Abstract
Objective
To define the relationship between Fuchs’ endothelial corneal dystrophy (FECD) severity and central corneal thickness (CCT).
Methods
Eyes from a subset of index cases, family members, and unrelated controls with normal corneas from the FECD Genetics Multi-Center Study (n=1610) were examined. To estimate the association between FECD severity grade (7-point severity scale based on guttae confluence) and CCT measured by ultrasonic pachymetry, a multivariable model was used that adjusted for eye, age, race, gender, glaucoma or ocular hypertension history, diabetes, contact lens wear, intraocular pressure and familial relationship to index case. An interaction between FECD severity grade and edema (stromal or epithelial) on slit lamp examination was used to investigate if the effect of FECD severity grade on CCT differed between those with and without edema.
Results
Average CCT was thicker in index cases for all FECD grades compared to unaffected controls (p ≤ 0.003) and in affected family members with an FECD grade of 4 or greater compared to unaffected family members (p ≤ 0.04). Similar results were observed for subjects without edema. Average CCT of index cases was greater than that of affected family members for grades 4, 5 and 6 (p ≤ 0.02). Intraocular pressure was also associated with CCT (p<0.01).
Conclusions
An increase in CCT occurs with increasing severity of FECD, including at lower FECD grades where clinically observable edema is not present. Monitoring corneal thickness changes serially could be a more sensitive measure of disease progression with surgical therapeutic implications.
Introduction
Corneal endothelial diseases, notably the commonly occurring Fuchs’ endothelial corneal dystrophy (FECD), influence central corneal thickness (CCT)1, 2 as do genetic determinants3, 4 and potentially intraocular pressure5–8. In healthy corneas, endothelial function is essential in maintaining normal thickness. The endothelium acts as a leaky fluid barrier between the aqueous humor and corneal stroma, enabling necessary nutrients to supply the cornea.9, 10 The endothelium also acts as an active transporter of ions across this cell layer creating an osmotic force that removes fluid from the corneal stroma. The balance between these two functions is a prime determinant of corneal thickness. Factors that impair the ability of the endothelium to perform these functions disrupt this balance with resultant corneal edema and an increase in thickness.
FECD is characterized by endothelial cell dysfunction that results in corneal edema. Thickening is believed to occur mainly in the later stages of FECD, manifesting as clinically apparent stromal and/or epithelial edema.11, 12 The relationship between earlier stages of FECD and CCT is less clear. Prior studies have been limited by small samples sizes, a lack of consistent definitions of FECD severity and by heterogeneous FECD study populations.1, 2, 13, 14 To further elucidate understanding of the pathogenesis of corneal thickening in FECD, the relationship between FECD severity and CCT in subjects from the FECD Genetics Multi-Center Study was examined.
Methods
Study Population
Subjects were selected from the FECD Genetics Multi-Center Study cohort, a study population recruited to identify genetic risk factors for FECD and previously described.15 In brief, families enriched for FECD were ascertained through severely affected probands, with an emphasis on identifying severely affected sibling pairs, although other family members, both affected and unaffected were also recruited. In addition, unrelated FECD cases and controls matched to be five years older than index cases were also collected. The control subjects have also been previously described15 and included pseudophakic eyes that were at least one year from their surgery. Written informed consent was obtained from all subjects following IRB approval of the study. Demographic information, ocular and systemic medical histories were obtained via a standardized questionnaire administered to the patient via interview, and each eye was evaluated separately for inclusion in the current study. Eyes were excluded for the purpose of this study if they: 1) had undergone penetrating or endothelial keratoplasty; 2) had cataract surgery within one year of the study exam; 3) had a history of blunt, penetrating or perforating trauma; and 4) had evidence of other corneal endothelial dystrophy. These exclusion criteria were chosen for their possible effect on corneal thickness, and thus potential to confound any relationship between FECD grade and CCT. Subject age, the time of examination, gender, self-reported diabetes, contact lens wear of any type, ocular and systemic medications, and self-reported prior history of ocular hypertension or glaucoma (either open or narrow angle) were recorded. A slit lamp biomicroscopic examination by a cornea-fellowship trained ophthalmologist was performed to determine the extent of corneal guttae as well as the presence of any stromal or epithelial edema, along with a manifest refraction and measurement of intraocular pressure by applanation tonometry. Each ophthalmologist was trained on a standardized protocol for assessing the FECD grading scale, provided with photographic examples of each grade, and were tested on live patient examples at the outset of the study to ensure consistency in grading across enrollment sites.15 The spherical equivalent was calculated from the manifest refraction for each eye.
