Abstract
Background
To generate clinical-grade dendritic cells (DCs) ex vivo for immunotherapy trials, peripheral blood monocytes are typically cultured in GM-CSF and IL-4, and then matured using one or more agents. Duration of the initial DC culture is one important variable that has not been systematically evaluated for its effect on the characteristics of the final mature DC product.
Study Design
DCs were generated from elutriated peripheral blood monocytes by incubation in medium containing 2000 units/ml each of GM-CSF and IL-4 for 3 to 7 days, followed by maturation with LPS and IFNγ. DC yield, viability, flow cytometric phenotype, and cytokine production were evaluated.
Results
The % yield and viability of mature DCs were similar after initial culture durations of 3 or 7 days. Mature DCs expressed abundant CD80, CD86, CD83, and CCR7 regardless of initial culture duration, but 3-day DCs expressed these antigens in a more consistent and homogenous manner. Mature DCs produced much more IL12 and less IL10 in response to restimulation with CD40L when the initial culture duration was 3 days, rather than 7 days. Analogous changes were observed in mature DCs prepared using lower concentrations of GM-CSF/IL4 or when the alternative maturation cocktails poly-(I:C)/IFNγ and CD40L/IFNγ were used.
Conclusion
Extended initial culture of DCs in GM-CSF/IL4 does not affect yield or viability of subsequently matured DCs, but can adversely affect their ability to homogeneously express high levels of costimulatory molecules and to produce IL12.
Keywords: Dendritic Cells, Immunotherapy, Tumor Vaccine, LPS, IL-12
Introduction
Dendritic cells (DCs) play a crucial role in transport and processing of antigens for presentation to T cells in vivo 1. Consequently, there has been considerable interest in using them as therapeutic agents to promote tumor and virus-specific T cell responses in patients with cancer or infection 2-5. In some cases, DCs for such clinical studies are retrieved directly from blood or generated from circulating CD34 cells 6,7. More commonly they are generated from peripheral blood monocytes by ex vivo culture with one or more differentiation agents to produce immature DCs, followed by incubation with one or more and maturation agents to produce mature DCs. Antigen-loaded DCs prepared in this manner have been demonstrated to induce T cell immune responses against tumor epitopes 8,9, but clinical responses in patients with cancer have been more elusive 3,4. Consequently, many current efforts focus on increasing the magnitude and impact of these immune responses through further refinements in the methods used to prepare, antigen load, and administer clinical grade DCs.
While the basic approach to DC preparation from monocytes is well established, the specific methods used for cell purification, culture, and maturation vary widely. Monocytes may be isolated from blood by adherence 8, elutriation 10, or positive selection using immunomagnetic beads 11. DC differentiation most commonly is induced using GM-CSF and IL4 12,13 but the doses of each reagent, the culture conditions (flask or closed plastic bag), the composition of the culture medium, the cocktail of reagents used to induce maturation, and the methods used to antigen-load DCs all vary substantially 5. Although any of these factors potentially could affect the quality of the final DC product, the sheer number of variables in a given manufacturing process makes direct comparisons of alternative methods difficult.
In addition to variability in reagents, investigators preparing clinical grade DCs also employ a variety of different culture schedules. In particular, monocytes have been cultured in GM-CSF/IL4 to induce a DC phenotype for as short as 2 days 14-16 or as long as 7 days 8,9. In the current study, we used a systematic approach to examine the effect of this initial monocyte culture on viability, yield, phenotype and cytokine producing activity of the final product. While DCs expressing a “mature” CD80+, CD83+, CD86+ phenotype by flow cytometry can be prepared using a wide variety of time schedules, we found that the expression of important surface molecules and production of cytokines may be adversely affected by extended initial DC incubation with GM-CSF/IL4. Our results suggest the need for caution in prolonging ex vivo incubation during the preparation of clinical grade DCs.
Materials and Methods
Human subjects
Normal healthy donors gave informed consent to leukapheresis procedures done as specified in clinical protocols approved by the Institutional Review Board of the Clinical Center of the National Institutes of Health.
Leukapheresis and counterflow centrifugal elutriation
Leukapheresis and elutriation procedures were done as previously described 17 using the Fenwal CS3000 Plus (Baxter Healthcare, Deerfield, IL), which processed a 5.0-7.5 L blood volume to collect a total of 5–10 ×109 mononuclear cells (MNC) 17,18. These were suspended in HBSS without magnesium, calcium, and phenol red (Cambrex), and processed by counterflow centrifugal elutriation (Elutra Cell Separation System, Gambro BCT, Lakewood, CO). Monocyte-rich fractions were collected and pooled to yield a product that contained 70-90% CD14+ monocytes. These cells were either placed directly into culture or cryopreserved by a controlled-rate freezing procedure using medium containing Plasmalyte A (Baxter Healthcare, Deerfield, IL) with 6% pentastarch (B. Braun, Irvine, CA), 4% human serum albumin (Buminate, Baxter Healthcare, Glendale, CA), 5% DMSO (Cryoserv, Research Industries, Salt Lake City, UT), 15 U/ml heparin (Fujisawa USA, Deerfield, IL), and 10 μg/ml DNAase (Dornase, Genentech, Emeryville, CA). Lymphocyte-enriched fractions were cryopreserved by the same method for subsequent use.
DC culture medium
The standard medium for DC culture was RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated autologous plasma or 10% human AB serum (both prepared in our facility) and 10 μg/ml of gentamycin (Cambrex).
