Abstract
Homeostatic adjustment of neuronal firing rates is considered a vital mechanism to keep neurons operating in their optimal range despite dynamically changing input. Two studies in Neuronprovide evidence for firing rate homeostasis in the neocortex of freely behaving rodents.
The nervous system has the remarkable ability to undergo adaptive changes in response to sensory experience during development and learning. Experience-dependent circuit refinements have been studied extensively in cortex, and are thought to rely heavily on synapse-specific, associative “Hebbian” plasticity mechanisms such as synaptic strengthening through long-term potentiation (LTP) and synaptic weakening through long-term depression (LTD). It has long been recognized that these Hebbian plasticity mechanisms, when left unchecked, could lead to saturation of synaptic strengthsandthus threaten the stability of neural networks. To solve this problem, non-Hebbian,“homeostatic”forms of plasticityhave been proposed to act in concert with Hebbian mechanisms,globally regulatingneuronal activity levels toward an optimal set point, and thus providingstability despite ongoing fluctuations in synaptic strength. In this issue of Neuron, Hengen et al. (2013) and Keck et al. (2013) provide the first glimpses that homeostatic mechanisms act to regulate firing rates within neocortical circuits in vivo.
Research over the past few decades has solidly established that cortical neurons possess mechanisms that maintain firing around a homeostatic stable pointin vitro (Turrigiano, 2011). One classic example of homeostatic regulation demonstrated that cultured neocortical neurons exposed to pharmacological activity blockade for prolonged periodsexhibit increased spontaneous firing rates when network activity is resumed. Reciprocally,neurons compensate after network activity is elevatedfor many hours, restoring firing rates to baseline. Notably, these activity manipulations induced bidirectional compensatory changes in the unit strength of synaptic inputs, globally increasing or decreasingthe strength of all synapses in a multiplicative manner referred to as “synaptic scaling”, thus allowing the preservation of information stored in the distribution of synaptic weights (Turrigiano et al., 1998).
More recently, focus has turned to whether and how homeostatic plasticityoperates in intact neocortex in vivo.Experiments to address these questions have monitored activity changes in response to sensory manipulations, using ex vivo electrophysiological recordings in acute slices or in vivo calcium or intrinsic signal imaging in anesthetized animals. One classic model of experience-dependent cortical plasticity has been the postnatal development of visual cortex (Levelt and Hubener, 2012).Original studies, primarily in cats, showed that depriving one eye of visual input (monocular deprivation, MD) during a critical period of development produces a loss in visual cortical responsiveness to inputs through the deprived eye, followed by a temporally delayed increase in responsiveness to inputs through the non-deprived eye. While the initial component of these shifts in ocular dominance have been shown to rely on LTD of excitatory synapses (Smith et al., 2009),several studies support that the second phase of the cortical response, namely the increase in responsiveness to the non-deprived eye, could be regulated by homeostatic forms of plasticity. Indeed, it has been shown that visual deprivation leads to global multiplicativescaling of mEPSC amplitudesin L2/3 and L4in visual cortical slicesex vivo (Desai et al., 2002; Goel and Lee, 2007). In addition, two-photon calcium imaging of visually evoked responses in visual cortex of anesthetized animals showeda delayed, presumably homeostatic, response potentiation following MD (Mrsic-Flogel et al., 2007). Furthermore, the increase of responsiveness following MD is dependent on TNFα, a molecule shown to be necessary for synaptic scaling in vitro (Kaneko et al., 2008).Yet the central hypothesis that homeostatic mechanisms act in the neocortex in vivo to regulate firing rates around a critical set point had never been tested.In this issue of Neuron,Hengen et al. (2013) and Keck et al. (2013) describe these long-awaited experiments, and in doing so provide several new insights into how cortical activity levels are regulated in freely behaving mice in response to sensory deprivation.
Hengen et al. set out to probe firing rate homeostasis in the neocortex using chronic multielectrode recordings in monocular visual cortex (mV1) to record neural activity prior to and following MD induced by lid suture in juvenile rats. Multiunit recordings of cells across all cortical layers in freely behaving animals were separated into putative parvalbumin (PV)-positive, fast-spiking inhibitory neurons (pFS) and regular spiking units (RSU), or putative excitatory pyramidal neurons. Hengen et al. observed an initial decrease in average ensemble firing rate of RSUs after two days of MD. Despite ongoing deprivation, firing ratesrestored to baseline within 24 hrs(Figure 1A), supporting homeostatic regulation. Remarkably, this homeostatic regulation of firing rates was observed across sleep and wake behavioral states. Interestingly, inhibitory pFS cells also underwent biphasic modulation following MD, although with a more rapid timescale. After one day of deprivation, pFS cells showed a significant drop in firing rate, followed by a rapid return to baseline by day two (Figure 1A). Thus, both excitatory and inhibitory neocortical neurons show homeostatic recovery of baseline firing rates following monocular deprivation.
