Abstract
We successfully deployed an in situ automatic chemical analyzer sensitive to manganese (Mn) in seawater for a period of 81 days for the first time on the deep seafloor of Sagami Bay along a convergent plate boundary south of Japan. The in situ Mn analyzer (GAMOS-IV) was connected to a submarine cable as a means to supply power and to relay real time data. During the observation period from April 5 till June 25, 2006, the amount of measured Mn was seen to increase abruptly up to 10 times that of the background level only on April 21, probably triggered by a M5.8 earthquake which occurred ∼7 km south-southwest of the observation site. This study demonstrates the suitability of submarine cables for the long-term geochemical monitoring of deep sea environments.
Keywords: submarine in situ Mn analyzer, plate boundary, temporal fluid venting, earthquake, submarine cable
Introduction
The in situ chemical measurement of seawater is an indispensable technique for detailed monitoring of chemical environments in the deep ocean,1)–5) especially if such environments are associated with short-term (hour to hour, or shorter time-scale) variations due to submarine volcanoes, earthquakes, etc. Although submarine chemical sensors (such as those for pH, O2, H2S, CH4, etc.) have been widely applied in in situ oceanographic studies,6)–8) lack of in situ calibration allows no lengthy time period for the collection of their high quality data, because chemical instruments consistently deteriorate and vary in their sensitivities due to dirt accumulation, biological activity, etc. on the sensor’s surfaces during long deployments in deep seawater.
A flow-through analyzer capable of self-calibration could overcome this problem,2),3),9) but its long-term operation requires large and heavy batteries as well as large amounts of analytical reagents. In the present study, we aimed to achieve a significant step forward in long-term chemical measurements by combining a submarine cable with an in situ flow-through Mn analyzer,10),11) electric power for which can be consistently supplied through the submarine cable. Another advantage of this combination is real-time data acquisition from the in situ instrument to land stations via the cable. In order to extend the in situ analysis time as long as possible, the analyzer was equipped with an extremely low flow rate pumping system11) to downsize the reagent consumption. Mn was used in this study because Mn is known to be a useful tracer for submarine hydrothermal activity on mid-oceanic ridges12) and for cold seepage at plate subduction zones.13)
We conducted a long-term deployment experiment in a cold seepage zone near the Sagami Trough plate boundary south of Japan, where previous data suggested a temporal Mn seepage from the seafloor.14) During the experiment, we happened to observe a spike in an Mn anomaly which occurred almost simultaneously with a submarine earthquake near the deployment site. This paper demonstrates i) the occurrence of tectonic fluid venting in the deep seafloor associated with a crustal deformation, and ii) the availability of the deep-sea cable network for the geochemical monitoring of submarine environments, as described in detail below.
Study area
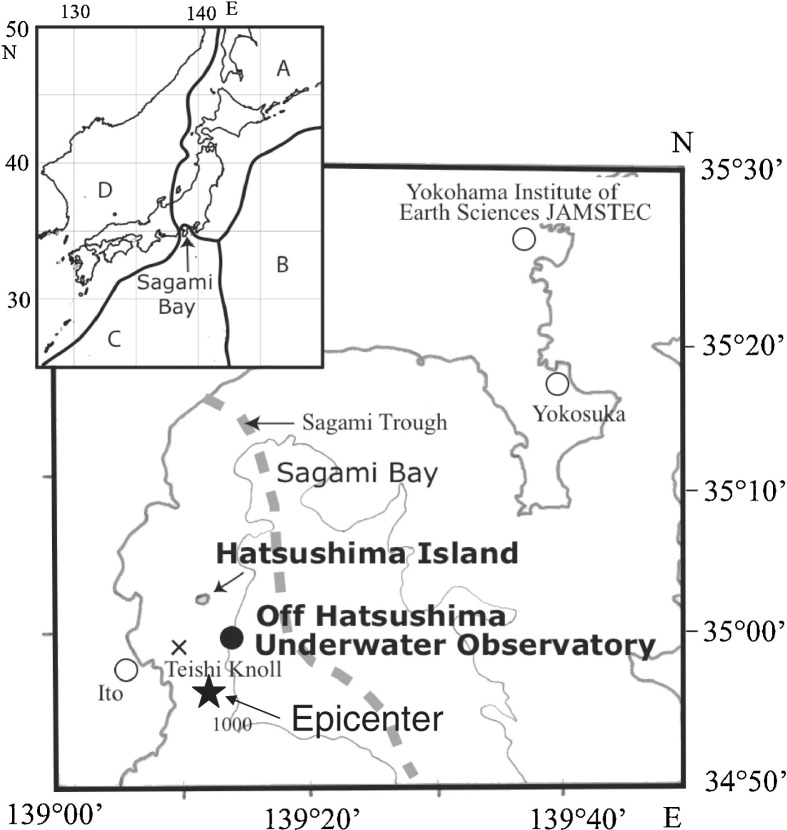