Dendritic cell preparation and maturation
Fresh or newly thawed donor monocytes were suspended at 1.5 million cells/ml in DC culture medium (described above) containing 2000 IU/ml of both human IL-4 (Schering-Plough, Kenilworth, NJ) and GM-CSF (Amgen, Thousand Oaks, CA). Cells were cultured in 25 cm2 polystyrene tissue culture flasks (Becton Dickinson, Franklin Lakes, NJ) at 37°C in a 5% CO2 incubator. Unless otherwise indicated an additional 2000 IU/ml of GM-CSF and 2000 U/ml of IL4 were added on day 2, and, for longer incubations, also on day 5.
To induce DC maturation, at varying times from day 2 to 7, a maturation “cocktail” was added. In most cases a mixture of LPS (100 ng/ml, Calbiochem, San Diego, CA) and IFNγ (1000 IU/ml, Peprotech, Rocky Hill, NJ) was used for this purpose 19, but in selected studies, alternative combinations of CD40L (Amgen, Thousand Oaks, CA) and IFNγ (1000 IU/ml) or poly(I:C) (20 μg/ml, Sigma, St. Louis, MO) and IFNγ (1000 IU/ml) were used 17,20,21.
DC Harvest
Eight to 42 h after addition of maturation cocktail, unbound and loosely adherent DCs were collected, and a dissociation buffer (Invitrogen) was added to each flask for 15 minutes at 4°C to retrieve more adherent cells. Cells loosened by this procedure were dislodged by additional pipetting, collected, and pooled with the previous collection. Harvested cells were pelleted, washed twice with HBSS, and resuspended in the DC culture medium.
The total cell concentration in each specimen was determined by manual counting using a hemocytometer. Viability was determined visually based on trypan blue dye exclusion. Viable DC yield was calculated by dividing the number of viable DCs recovered by the total number of cells placed into the initial culture, and multiplying by 100%.
Flow cytometric analysis
Samples of 100 μL containing 100,000 to 200,000 cells were incubated for 10 minutes with 100 μL of an ice-cold 1:5 dilution of reconstituted IVIG (Sandoglobulin, Sandoz, East Hanover, NJ) in HBSS. Cells were stained with phycoerythrin-labeled antibodies against CD80, CD83, CD86, and Class I MHC products (W6/32) (Becton Dickinson, San Jose, CA), CCR7 (R&D Systems, Minneapolis, MN), or with PE-labeled isotype-matched control antibodies. To exclude nonviable cells during flow cytometry, 7AAD (Pharmingen, San Diego, CA) was added to each sample 10 minutes before acquisition. Flow cytometry acquisition was performed using a FACScan with CellQuest software (Becton Dickinson, Mountain View, CA). Data analysis was performed using FlowJo software (Tree Star, Ashland, OR).
Dendritic cell cytokine generation
IL-12 and IL-10 release during maturation was measured in the culture medium collected at the time of DC harvest. To measure DC cytokine production after restimulation, harvested washed DCs (100,000/well) were added in a total volume of 500 μl to a 48-well plate containing 50,000 adherent mouse LTK fibroblasts transfected with CD40L (CD40L-LTK). This cell line was kindly provided by C.van Kooten, Ph.D. (Leiden, the Netherlands). The supernatant medium was collected 24 hours later and assayed for human IL-12 and IL-10 expression using commercial ELISA kits (eBioscience, San Diego, CA). In all cases, DCs added to wells in the absence of CD40L did not secrete measurable IL-10 or IL-12.
Statistical methods
The mean and SD were used to express group results for cell viability and % recovery and the Student's t-test was used to test the significance of group differences. Cytokine production values were not normally distributed; hence the significance of differences were assessed using the paired Wilcoxon Signed-Rank Test.
Results
Design for studying the effect of immature DC culture duration on characteristics of mature DCs
Matched flasks of monocytes were cultured for 3 versus 7 days to generate immature DCs, and then matured by an additional 18 h exposure to 100 ng/ml of LPS and 1000 IU/ml of IFNγ. Cell yield, viability, surface phenotype, and cytokine secretion were then measured in both groups.
Effect of immature DC culture duration on viability and yield of mature DCs
There were no significant differences in cell viability or % yield of mature DCs generated after 3 or 7 days of immature DC culture in GM-CSF/IL4. In an analysis of 7 experiments, trypan blue viability at harvest (85% for 3-day versus 83% for 7-day cultured cells) and yield (27% versus 23% of initial cultured monocyte number) were similar for both groups (Table 1).
Table 1.
Effect of Immature DC Culture Duration on Viability and % Yield of Mature DCs
| Immature DC Culture Duration (days) | Viability of Mature DCs | % Yield of Mature DCs |
|---|---|---|
| 3 | 85 ± 10 % | 27.4 ± 4.6 % |
| 7 | 83 ± 13 % | 24.2 ± 5.6 % |
Monocytes were incubated with GM-CSF/IL4 for 3 or 7 days to generate immature DCs, which were then matured by incubation for 18 h with LPS/IFNƔ. These results represent the mean ± SD for 7 paired experiments.
