Abstract
Hypertensive pregnancy disorders (HPD) are important causes of maternal and fetal morbidity and mortality worldwide. In addition, a history of HPD has been associated with an increased risk for maternal cardiovascular disease later in life, possibly due to irreversible vascular and metabolic changes that persist beyond the affected pregnancies. Therefore, treatment of HPD may not only improve immediate pregnancy outcomes, but also the maternal long-term cardiovascular health. Unlike the recommendations for hypertension treatment in the general population, treatment recommendations of HPD have not changed substantially for more than two decades. This is particularly true for mild to moderate hypertension in pregnancy, defined as a blood pressure of 140–159/90–109 mm Hg.
This review focuses on the goals of therapy, treatment strategies, and new developments in the field of HPD that should be taken into account when considering blood pressure targets and pharmacological options for treatment of hypertension in pregnant women.
Keywords: Hypertension, Treatment, Pregnancy induced, Antihypertensive agents, Cardiovascular diseases in women, Blood pressure medications
Introduction
Hypertension is the most common medical condition encountered during pregnancy, occurring in approximately 6–8% of pregnancies [1]. The hypertensive disorders of pregnancy cover a spectrum of conditions, including preeclampsia-eclampsia, gestational hypertension and chronic hypertension. The pooled incidence of preeclampsia in developing countries is reported to be around 3.4% and, in developed countries, ranges from 0.4–2.8% [2]. In the US, the rates of hypertensive pregnancy disorders (HPD) have risen steadily over the last 3 decades, with the most recently reported rates of preeclampsia and gestational hypertension of 29.7 and 32.1 per 1000 deliveries, respectively [3]. As a group, HPD represent the most common direct cause of maternal mortality in both developed countries (16% of all maternal deaths) and developing countries (9–25% of all maternal deaths) [4]. Hypertension in pregnancy is one of the major causes of maternal mortality in the United States (similar to other industrialized countries), accounting for 12.3% of the maternal deaths between 1998 and 2005 [5]. Even in the modern era, hypertension in pregnancy imparts a significant increase in maternal morbidity. In 36,537,061 delivery discharges between 1998 and 2006, as identified by the Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project, there was an increased risk for obstetric complications, such as acute renal failure, pulmonary edema, need for ventilator support, and cerebrovascular complications, for every category of hypertensive pregnancy including mild preeclampsia [6].
Hemodynamic changes in normal pregnancy
Changes in blood pressure (BP) during normal pregnancy are related to alterations in cardiac output and systemic vascular resistance (SVR). Systemic vasodilation is induced by pregnancy hormones such as estrogen, progesterone, prolactin and relaxin [7], along with a decreased responsiveness to pressor hormones, such as angiotensin II and vasopressin [8]. This systemic vasodilation, combined with the low resistance system of the uteroplacental circuit, results in a marked reduction in SVR. In response to this, there is a gradual increase in plasma volume, accomplished through an increase in plasma renin, accompanied by reduced atrial natriuretic peptide levels [9]. Heart rate increases, mainly due to systemic vasodilation. The overall effect is of increased cardiac output [10]. The sum effect of these hemodynamic changes is an initial decrease in systemic arterial BP by 10 – 15 mmHg in early pregnancy. A nadir in BP usually occurs towards the end of the second trimester. Beginning in the third trimester, BP rises by about 10 mmHg, and returns to the individual’s baseline value by the end of pregnancy [11].
Pathophysiology of preeclampsia
In preeclampsia, the placental spiral arteries fail to lose their musculoelastic layers ultimately leading to decreased placental perfusion [12, 13]. Placental hypoxia is frequently viewed as an early trigger of placental production of soluble factors resulting endothelial dysfunction [14], which may play a central role in the pathogenesis of the maternal syndrome of preeclampsia. Recent studies of vascular endothelial growth factor (VEGF) and its receptors have suggested that down-regulation of VEGF may be the missing link between the ischemic placenta and maternal endothelial dysfunction [15]. Other mechanisms implicated in the pathophysiology of preeclampsia include, oxidative stress, placental steroidogenesis, formation of agonist auto-antibodies against the angiotensin II receptor, exaggeration of the hypercoagulability of pregnancy, and insulin resistance [16, 17]. The end result of this complex interplay between maternal and placental mechanisms is a maternal multi-system disorder, characterized by hypertension, proteinuria, and, in severe cases, multi-organ dysfunction.
Kidney biopsies have revealed generalized swelling and vacuolization of endothelial cells. Renal vasoconstriction decreases renal plasma flow; while increasing creatinine levels and causing oliguria. Small vessel endothelial injury leads to activation of the coagulation system, with formation of platelet and fibrin thrombi in the microvasculature. This may lead to thrombocytopenia, microangiopathic hemolytic anemia and hemoconcentration from capillary leakage. Similar effects are noted in the liver, leading to periportal and sinusoidal fibrin deposition, reduced hepatic blood flow and periportal hemorrhage, resulting in capsular pain and abnormal liver enzymes. The triad of hemolysis, elevated liver enzymes, and low platelet count, known as HELLP syndrome, is one of the most severe forms of preeclampsia (Table 1). In the cerebrovascular system, vasculopathy, microinfarcts and hemorrhage, and cerebral edema are noted. These findings are possibly due to vasospasm of the cerebral vasculature in response to hypertension, or from the loss of cerebrovascular autoregulation- with areas of both vasoconstriction and forced vasodilation. This may manifest clinically as headache, altered mental status and seizures. It is thought that this may represent a form of posterior reversible leukoencephalopathy syndrome (PRES) [18], a clinical syndrome of neurological signs and symptoms coupled with neuroimaging findings of vasogenic edema, observed predominantly in the posterior circulation. Fetal effects include growth restriction and fetal loss.