FECD grade and central corneal thickness determination
The FECD grade was determined on a semi-quantitative scale from 0–6, modified from a previous severity scale.15 The grade scale was as follows: 0 (no guttae); 1 (1–12 central or paracentral non-confluent guttae); 2 (>12 central/paracentral non-confluent guttae); 3 (1–2 mm of confluent central/paracentral guttae; 4 (>2 to 5 mm of confluent central/paracentral guttae); 5 (>5mm confluent central/paracentral guttae); and 6 (> 5 mm of confluent central/paracentral guttae with stromal and/or epithelial edema). Cases in which stromal or epithelial edema was observed overlying regions of focally dense guttae were graded according to the diameter of the area of guttae with edema independently recorded. CCT was measured by a technician masked to the FECD grade of the subject and instructed to obtain measurements at the center of the cornea and centered over the pupil. Pachymeters were used from the following manufacturers (Accutome, Malvern PA; Bausch and Lomb Surgical, Rancho Cucamonga, CA; DGH Technology, Exton PA; KMI Surgical, Downington, PA; Eye Technology, Inc., Ardmore, PA; Haag-Streit, Mason, OH; Sonogage, Cleveland, OH; Sonomed, New Berlin, WI; and Tomey, Phoenix, AZ). Each instrument internally calibrates and takes repeated measurements to determine the thickness ultrasonically. Given the difficulty in defining the exact center of the cornea, three separate readings were obtained immediately after each other and the mean of these measurements used as the CCT. Eyes with any CCT measurement less than 100 um was excluded from further analysis (n=4).
Statistical Analyses
The eyes from subjects were divided into four categories for analysis: 1) proband and unrelated cases (hereafter referred to as index cases); 2) affected family members (FECD grade greater than 0); 3) unaffected family members (FECD grade of 0); and 4) unrelated controls with normal corneas. Enrollment under the genetic study design emphasized severely affected probands and affected siblings with FECD grades greater than 4 in at least one eye, resulting in small sample sizes in groups with FECD grade 1–3. As such, these eyes were combined into a single group for both the index cases and affected family member categories. Using a generalized estimating equations (GEE) approach that accounted for correlation between eyes using an exchangeable working correlation structure, two multivariable models were fit to estimate the effect of FECD grade on CCT. The first model adjusted for eye (OD vs. OS), age, race (Caucasian vs. non-Caucasian), presence of glaucoma/ocular hypertension, diabetes, contact lens wear (ever vs. never) and intraocular pressure. FECD grade and relationship to the index case were also included as categorical variables with an interaction between them. The second model adjusted for covariates from the first model as well as spherical equivalent for each eye, for the time of the evaluation (morning vs. afternoon), and whether the subject reported symptoms of blurred vision in the morning for the eye. The second model was used to estimate the effects of these three variables on CCT because they had limited data compared to the other variables included in the first model.
Because clinically evident edema was observed in several subjects at FECD grades where it was not a defining grading criteria, an interaction between FECD grade and presence or absence of clinically apparent stromal or epithelial edema was also included to investigate if the effect of FECD grade on CCT differed between those subjects with and without edema.
Results
In total, 3118 eyes from 1559 subjects were considered for this study, with 1610 eyes from 969 subjects meeting inclusion criteria. Of the excluded eyes, 945 eyes underwent prior keratoplasty, 273 eyes had known cataract surgery within 1 year of study enrollment, 77 eyes had a history of trauma and 11 eyes had findings consistent with an additional corneal dystrophy. Eyes were also excluded if data for CCT, FECD grade or variables included in the analysis models were missing. 18% of eyes were from index cases, 40% from affected family members, 34% from controls and 8% from unaffected family members. The cohort was predominantly Caucasian (98% of eyes) and female (65%), similar to our larger cohort.15 Index cases were slightly older than affected family members (mean 68.9 ± 11.4 vs. 63.2 ± 12.8 years) (Table 1). Given the matching practice of the primary study of selecting controls five years older than corresponding cases,15 controls were older than the index cases (mean 71.2 ± 7.6 years). Unaffected family members were the youngest group with a mean age of 52.2 ± 13.6 years. The proportion of females was greatest in the affected family member group (73%) compared to the index case, control and unaffected family member groups (62% vs. 60% vs. 66%, respectively). A greater proportion of eyes from index cases were noted to have either epithelial or stromal edema than eyes from affected family members (46% vs. 21%) (Table 2). Eyes from index cases had a higher prevalence of a history of glaucoma or ocular hypertension than eyes from affected family members or controls (11% vs. 7% vs. 6%, respectively). No unaffected family members reported a history of glaucoma or ocular hypertension. Mean intraocular pressure was greatest in the controls (15.8 ± 3.2) and lowest in index cases (14.9 ± 3.1) (Table 2). Among patients that contributed both eyes to our sample of 1610 eyes, the Pearson correlation for CCT between eyes was 0.92.