Effect of culture duration on DC phenotype
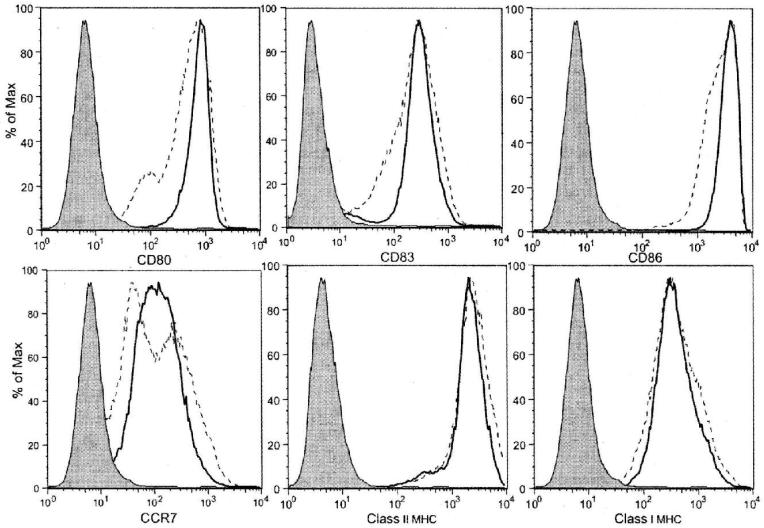
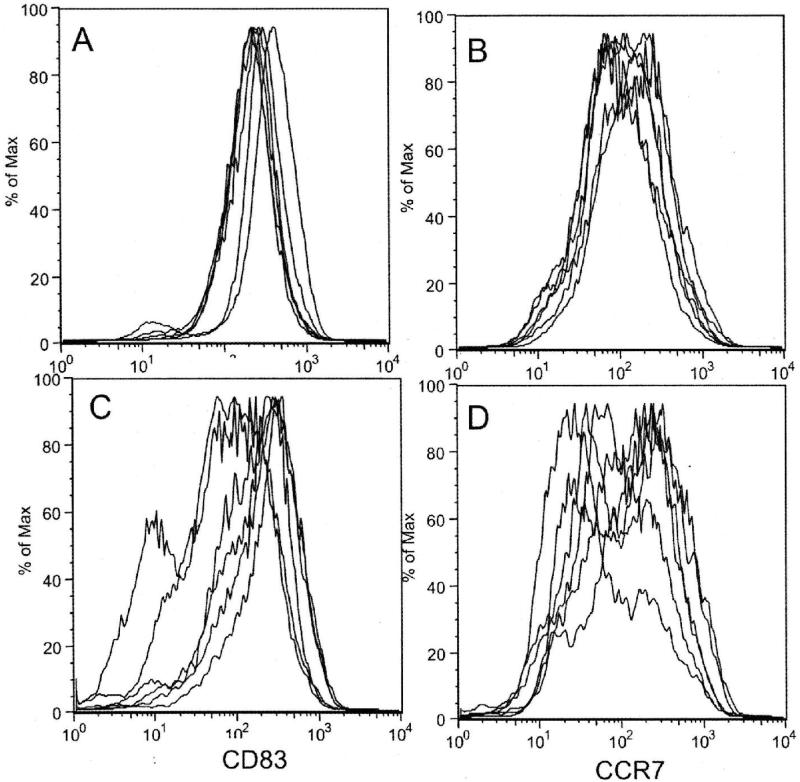
Comparison of flow cytometric phenotype showed that immature DCs cultured for 3 days usually demonstrated slightly less forward scatter than those cultured for 7 days, presumably an indication of smaller cell size, but there were no consistent differences in the level of CD80, CD83, CD86, or CCR7 expression (data not shown). Eighteen hours of maturation with LPS/IFNγ markedly enhanced the expression of CD80, CD83, CD86 and CCR7, and class II MHC in both the 3- and 7-day cultured DCs (Figure 1). Comparison of fluorescence intensity from 6 matched experiments showed that, after maturation, 3-day cultured DCs expressed slightly higher median channel fluorescence, particularly for CD83 and CCR7, than 7-day cultured DCs, but these differences did not achieve statistical significance. Direct inspection of overlay plots of CD83, and CCR7 expression, however, did reveal noteworthy differences in pattern. CD83 and CCR7 expression plots for 3-day cultured DCs after maturation were consistent across experiments, with a single dominant peak of expression. The corresponding peaks for 7-day cultured DCs after maturation were much more variable, in some cases displaying a distinct shoulder or even a second peak of cells expressing lower antigen levels (Figure 2). Similar, but less pronounced, patterns were noted for CD80, CD86, and Class II MHC product expression.
Figure 1.
Cell surface marker expression on mature DCs generated from monocytes after 3- (bold solid line) or 7-day (dashed line) culture in GM-CSF/IL4 followed by 18 hours of exposure to LPS/IFN-γ. Similar findings were obtained in each of three independent phenotypic studies. Shaded curves represent the isotype control.
Figure 2.
Overlay flow cytometric plots from 6 independent experiments comparing CD83 (panels A and C) and CCR7 (panels B and D) expression by LPS/IFNγ-treated DCs prepared after 3 days (Panel A and B) or 7 days (Panel C and D) of culture in GM-CSF and IL4. Mature DCs generated after 3 days of initial culture typically demonstrated a single dominant peak of antigen expression, whereas those generated after 7 days of initial culture often showed shoulders or second peaks, indicating populations with lower levels of antigen expression.