The project was conducted at a cable-connected submarine observatory (35°00.18′N, 139°13.48′E; Depth = 1,175 m, water temperature = 2.9°C) in Sagami Bay, south of Japan. Fig. 1 shows the location of this observatory, which is off the Hatsushima Underwater Observatory of JAMSTEC (Japan Agency for Marine-Earth Science and Technology).15) The bottom immediately below and around the observatory is known to be an active fault zone close to the Sagami Trough (Fig. 1), a boundary between the North American plate and the Philippine Sea plate, characterized by occasional swarm earthquakes and active cold seepage providing an environment for the development of large colonies of autotrophic chemosynthetic Vesicomyid clams.16)–19) The chemical characteristics of the seepage fluids have been previously investigated.16),17),19),20) In addition, approximately 8.5 km west of the observatory there exists the small submarine volcano “Teishi Knoll” (Depth = 120 m) which erupted in July 1989 (ref. 21).
Fig. 1.
Location of Off Hatsushima Underwater Observatory in Sagami Bay. The Japanese Islands are associated with four plates: The North American Plate (A), The Pacific Plate (B), The Philippine Sea Plate (C), and The Eurasia Plate (D). Sagami Bay fronts the southeastern side of the Islands. In the middle of Sagami Bay is a plate boundary (Sagami Trough) between plates A and C. A cross and a star marks show the location of the submarine volcano “Teishi Knoll” and the epicenter of the M5.8 earthquake on April 21, 2006.
A double-armored electro-optical submarine cable (length: 9 km) for data transmission and to privide a DC power supply was laid between the observatory and a land station on Hatsushima Island (Fig. 1). The observatory has been playing important roles as a multiplexer combined with various in situ instruments such as TV camera systems, CTD (Conductivity, Temperature, and Depth sensors) systems, a current meter, a seismometer, etc. in long-term geophysical and ecological studies.22)–25) The present design and schematic diagram of the observatory-land station system have been described in detail by Iwase et al.15)
Materials and method
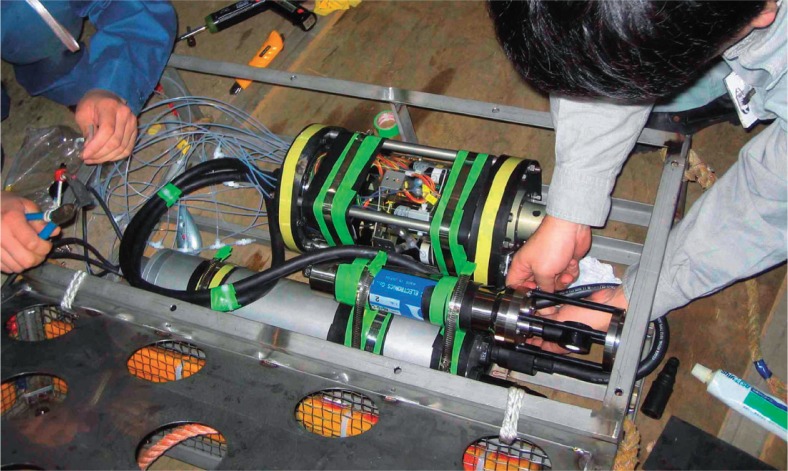
We deployed a submersible automatic Mn analyzer GAMOS-IV (Geochemical Anomaly MOnitoring System-IV, Kimoto Electric Co. Ltd., 26 kg weight in seawater, shown in Fig. 2).10),11) The GAMOS-IV constantly draws seawater through a Teflon tube inlet (1 mm in inner diameter, covered with a 10 μm mesh screen for particle filtration) to measure acid (pH:5)-dissolvable Mn by means of an H2O2-luminol chemiluminescence method.10) The flow-through analyzing unit is situated in an oil-filled vessel and is equipped with metal-free micro-diaphragm pumps to adjust the flow rate of the agents and seawater sample as slow as 60 μL min−1 (ref. 11) for the purpose of minimizing reagent consumption. It takes about 50 minutes for the seawater sample to reach the detector (photomultiplier), which accumulates the Mn concentration every 5 seconds and stores it in a pressure housing (pressure rating of 5.4 ×107 Pa, or 5200 m depth) which is equipped with a CPU and a data logger. The system is able to perform in situ self-calibration by periodically analyzing standard and blank solutions instead of seawater. The analyzer showed a 98% response time of approximately 6 minutes. Laboratory experiments confirmed the detection limit of Mn as low as 0.33 nM.11) It was also shown that the detector responses linearly with the Mn concentration of up to 10 μM at temperatures ranging between 2 and 30°C.11)
Fig. 2.