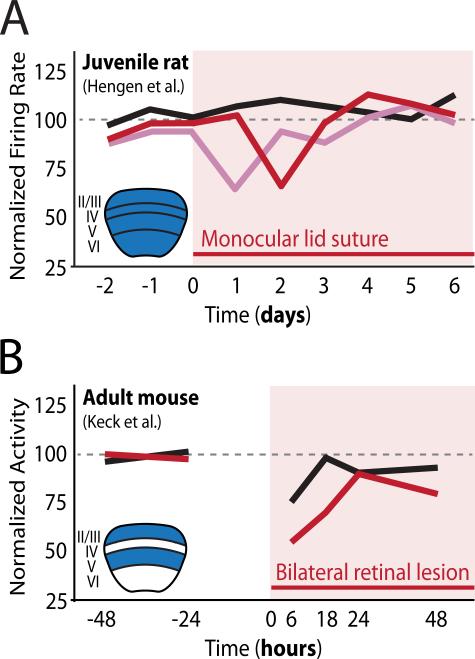
Figure 1. Schematic representation of evidence for firing rate homeostasis in vivo.
A. Hengen et al. used chronic multielectrode recordings fromall layers of the visual cortex offreely behaving juvenile rats to show that firing rates of inhibitory neurons (pink line) and putative pyramidal neurons (red line),which decreased following monocular lid suture, returned to baseline 24 hrs later despite continued visual deprivation. Controls (black line) were unchanged.
B. Keck et al. used calcium imaging of GCaMP signals from neurons in L2/3 and L5 ofthe visual cortex ofawake adult mice to show that overall activity levels, which decreased withinsix hrs of bilateral retinal lesions (red line), returned to mock-lesioned control levels (black line) within 24 hrs, despite the irreversible loss of visual input.
It may seem surprising thatHengen and colleagues did not observe a drop in firing rate of putative excitatory neurons until the second day after monocular deprivation. The authors suggest that a drop in firing rates might be masked by a release from inhibition due to decreased firing rates of pFS cells 24 hrs after MD. Consistent with this hypothesis, Hengen et al. observed a significant anti-correlation between firing rates of inhibitory and excitatoryneurons from the same electrode, suggesting indeed that the inhibitory neurons were suppressing firing of the excitatory neurons. Notably, a recent study reported a drop in visually-evoked firing rates of PV neurons L2/3 in vivoafter one day of MD, leading to a doubling of visually-evoked monocular responses and an overall conservation of firing rate (Kuhlman et al., 2013).
Whichcellular mechanisms support the homeostatic recovery of firing rates in these putative pyramidal neurons? Hengen et al. hypothesized that the recovery of firing rates could involve homeostatic scalingof mEPSC amplitudes. To test this possibility, the authors measured mEPSC amplitudes on layer 2/3 pyramidal neurons in acute slices of mV1 following 2, 4, or 6 days of MD.They found that mEPSC amplitudes were depressed after two days of MD, rebounded to baseline by day four, and were elevated above baseline by day six. These changes matched the time course of RSU response measured across all cortical layers, and suggest that synaptic scaling could be one of the mechanisms at play to support firing rate homeostasis in the neocortex in vivo.
Keck et al.used the latest technological approachesto examineneocortical activity levels in awake, behaving animals in response to sensory deprivation. In these experiments, the authors probed changes in the activity of neocortical neuronsin adult mice following bilateral retinal lesionusing two-photon calcium imaging of GCaMP3 or GCaMP5 in L2/3 and L5 cells of mV1. Notably, imaging data were obtained as the animalsexperienced virtual environments while moving on a spherical treadmill, as recent studies have shown that locomotion affects the gain of cortical responses in primary visual cortex (Niell and Stryker, 2010). Keck and colleagues observed that activity of excitatory neurons in mV1 was rapidly decreased by 50-60% within 6 hrs of lesioning. Remarkably, despite the irreversible retinal lesions, neuronal activity levels were restored to baseline within 24 hrs post-lesion(Figure 1B), supporting homeostatic adjustment of firing ratesin the neocortex of adult mice in vivo.
