Abstract
Methanol (CH3OH) fluxes were quantified above a managed temperate mountain grassland in the Stubai Valley (Tyrol, Austria) during the growing seasons 2008 and 2009. Half-hourly methanol fluxes were calculated by means of the virtual disjunct eddy covariance (vDEC) method using 3-dimensional wind data from a sonic anemometer and methanol volume mixing ratios measured with a proton-transfer-reaction mass spectrometer (PTR-MS). During (undisturbed) mature and growing phases methanol fluxes exhibited a clear diurnal cycle with close-to-zero fluxes during nighttime and emissions, up to 10 nmol m−2 s−1, which followed the diurnal course of radiation and air temperature. Management events were found to represent the largest perturbations of methanol exchange at the studied grassland ecosystem: Peak emissions of 144.5 nmol m−2 s−1 were found during/after cutting of the meadow reflecting the wounding of the plant material and subsequent depletion of the leaf internal aqueous methanol pools. After the application of organic fertilizer, elevated methanol emissions of up to 26.7 nmol m−2 s−1 were observed, likely reflecting enhanced microbial activity associated with the applied manure. Simple and multiple linear regression analyses revealed air temperature and radiation as the dominant abiotic controls, jointly explaining 47 % and 70 % of the variability in half-hourly and daily methanol fluxes. In contrast to published leaf-level laboratory studies, the surface conductance and the daily change in the amount of green plant area, used as ecosystem-scale proxies for stomatal conductance and growth, respectively, were found to exert only minor biotic controls on methanol exchange.
Keywords: disjunct eddy covariance, flux, methanol, volatile organic compounds, management, grassland, PTR-MS
1 Introduction
Volatile organic compounds (VOCs) have received increased attention in recent years due to their role in atmospheric chemistry [Chameides et al., 1988]. The emission of VOCs from plants to the atmosphere constitutes a significant source of reactive non-methane hydrocarbons [Cojocariu et al., 2005] and therefore strongly affects the physical/chemical properties of the atmosphere and thus climate [Arey et al., 1991; Bermejo et al., 2003]. In the presence of NOx (NO, NO2) and sunlight, VOCs react to form tropospheric ozone [Atkinson, 2000], which is a greenhouse gas and represents a risk to plants and human health [Bernard et al., 2001]. VOCs react with the hydroxyl radical (OH) and thus decrease its concentration which in turn affects the atmospheric lifetime and hence the concentration of greenhouse gases such as methane. Finally, VOCs directly and indirectly affect the radiative balance of the earth-atmosphere system through contributing to the formation and growth of primary and secondary organic aerosols [Kulmala et al., 2004; Ramanathan et al., 2001]. Changes in the emission and uptake of VOCs from/to the earth’s surface thus have the potential to modify climate [Kanakidou et al., 2005].
Methanol (CH3OH) is the second most abundant VOC in the troposphere after methane [Custer and Schade, 2007; Jacob et al., 2005] and represents nearly 20 % of total VOC emissions [Guenther et al., 1995], with typical concentrations in the range of 1-10 ppbv in the continental boundary layer [Singh et al., 1995; Heikes et al., 2002]. Methanol is known to play a crucial role in atmospheric chemistry [Folberth et al., 2006; Folkins and Chatfield, 2000; Lary and Shallcross, 2000; Singh et al., 1995; Sommariva et al., 2005]. It is a significant atmospheric source of formaldehyde [Riemer et al., 1998; Palmer et al., 2003] and carbon monoxide [Duncan et al., 2007] and plays a minor role in the carbon cycle [Heikes et al., 2002]. Compared to other volatiles, the lifetime of methanol (5-12 days) is relatively long [Galbally and Kirstine, 2002; Heikes et al., 2002; Tie et al., 2003; Jacob et al., 2005], and can play a significant role in controlling tropospheric oxidants in the upper troposphere [Tie et al., 2003].