Assembly of a package of the GAMOS-IV system before the long-term deploying experiment.
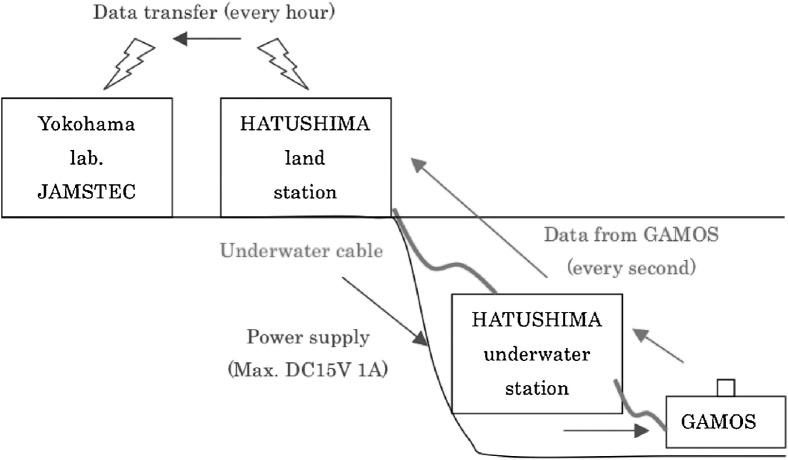
On April 5, 2006, the 3,000 m-type ROV Hyper Dolphin of JAMSTEC took the GAMOS-IV to the bottom of Sagami Bay, connecting it with a RS-232C port at the observatory via an underwater mateable connector (Ocean Design, Inc.). The distance between the observatory and the GAMOS-IV was 1–2 meters. Fig. 3 shows a schematic diagram of the instrument network used in this study. The GAMOS-IV had five flexible plastic tanks including three analytical reagents (pH 5 buffer, H2O2, and NH4OH with luminol solutions), a blank solution (([Mn2+] = 0 nM) and a standard solution ([Mn2+] = 100 nM), the total volume of which was ∼20 L, which was sufficient for the 3-month operation. The GAMOS-IV needs electric power (DC15 V, 0.5 A on the average, 1 A at the maximum) for running its diaphragm pumps in order to maintain steady flows of seawater sample, standard solutions, and analytical reagents. The power was supplied from the Hatsushima land station through the submarine cable (Fig. 3). The GAMOS-IV measured Mn concentration continuously from April 5 through June 25, 2006, when another dive of the Hyper Dolphin detached the underwater connection to recover the GAMOS-IV on board the tendership Natsushima. Throughout the 81 days of deployment, in situ raw data from the GAMOS-IV were sent to a computer at the Hatsushima land station through the cable in real time (Fig. 3).
Fig. 3.
Schematic diagram of the connection of GAMOS with the land station by submarine cables. Electric power was supplied from the land station to the GAMOS, and observed data was sent from the GAMOS to the land station.