Table 1.
Definitions of Hypertensive Pregnancy, adapted from the Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy (NHBPEP) and American College of Gynecology practice guidelines
| Features | Timing | Comments | |
|---|---|---|---|
| Chronic hypertension | BP ≥ 140 mm Hg systolic, or ≥ 90 mm Hg diastolic on 2 occasions, 6 hours apart | Diagnosed pre-pregnancy, or before 20 weeks gestation, or persists beyond 12 weeks postpartum | Mild: SBP 140–159 mmHg, DBP 90–109 mmHg Severe: SBP ≥ 160 mmHg DBP ≥ 110 mmHg |
|
| |||
| Preeclampsia-eclampsia | Hypertension plus proteinuria, defined as:
|
|
|
|
|
|||
|
|
|
|
|
|
|||
|
|||
|
| |||
| Preeclampsia superimposed on chronic hypertension | New proteinuria (or significant worsening in those with pre-existing proteinuria) in patient with chronic hypertension | Hypertension diagnosed pre-pregnancy, before 20 weeks gestation, or persists beyond 12 weeks postpartum | Consider also in new onset hyperuricemia, thrombocytopenia and other end-organ effects of preeclampsia |
|
| |||
| Gestational hypertension | Normal BP before pregnancy. This category encompasses 3 groups, with the final differentiation being possible only retrospectively, i.e., postpartum | Hypertension occurring in the second trimester in the absence of proteinuria |
|
BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure
Classification and definitions of hypertensive pregnancy disorders
The National High Blood Pressure Education Program (NHBPEP) Working Group on High Blood Pressure in Pregnancy defines hypertension in pregnancy as two BP measurements of ≥ 140/90 mmHg measured ≥ six hours apart. The HPD are divided into four classes: chronic hypertension, preeclampsia-eclampsia, preeclampsia superimposed on chronic hypertension and gestational hypertension. (Table 1) [1].
Contemporary management of hypertension
Management of chronic hypertension in the non-pregnant versus pregnant population
Hypertension remains the most common medical condition in both the non-pregnant and pregnant populations. As late as the 1940–50s, an elevated BP in the non-pregnant population was considered to be necessary for adequate organ perfusion. It was believed that BP values of 180/110 mm Hg or even higher could be observed without therapy, as long as there was no evidence of cardiac involvement [19]. Subsequent clinical trials demonstrated that treatment of hypertension was associated with a decreased incidence of stroke, myocardial infarction and heart failure. As of 2003, the Joint National Committee (JNC) on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure has published seven reports reflecting increasing levels of aggressiveness in the management of hypertension as more research and evidence of efficacy of therapy have become available. Therapy is now indicated for sustained BP levels above 140/90 mm Hg, and close observation and lifestyle management for pre-hypertension, i.e. BP values between 120/80 and 139/89 mmHg) [20].
The treatment recommendations for hypertension in pregnancy have not evolved similarly. The initial guidelines on the management of hypertension in pregnancy, published in 1990 by the NHBPEP Working Group Report on High Blood Pressure in Pregnancy [21], were updated, but not significantly modified, in 2000 [1]. These guidelines represent the most contemporary guidelines available in the US and were upheld by the American Society of Hypertension’s position article on Hypertension in Pregnancy [22]. Recommendations for the treatment of chronic hypertension in pregnancy support the use of anti-hypertensive therapy for BP levels of ≥ 160/110 mm Hg or in the presence of target organ damage, such as left ventricular hypertrophy and renal insufficiency [1]. Notably, there is a discrepancy between the NHBPEP Working Group Report on High Blood Pressure in Pregnancy and JNC 7 guidelines: mild to moderate hypertension in pregnancy is defined as a BP 140–159/90–109 mm Hg, whereas, according to JNC-7, a diastolic BP ≥ 100 mm Hg is stage 2 hypertension. In addition, there is significant heterogeneity in the recommendations for treatment among different expert panels. The United Kingdom’s National Institute for Health and Clinical Excellence (NICE) clinical guidelines recommend slightly more aggressive treatment, i.e. therapy for all classes of HPD with BP ≥ 150/100 mmHg, and keeping a BP lower than140/90 mm Hg in pregnant women with target-organ damage (such as renal disease) [23]. Similarly, the European Society of Cardiology (ESC) endorses different nomenclature, treatment thresholds and targets [24]. These significant differences among the expert panels reflect an ongoing controversy as to when, if at all, to treat mild to moderate chronic hypertension in pregnancy. The discussion that follows aims to present new developments within the field of hypertension, in general, and in pregnant women, in particular, which may influence contemporary BP treatment thresholds and targets, and the use of specific anti-hypertensive medications in pregnancy.