Sources of methanol are thought to be primarily biogenic (40 - 80%, Millet et al., 2008; Heikes et al., 2002; Jacob et al., 2005] and numerous studies have reported green plant and soil sources of methanol [Brunner et al., 2007; Cojocariu et al., 2004; Custer and Schade, 2007; de Gouw et al., 2000; Fukui and Doskey, 1998; Karl et al., 2001a, 2001b, 2002, 2003, 2005; Kirstine et al., 1998; Schade and Goldstein, 2001; Spirig et al., 2005; Warneke et al., 1999, 2002; Rinne et al., 2005; Schade and Custer, 2004; Baker et al., 2001]. Estimates of the global terrestrial plant source of methanol ranging from 77 to 312 Tg a−1 are still highly uncertain [Jacob et al., 2005; Millet et al., 2008].
Plants are thought to release methanol mainly as a by-product of pectin demethylation during leaf growth [Fall and Benson, 1996; Galbally and Kirstine, 2002; Hüve et al., 2007], resulting in significant seasonal variations in atmospheric methanol concentrations [Tie et al., 2003]. In accordance with these findings, MacDonald and Fall [1993] reported high emission rates for young growing leaves as opposed to mature ones. Note that while the substrate, pectin, is the same, this process is different from the recently reported emission of methane (CH4) under oxic environmental conditions after pectin irradiation by UV, where ester methyl groups of pectin can serve as a precursor of CH4 [Keppler et al., 2008].
Methanol emissions are temperature and light dependent; in addition stomatal opening plays an important role in explaining observed emission patterns [Folkers et al., 2008; Hüve et al., 2007; Custer and Schade, 2007; Brunner et al., 2007; Karl et al., 2005; Schade and Goldstein, 2001]. The sensitivity of different VOCs to changes in stomatal conductance (gs) depends on the equilibrium gas/liquid-phase distribution coefficient H (Henry’s law constant, Pa m3 mol−1) which is compound-specific and temperature dependent. Compounds with high water solubility like methanol have a low H value, therefore large increases in aqueous-phase concentrations are needed to support an increase in gas-phase concentrations [Niinemets and Reichstein, 2003]. Accordingly, stomata can constrain the emission of more soluble compounds like methanol over a longer time period than the emission of less soluble volatiles, resulting in a direct effect of gs on the efflux rate of methanol [Nemecek-Marshall et al., 1995].
Sinks of methanol are highly uncertain as well and are estimated to be in the range of 107 to 284 Tg a−1 [Jacob et al., 2005], with the main sink being the gas-phase oxidation by OH [Schade et al., 2008], which takes place on a timescale of approximately 10 days [Millet et al., 2008]. Minor contributions are from the uptake of methanol by the ocean [Sinha et al., 2007; Mao et al., 2006] for which recent studies estimate a 10 to 15 Tg a−1 sink [Singh et al., 2004; Jacob et al., 2005] and wet and dry deposition to land [Karl et al., 2004, 2005; Jacob et al., 2005; Talbot et al., 2005; Mao et al., 2006], which may only be observable at night as methanol emissions are regulated by gs [Schade et al., 2008]. The processes responsible for the uptake of methanol in plant canopies are still unclear, but generally trace gas uptake in soils is microbially mediated when methanol diffuses to active methylotrophic microorganism sites in the soil, a process which in turn is affected by physical parameters such as soil texture and water filled pore space (e.g. Smith et al., 2003; Yonemura et al., 2000). Methylotrophic bacteria can also be found on leaf surfaces [Fall and Benson, 1996] and may contribute to observed methanol deposition fluxes.