Time course of CD83 and CCR7 upregulation during DC maturation with LPS/IFNγ
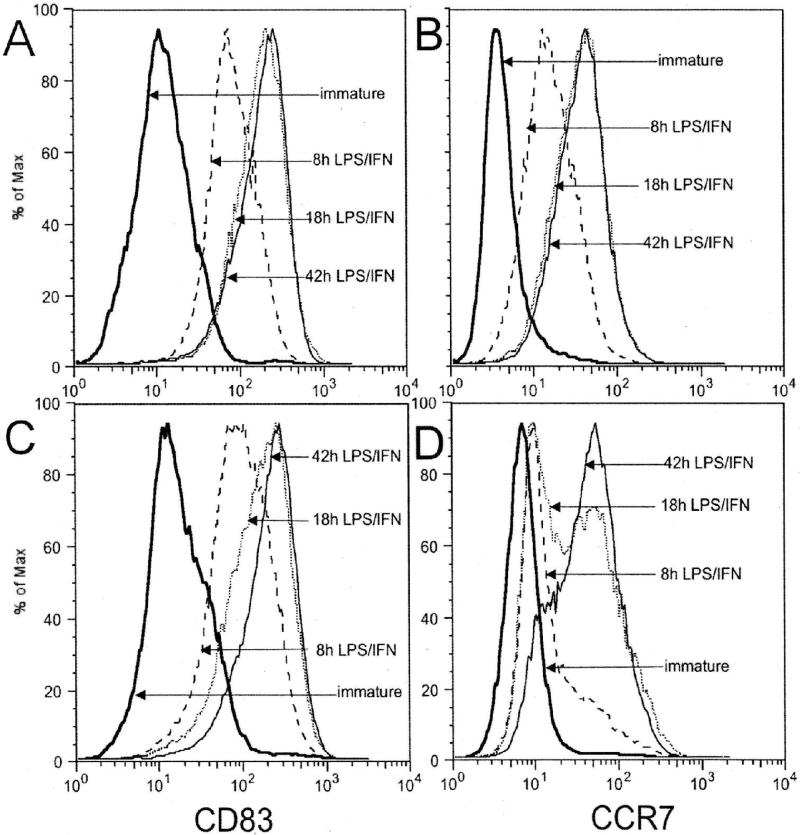
To compare the time course of costimulatory molecule upregulation in response to LPS/IFNγ, we measured CD83 and CCR7 expression in matched 3-d and 7d-GM-CSF/IL4 treated cells after 8,18, and 42 h of LPS/IFNγ exposure. Both 3 day- and 7-day cultured DCs showed substantially increased expression of the costimulatory molecules CD83 and CCR7 within 8 h of incubation in LPS/IFNγ, but the 3-day DCs reached peak levels more quickly. Whereas 3-day DCs achieved peak levels of CD83 and CCR7 expression within 18 h, 7-day cells required 42 h to reach comparable levels of expression (Figure 3).
Figure 3.
Time course of CD83 (Panel A and C) and CCR7 (Panel B and D) expression by DCs after treatment with LPS/IFN-γ. Immature DCs generated from monocytes by incubation with GM-CSF/IL4 for 3- (Panels A and B) or 7-days (Panel C and D), were phenotyped before maturation (solid double line), and 8 (dashed), 18 (dotted), or 42 (solid) hours after addition of LPS/IFN-γ. The 3-day DCs achieved peak levels of CD83 and CCR7 expression within 18 h, but 7-day treated cells required 42 h to reach comparable levels.
Effect of DC culture duration on IL12 and IL10 production
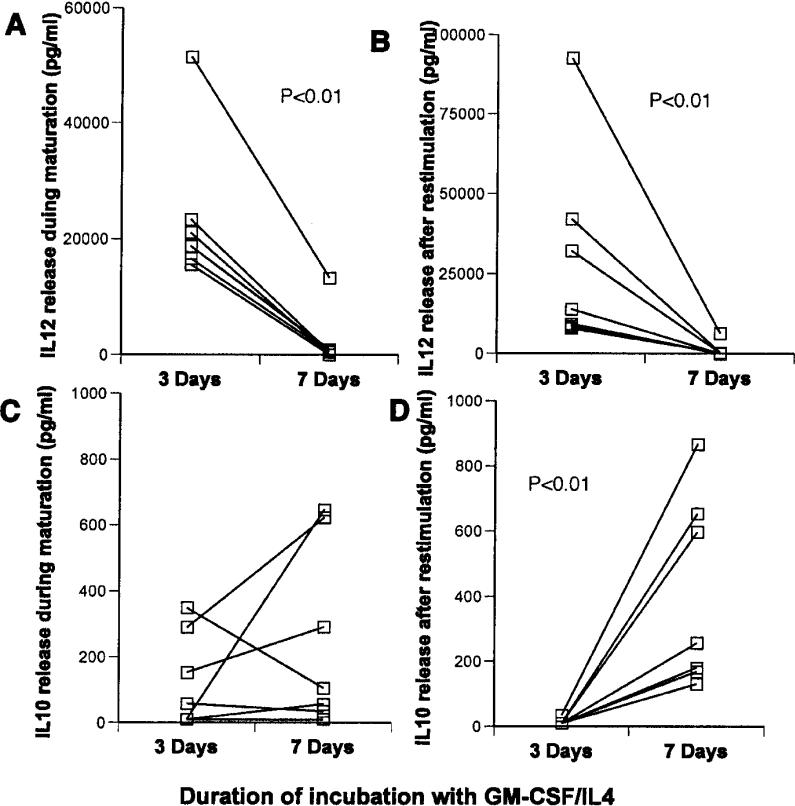
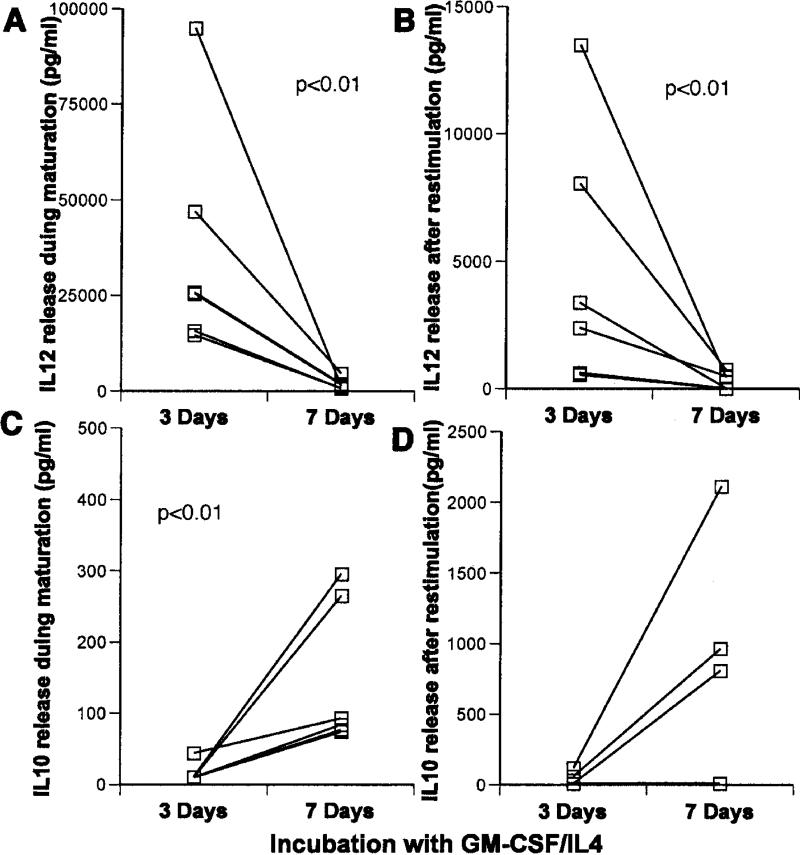
Mature DCs prepared after 3-day culture in GM-CSF/IL4 made much more IL12 both during maturation with LPS/IFNγ (median value of 18,800 versus 490 pg/ml with a p<0.01 Figure 4A), and after restimulation with CD40L (13,000 versus 280 pg/ml with a