Table 1.
Baseline characteristics by subject for the four study analysis groups.
| Characteristic | Index Cases (n=247) | Affected Family (n=358) | Controls (n=300) | Unaffected Family (n=64) | p-value |
|---|---|---|---|---|---|
|
| |||||
| 1 eye1 (OD) | 97 (39.3) | 21 (5.9) | 29 (9.7) | 1 (1.6) | <0.001 |
| 1 eye1 (OS) | 105 (42.5) | 48 (13.4) | 20 (6.7) | 7 (10.9) | <0.001 |
| 2 eyes1 | 45 (18.2) | 289 (80.7) | 251 (83.7) | 56 (87.5) | <0.001 |
|
| |||||
| Age2 | 68.9 ± 11.4 | 63.2 ± 12.8 | 71.2 ± 7.6 | 52.2 ± 13.6 | <0.001 |
|
| |||||
| Caucasian1 | 239 (96.8) | 352 (98.3) | 291 (97.0) | 64 (100.0) | 0.31 |
|
| |||||
| Male1 | 95 (38.5) | 98 (27.4) | 121 (40.3) | 22 (34.4) | 0.003 |
|
| |||||
| Diabetes (ever)1 | 31 (12.6) | 41 (11.5) | 31 (10.3) | 2 (3.1) | 0.18 |
|
| |||||
| FECD exam time (PM)2 (n=906) | 140 (57.1) (n=245) | 188 (53.7) (n=350) | 100 (40.0) (n=250) | 29 (47.5) (n=61) | <0.001 |
Results are presented using the frequency and percentage.
Results are presented using the mean and standard deviation.
Table 2.
Baseline characteristics by eye for the four analysis groups.
| Characteristic | Index Cases (n=292) | Affected Family (n=646) | Controls (n=551) | Unaffected Family (n=121) | p-value |
|---|---|---|---|---|---|
| Glaucoma1,3 | 25 (8.6) | 31 (4.8) | 15 (2.7) | 0 (0) | <0.001 |
| Ocular hypertension1,3 | 6 (2.1) | 12 (1.9) | 18 (3.3) | 0 (0) | 0.13 |
| Contact lens wear1 | 40 (13.7) | 152 (23.5) | 111 (20.1) | 57 (47.1) | <0.001 |
| Spherical equivalence2 (n=1506) | −0.28 ± 2.31 (n=276) | −0.20 ± 2.37 (n=613) | −0.02 ± 2.55 (n=504) | −1.77 ± 2.88 (n=113) | <0.001 |
| Intraocular pressure2 | 14.9 ± 3.1 | 15.4 ± 3.1 | 15.8 ± 3.2 | 15.5 ± 3.5 | <0.001 |
| Epithelial edema1,3 | 27 (9.8) | 23 (3.6) | 0 (0) | 0 (0) | <0.001 |
| Stromal edema1,3 | 113 (41.1) | 122 (19.3) | 0 (0) | 0 (0) | <0.001 |
| Epithelial or stromal edema1,3 | 134 (45.9) | 138 (21.4) | 0 (0) | 0 (0) | <0.001 |
Results are presented using the frequency and percentage.
Results are presented using the mean and standard deviation.
P-values are based on logistic regression models using Firth’s method34 to account for the presence of zero cell frequencies (eyes are assumed independent).
An estimate of the effect of several covariates on CCT was also examined (Table 3). We found that for each increase in intraocular pressure by 1 mm Hg, the cornea was on average 1.0μ thicker (95% CI: (0.2, 1.8); p = 0.01). Blurred vision in the morning was also significantly associated with CCT (p = 0.04). Symptomatic eyes had corneas 8.1μ thicker (95% CI: (0.3, 16.0)) on average than eyes without this symptom. Age and gender also appeared to have a borderline statistically significant association with CCT. No significant relationship was found between CCT and eye, race, reported glaucoma or ocular hypertension, a history of diabetes, contact lens wear, spherical equivalence and time of exam (p > 0.05).
Table 3.
Effects of covariates on central corneal thickness.