Globally, grasslands cover around 40% of the ice-free terrestrial surface [Ramankutty and Foley, 1999]. In the Alps, grassland farming (grazing by domestic ungulates and cutting for hay production) is the dominant agricultural practice consuming >85% of the available agricultural land in 75% of the municipalities [Tappeiner et al., 2008]. In contrast to many forest ecosystems, whose VOC fluxes are dominated by terpenoids [Rinne et al., 2007], methanol is the major VOC emitted from grasslands not only after cutting when large amounts of various VOCs escape to the atmosphere from the severed plant material [Davison et al., 2008; Ruuskanen et al., 2010], but also during undisturbed conditions [Fukui and Doskey, 1998; de Gouw et al., 2000; Karl et al., 2001a, 2001b; Warneke et al., 2002; Karl et al., 2005; Brunner et al., 2007; Bamberger et al., 2010].
The uncertainties in global sources and sinks of methanol [Millet et al., 2008] are in apparent contradiction to the well-established understanding of the processes and pathways involved in the production of methanol in and its emission from leaves [Fall and Benson, 1996] which reflects difficulties in transferring leaf-level laboratory studies to field conditions and the ecosystem level [Niinemets et al., 2010]. The objective of the present paper is thus to test whether the controls on methanol fluxes reported at leaf level and often under controlled environmental conditions are applicable under in situ conditions at ecosystem scale. To this end we quantified the diurnal, seasonal and inter-annual variability of methanol exchange and analyzed the data with regard to the major biotic and abiotic controls. Based on the above-mentioned leaf-level studies we hypothesized that (i) air temperature would represent the major abiotic driver of methanol emissions and (ii) gs and plant growth would constitute the key biotic controls on the emission of methanol. As previous studies over grassland (e.g. Karl et al., 2001a,b) have shown emissions of various compounds to be elevated immediately after cutting we further hypothesized that (iii) during cutting (leaf wounding) events and subsequent drying high amounts of methanol are emitted into the atmosphere. We selected the study site Neustift, a temperate mountain grassland in Austria, because it is cut three times per year for hay production and has been described extensively in terms of the effects of cutting on the net ecosystem CO2 and energy exchange (Hammerle et al., 2008; Wohlfahrt et al., 2008).
2 Methods
2.1 Site description
The study site is located at an intensively managed meadow in the middle of the flat valley bottom at an elevation of 970 m a.s.l. near the village of Neustift (47°07′N, 11°19′E) in the Stubai Valley (Austria). The fetch is homogeneous up to 300 m to the north-north-east and 900 m to the south-south-west of the instrument tower, parallel to the Valley’s orientation and the dominant daytime and nighttime wind directions, respectively. During the night wind velocities are usually low and the atmosphere shows stable stratification. This resulted in a larger footprint than during daytime, where conditions were typically unstable and wind velocities were higher [Bamberger et al., 2010].
The climate in Stubai valley is humid continental with alpine influences characterized by an average annual temperature of 6.5 °C and an average annual precipitation of 852 mm. The vegetation of the meadow consists mainly of only a few dominant graminoids (Dactylis glomerata, Festuca pratensis, Phleum pratensis, Trisetum flavescens) and forbs (Ranunculus acris, Taraxacum officinale, Trifolium repens, Trifolium pratense, Carum carvi), while coniferous forest is the predominant vegetation type on the slopes of the surrounding mountains. The meadow is cut and harvested three times a year, with cuts taking place at the beginning of June and August and at the end of September. Fertilization takes place once per year, typically at the end of October by manure spreading.
Measurements were conducted from 22 May until 20 November 2008 (182 days), and continued the following year starting on 19 March 2009, some days before snowmelt, until 11 December 2009 (267 days).
