderl S, Ravandi S, Garcia-Manero G, et al. Frontline therapy for older patients (pts) with acute myeloid leukemia (AML): clofarabine plus low-dose cytarabine induction followed by prolonged consolidation with clofarabine plus low-dose cytarabine alternating with decitabine. Blood. 2010;116 doi: 10.1002/cncr.27429. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Garcia-Manero G, O’Brien S, et al. Phase I clinical and pharmacokinetic studhy of oral sapacitabine in patients with acute leukemia and myelodys-plastic syndrome. J Clin Oncol. 2010;28:285–91. doi: 10.1200/JCO.2009.25.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green SR, Choudhary AK, Fleming IN. Combination of sapacitabine and HDAC inhibitors stimulates cell death in AML and other tumour types. Br J Cancer. 2010;103:1391–9. doi: 10.1038/sj.bjc.6605922. [DOI] [PMC free article] [PubMed] [Google Scholar]