Severe versus mild to moderate hypertension in pregnancy
With respect to severe hypertension (commonly defined as a diastolic BP of ≥ 110 mm Hg), there is consensus that anti-hypertensive therapy may reduce the risk of end-organ complications, including abruptio placentae, stroke and pulmonary edema [25, 26]. Initiation of drug therapy for mild-moderate, or less severe, hypertension in pregnancy (<160/110 mm Hg) in women without other co-morbidities is still a controversial topic with contrasting recommendations. Central to this controversy is the concern that pharmacological therapy may provide no significant maternal benefit while, on the other hand, intrauterine fetal exposure may increase fetal risks related to both adverse medication effects, and growth restriction due to the lowering of BP and subsequent reduction in uteroplacental blood flow. This controversy is fueled by the absence of well-designed and adequately powered studies of the risks and benefits of the treatment of mild to moderate hypertension in pregnancy. In contrast, in the general population, the decision to start medical therapy for the same degree of hypertension is clearly accepted. The higher threshold of BP recommended for the initiation of anti-hypertensive therapy in pregnant women, compared to the general population, may be due to the following:
lack of evidence that treatment of mild hypertension in pregnancy leads to improved maternal outcomes
assumption that mild hypertension of 4–5 months duration does not adversely affect immediate and long-term cardiovascular disease (CVD) risks
concern that decreased maternal BP may compromise uteroplacental and fetal circulation, thus resulting in small-for-gestational–age (SGA) infants
potential increase in risk for fetal adverse effects due to exposure to potentially harmful medications in utero
However, what is the evidence that supports the above notions and how may these issues influence our approach in treating a hypertensive pregnant patient?
Several observational studies have suggested that treatment of mild hypertension in pregnancy may not be associated with improved maternal outcomes [27–29]. However, treatment of chronic hypertension has been shown to prevent progression to severe hypertension [30, 31]. A recent study indicated that adverse pregnancy outcomes are more frequent with mild-to-moderate BP elevations (140–159/90–109 mm Hg) than with normal baseline blood pressures. The risks for these adverse pregnancy outcomes, a primary composite outcome (perinatal death, preterm birth, placental abruption, and severe preeclampsia), and SGA infants, were further increased with increasing BP [32]. Of note, this was a secondary analysis of a cohort of women with chronic hypertension who were initially enrolled in a National Institute of Child Health & Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network randomized controlled trial designed to evaluate aspirin use for preeclampsia prevention [33]. These results are consistent with previous reports of an association between chronic hypertension and increased maternal and fetal risks, such as perinatal mortality and placental abruption [34]. In the absence of well-designed, adequately powered studies, and with a growing body of evidence demonstrating serious maternal and fetal complications of untreated/inadequately treated hypertension in pregnancy, most experts in the field now agree that treatment of chronic hypertension in pregnancy should be initiated for a BP of ≥ 150/90 mm Hg [35]; in the presence of renal disease or other target organ complications, anti-hypertensive therapy is initiated for a diastolic BP ≥ 90 mm Hg. But, are there reasons to further change these criteria for treatment?
The assumption that mild hypertension of 4–5 months duration does not adversely affect immediate and long-term CVD risks needs to be re-visited in the setting of the changing demographics with respect to the age and the overall health of pregnant women. Most notably, a trend toward advanced age at first pregnancy, particularly in developed countries, coupled with sophisticated measures of assisted reproduction (such as in-vitro fertilization), may increase the number of women who, in addition to hypertension, may have other risk factors, such as renal disease, diabetes, polycystic ovary syndrome, all of which may result in either clinical or subclinical evidence of vascular damage. Treatment of hypertension during pregnancy in these women may decrease their overall cardiovascular risk. This would be consistent with recent studies showing a significant correlation between better cardiovascular outcomes with earlier and more effective BP treatment [36]. As the findings of the studies performed in the general population are not directly applicable to pregnant patients, further research should focus on immediate and long-term cardiovascular outcomes as functions of hypertension control over the course of pregnancy.
The concern with respect to the possible relationship between fetoplacental growth and the use of anti-hypertensive therapy is based on a meta-analysis of clinical trials of anti-hypertensive agents published in 2000, which reported that a 10 mm Hg decrease in mean arterial pressure was associated with a 145-gram decrease in birth-weight [37]. This meta-analysis might have been subject to selection bias [38]. For example, a study that compared nicardipine to metoprolol and showed increased birth weight in the nicardipine treated group, despite an improved anti-hypertensive effect of the medication, was not included [39]. In addition, this meta-analysis included studies performed up to the year 2000. Notably, patients with mild to moderate hypertension were, more likely, not treated, based on the guidelines within that time-period. This raises the possibility of selection bias towards the patients who, in addition to hypertension, might have had other medical conditions and co-morbidities which may have predisposed them to delivering SGA infants (e.g., renal disease). A recent study further characterized the outcome of hypertensive pregnancy, reporting that chronic hypertension was associated with adverse fetal outcomes regardless of treatment [27], suggesting that the low birth weight and growth restriction seen in trials of anti-hypertensive therapy may be an example of confounding by indication [40], i.e. the effects observed may be due to the underlying disease itself, rather than treatment of the disease.