2.2 Eddy covariance measurements
The net ecosystem methanol exchange was measured using the virtual disjunct eddy covariance (vDEC; Karl et al., 2002) method that is based on the eddy covariance method [Baldocchi et al., 1988]. The three wind components and the speed of sound were measured with a three-dimensional sonic anemometer (R3IA, Gill Instruments, Lymington, UK) 2.5 m above ground, while methanol volume mixing ratios were simultaneously detected by a PTRMS at m/z 33. The sample inlet was 0.1 m below the anemometer. Sample air went through a particulate filter (1-2 μm, PTFE), was drawn through a 16 m (2008) / 12 m (2009) PFA Teflon tube with 0.004 m inner diameter and was finally analyzed for VOC concentrations by the PTR-MS. To minimize interactions between tube walls and sample air, the inlet line was heated to 40 °C (2008) / 35 °C (2009). The flow rate was held constant at 8 sl min−1 (standard liter per minute; air volume normalized to standard temperature and pressure conditions: 273 K, 1013 hPa). The 20 Hz sonic anemometer data were stored to a hard drive of a personal computer using the Eddymeas software (O. Kolle, Max Planck Institute for Biogeochemistry, Jena, Germany).
2.3 PTR-MS setup
For the measurement of selected VOCs a high-sensitivity PTR-MS was deployed in a container next to the field site during the measurement campaigns. The PTR-MS method is described in detail by Hansel et al. [1995] and Lindinger et al. [1998] and was used to analyze ambient air for a number of VOCs. In 2008, 13 species of VOCs were measured sequentially, resulting in a repetition rate of 2.82 s until 10 July, after which it was changed to 3.00 s (15 species) until 6 November 2008 and after that to 1.80 s (8 species) until 20 November. In 2009 the repetition rate was 2.25 s until 6 April (12 species) and then 2.35 s until 11 December (13 species). The dwell time for methanol was 0.5 s / 0.2 s during 2008 / 2009. In addition to the air sampling, a pump continuously flushed 500 ml of ambient air through a home-built catalytic converter (350 °C) to produce VOC-free zero-air, which was switched into the PTR-MS during the last five minutes of every half-hour period to determine the instrumental background (zero calibration). Sensitivities of the PTR-MS were calibrated once a week in 2008 and every 50 hours in 2009 by adding a multi-component gas standard containing VOCs in N2 (Apel Riemer Inc., USA) to VOC-free air at ambient humidity. An automated routine calibrated the PTR-MS with the flow of the gas standard adjusted to 1 sccm (standard cubic centimeters per minute), 2.5 sccm, 5 sccm and 7.5 sccm, respectively, and diluted with 500 sccm scrubbed ambient air. The known calibration gas mixture was analyzed by the PTR-MS and sensitivities were determined from linear regression of the known VOC volume mixing ratios and the respective signal intensities (see Bamberger et al., 2010). Typical calibration factors for methanol were 12 ncps (normalized counts per second) ppv−1. The PTR-MS was operated at a drift tube pressure of 2.15 mbar / 2.3 mbar and a drift voltage of 550 V / 600V during 2008 / 2009. Data were stored in 30-min files and processed to VOC volume mixing ratios in ppbv using a homemade program based on MATLAB 7.4.0 (R2007a, The MathWorks, Inc, USA). Detailed information regarding setup, calibration and operation of the instrument was presented by Bamberger et al. [2010].
2.4 Flux calculations
Due to the zero calibration, half-hourly flux calculations are based on measurements over the first 25 minutes of each 30 minute period. Methanol fluxes were calculated as the covariance between the turbulent departures of the vertical wind speed and the methanol volume mixing ratios using the post-processing software EdiRe (University of Edinburgh). Means and turbulent departures were calculated by Reynolds (block) averaging.
Because of the sequential measurement of VOCs by the PTR-MS the concentration time series of a specific compound is disjunct and its time resolution lower than that of the 20 Hz wind data. Fluxes were therefore calculated by means of the vDEC method [Karl et al., 2002] by using a subsample of the horizontal wind data as given by the sampling rate of the PTRMS. As shown by Hörtnagl et al. [2010] the vDEC method does not result in a systematic bias, but causes the random flux uncertainty to increase as compared to 20 Hz sampling. The random methanol flux uncertainty, based on the methods of Hollinger and Richardson [2005], has been presented previously by Bamberger et al. [2010].