Results and discussion
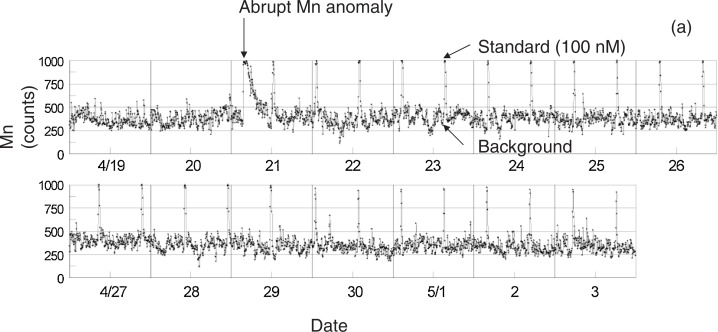
We confirmed that the GAMOS operated normally throughout the 81 days observation period, except that the analysis of the standard solution was neglected during the first 15 days of the experiment (between 5 and 20 of April) due to unknown reasons. Fig. 4 shows a part of the continuous Mn data of bottom seawater (averaged every 5 minutes) between April 19 and May 3. Since April 20, the GAMOS-IV repeated sequential measurements of seawater samples (12 hours), the blank solution (30 minutes), and the standard solution (30 minutes). Besides the blank and standard signals as well as a significant peak on April 21, Mn concentration is characterized by very frequent fluctuation throughout the measurement period, the significance of which is now under examination.
Fig. 4.
In situ bottom Mn data measured by the Gamos-IV between April 19 and May 3, 2006. The vertical axis shows raw count numbers (relative intensity) of Mn output averaged every 5 minutes. Just after the earthquake on April 21, the normal sampling interval of the background (30 min), the 100 nM standard (30 min), and the seawater sample (12 hr) was started. The horizontal value (date and time) should be adjusted by subtracting the delay time of 50 minutes which is necessary for the flow-through Mn determination (see text).
It is noteworthy that in Fig. 4 the Mn concentration abruptly increased at ∼3:40 a.m. April 21 (JST: Japan Standard Time), maintained the averaged value of 100.0 ± 1.3 nM for an hour (the instantaneous maximum of 102 nM was recorded), and then decreased exponentially to the background level in the evening of that day. In the early morning of the same day (2:50 JST of April 21), a submarine earthquake with a magnitude (M) of 5.8 occurred on the western side of Sagami Bay (34°56.4′N, 139°11.7′E), about 7 km south-southwest of the observation site. This was the first earthquake with a magnitude of >4 after the GAMOS-IV was deployed on April 5. Since the GAMOS-IV data has a delay of ∼50 minutes as mentioned earlier, the actual initial time of the abrupt Mn increase was almost the same as that of the earthquake.
There are two possible reasons for the abrupt Mn increase: one is a sudden fluid burst with a much higher Mn concentration than seawater, possibly through local faults or fissures formed by the earthquake (although we have no morphological evidence for this based on observation), and the other is an artificial Mn dissolution inside the GAMOS-IV from sampled seawater containing fine mud particles (<10 μm) due to resuspension of bottom sediment by the earthquake shakes. In fact, two video cameras attached to the observatory recorded turbid mud flow a few minutes after the earthquake.26)
In order to evaluate the latter possibility by means of a laboratory experiment, following the earthquake we collected a block of bottom mud and three bottom seawater samples close to the observatory. We actuated the GAMOS-IV in the same way as it had been on the bottom, measuring Mn concentration for the collected bottom seawater mixed with the bottom mud. We added 30 g of bottom mud to 100 cm3 of bottom seawater, stirring the mixture for 10 minutes to disperse the mud particles uniformly in order to simulate the resuspension of bottom sediment. The Mn concentration was measured in the seawater samples after passing them through 10 μm mesh filters. The Mn concentrations measured by the GAMOS-IV were 10, 15, and 12 nM (12 ± 2 nM on the average). These are slightly higher than the original seawater value of 8.2 nM, which is an order of magnitude lower than the Mn peak (102 nM) observed in situ on 21st April (Fig. 4).
We would therefore regard the Mn anomaly observed on April 21 as being attributed not to the resuspension of bottom sediment but to a sporadic fluid supply rich in Mn from the seafloor, which may have occurred almost synchronously with the earthquake. It is also worth noting that several earthquakes occurred in the same area between April 22 and May 2 (M4.0 to 5.1) causing little Mn anomalies, suggesting that the large-scale eruption of Mn-enriched pore fluid occurred only once, immediately at the time of the first main earthquake (M5.8) on April 21.