With respect to preeclampsia, the current guidelines recommend treatment of diastolic BP levels >105 mmHg or lower in high risk circumstances, such as teenagers with recent diastolic pressures <70 mm Hg, or evidence of cardiac or cerebral decompensation; the level of systolic BP at which anti-hypertensive therapy is indicated was not defined [1]. A study of 29 women who developed a stroke in the setting of severe preeclampsia and eclampsia showed that the diastolic BP was ≥ 105 mm Hg in only 20% of patients, while all patients had a systolic BP >155 mm Hg [41]. The report called for a paradigm shift towards considering anti-hypertensive therapy for these patients when the systolic BP reaches or exceeds 155–160 mm Hg. In addition, PRES seems to occur at lower peak systolic BP values in pregnant compared to non-pregnant patients [42, 18]. In summary, the findings of these studies support medical treatment for a systolic BP ≥ 150 mm Hg in women who develop hypertension during pregnancy, and continuation of therapy in women with chronic hypertension on adequate therapy prior to becoming pregnant. Current clinical practice, for the most part, does not reflect published guidelines. Most investigators agree that anti-hypertensive therapy in a preeclamptic patient should be initiated for a diastolic BP approaching 100 mm Hg, and for BP ≥ 150–160/100 mm Hg [35]. But should this still be the recommended level for initiation of antihypertensive medications?
An alternative approach, which would be similar to hypertension treatment in the general population, would be to initiate anti-hypertensive therapy when BP rises to levels of 140/90 mmHg or more in pregnant women, regardless of the presence of proteinuria or other signs of pre-eclampsia. Early BP control may prevent progression to severe hypertension, maternal complications (such as a cerebrovascular hemorrhage and heart failure), improve fetal maturity by permitting prolongation of pregnancy, even in women with secondary hypertension (Figure 1) who may be at a particularly high risk for adverse pregnancy outcomes. Several methods are available that can be used to monitor clinically fetal well-being and safety during both the introduction and titration of antihypertensive medications.
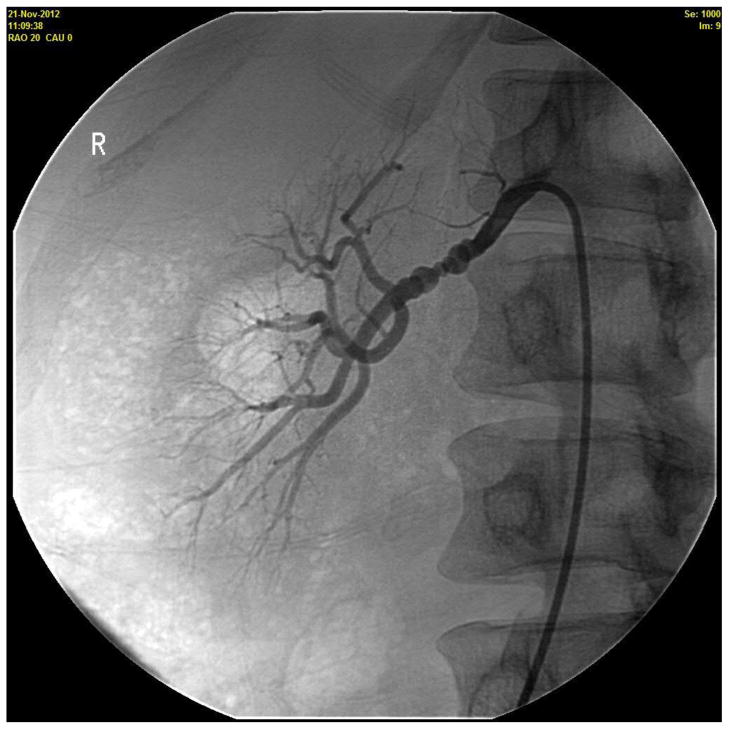
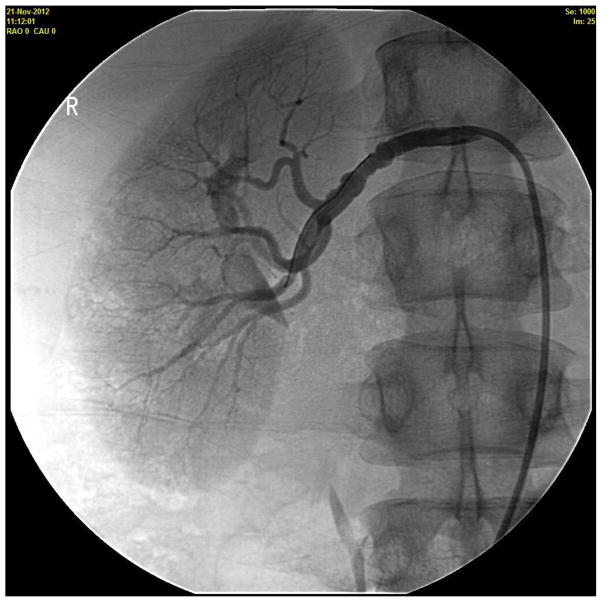
Figure 1.