A three axis coordinate-rotation was performed by aligning the coordinate system’s vector basis with the mean wind streamlines [Kaimal and Finnigan, 1994]. The tubing induced time delay of the VOC signals was determined by optimizing the correlation coefficient with the vertical wind velocity [McMillen, 1988] within a time window of +/− 50 s. For methanol the determination of the time delay worked well and the frequency distribution of the lag times showed a peak around 1.5 s, which is slightly longer than the expected time delay based on tube length, diameter and flow rate. A second lag window of ± 3 s around the peak of the distribution was defined. If the inferred lag time was outside this time window, it was set to the peak value of the frequency distribution (i.e. 1.5 s). As shown by Bamberger et al. [2010] the raw methanol fluxes were then corrected for high-pass (block averaging) and low-pass (lateral sensor separation, dynamic frequency response, scalar and vector path averaging, frequency response mismatch and the attenuation of concentration fluctuations down the sampling tube) filtering according to Moore [1986], Massman [2000] and Aubinet et al. [2000]. Frequency-response corrections were based on a site-specific model cospectrum described by Wohlfahrt et al. [2005]. Instrumentation, data treatment and quality control of CO2, sensible and latent heat fluxes have been described by Wohlfahrt et al. [2008] and Hammerle et al. [2008]. The surface conductance to water vapor (gsurf) was calculated after Wohlfahrt et al. [2009] and is used as a proxy for the canopy-integrated stomatal conductance in the following.
2.5 Quality control
Half-hourly methanol fluxes were subjected to several quality control tests. Data were excluded from further analysis if (i) the third rotation angle exceeded 10° [McMillen, 1988], (ii) the stationarity test for methanol fluxes exceeded 60 % [Foken and Wichura, 1996], (iii) the deviation of the integral similarity characteristics was larger than 60 % [Foken and Wichura, 1996], (iv) the maximum of the footprint function [Hsieh et al., 2000] was outside the boundaries of the meadow, (v) the measured background signal of methanol was higher than its ambient concentration (averaged over half an hour) and (vi) the background drift wasgreater than the sum of the standard deviations of the two adjacent background concentration measurements within an hour. If the difference between a specific data point and the averaged signal of a certain half-hour was higher than 20 times the theoretical standard deviation (noise) of the signal, the data point was defined as an outlier. Half-hours with more than five outliers were flagged and not used for further analysis, except for during and one day after cutting events, when large fluctuations in methanol concentrations were found to be physically realistic. The flux detection limit was calculated according to Karl et. al. [2002] on a half-hourly basis and used as a post-processing quality control criterion, i.e. half-hourly fluxes were rejected if below the detection limit. All fluxes that passed the above-mentioned quality criterion exceeded the flux detection limit. During typical daytime and nighttime conditions the flux detection limit amounted to 0.07 and 0.02 nmol m−2 s−1, respectively. Over the course of the two measurement campaigns 17757 half-hourly fluxes for methanol were recorded, of which 13880 (78 %) passed all quality tests and were used in the subsequent analysis.
2.6 Ancillary data
Meteorological measurements were collected continuously by a data logger (CR10X, Campbell Scientific, Logan, UT, USA) and included total and diffuse photosynthetically active radiation (PAR) (BF3H, Delta-T, Cambridge, UK), air temperature (Tair) and humidity at 2 m height measured by the means of a combined temperature/humidity sensor (RFT-2, UMS, Munich, Germany), soil temperature (Tsoil) at 0.05 m depth (TCAV thermocouple, Campbell Scientific, Logan, UT, USA), volumetric soil water content (ML2x, Delta-T Devices, Cambridge, UK) and precipitation (52202, R. M. Young, Traverse City, MI, USA). The green plant area index (GAI) was assessed (i) in a destructive fashion by clipping of square plots of 0.09 m2 (3-5 replicates) and subsequent plant area determination (Li-3100, Li-Cor, Lincoln, NE, USA) and (ii) from measurements of canopy height which was related to destructively measured GAI [Wohlfahrt et al., 2008]. Continuous time series of the GAI were derived by fitting appropriate empirical functions, separately for each growing phase, to measured data. The daily change in GAI, referred to as dGAI, is the difference between subsequent daily values and used as a surrogate for growth. For a more detailed list of all auxiliary parameters measured at this site see Hammerle et al. [2008] and Wohlfahrt et al. [2008].