p<0.01-Figure 4B) than 7-day-cultured cells. Although IL10 production was not significantly different between groups during maturation, the 3-day DCs also made significantly less IL10 (23 versus 760 pg/ml respectively p<0.01) after restimulation than the older DCs (Figure 4C and D).
Figure 4.
Comparison of cytokine production by 3-day and 7-day, GM-CSF/IL4-treated DCs in response to LPS/IFN-γ. Immature DCs in each case were exposed to LPS/IFNγ for 18 h. IL12 (A and B) and IL10 (C and D), were measured in culture medium collected immediately after maturation (A and C) or after harvest and restimulation with CD40L (B and D). Statistically significant differences (p<0.01) were noted in Panels A, B, and D.
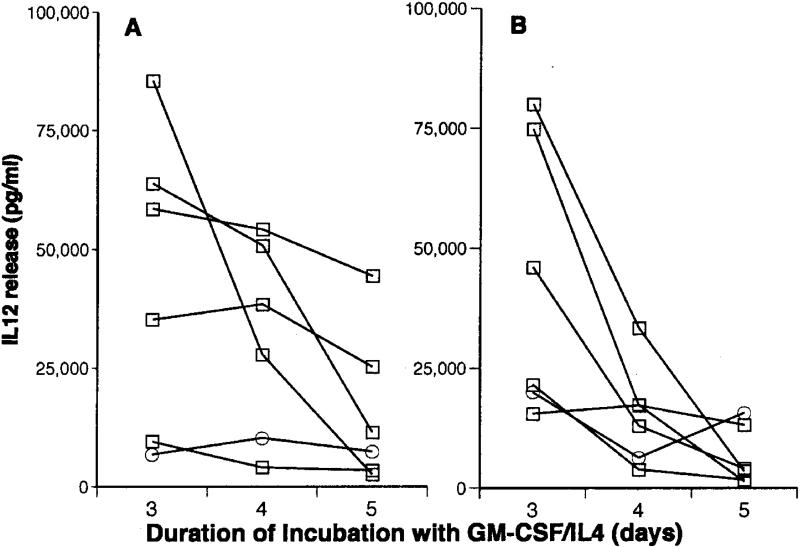
To determine with greater resolution how soon after day 3 cells incubated with GM-CSF/IL4 begin to downregulate IL12 production towards day 7 levels, we measured cytokine levels in matched cells incubated with GM-CSF/IL4 for 3-5 days before maturation ((Figure 5). In 4 of 6 experiments IL12 production after restimulation diminished >90% between day 3 and 5. The rate of change was less dramatic in the other 2 experiments, but even in these instances production was reduced 15-25% during this time period (Figure 5).
Figure 5.
Effect of variations in the duration of initial DC culture in GM-CSF/IL4 on IL12 production. After incubation with GM-CSF/IL4 for 3, 4, or 5 days, immature DCs were matured with LPS/IFN-γ for 18 h. IL12 release was then measured in culture medium collected immediately after maturation (A), or after restimulation with CD40L (B).