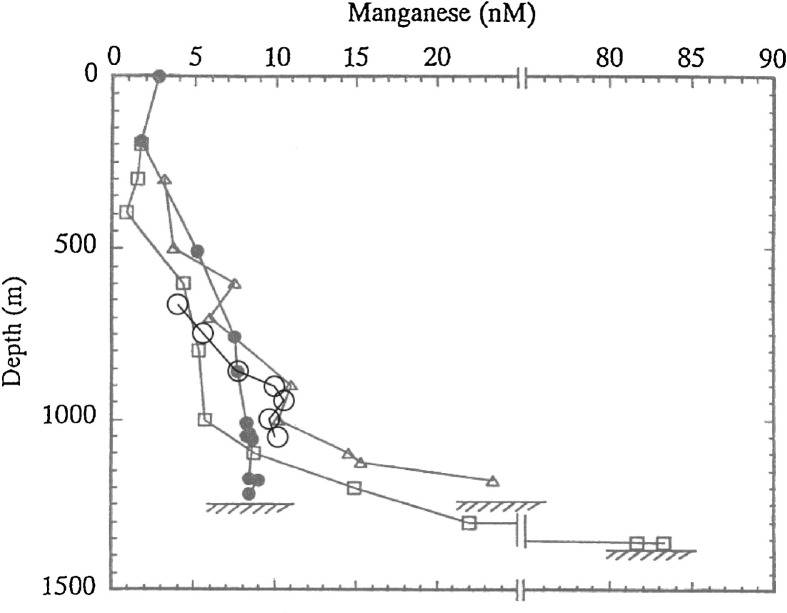
The Mn peak detected on April 21 reminds us of previously reported Mn anomalies in the bottom water of the same area. Nakayama et al.14),27) observed Mn concentrations of up to 83 nM in seawater 5∼10 m above the bottom, much higher than the background level of ∼10 nM (Fig. 5), just after the eruption of the submarine volcano Teishi Knoll in western Sagami Bay (Fig. 1). The maximum Mn value of 83 nM was almost the same level as that observed on April 21 (102 nM) in this study. Mn concentrations so far observed in the deep and bottom waters of the western Sagami Bay since 1988 (Figs. 4 and 5) strongly suggest that the background Mn concentration (∼10 nM or less) has been occasionally increased by an order of magnitude due to earthquakes or submarine eruptions. It may be probable that the eruption of Teishi Knoll caused a similar crustal deformation to trigger fluid venting rich in Mn. Furthermore, Nakayama et al.27) observed remarkable enrichments of Mn and Fe in the bottom waters (within ∼70 m above the bottom) of the Japan Trench subduction zone (40°24.9′N, 144°32.7′E; Depth = 7390 m), ∼70 km east of the epicenter of the 1994 Sanriku-Haruka Oki Earthquake (M7.5)28) that occurred two months after the observation. The maximum concentrations of Mn and Fe were 12 nM (>10 times higher than the background level in the Japan Trench) and 110 nM (50 to 100 times higher than the background level), respectively.
Fig. 5.
Historical Mn profiles (between 1988 and 1995) around the observatory. Filled and open circles (34°59.8′N, 139°15.8′E, and 34°59.9′N, 139°13.3′E, respectively) show background profiles measured in February 1988 and March 1995, respectively. Data indicated by open triangles (34°59.7′N, 139°14.0′E) and open squares (34°59.8′N, 139°15.8′E) show the Mn profiles at almost the same time as the eruption of a submarine volcano Teishi Knoll (13 July, 1989), and 2 days after the eruption, respectively. Data in 1988 and 1989 are from Nakayama et al.,14),27) and the data in 1995 are unpublished ones.