Renovascular hypertension during pregnancy. A 36 year old woman presented at 14 weeks in her first pregnancy for management of hypertension. She was started on Labetalol 100 mg twice a day. Her follow up blood pressure (BP) was 184/114 mm Hg and evaluation for secondary causes of hypertension was initiated. A Doppler study of the renal arteries showed markedly elevated velocities in the mid-distal right renal artery, peak systolic velocity (PSV) of 533cm/sec, consistent with fibromuscular dysplasia (FMD) causing a high-grade right renal artery stenosis, and borderline elevated velocities in the left renal artery (PSV 199cm/sec), suggesting probable moderate stenosis caused by FMD of the left renal artery. The decision was made to optimize her medical management, with intervention to be considered only if she were to fail pharmacotherapy. The dose of labetalol was gradually increased to 200 mg four times a day and, ultimately, nifedipine XL 90 mg was added. On that regimen, her systolic BP averaged 122–144 mm Hg, diastolic 78–92 mm Hg for the remainder of her pregnancy. At 38 weeks of gestation (BP of 146/94 mm Hg), she delivered, by Caesarean section, a healthy 2.8 kg baby boy with Apgar score of 8 and 9 at 1 and 5 minutes, respectively. Six-months postpartum, she underwent a renal angiogram with successful bilateral angioplasty (Figure 1A and 1B, right renal artery, before and after angioplasty, respectively.). She is currently normotensive and off all BP medications.
Anti-hypertensive medications and pregnancy
The choice of anti-hypertensive medication in pregnancy has been limited to those that are considered relatively safe, have a long history of clinical use, and have side-effect profiles that physicians have found to be acceptable (Table 2). The NHBPEP recommends α-methyldopa or hydralazine as initial pharmacologic agents. Methyldopa has a long track record of safety and has been shown to have no adverse effects in children followed up to 7.5 years from their exposures in utero [43]. Unfortunately, the side-effect profiles of these drugs, including severe somnolence at the doses of methyldopa often required for an anti-hypertensive effect, and the need for multi-dose daily regimens (hydralazine), may result in non-adherence, thus limiting their usefulness. BP is often not reduced to normotensive levels with these agents, and subsequently they are rarely used for treatment of elevated BP in the general population.
Table 2.
Common antihypertensive drugs
| Drug | FDA Pregnancy Category | Comments |
|---|---|---|
| Central α-agonist | ||
|
| ||
| Methyldopa | B | Long track record of use. Maternal sedation common at higher doses which are often necessary if used as single agent. Initial drug of choice in NHBPEP guidelines |
| Clonidine | C | Limited data: use only if clearly indicated |
|
| ||
| β-blockers | Concern for fetal bradycardia and reduced placental blood flow | |
|
| ||
| Atenolol | D during 1st and 2nd trimesters | Definite association with intrauterine growth restriction if used before third trimester. |
| Metoprolol | C | Limited data: use only if clearly indicated; likely same considerations as atenolol |
|
| ||
| α-β blockers | Preserved placental blood flow compared to β-blockers | |
|
| ||
| Labetalol | C | Initial drug of choice on NICE guidelines. Oral or IV administration |
| Carvedilol | C | Not as well studied in pregnancy |
|
| ||
| Calcium channel blockers (CCBs) | Not as much experience. Possible decreased risk for growth restriction compared to β-blockers. Possible interaction with magnesium (bradycardia), especially nondihydropyridines | |
|
| ||
| Dihydropyridines, e.g. Nifedipine | C | In extended release form, nifedipine is widely used in pregnant population. Do not use immediate release form for management of severe hypertension due to risk of precipitous fall in BP. Amlodipine (very common in nonpregnant population), not well studied in pregnancy |
| Nondihydropyridine e.g. Verapamil, Diltiazem | C | Not as well studied |
|
| ||
| Vasodilators | ||
|
| ||
| Hydralazine | C | Associated with reflex tachycardia and fluid retention: need to use CCB or β-blocker and diuretic. Can be administered intravenously, but effect not as predictable as other agents |
| Nitroprusside | C | IV administration only in emergency settings if other agents unavailable. Associated with cyanide and thiocyanate accumulation and fetal toxicity when used for ≥ 4 hours. Labetalol preferred |
|
| ||
| Diuretics | Theoretic concern for volume contraction. Generally shown to be safe (except spironolactone). Useful in combination with vasodilator | |
|
| ||
| HCTZ | B | Long track record of use. |
| Furosemide | C | Not diuretic of choice except in cases of true volume overload or renal impairment |
| Spironolactone | (C) | Limited data, possible fetal anti-androgenic effects: Contraindicated in pregnancy |
|
| ||
| Angiotensin Converting Enzyme Inhibitors | D | Contraindicated in pregnancy |
| Angiotensin Receptor Blockers | D | Contraindicated in pregnancy |
| Direct Renin Inhibitors | D | Contraindicated in pregnancy |
FDA: United States’ Food and Drug Administration; IV: intravenous; NHBPEP: National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy; NICE: United Kingdom’s National Institute for Health and Clinical Excellence
Labetalol, diuretics and calcium channel blockers are now used more frequently than methyldopa. These are acceptable alternatives that are well tolerated and are often prescribed in the non-pregnant population (unlike methyldopa), and can be continued in women with previously diagnosed hypertension [44]. Angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and by extrapolation, renin inhibitors, are contraindicated in pregnancy because of their risks for fetal toxicity [45–47], and should be stopped prior to conception or as soon as pregnancy is diagnosed. The use of diuretics in the 1970s was discouraged, primarily due to theoretical concerns with respect to their potential adverse effects on placental blood flow. A subsequent randomized trial of women with chronic hypertension in pregnancy showed no adverse pregnancy outcomes, despite reductions in plasma volumes [48]. Current guidelines encourage women to continue diuretics if they were on that regimen before pregnancy. Some physicians would opt to discontinue diuretics for those who develop premonitory signs of preeclampsia, due to the concern that their continuous use may further aggravate the hypovolemic state, which is characteristic for preeclampsia, thus stimulating renin-angiotensin system, and potentially resulting in worsening of hypertension.