2.7 Statistical analyses
Statistical analyses were performed using Statistica 9 (StatSoft, Inc.) and SigmaPlot 11 (Systat Software, Inc.). The partial correlation reported in the present study is a measure of the correlation between two variables that remains after controlling for the effects of one or more other predictor variables. The tolerance of a variable as given in Table 2 is defined as 1 minus the squared multiple correlation of this variable with all other independent variables in the regression equation and is a measure of how redundant the contribution of a variable is as compared to the contribution of the other independent variables. The higher the tolerance of a variable, the more unique is its contribution to the regression equation.
Table 2. Regression analysis of the methanol flux a.
| simple linear regression |
multiple linear regression |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Half-hourly |
Daily Average |
Half-hourly |
Daily Average |
|||||||||||||||
| N | r | p | N | r | p | partial correlation | tolerance | p | partial correlation | tolerance | p | |||||||
| NEE | 5503 | −0.44 | *** | 228 | −0.08 | ns | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| latent heat flux | 5503 | 0.63 | *** | 219 | 0.71 | *** | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| sensible heat flux | 5503 | 0.32 | *** | 235 | −0.01 | ns | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| GAI | 5503 | -- | -- | 321 | 0.39 | *** | -- | -- | -- | -- | -- | -- | 0.10 | (0.11) | 0.64 | (0.64) | ns | (ns) |
| dGAI | 5503 | -- | -- | 321 | 0.53 | *** | -- | -- | -- | -- | -- | -- | 0.12 | (0.18) | 0.51 | (0.51) | ns | (*) |
| SWC | 5503 | −0.36 | *** | 321 | −0.60 | *** | −0.12 | (0.00) | 0.69 | (0.59) | *** | (ns) | 0.00 | (0.18) | 0.51 | (0.46) | ns | (*) |
| fdif | 5503 | −0.43 | *** | 321 | −0.25 | *** | 0.10 | (0.11) | 0.32 | (0.32) | *** | (***) | 0.20 | (0.14) | 0.21 | (0.21) | * | (ns) |
| b gsurf | 3954 | 0.34 | *** | 219 | 0.35 | *** | 0.08 | (0.15) | 0.79 | (0.75) | *** | (***) | 0.00 | (0.15) | 0.79 | (0.73) | ns | (ns) |
| VPD | 5503 | 0.50 | *** | 321 | 0.68 | *** | −0.25 | (-0.17) | 0.20 | (0.19) | *** | (***) | 0.04 | (0.06) | 0.14 | (0.14) | ns | (ns) |
| b Tsoil | 5503 | 0.45 | *** | 321 | 0.72 | *** | −0.06 | (0.00) | 0.24 | (0.23) | *** | (ns) | 0.01 | (0.11) | 0.12 | (0.11) | ns | (ns) |
| b Tair | 5503 | 0.60 | *** | 321 | 0.82 | *** | 0.32 | (0.19) | 0.10 | (0.08) | *** | (***) | 0.29 | (0.11) | 0.07 | (0.06) | *** | (ns) |
| b PAR | 5503 | 0.60 | *** | 321 | 0.63 | *** | 0.36 | (0.39) | 0.29 | (0.29) | *** | (***) | 0.28 | (0.16) | 0.14 | (0.13) | *** | (*) |
| methanol VMR | 5503 | 0.41 | *** | 321 | 0.80 | *** | -- | (0.30) | -- | (0.52) | -- | (***) | -- | (0.49) | -- | (0.28) | -- | (***) |
|
|
|
|
||||||||||||||||
| whole model r2 | 0.52 (0.56) | 0.74 (0.80) | ||||||||||||||||
Correlation coefficients of a simple linear regression analysis and partial correlations, tolerance and significance of a multiple linear regression analysis, using half-hourly and daily average values of net ecosystem CO2 exchange (NEE), latent and sensible heat flux, green plant area index (GAI), change in green plant area index (dGAI), soil water content (SWC) at 0.05 m soil depth, fraction of diffuse radiation (fdif), surface conductance (gsurf), vapor pressure deficit (VPD), soil temperature (Tsoil) at 0.05 m soil depth, air temperature (Tair), photosynthetically active radiation (PAR) and methanol volume mixing ratio (VMR) as independent variables. For the multiple regression analysis 3154 half hourly and 169 daily average data points were used, respectively. Numbers in parenthesis refer to the analysis with the methanol concentration included in the multiple regression equation. Management events were excluded from the analysis.