Submarine crustal deformations induced by volcanoes and/or earthquakes, therefore, might play an important role in supplying a temporal chemical flux from the seafloor by transferring pore fluids from deep anoxic environments where Mn(IV) and Fe(III) in volcanic rocks should be reduced to soluble Mn(II) and Fe(II).27) Claesson et al.29) observed significant Cu, Zn, Mn, and Cr anomalies in meteoric waters from a 1500 m-deep borehole before a submarine earthquake (M5.8) occurred north of Iceland. Blanc et al.13) confirmed high Mn concentrations of up to 500 μM in sedimentary interstitial waters in the décollement and thrust faults of the Barbados accretionary complex, where Mn-rich fluids are thought to originate from a reduction of MnO2 associated with the oxidation of organic carbon. The occasional chemical flux or “tectonic pumping” observed in this study may be a useful precursor or signature for large earthquakes or volcanic eruptions, and should be clarified more quantitatively using long-term in situ chemical analyzers and sensors.
The submarine cable network will be an important and convenient tool for long-term multi-disciplinary monitoring of the oceans, as already planned by NEPTUNE (North-East Pacific Time-series Undersea Networked Experiments) and ESONET (European Sea Floor Observatory Network) programs.1) Although most time-series monitoring experiments have so far been restricted to those using geophysical tools such as ocean bottom seismometers, the present study demonstrates that the submarine cable is also quite useful and applicable for geochemical observation. Further technological advancement of in situ chemical analysis will play a key role in elucidating geochemical cycles between seawater and the seafloor, which are characterized by a large spatial and temporal variability. The success of the 3-month experiment involving in situ chemical measurement in combination with a deep-sea cable network as described in this study will in the near future ensure a technological enhancement that will allow longer-term operation of up to a year.
Acknowledgments
We thank the operation team of the ROV Hyper Dolphin, the crew members of the R/V Natsushima, the technical staff on board the ship, and Kimoto Electric Co. Ltd. for their valuable collaboration during the NT06-06 and NT06-12 cruises. Thanks are also due to two anonymous reviewers who gave us many useful comments to improve the manuscript. This study was partly supported by a grant-in-aid for exploratory research (No. 17651004) and that for Scientific Research (A) (No. 19253006) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Copley, J. (2004) Nature 427, 10–12 [DOI] [PubMed] [Google Scholar]
- 2.Johnson, K. S., Beehler, C. L., Sakamoto-Arnold, C. M. and Childress, J. J. (1986) Science 231, 1139–1141 [DOI] [PubMed] [Google Scholar]
- 3.Coale, K. H., Chin, C. S., Massoth, G. J., Johnson, K. S. and Baker, E. T. (1991) Nature 352, 325–328 [Google Scholar]
- 4.Jannasch, H. W., Johnson, K. S. and Sakamoto, C. M. (1994) Anal. Chem. 66, 3352–3361 [Google Scholar]
- 5.Massoth, G. J. and Milburn, H. B. (1997) Proc. Mar. Anal. Chem. for Monitoring & Oceanogr. Res., Plouzane, France, 17–19 Nov., pp. 80–87 [Google Scholar]
- 6.Ding, K., Seyfried, W. E., Tivey, M. K. and Bradley, A. M. (2001) Earth Planet. Sci. Lett. 186, 417–425 [Google Scholar]
- 7.Gamo, T., Masuda, H., Yamanaka, T., Okamura, K., Ishibashi, J., Nakayama, E., Obata, H., Shitashima, K., Nishio, Y., Hasumoto, H.et al. (2004) Geochem. J. 38, 527–534 [Google Scholar]