| Covariate | Model 1 (N=1610) | Model 2 (N=1395) | ||
|---|---|---|---|---|
| Effect size (95% CI) | P-value | Effect size (95% CI) | P-value | |
| Eye (OD) | 0.9 (−0.5, 2.3) | 0.21 | 1.1 (−0.5, 2.7) | 0.20 |
| Age (per year increase) | −0.3 (−0.6, 0.0) | 0.03 | −0.3 (−0.6, 0.0) | 0.06 |
| Caucasian | 11.8 (−6.9, 30.5) | 0.22 | 13.9 (−4.7, 32.6) | 0.14 |
| Male | 6.1 (−0.1, 12.2) | 0.06 | 7.0 (0.3, 13.6) | 0.04 |
| Glaucoma or ocular hypertension (ever) | 0.2 (−10.1, 10.5) | 0.96 | −3.5 (−14.0, 6.9) | 0.51 |
| Diabetes (ever) | 4.2 (−4.9, 13.4) | 0.36 | 4.6 (−5.0, 14.2) | 0.35 |
| Contact lens wear (ever) | −6.4 (−13.8, 1.1) | 0.09 | −6.0 (−14.0, 1.9) | 0.13 |
| Intraocular pressure (per mmHg increase) | 1.0 (0.2, 1.8) | 0.01 | 1.0 (0.2, 1.8) | 0.01 |
| Spherical equivalent (per diopter increase) | -- | -- | 0.1 (−1.0, 1.3) | 0.80 |
| FECD exam time (PM) | -- | -- | 1.1 (−4.9, 7.2) | 0.71 |
| Blurred AM vision | -- | -- | 8.1 (0.3, 16.0) | 0.04 |
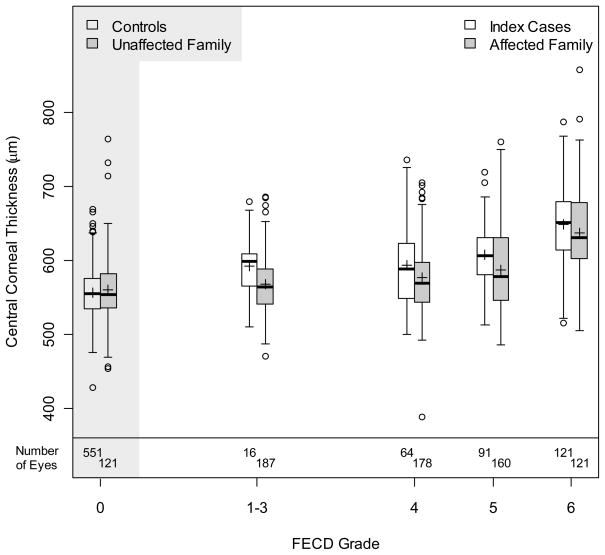
We also found that mean CCT increased as the FECD severity grade worsened from 1–3 to 6 in both the index cases and affected family member groups (Figure 1). The mean CCT of eyes from index cases was significantly thicker at all grade levels than eyes from controls, including the group containing eyes with FECD grades from 1–3 (p ≤ 0.003) (Table 4). Similarly, the mean CCT of eyes from affected family members was thicker than unaffected family members for all grades (p ≤ 0.04). Index case corneas were on average thicker than the corresponding corneas of affected family members for grade categories 4, 5 and 6 (p ≤ 0.02); there was no difference in thickness in the grade 1–3 groups (p=0.12). Mean CCT in corneas from controls and unaffected family members were not significantly different (p = 0.97).
Figure 1.
Boxplots of central corneal thickness for index cases (FECD grade=1–3, 4, 5, 6), affected family members (FECD grade=1–3, 4, 5, 6), unaffected family members and controls. Means are represented by ‘+’.
Table 4.
Model 1 estimates and comparisons of mean central corneal thickness.
| Mean (95% CI) | P-value1 | Mean (95% CI) | P-value2 | Mean (95% CI) | P-value3 | |
|---|---|---|---|---|---|---|
| FECD Grade | Controls | Unaffected family | Difference | |||
| 0 | 558 (554, 562) | -- | 558 (547, 569) | -- | 0 (−12, 12) | 0.97 |
| Index Cases | Affected family | Difference | ||||
| 1–3 | 586 (567, 605) | 0.003 | 571 (564, 577) | 0.04 | −16 (−36, 4) | 0.12 |
| 4 | 596 (584, 607) | <0.001 | 574 (564, 584) | 0.03 | −21 (−37, −6) | 0.005 |
| 5 | 612 (604, 620) | <0.001 | 595 (584, 607) | <0.001 | −17 (−31, −3) | 0.02 |
| 6 | 648 (639, 658) | <0.001 | 630 (618, 642) | <0.001 | −19 (−34, −4) | 0.01 |
P-value associated with comparing the mean CCT between each index FECD grade group to controls.
P-value associated with comparing the mean CCT between each affected family member FECD grade group to unaffected family members.
P-value associated with comparing the mean CCT between affected family members to index cases or unaffected family members to controls.