number of cases>
correlation coefficient
significance level:
…p < 0.05
…p < 0.001, ns…not significant
…linearized
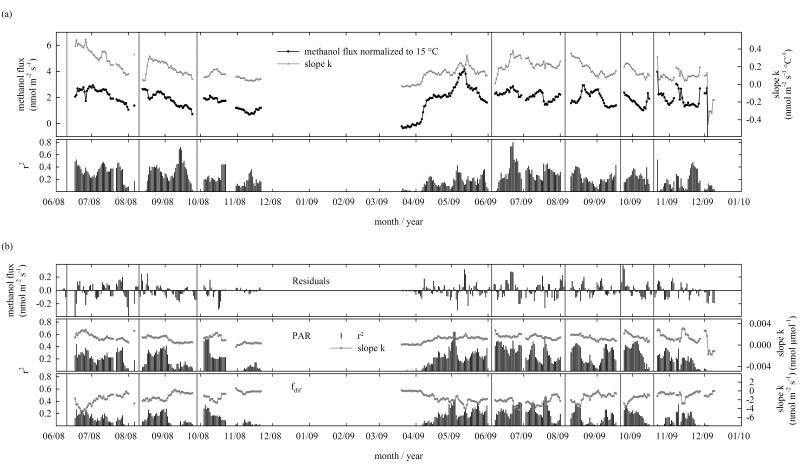
For the regression analyses in Table 2 some independent variables (gs, Tsoil, Tair, PAR) were linearized using the equation y = xn [Lozán, 1992]. In order to linearize data for the analysis shown in Figure 5 (i.e. the relationship between air temperature and methanol flux), the natural logarithm of the observed methanol flux was calculated, and data were then fitted using the equation ln(Fmethanol) = k Tair + d.
Figure 5. Seasonal and interannual variability in the temperature sensitivity of the methanol flux for moving time windows of 7 days, with each following data point being shifted by one day.
(a) Methanol flux normalized to an air temperature (Tair) of 15 °C, the slope of a linear regression between air temperature and the log-transformed methanol flux (k) and r2 which shows the variance of the observed methanol flux that can be explained by Tair. (b) The residuals show the difference between the observed and the predicted (based on Tair) flux, while r2 shows to what extent the observed variance of the residuals can be explained in a linear regression by photosynthetically active radiation (PAR) or fraction of diffuse radiation (fdif), other independent variables were not significant. Vertical lines show management dates.