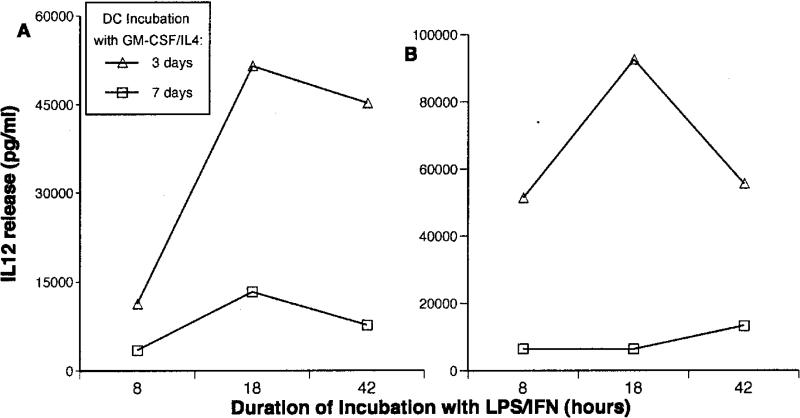
To be certain the differences in IL12 production we observed after maturation were not attributable simply to a slower rate of IL12 release by older cells, cytokine production by 3- and 7-day DCs was compared after either 8, 18, or 42 h of incubation with LPS/IFNγ. In fact, the same pattern of increased IL12 production by 3-day DCs after maturation was observed at all 3 time points (Figure 6A). More important, a similar pattern was also observed when the matured cells were washed and restimulated with CD40L (Figure 6B). Thus the differences in production at 18 h reflect a difference in IL12 producing capacity and not a change in the kinetics of its release.
Figure 6.
Time course of IL12 production in 3- and 7-day cultured DCs after maturation with LPS/IFNγ. Immature DCs prepared by 3-day or 7-day incubation with GM-CSF/IL4 were matured with LPS/IFN-γ for either 8, 18, or 42 h. IL12 release during maturation (panel A) and after restimulation with CD40L (panel B) were measured. The markedly higher IL12 produced by 3-day DCs (noted in Figure 4) was apparent at 8 h and persisted even after 42 h of stimulation.
Effect of GM-CSF and IL4 Concentration on 7-Day DCs
Because DC preparation protocols often use lower concentrations of GM-CSF and/or IL4 than those routinely used in our facility 22-25, IL12 production by DCs generated after 7-day culture with half or one quarter our usual concentrations of GM-CSF/IL4 was also assessed. In the latter instance we also used half our usual starting cell concentration, to exclude any possible role of cell crowding or medium depletion in the downregulation of IL12 production. Using either of these modifications in culture conditions, 7-day DCs produced substantially less IL12 than matched DCs incubated for 3 days with our conventional dose of GM-CSF/IL4 (Table 2).
Table 2.
Effect of variations in culture conditions on DC yield and IL12 production after maturation with LPS/IFN-Ɣ
| Culture duration with GM-CSF/IL4 | Initial cell concentration | GM-CSF/IL4 dose | Refeeding times | Cell yield | IL12 release (pg/ml) | |
|---|---|---|---|---|---|---|
| (days) | million/ml | IU/ml | (days) | % | during mat. | restimulated |
| 3 | 1.5 | 2000/2000 | 2 | 36 | 12500 | 5060 |
| 7 | 1.5 | 2000/2000 | 2, 5 | 17 | 210 | 40 |
| 7 | 1.5 | 2000/2000 | 2, 4, 6 | 19 | 1030 | 120 |
| 7 | 1.5 | 1000/1000 | 2, 5 | 12 | 610 | 80 |
| 7 | 0.75 | 500/500 | 2, 5 | 9 | 2160 | 250 |
Effect of maturation with CD40L/IFNγ and Poly(I:C)/IFNγ on IL12 and IL10 production of 3- and 7-day DCs
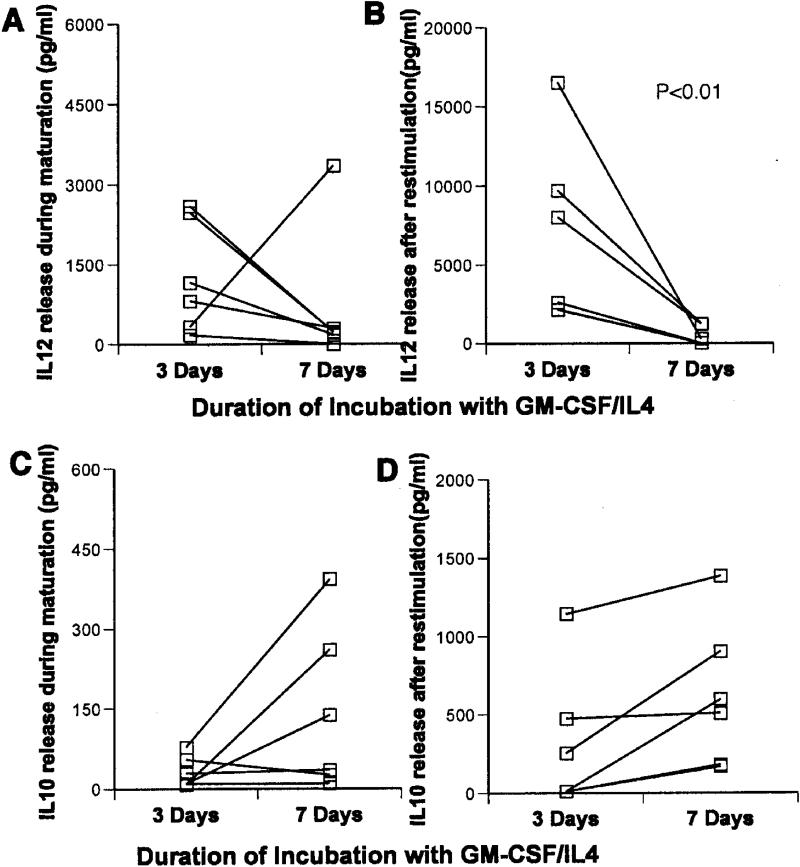
To assess how variations in the duration of GM-CSF/IL4 incubation affects DCs matured with other maturation cocktails known to highly stimulate IL12 production 17,20,21, we compared phenotype and cytokine production in DCs matured using CD40L/IFNγ or poly-(I:C)/IFNγ after 3- or 7-days of GM-CSF/IL4 treatment. A similar pattern of increased heterogeneity in maturation marker expression (analogous to figure 4) was observed using either maturation cocktail. Younger DCs matured with CD40L/IFNγ again produced significantly more IL12 during maturation (median 26,600 vs. 2880 pg/ml) and during restimulation (1430 vs 230 pg.ml) than older cells. On the other hand, they made significantly less IL10 during maturation (90 vs 410 pg/ml- Figure 7). Consistent differences in cytokine production between younger and older DCs were not observed during maturation with poly-(I:C)/IFNγ, but 3-day treated DCs again made significantly more IL12 than older cells after restimulation with CD40L (5300 vs 130 pg/ml - Figure 8).