As anticipated, corneas with slit-lamp-observed edema were thicker than corneas without edema (Table 5). Mean CCT in index cases without edema was thicker than in controls for FECD grades 4 and 5 (p < 0.001) while mean CCT in affected family members without edema was thicker than unaffected family members in the FECD grade 5 group (p < 0.001). By definition, FECD grade 6 cases had edema on exam and so they could not be included in these results. Comparisons between index cases and affected family members without edema demonstrated significantly thicker corneas in the index group for subjects with FECD grades of 4 and 5 (p = 0.02). Alternatively, in the subjects with edema, the mean CCT in index cases was thicker than affected family members only for grade 6 (p = 0.01). Subjects with a grade 5 guttae diameter and also observed to have edema were, by definition, classified as grade 6 and were not included in these results. Note that after adjustment for an interaction between edema and FECD grade, there was evidence of an association between intraocular pressure, age and contact lens wear on CCT (p ≤ 0.045, results not shown).
Table 5.
Estimates and comparisons of central corneal thickness for subjects without and with edema.
| Without Edema | With Edema | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) | P-value1 | N | Mean (95% CI) | P-value2 | Mean (95% CI) | P-value3 | N | Mean (95% CI) | N | Mean (95% CI) | Mean (95% CI) | P-value 4 | |
| FECD | Controls | Unaffected family | Difference | Controls | Unaffected family | Difference | ||||||||
| 0 | 551 | 558 (554, 562) | -- | 121 | 558 (548, 569) | -- | 0 (−11, 12) | 0.96 | 0 | -- | 0 | -- | -- | -- |
| Index Cases | Affected family | Difference | Index Cases5 | Affected family5 | Difference | |||||||||
| 1 to 3 | 12 | 576 (557, 595) | 0.06 | 182 | 570 (563, 576) | 0.06 | −7 (−27, 14) | 0.52 | 4 | 629 (586, 672) | 5 | 592 (575, 608) | −37 (−83, 9) | 0.11 |
| 4 | 55 | 589 (577, 600) | <0.001 | 166 | 571 (560, 581) | 0.09 | −18 (−33, −3) | 0.02 | 9 | 628 (595, 660) | 12 | 626 (593, 659) | −1 (−48, 45) | 0.95 |
| 5 | 91 | 612 (603, 620) | <0.001 | 160 | 595 (583, 607) | <0.001 | −17 (−31, −2) | 0.02 | 0 | -- | 0 | -- | -- | -- |
| 6 | 0 | -- | -- | 0 | -- | -- | -- | -- | 121 | 649 (640, 658) | 121 | 630 (619, 641) | −19 (−33, −4) | 0.01 |
P-value associated with comparing the mean CCT between each index FECD grade group to controls for subjects without clinically apparent edema.
P-value associated with comparing the mean CCT between each affected family member FECD grade group to unaffected family members for subjects without clinically apparent edema.
P-value associated with comparing the mean CCT between affected family members to index cases or unaffected family members to controls for subjects without clinically apparent edema.
P-value associated with comparing the mean CCT between affected family members to index cases for subjects with clinically apparent edema.
Subjects with edema at FECD grades 1–4 represent individuals with focally dense guttae observed to also have clinically evident edema on slit lamp examination. Subjects scored as FECD grade 5 and also noted to have clinically observed edema were equivalent to a FECD grade 6 and were classified accordingly.
Comment
General understanding based on compensatory mechanisms in the deteriorating endothelium in FECD is that CCT remains normal until the late stages of the disease when there are extensive confluent guttae.11 Another possibility, however, is that a gradual increase in CCT arises as FECD progresses. Studies have been limited in their ability to distinguish between these mechanisms due to a lack of standardization of grading criteria, small sample sizes and a lack of prospective studies. This current study suggests that there is a gradual increase in CCT as FECD progresses clinically. Of note, significant differences in CCT were even detectable at early grades of FECD, as compared to normal controls. Our results indicate subjects with as few as 1–2 mm of confluent guttae may begin to develop central corneal thickening, pointing to a gradual process of endothelial dysfunction rather than an acute ‘tipping point’ of endothelial decompensation as a likely mechanism of corneal edema in FECD. Importantly, our findings were not limited to one subset of our cohort, but rather were observed in the affected family members as well as index cases, indicating this is not an isolated phenomenon.
Corneal hydration is mainly regulated by endothelial barrier function and ionic gradients set up by Na/K-ATPases. The physiological basis for corneal edema in FECD has been attributed to alterations in Na/K ATPase pump site density and/or breakdown in barrier function. There is a possible increase in Na/K-ATPase pump site density in early FECD stages16 with a gradual decline as the disease progresses17, 18. Conversely, one of the earliest defects in FECD is a breakdown in barrier function that results in the increased permeability of corneal endothelium to solutes and increased corneal thickening.1 Although our study did not specifically study the mechanisms of corneal edema, it corroborates the above studies by showing that corneal hydration could be affected in early stages of the disease process. Unfortunately ultrasonic pachymetry only measures total corneal thickness and cannot distinguish changes in thickness by individual corneal layer like other methods such as confocal microscopy;19 however, it seems feasible that subclinical thickening of the stroma may occur at lower FECD grades as endothelial function and compensatory mechanisms become impaired.