- 8.Marinaro, G., Etiope, G., Bue, N. L., Favali, P., Papatheodorou, G., Christodoulou, D., Furlan, F., Gasparoni, F., Ferentinos, G., Masson, M.et al. (2006) Geo-Mar. Lett. 26, 297–302 [Google Scholar]
- 9.Gamo, T., Sakai, H., Nakayama, E., Ishida, K. and Kimoto, H. (1994) Anal. Sci. 10, 843–848 [Google Scholar]
- 10.Okamura, K., Kimoto, H., Saeki, K., Ishibashi, J., Obata, H., Maruo, M., Gamo, T., Nakayama, E. and Nozaki, Y. (2001) Mar. Chem. 76, 17–26 [Google Scholar]
- 11.Okamura, K., Hatanaka, H., Kimoto, H., Suzuki, M., Sohrin, Y., Nakayama, E., Gamo, T. and Ishibashi, J. (2004) Geochem. J. 38, 635–642 [Google Scholar]
- 12.Klinkhammer, G., Bender, M. and Weiss, R. F. (1977) Nature 269, 319–320 [Google Scholar]
- 13.Blanc, G., Boulegue, J. and Gieskes, J. M. (1991) Oceanol. Acta 14, 33–49 [Google Scholar]
- 14.Nakayama, E., Sohrin, Y. and Isshiki, K. (1993) InDeep Ocean Circulation, Physical and Chemical Aspects (ed. Teramoto, T.). Elsevier Science Publication, Amsterdam-London-New York-Tokyo, pp. 199–208 [Google Scholar]
- 15.Iwase, R., Asakawa, K., Mikada, H., Goto, T., Mitsuzawa, K., Kawaguchi, K., Hirata, K. and Kaiho, Y. (2003) Proc. 3rd International Workshop on Scientific Use of Submarine Cables and Related Technologies, Tokyo, pp. 31–34 [Google Scholar]
- 16.Sakai, H., Gamo, T., Endow, K., Ishibashi, J., Ishizuka, T., Yanagisawa, F., Kusakabe, M., Akagi, T., Igarashi, G. and Ohta, S. (1987) Geochem. J. 21, 227–236 [Google Scholar]
- 17.Gamo, T., Ishibashi, J., Shitashima, K., Kinoshita, M., Watanabe, M., Nakayama, E., Sohrin, Y., Kim, E.-S., Masuzawa, T. and Fujioka, K. (1988) Geochem. J. 22, 215–230 [Google Scholar]
- 18.Hashimoto, J., Ohta, S., Tanaka, T., Hotta, H., Matsuzawa, S. and Sakai, H. (1989) Palaeogeogr. Palaeoclim. Palaeoecol. 71, 179–192 [Google Scholar]
- 19.Masuzawa, T., Handa, N., Kitagawa, H. and Kusakabe, M. (1992) Earth Planet. Sci. Lett. 110, 39–50 [Google Scholar]
- 20.Tsunogai, U., Ishibashi, J., Wakita, H., Gamo, T., Masuzawa, T., Nakatsuka, T., Nojiri, Y. and Nakamura, T. (1996) Earth Planet. Sci. Lett. 138, 157–168 [Google Scholar]
- 21.Yamamoto, T., Soya, T., Suto, S., Uto, K., Tanada, A., Sakaguchi, K. and Ono, K. (1991) Bull. Volcanol. 53, 301–308 [Google Scholar]
- 22.Momma, H., Iwase, R., Mitsuzawa, K., Kaiho, Y. and Fujiwara, Y. (1989) Phys. Earth Planet. Interior 108, 263–274 [Google Scholar]
- 23.Fujiwara, Y., Tsukahara, J., Hashimoto, J. and Fujikura, K. (1998) Deep-Sea Res. I, 45, 1881–1889 [Google Scholar]
- 24.Kitazato, H., Nakatsuka, T., Shimanaga, M., Kanda, J., Soh, W., Kato, Y., Okada, Y., Yamaoka, A., Masuzawa, T., Suzuki, K.et al. (2003) Prog. Oceanogr. 57, 3–16 [Google Scholar]
- 25.Watanabe, T., Goto, T., Araki, E., Mikada, H., Mitsuzawa, K. and Asakawa, K. (2003) Proc. 3rd International Workshop on Scientific Use of Submarine Cables and Related Technologies, Tokyo, pp. 54–57 [Google Scholar]
- 26.Iwase, R., Goto, T., Kikuchi, T. and Mizutani, K. (2006) EOS Trans. AGU, 87(52), Fall Meet. Suppl. OS43C-0670. [Google Scholar]
- 27.Nakayama, E., Maruo, M., Obata, H., Isshiki, K., Okamura, K., Gamo, T., Kimoto, H., Kimoto, T. and Karatani, H. (2002) InMarine Environment: the past, present and future (ed. Chen, C.-T. A.). The Fuwen Press, Kaohsiung (Taiwan), pp. 345–355 [Google Scholar]
- 28.Sato, T., Imanishi, K. and Kosuga, K. (1996) Geophys. Res. Lett. 23, 33–36 [Google Scholar]
- 29.Claesson, L., Skelton, A., Graham, C., Dietl, C., Mörth, M., Torssander, P. and Kochum, I. (2004) Geology 32, 641–644 [Google Scholar]