Couldsynaptic scaling also support homeostatic regulation of activity levels in adult neocortex? Earlier studies usingacute slices from dark-reared adult mice found that cells of layer 2/3 retain a form of synaptic scaling into adulthood (Goel and Lee, 2007). However, Ranson et al.(2012) showed that open eye response potentiation following MD persists in adult TNFα KOs animals, suggesting that TNFα –mediated synaptic scaling is not required.To examine a role for synaptic scaling, Keck et al. measured mEPSC amplitudes from L5 pyramidal neurons in acute slices of mV1 from animals with bilateral retinal lesions or mock-lesioned controls. They found that mEPSC amplitudes were unchanged at 6 and 18 hrs post-lesion, but then increased at 24 and 48 hrs, closely matching the time course of activity rate homeostasis.Because spine size iscorrelated with synaptic strength, and changes in a predictable manner when circuits are weakened or strengthened in response to MD in vivo (Hofer et al., 2009), Keck and colleagues hypothesized that in vivo scaling of synaptic strengths should have a structural correlate in altered dendritic spine size. Remarkably, they indeedfound that spine size on L5 pyramidal neurons increased 24 hrs after the retinal lesion, and was maintained at 48 hrs, thus following the same time-course as the changes in mEPSC amplitude and cortical activity in vivo. Altogether, these data and those obtained by Hengen et al. are consistent with the hypothesis that synaptic scaling could underlie homeostatic adjustments in neocortical firing rates in vivo.
Thestudies by Hengen et al. and Keck et al. provide much anticipated evidence supporting that neuronal activity levels are homeostatically regulated in the neocortex in vivo. While both studies report an initial drop in activity levels in response to sensory deprivation,followed by a subsequent rebound, the time courses of the two observations are dramatically different. Interestingly, the rapid sensory deprivation induced drop in overall activity levels observed by Keck and colleagues recovered to control levels within 24 hrs, which is when Hengen et al. obtained their first measurements also showing baseline firing rates in excitatory neurons. Discrepancies between the two studies are evident only at 48 hrs, when Hengen et al. see significant depression of firing rates in excitatory neurons, whereas Keck and colleagues observe baseline activity levels. Most likely, differences are due to the widely diverse experimental conditions in the two studies – including deprivation protocols (monocular lid suture versus binocular retinal lesion), species (rat versus mouse), and ages (juvenile versus adult; Figure 1). Future experiments utilizing similar paradigms, while independently varying the individual parameters, will shed light on the mechanisms and origins of these differences.
Several testable predictions arise from these studies and lead to exciting new avenues of research.While these studies support that synaptic scaling could be responsible for homeostatic regulation of firing rates in the neocortex, they do not exclude that alternative mechanisms of synaptic plasticity, such as plasticity of intrinsic excitability, anti-Hebbian mechanisms or Hebbian modifications of excitatory or inhibitory synapses,are also at play. One prediction is that a homeostatic set point should operate bidirectionally; and consequently, enhanced firing rates due to sensory overstimulation should be homeostatically downregulated. Clearly, bilateral retinal lesions cannot be bidirectional; however, lid suture can be reversed, and firing rates immediately after eye re-opening are expected to beheightened above normal. Similar approaches utilizing other sensory modalities (auditory, somatosensory) that are potentially more amenable to bidirectional manipulations would provide further support and also establishhow generalizable are the findings. The hypothesis thatsynaptic scaling is responsible for homeostatic regulation of firing rates in vivoleads to the prediction that knockouts that interrupt synaptic scaling in response to monocular deprivation (Kaneko et al., 2008) would also be expected to interrupt firing rate homeostasis in vivo. Ultimately, the utilization of patterned optogenetic stimulation (Wyatt et al., 2012) of identified cells in the LGN or V1 should provide a wealth of information that will help elucidate the activity patterns, combinations of inputs and plasticity mechanisms leading to firing rate homeostasis in vivo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Nat. Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Goel A, Lee HK. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Neuron. 2013;XX:xx–xx. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka R, Stryker MP. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hubener M. NeuronXX. 2013:xx–xx. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. Nature. 2013;501:543–549. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Annu. Rev. Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson A, Cheetham CEJ, Fox K, Sengpiel F. Proc. Nat. Acad. Sci. USA. 2012;109:1311–1316. doi: 10.1073/pnas.1112204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Phil. Trans. R. Soc. B. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Annu. Rev. Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Wyatt RM, Tring E, Trachtenberg JT. Nat. Neurosci. 2012;15:949–951. doi: 10.1038/nn.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]