Average CCT in our two groups of unaffected subjects is consistent with the current understanding of normal corneal thickness measured by ultrasonic pachymetry.20 The lack of a significant difference in mean CCT between explicitly recruited controls with normal corneas by slit lamp biomicroscopy and unaffected family members collected during familial recruitment is reassuring. Interestingly, affected family members had on average thinner corneas at a given FECD grade than the index cases despite identical grading criteria for both groups. We hypothesized that index cases, being the subject that brought the family to the attention of the study, were more likely to be symptomatic and, thus, more likely to have greater corneal thickening, regardless of the extent of guttae. Our results show that index cases did indeed have a higher rate of blurred vision in the morning than affected family (50% vs. 36%) but adjusting for this symptom in the model had no effect on the observed difference between the two groups. Either this symptom as measured in our study is not a sensitive enough “symptomatic” marker to adequately account for the effect, or other hypotheses for the phenomenon should be considered. Recent work has examined the progressive loss of endothelial cell density in corneas with guttae without clinical edema and found that these eyes could be classified as likely asymptomatic guttae, borderline guttae that may progress to development of corneal edema and guttae likely to be a preliminary form of FECD (as defined by the development of stromal or epithelial edema or other late stage complications) based on changes in cell density21. Our observed difference in CCT between index cases and affected family members may represent a greater proportion of index case eyes falling into the latter two categories with resultant endothelial dysfunction. As the other eye of each index case must have been severely affected for the subject to have initially qualified for the study, it would not be surprising that their companion eye would be likely to progress to late stage FECD.
Also of interest was the identification of a small subgroup of subjects with focally dense guttae that resulted in clinically evident edema, with a greater proportion of index cases than affected family members encompassed in this subgroup. These subjects do not entirely account for the differences in CCT between the index cases and affected family members as analyses excluding subjects with edema still identify differences in CCT. These subjects may partially contribute to the findings of increased CCT at lower FECD grades, as we observed the loss of a significant difference in CCT between index cases and controls for FECD grades 1–3 and affected and unaffected family members for FECD grade 1–3 and 4 when these subjects were excluded, although a trend towards an effect remained. It is possible that the same factors which underlie the difference in CCT between index cases and their affected familial counterparts may also underlie the development of edema in the presence of only focally dense guttae.
Several factors, including intraocular pressure and contact lens wear, have been previously implicated in affecting corneal thickness and were examined as potential confounders in the current study. There is a known association between increased intraocular pressure and greater CCT.5–8 Multiple studies have also shown that subjects with ocular hypertension have increased CCT7, 22 and there is some evidence that chronic elevation of IOP contributes to this increase.23 Within our cohort, increases in intraocular pressure were significantly associated with increased CCT, although the causal relationship remains unclear. Whether this represents the known association between intraocular pressure and CCT or if FECD itself may have a relationship with intraocular pressure requires further study.
Prior research has demonstrated an association between corneal thinning and long term extended contact lens wear,24, 25 although there appears to be significant individual variability in the corneal response to extended contact lens wear usage26. Our models show some evidence of lower CCT among those who have worn contacts (p= 0.09 in our main model and 0.045 in the model that includes an interaction between group and edema). Since our study is limited by the lack of complete data on the type, duration, and time interval of contact lens wear, it is feasible that the contact lens effect in our study would be diluted compared to what has been shown previously in the literature and could explain why the association is inconclusive despite the large sample size.
Numerous previous studies have examined the correlation between age and corneal thickness without any obvious trends emerging.20, 27 Our models show some evidence of lower CCT being associated with increased age (p=0.06 in our main model and 0.02 in the model that includes an interaction between group and edema), consistent with the ambiguity in the literature. Also, since our population was predominantly older with an average age of 66 years, it is difficult to draw conclusions about age and CCT that could be generalized to a broader population.