Hypertensive pregnancy disorders as a risk factor for future cardiovascular disease
Hypertensive pregnancy disorders have been associated with an increased risk of development of CVD [49–52], with the most commonly studied outcomes being ischemic heart disease, stroke, venous thromboembolism and cardiovascular death. A recent intriguing study of 75, 242 women in Ontario, Canada, demonstrated an increase in the risks for heart failure and arrhythmias long after hypertensive pregnancy, with a hazard ratio of 1.5, even after adjusting for hypertension and coronary disease, as well as other cardiovascular risk factors [53].
Echocardiographic studies may contribute towards the understanding of the findings of this study. Studies conducted in normal pregnancy demonstrate an increase in the size of all cardiac chambers, which decline to pre-pregnancy levels after delivery [54]. This is associated with eccentric hypertrophy, and a decrease in LV systolic strain, but stroke volume and stroke work increase, suggesting that the physiologic increase in cardiac output is partly maintained by the increase in chamber dimensions [55]. In hypertensive pregnancy, the myocardial remodeling that is observed in normal pregnancy becomes exaggerated as a result of exposure to increased afterload [56]. Features of hypertensive pregnancy include concentric remodeling, concentric and eccentric hypertrophy, biventricular diastolic dysfunction and myocardial impairment [57–59]. Unlike normal pregnancy, however, these effects may persist both in the short term (up to two years post pregnancy) [60] and long-term (13–18 years post pregnancy) [61], and may contribute to the increased risk of heart failure and arrhythmias reported by Ray et al [53].
These findings suggest that hypertensive pregnancy may be more than simply a stress test, and may itself have long lasting effects on the cardiovascular system. A recent study from Finland seems to support this. The authors found that hypertensive pregnancy, regardless of classification, including isolated systolic or diastolic hypertension (even in the absence of known CVD risk factors), was associated with a higher risk of later CVD, chronic kidney disease, and diabetes mellitus when compared to normotensive pregnancy [62]. Whether treatment of hypertension in pregnancy to the BP goals established for the general population affects future cardiovascular outcomes in affected women, remains to be determined in future studies.
Conclusions
Small, underpowered trials and observational studies have proven to be equivocal in guiding the practitioner managing mild-moderate hypertension in pregnancy. Evidence is mounting, however, that hypertension occurring in pregnancy may itself be detrimental to both immediate and long-term maternal health. The increasing prevalence of hypertensive pregnancy due to increasing maternal age, and increase in risk factors such as obesity, mandate that efforts be made to decrease this impact. Furthermore, there is no convincing evidence that appropriate treatment of HPD is detrimental to fetal wellbeing. Rather, one must further consider that major guidelines use the level of BP as a major criterion for induction of delivery and, since antihypertensive treatment may prevent progression to severe hypertension, if BP elevations were treated earlier, with appropriate medications, fewer earlier terminations of pregnancy would result.
Currently, several intervention trials for HPD are in progress [44] to address some of these issues. While awaiting the results of these trials, a different approach to managing hypertension in pregnancy should be considered, one that should protect mother from both immediate and long-term cardiovascular events and one that may improve fetal maturity and outcome by allowing for continuation of pregnancy. When BP rises to ≥ 140/90 mmHg, anti-hypertensive treatment, coupled with close fetal monitoring, may result in both improved fetal outcome, as well as decreasing immediate maternal complications (i.e., stroke, heart failure) and permanent vascular injury, impacting CVD rates years into the future.
Acknowledgments
The project described was supported by Award Number P-50 AG44170 (V.D. Garovic) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The writing of the manuscript and the decision to submit it for publication were solely the authors’ responsibilities.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Dawn C. ScantleburyGary L. Schwartz, Letitia A. Acquah, Wendy M. White, and Marvin Moser declare that they have no conflict of interest.
Vesna D. Garovic has patents filed, but not licensed.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Dawn C. Scantlebury, Email: scantlebury.dawn@mayo.edu.
Gary L. Schwartz, Email: schwartz.gary@mayo.edu.
Letitia A. Acquah, Email: acquah.letitia@mayo.edu.
Wendy M. White, Email: white.wendy@mayo.edu.