Figure 7.
IL12 and IL10 production by 3-day and 7-day GM-CSF/IL4 cultured DCs in response to CD40L/IFN-γ. DCs in each case were matured by 18 h exposure to CD40L/IFN-γ. IL12 (A and B) and IL10 (C and D) were measured in culture medium collected immediately after maturation (A and C) or after restimulation with CD40L (B and D). Statistically significant differences (p<0.01) were noted as indicated in panels A, B, and C.
Figure 8.
IL12 and IL10 production by 3-day and 7-day GM-CSF/IL4 cultured monocytes in response to Poly(I:C)/IFN-γ. DCs in each case were matured by 18 h exposure to the maturation cocktail. IL12 (A and B) and IL10 (C and D) were measured in culture medium collected immediately after maturation (A and C) or after harvest and 18 h restimulation with CD40L (B and D). Statistically significant reductions in IL12 production after restimulation were noted.
Discussion
These studies demonstrate that surface expression of the costimulatory molecules CD83 and CCR7 and production of the cytokines IL12 and IL10 by mature DCs derived from peripheral blood monocytes can be substantially affected by the duration of initial culture in GM-CSF/IL4.
The use of a 6- to 7-day incubation with GM-CSF and IL4 to promote differentiation of monocytes into DCs was first described in 1994 12,13, and this approach is still commonly used to generate DCs for clinical trials. While such prolonged incubations may be required for the generation of morphologically “classic” large DCs, more recent studies have established that monocytes can express DC surface markers, produce abundant IL12 in response to stimulation, and effectively stimulate antigen-specific CD8 cells after as short a duration as 1-2 days in culture with GM-CSF/IL4 14,26-29. These abbreviated protocols for DC preparation are an attractive alternative, particularly for cell processing facilities interested in increasing the efficiency and convenience of DC generation, while decreasing the period for which the cellular product is at risk for microbial contamination. Still, in the absence of controlled clinical trials, the relative merits of DCs produced using short versus “standard” 6-7 day GM-CSF/IL4 incubations remain unclear.
Although a variety of prior studies have examined costimulatory molecule, cytokine receptor, and cytokine production by young 19,20,30 and older DCs 17,19,20,31,32, variations in the conditions used to generate, stimulate, and monitor DC responses make quantitative comparisons between studies difficult. By specifically comparing 3-day and 7-day DCs from the same donor generated under otherwise identical conditions, the current studies clearly demonstrate that extending DC incubation with GM-CSF/IL4 beyond 3-4 days before maturation can significantly affect their phenotype and function. After maturation, 3-day DCs expressed CD83 and CCR7 more rapidly, uniformly, and consistently, produced significantly more IL12 during and after maturation and less IL10 after maturation, compared to 7-day DCs.
These changes may have significant impact on DC function as APCs. CCR7 33 and CD83 34,35 each play an important role in DC-T cell interaction. IL12 is critical both in promoting type 1 T cell polarization and in enhancing the functional avidity of T cell responses 14,36, and IL10 is a potent suppressor of T cell function. Taken together, a pattern of reduced CCR7, CD83 and IL12 expression in association with an increase in IL10 production might be predicted to reduce the ability of older DCs to stimulate type 1 T cell responses. Clinical comparisons are not available, but recent in vitro studies comparing T cell responses induced using DCs prepared much like ours, report stronger responses using DCs with shorter culture duration 37. The changes in costimulatory molecule and cytokine expression we observe may help explain this finding.
This study focused most thoroughly on a maturation cocktail of LPS and IFNγ However, to examine the relevance of our finding under different culture conditions, we also compared 3-day and 7-day DCs matured using CD40L/IFNγ and poly(I:C)/IFNγ, two alternative maturation cocktails used to generate mature DCs with robust IL12 production 17,20,21. Using either maturation cocktail, we observed similar culture duration-related changes in CD83 and CCR7 expression in the mature DC product. Perhaps more importantly, IL12 production, particularly after restimulation post maturation, was again significantly reduced in both instances. Like LPS/IFNγ, CD40L/IFNγ, but not Poly(I:C)/IFNγ, a cocktail which does not stimulate extensive IL-10 production 32, also increased IL10 release from older DCs. Although most of our studies were performed using 2000 units/ml of GM-CSF and IL4, we noted similar changes in DCs generated using lower concentrations of GM-CSF and IL4 comparable to the concentrations more often used in other centers. Although our studies cannot address the myriad of other possible culture conditions, the persistent pattern does suggest our observations may have more general relevance. The findings could simply reflect cell damage and/or senescence during extended culture. Alternatively, they may be attributable to a more physiologic change in cell phenotype related to cell age per se, or to the presence of GM-CSF, IL4 and/or other components of our culture medium. Additional studies comparing mRNA and/or protein expression may be helpful both in providing a broader picture of the changes in phenotype and in understanding the etiology of these changes.