The cross-sectional nature, the method chosen for assessing CCT, and the measurement of thickness only in the central cornea are limitations to this study. Within the general population, there is a normal variation of corneal thickness20 and it is upon this variability that the effects of FECD are superimposed. Our study did not follow subjects longitudinally, since the primary objective of the study was to assess the genetic factors associated with advanced late onset FECD; thus, the change in CCT over time with advancing FECD and genetic factors associated with this phenotype by individual, as studied in several late-onset FECD families,28, 29 was not examined. Some subjects may have corneas thinner than 500μ prior to development of advanced FECD30, and, therefore, may never develop CCT values above 700μ, usually considered abnormally thick, even with advanced disease. The spread in normal CCT prior to disease onset was most likely reflected in the CCT spread of our own FECD grade 6 index and affected family member cases where some subjects had a CCT less than the average CCT for controls and unaffected family members (Figure 1). As such, an individual’s overall change in CCT will likely be most useful in clinical management rather than a comparison with CCT from others with FECD. This observation particularly impacts decision making regarding cataract surgery in the setting of FECD. The decision to do cataract surgery alone, cataract surgery with keratoplasty, or keratoplasty alone should be based on a number of factors including the type and degree of cataract, density and location of the guttae which may cause light scattering and a decrease in visual acuity directly31, 32, as well as based on our findings, the change in CCT between visits and clinical presence of stromal and/or epithelial edema rather than the absolute CCT value.33 With the advent of earlier surgical intervention in FECD with endothelial keratoplasty, the application of these proposed principles and their role in the management of earlier stages of FECD becomes even more important.
Our method for assessing CCT, ultrasound pachymetry, was chosen as it is commonly used clinically and was thus available across the many sites enrolling subjects for the genetic study. In using ultrasonic pachymetry, we were unable to examine the individual layers of the cornea for changes contributing to increased thickness. Additionally, thickness was solely measured in the center of the cornea for consistency and thus our conclusions can only apply to this measurement. The measurement of CCT may be an underestimate of the thickest area of the cornea when more severe disease is located in the paracentral region. However, paracentral measurement is confounded by the increasing thickness of the cornea from center to periphery and variability from subject to subject for a defined paracentral location. Despite these limitations, our study provides evidence from a large cohort of subjects that changes in CCT occur in FECD patients prior to clinically apparent edema.
Our findings provide evidence of utilizing CCT as a quantitative parameter in following FECD progression in addition to more subjective modalities such as slit lamp examination. A clear connection between CCT values and FECD severity grade points to the potential use of CCT in guidance of treatment decisions and prognostication for surgical intervention. The cross-sectional design of our study limits our ability to examine this question in the current cohort; however, future prospective, longitudinal studies could do so. Our findings also highlight the benefit of collecting additional clinical data in a cohort initially assembled to investigate genetic risk factors for FECD. Our insights into the pathology of earlier stages of FECD enhance the current clinical paradigm and add to the disease model through which the results of any future studies, genetic analyses or otherwise, will be interpreted.
Acknowledgments
Support provided by R01EY16482, R21 EY015145, P30 EY11373, Research to Prevent Blindness and the Ohio Lions Eye Research Foundation. The FECD Genetics Multi-Center Study group list has been previously published.15 We also acknowledge Dr. David Musch for his advice on study design. Drs. Kopplin, Iyengar and Lass had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Burns RR, Bourne WM, Brubaker RF. Endothelial function in patients with cornea guttata. Invest Ophthalmol Vis Sci. 1981;20(1):77–85. [PubMed] [Google Scholar]
- 2.Mandell RB, Polse KA, Brand RJ, Vastine D, Demartini D, Flom R. Corneal hydration control in Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 1989;30(5):845–852. [PubMed] [Google Scholar]
- 3.Lu Y, Dimasi DP, Hysi PG, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010;6(5):e1000947. doi: 10.1371/journal.pgen.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitart V, Bencic G, Hayward C, et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum Mol Genet. 2010;19(21):4304–4311. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53(1):34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 6.Eysteinsson T, Jonasson F, Sasaki H, et al. Central corneal thickness, radius of the corneal curvature and intraocular pressure in normal subjects using non-contact techniques: Reykjavik Eye Study. Acta Ophthalmol Scand. 2002;80(1):11–15. doi: 10.1034/j.1600-0420.2002.800103.x. [DOI] [PubMed] [Google Scholar]