Marvin Moser, Email: moserbp@aol.com.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 2.Dolea C, AbouZahr C. Evidence and Information for Policy (EIP) World Health Organization; 2003. Global burden of hypertensive disorders of pregnancy in the year 2000. http://www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpregnancy.pdf. [Google Scholar]
- 3.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. American Journal of Hypertension. 2008;21(5):521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 5.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 6*.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–306. doi: 10.1097/AOG.0b013e3181a45b25. This paper examines recent trends in the rates of HPD in the United States and immediate maternal morbidity. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe J, Ueland K. Maternal cardiovascular adjustments to pregnancy. Prog Cardiovasc Dis. 1974;16(4):363–74. doi: 10.1016/0033-0620(74)90028-0. [DOI] [PubMed] [Google Scholar]
- 8.Curran-Everett D, Morris KG, Jr, Moore LG. Regional circulatory contributions to increased systemic vascular conductance of pregnancy. Am J Physiol. 1991;261(6 Pt 2):H1842–7. doi: 10.1152/ajpheart.1991.261.6.H1842. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056–63. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard JA. Changes in the Blood Volume during Pregnancy and Delivery. Anesthesiology. 1965;26:393–9. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol. 2000;96(5 Pt 2):849–60. doi: 10.1016/s0029-7844(00)00938-8. [DOI] [PubMed] [Google Scholar]
- 12.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. British Journal of Obstetrics and Gynaecology. 1994;101(8):669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 13.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British Journal of Obstetrics and Gynaecology. 1986;93(10):1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 14.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of clinical investigation. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Herse F, Lamarca B. Angiotensin II Type 1 Receptor Autoantibody (AT1-AA)-Mediated Pregnancy Hypertension. American journal of reproductive immunology. 2013;69(4):413–8. doi: 10.1111/aji.12072. This recent review summarizes evidence for the role of the AT1 receptor antibody in the pathogenesis of hypertensive pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner SJ, Craici IM, Grande JP, Garovic VD. From placenta to podocyte: vascular and podocyte pathophysiology in preeclampsia. Clinical nephrology. 2012;78(3):241–9. doi: 10.5414/cn107321. [DOI] [PubMed] [Google Scholar]
- 18*.Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clinic Proceedings. 2011;86(9):851–6. doi: 10.4065/mcp.2011.0090. This report demonstrates that PRES and eclampsia may develop at relatively modest BP elevations, further emphasizing the need to readress the treatment targets in HPD guidelines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser M. Historical perspectives on the management of hypertension. J Clin Hypertens (Greenwich) 2006;8(8 Suppl 2):15–20. doi: 10.1111/j.1524-6175.2006.05836.x. quiz 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA: the journal of the American Medical Association. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 1990;163(5 Pt 1):1691–712. doi: 10.1016/0002-9378(90)90653-o. [DOI] [PubMed] [Google Scholar]
- 22.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2010;4(2):68–78. doi: 10.1016/j.jash.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Redman CW. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97(23):1967–9. doi: 10.1136/heartjnl-2011-300949. [DOI] [PubMed] [Google Scholar]
- 24.European Society of G, Association for European Paediatric C, German Society for Gender M, Authors/Task Force M. Regitz-Zagrosek V, Blomstrom Lundqvist C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(24):3147–97. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 25.Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S, et al. How to manage hypertension in pregnancy effectively. British Journal of Clinical Pharmacology. 2011;72(3):394–401. doi: 10.1111/j.1365-2125.2011.04002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sibai BM, Anderson GD. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstetrics and Gynecology. 1986;67(4):517–22. [PubMed] [Google Scholar]
- 27**.Orbach H, Matok I, Gorodischer R, Sheiner E, Daniel S, Wiznitzer A, et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.11.011. This very recent report presents evidence for increasing fetal morbidity with chronic hypertension, regardless of treatment status. [DOI] [PubMed] [Google Scholar]
- 28.Su CY, Lin HC, Cheng HC, Yen AM, Chen YH, Kao S. Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PLoS One. 2013;8(2):e53844. doi: 10.1371/journal.pone.0053844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray JG, Vermeulen MJ, Burrows EA, Burrows RF. Use of antihypertensive medications in pregnancy and the risk of adverse perinatal outcomes: McMaster Outcome Study of Hypertension In Pregnancy 2 (MOS HIP 2) BMC pregnancy and childbirth. 2001;1(1):6. doi: 10.1186/1471-2393-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. American Journal of Obstetrics and Gynecology. 1990;162(4):960–6. doi: 10.1016/0002-9378(90)91297-p. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 31.Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;(1):CD002252. doi: 10.1002/14651858.CD002252.pub2. [DOI] [PubMed] [Google Scholar]
- 32*.Ankumah N, Tita A, Cantu J, Chapman-Jauk V, Biggio J, Hauth J, et al. Pregnancy outcome vary by blood pressure level in women with mild-range chronic hypertension. American Journal Of Obstetrics And Gynecology. 2013;208(1):S261. Although only in abstract form, these data are based on the best characterized cohort of women with chronic hypertension in pregnancy and present important evidence of increased morbidity with mil to moderate hypertension in pregnancy. [Google Scholar]