In light of the complexity of antigen presentation in vivo, no single set of surrogate markers can be expected to fully predict the fitness of DCs to induce T cell responses against tumor or microbial pathogens in vivo. The observed reductions in expression of bioactive molecules associated with prolonged in vitro incubation merit concern, both for their direct impact on cell function and also as a potential indicator of more generalized reductions in the cellular “reserves” of older cells. DCs generated using other combinations of culture conditions, differentiation agents, and maturation agents may be affected differently, but unless specific benefits attributable to prolonged ex vivo incubation can be identified, it would seem most prudent to avoid prolonged incubation of DCs with GM-CSF/IL4 and other differentiation cocktails.
Acknowledgments
The authors acknowledge a tremendous debt to Charles Carter (deceased) who played an invaluable role in conceiving, initiating, and facilitating these experiments. We also appreciate the advice and assistance provided by Vicki Fellowes.
This work was supported by the Departments of Laboratory Medicine and Transfusion Medicine of the NIH Clinical Center.
References
- 1.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 2.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 5.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, Taidi B, Rajapaksa R, Caspar CB, Okada CY, van Beckhoven A, Liles TM, Engleman EG, Levy R. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 7.Fay JW, Palucka AK, Paczesny S, Dhodapkar M, Johnston DA, Burkeholder S, Ueno H, Banchereau J. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother. 2006;55:1209–1218. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 9.Dhodapkar MV, Steinman RM, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe SM, Dunbar PR, Cerundolo V, Nixon DF, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger TG, Strasser E, Smith R, Carste C, Schuler-Thurner B, Kaempgen E, Schuler G. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods. 2005;298:61–72. doi: 10.1016/j.jim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Babatz J, Rollig C, Oelschlagel U, Zhao S, Ehninger G, Schmitz M, Bornhauser M. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: a phase I study. J Hematother Stem Cell Res. 2003;12:515–523. doi: 10.1089/152581603322448222. [DOI] [PubMed] [Google Scholar]
- 12.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor ? J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 15.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 16.Koski GK, Lyakh LA, Rice NR. Rapid lipopolysaccharide-induced differentiation of CD14(+) monocytes into CD83(+) dendritic cells is modulated under serum-free conditions by exogenously added IFN-gamma and endogenously produced IL-10. Eur J Immunol. 2001;31:3773–3781. doi: 10.1002/1521-4141(200112)31:12<3773::aid-immu3773>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Kurlander RJ, Tawab A, Fan Y, Carter CS, Read EJ. A functional comparison of mature human dendritic cells prepared in fluorinated ethylene-propylene bags or polystyrene flasks. Transfusion. 2006;46:1494–1504. doi: 10.1111/j.1537-2995.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong EC, Maher VE, Hines K, Lee J, Carter CS, Goletz T, Kopp W, Mackall CL, Berzofsky J, Read EJ. Development of a clinical-scale method for generation of dendritic cells from PBMC for use in cancer immunotherapy. Cytotherapy. 2001;3:19–29. doi: 10.1080/146532401753156377. [DOI] [PubMed] [Google Scholar]
- 19.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 20.Mosca PJ, Hobeika AC, Clay TM, Nair SK, Thomas EK, Morse MA, Lyerly HK. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–3504. [PubMed] [Google Scholar]
- 21.Rouas R, Lewalle P, El Ouriaghli F, Nowak B, Duvillier H, Martiat P. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int Immunol. 2004;16:767–773. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 22.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michiels A, Tuyaerts S, Bonehill A, Corthals J, Breckpot K, Heirman C, Van Meirvenne S, Dullaers M, Allard S, Brasseur F, van der Bruggen P, Thielemans K. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 2005;12:772–782. doi: 10.1038/sj.gt.3302471. [DOI] [PubMed] [Google Scholar]
- 25.Su Z, Vieweg J, Weizer AZ, Dahm P, Yancey D, Turaga V, Higgins J, Boczkowski D, Gilboa E, Dannull J. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–5048. [PubMed] [Google Scholar]
- 26.Dauer M, Schad K, Herten J, Junkmann J, Bauer C, Kiefl R, Endres S, Eigler A. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J Immunol Methods. 2005;302:145–155. doi: 10.1016/j.jim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Dauer M, Herten J, Bauer C, Renner F, Schad K, Schnurr M, Endres S, Eigler A. Chemosensitization of pancreatic carcinoma cells to enhance T cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic cells. J Immunother. 2005;28:332–342. doi: 10.1097/01.cji.0000164038.41104.f5. [DOI] [PubMed] [Google Scholar]
- 28.Alldawi L, Takahashi M, Narita M, Ayres F, Tsukada N, Osman Y, Furukawa T, Aizawa Y. Effect of prostaglandin E2, lipopolysaccharide, IFN-gamma and cytokines on the generation and function of fast-DC. Cytotherapy. 2005;7:195–202. doi: 10.1080/14653240510018127. [DOI] [PubMed] [Google Scholar]
- 29.Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-g as potent immune enhancers. J Immunother. 2006;29:67–77. doi: 10.1097/01.cji.0000183093.77687.46. [DOI] [PubMed] [Google Scholar]
- 30.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: Impact on priming of TH1,TH2 and nonpolarized T cells. Nature Immunology. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 31.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114:204–212. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 33.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano N, Butler MO, Xia Z, Ansen S, von Bergwelt-Baildon MS, Neuberg D, Freeman GJ, Nadler LM. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528–1536. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol. 2007;178:5454–5464. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 37.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]