- 7.Stodtmeister R. Applanation tonometry and correction according to corneal thickness. Acta Ophthalmol Scand. 1998;76(3):319–324. doi: 10.1034/j.1600-0420.1998.760313.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolfs RC, Klaver CC, Vingerling JR, Grobbee DE, Hofman A, de Jong PT. Distribution of central corneal thickness and its association with intraocular pressure: The Rotterdam Study. Am J Ophthalmol. 1997;123(6):767–772. doi: 10.1016/s0002-9394(14)71125-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman PL, Alm A, Adler FH. Adler’s physiology of the eye: clinical application. 10. St. Louis: Mosby; 2003. [Google Scholar]
- 10.Waring GO, 3rd, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982;89(6):531–590. [PubMed] [Google Scholar]
- 11.Eghrari AO, Gottsch JD. Fuchs’ corneal dystrophy. Expert Rev Ophthalmol. 2010;5(2):147–159. doi: 10.1586/eop.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh KT, Weil LJ, Oh DM, Mathers WD. Corneal thickness in Fuchs’ dystrophy with and without epithelial oedema. Eye (Lond) 1998;12 (Pt 2):282–284. doi: 10.1038/eye.1998.65. [DOI] [PubMed] [Google Scholar]
- 13.Polse KA, Brand RJ, Vastine DW, Demartini DR, Sanders TL. Clinical assessment of corneal hydration control in Fuchs’ dystrophy. Optom Vis Sci. 1991;68(11):831–841. doi: 10.1097/00006324-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SE, Bourne WM, O’Brien PC, Brubaker RF. Endothelial function and aqueous humor flow rate in patients with Fuchs’ dystrophy. Am J Ophthalmol. 1988;106(3):270–278. doi: 10.1016/0002-9394(88)90360-1. [DOI] [PubMed] [Google Scholar]
- 15.Louttit MD, Kopplin LJ, Igo RP, et al. A multi-center study to map genes for Fuchs’ endothelial corneal dystrophy: baseline characteristics and heritability. Cornea. 2011 doi: 10.1097/ICO.0b013e31821c9b8f. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geroski DH, Matsuda M, Yee RW, Edelhauser HF. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology. 1985;92(6):759–763. doi: 10.1016/s0161-6420(85)33973-8. [DOI] [PubMed] [Google Scholar]
- 17.McCartney MD, Robertson DP, Wood TO, McLaughlin BJ. ATPase pump site density in human dysfunctional corneal endothelium. Invest Ophthalmol Vis Sci. 1987;28(12):1955–1962. [PubMed] [Google Scholar]
- 18.McCartney MD, Wood TO, McLaughlin BJ. Moderate Fuchs’ endothelial dystrophy ATPase pump site density. Invest Ophthalmol Vis Sci. 1989;30(7):1560–1564. [PubMed] [Google Scholar]
- 19.Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129(5):555–561. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 21.Hatou S, Shimmura S, Shimazaki J, et al. Mathematical projection model of visual loss due to Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-8040. [DOI] [PubMed] [Google Scholar]
- 22.Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108(10):1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 23.Herman DC, Hodge DO, Bourne WM. Changes in corneal thickness in patients with treated and untreated ocular hypertension. Cornea. 2006;25(6):639–643. doi: 10.1097/01.ico.0000214231.28862.03. [DOI] [PubMed] [Google Scholar]
- 24.Holden BA, Sweeney DF, Vannas A, Nilsson KT, Efron N. Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci. 1985;26(11):1489–1501. [PubMed] [Google Scholar]
- 25.Liu Z, Pflugfelder SC. The effects of long-term contact lens wear on corneal thickness, curvature, and surface regularity. Ophthalmology. 2000;107(1):105–111. doi: 10.1016/s0161-6420(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 26.Schoessler JP, Barr JT. Corneal thickness changes with extended contact lens wear. Am J Optom Physiol Opt. 1980;57(10):729–733. doi: 10.1097/00006324-198010000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Prasad A, Fry K, Hersh PS. Relationship of age and refraction to central corneal thickness. Cornea. 2011;30(5):553–555. doi: 10.1097/ICO.0b013e3181fb880c. [DOI] [PubMed] [Google Scholar]
- 28.McGlumphy EJ, Yeo WS, Riazuddin SA, et al. Age-severity relationships in families linked to FCD2 with retroillumination photography. Invest Ophthalmol Vis Sci. 2010;51(12):6298–6302. doi: 10.1167/iovs.10-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meadows DN, Eghrari AO, Riazuddin SA, Emmert DG, Katsanis N, Gottsch JD. Progression of Fuchs corneal dystrophy in a family linked to the FCD1 locus. Invest Ophthalmol Vis Sci. 2009;50(12):5662–5666. doi: 10.1167/iovs.09-3568. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed KA, McLaren JW, Baratz KH, Maguire LJ, Kittleson KM, Patel SV. Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Am J Ophthalmol. 2010;150(4):490–497. doi: 10.1016/j.ajo.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 31.van der Meulen IJ, Patel SV, Lapid-Gortzak R, Nieuwendaal CP, McLaren JW, van den Berg TJ. Quality of Vision in Patients With Fuchs Endothelial Dystrophy and After Descemet Stripping Endothelial Keratoplasty. Arch Ophthalmol. 2011 doi: 10.1001/archophthalmol.2011.247. [DOI] [PubMed] [Google Scholar]
- 32.Seitzman GD. Cataract surgery in Fuchs’ dystrophy. Curr Opin Ophthalmol. 2005;16(4):241–245. doi: 10.1097/01.icu.0000172828.39608.7c. [DOI] [PubMed] [Google Scholar]
- 33.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs’ corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112(3):441–446. doi: 10.1016/j.ophtha.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]