- 33.Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. New England Journal of Medicine. 1998;338(11):701–5. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstetrics and Gynecology. 2000;96(5 Pt 2):849–60. doi: 10.1016/s0029-7844(00)00938-8. [DOI] [PubMed] [Google Scholar]
- 35.August P. Preeclampsia: New Thoughts on an Ancient Problem. Journal of Clinical Hypertension. 2000;2:115–23. [PubMed] [Google Scholar]
- 36.Gradman AH, Basile JN, Carter BL, Bakris GL. Combination therapy in hypertension. Journal of the American Society of Hypertension. 2010;4(1):42–50. doi: 10.1016/j.jash.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 37.von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355(9198):87–92. doi: 10.1016/s0140-6736(98)08049-0. [DOI] [PubMed] [Google Scholar]
- 38.de Swiet M. Maternal blood pressure and birthweight. Lancet. 2000;355(9198):81–2. doi: 10.1016/S0140-6736(99)00288-3. [DOI] [PubMed] [Google Scholar]
- 39.Jannet D, Carbonne B, Sebban E, Milliez J. Nicardipine versus metoprolol in the treatment of hypertension during pregnancy: a randomized comparative trial. Obstet Gynecol. 1994;84(3):354–9. [PubMed] [Google Scholar]
- 40.Macones GA, Odibo A, Cahill A. Discussion: ‘Hypertension and antihypertensives in pregnancy,’ by Orbach H et al. American Journal of Obstetrics and Gynecology. 2013 doi: 10.1016/j.ajog.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Martin JN, Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstetrics and Gynecology. 2005;105(2):246–54. doi: 10.1097/01.AOG.0000151116.84113.56. [DOI] [PubMed] [Google Scholar]
- 42.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. New England Journal of Medicine. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 43.Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1(8273):647–9. doi: 10.1016/s0140-6736(82)92202-4. [DOI] [PubMed] [Google Scholar]
- 44.Moser M, Brown CM, Rose CH, Garovic VD. Hypertension in pregnancy: is it time for a new approach to treatment? J Hypertens. 2012;30(6):1092–100. doi: 10.1097/HJH.0b013e3283536319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaefer C. Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Birth defects research Part A, Clinical and molecular teratology. 2003;67(8):591–4. doi: 10.1002/bdra.10081. [DOI] [PubMed] [Google Scholar]
- 46.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 47.Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. The American journal of medicine. 1994;96(5):451–6. doi: 10.1016/0002-9343(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 48.Sibai BM, Grossman RA, Grossman HG. Effects of diuretics on plasma volume in pregnancies with long-term hypertension. American Journal of Obstetrics and Gynecology. 1984;150(7):831–5. doi: 10.1016/0002-9378(84)90458-7. [DOI] [PubMed] [Google Scholar]
- 49.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. American Heart Journal. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 50.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51. doi: 10.1161/hypertensionaha.109.130765. [DOI] [PubMed] [Google Scholar]
- 51.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–803. doi: 10.1016/s0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 53**.Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98(15):1136–41. doi: 10.1136/heartjnl-2011-301548. This very large population based study is the first to report the association between maternal placental syndromes, including preeclampsia, and an increased risk of HF and arrhythmias. [DOI] [PubMed] [Google Scholar]
- 54.Campos O. Doppler Echocardiography During Pregnancy: Physiological and Abnormal Findings. Echocardiography. 1996;13(2):135–46. doi: 10.1111/j.1540-8175.1996.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 55.Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I, Popescu BA, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circulation Cardiovascular imaging. 2012;5(3):289–97. doi: 10.1161/CIRCIMAGING.111.970012. [DOI] [PubMed] [Google Scholar]
- 56.Zanati Bazan SG, Borges VM, Martin LC, Magalhaes CG, Hueb JC, de Arruda Silveira LV, et al. Disproportionate Pregnancy-Induced Myocardial Hypertrophy in Women With Essential Hypertension. Am J Hypertens. 2013 doi: 10.1093/ajh/hpt023. [DOI] [PubMed] [Google Scholar]
- 57*.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57(1):85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. This studiy explorse the effects of hypertensive pregnancy on maternal cardiac structure and function. [DOI] [PubMed] [Google Scholar]
- 58.Ingec M, Yilmaz M, Gundogdu F. Left atrial mechanical functions in pre-eclampsia. J Obstet Gynaecol Res. 2005;31(6):535–9. doi: 10.1111/j.1447-0756.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 59.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31(4):454–71. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 60.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 61*.Strobl I, Windbichler G, Strasak A, Weiskopf-Schwendinger V, Schweigmann U, Ramoni A, et al. Left ventricular function many years after recovery from pre-eclampsia. Bjog. 2011;118(1):76–83. doi: 10.1111/j.1471-0528.2010.02780.x. This is an echocardiographic study investigating the effect of hypertensive pregnancy on the maternal heart longterm. [DOI] [PubMed] [Google Scholar]
- 62**.Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90. doi: 10.1161/CIRCULATIONAHA.112.128751. This report demonstrates that all types of hypertensive pregancy, not only preeclampsia, are associated with longterm maternal cardiac morbidity, even in the absence of other known cardiovascular disease risk factors. This further underscores the impact of hypertension in pregnancy itself as a risk factor for CVD. [DOI] [PMC free article] [PubMed] [Google Scholar]