3 Results
3.1 Environmental conditions
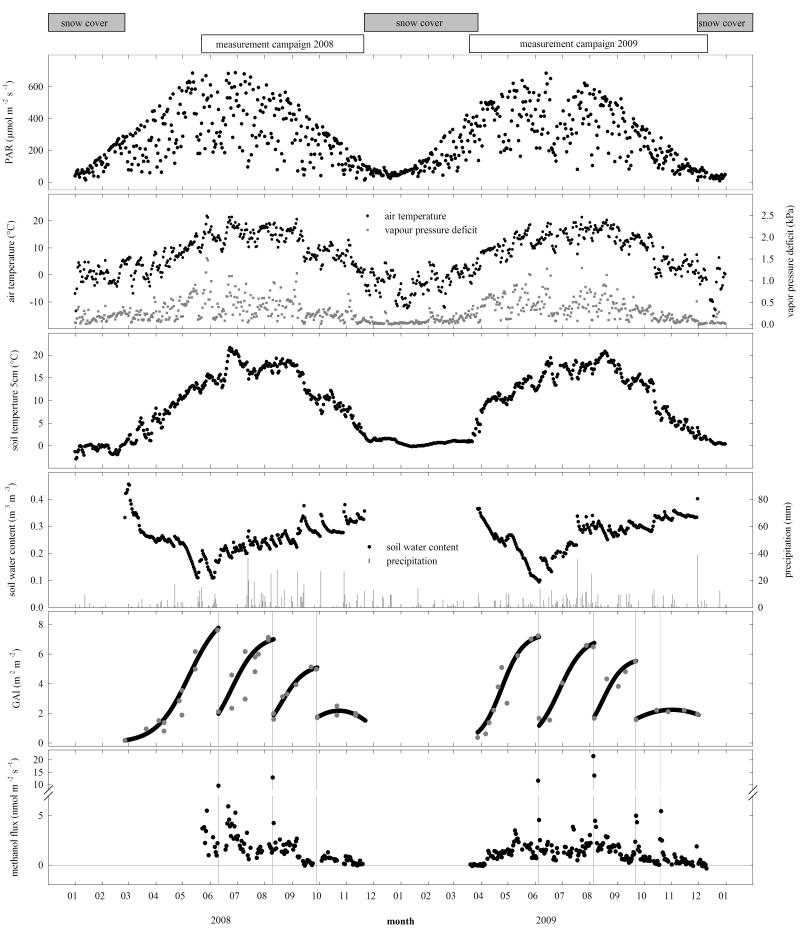
With annual averages of 7.1 °C and 6.8 °C in 2008 and 2009, respectively, Tair was close to the 2001-2007 average of 6.7 °C. Total precipitation was 648 mm a−1 in 2008 and 638 mm a−1 in 2009, well below the 2001-2007 average of 765 mm a−1. Over the course of the measurement campaigns, precipitation was registered on 77 and 124 days in 2008 and 2009, respectively, with a total precipitation of 458 and 498 mm (Figure 1). Monthly Tair was very similar to the long-term trend, with the exception of March 2009 being cooler and April and November 2009 being warmer than the average. Differences between the two measurement years were much more evident for rainfall. June 2008 was exceptionally dry and showed only one third of the rainfall compared to the long-term average, while the amount of precipitation recorded in June 2009 was 50 % above the average (Figure 1). Rainfall in September and October 2008 as well as in November 2009 was higher by 30 %, while March to May and August 2009 received only half of the average rainfall. PAR was very similar in both years with maximum daily sums of 59 mol m−2 d−1. A notable difference was a strong decrease of PAR at the end of June 2009 that showed maximum daily sums of around 20 mol m−2 d−1, whereas values were around 50 mol m−2 d−1 in June 2008 (Figure 1). Daily average Tsoil were around 0 °C during snow cover and increased rapidly by up to 3 °C per day right after snowmelt in 2009 (Figure 1), while the increase was less steep and occurred about one month earlier in 2008. The drying phase of the soil before the 1st cut was similar in both years.
Figure 1. Daily averages of photosynthetically active radiation (PAR), air temperature, vapour pressure deficit, soil temperature and water content at 0.05 m soil depth, precipitation, green plant area index (GAI) and measured methanol flux over the whole measurement campaign during 2008 and 2009.
Vertical lines show management dates.
The maximum values of GAI decreased from the 1st to the 3rd cut in both years. GAI was close to zero right after snowmelt, increased to up to 7.8 m−2 m−2 before cutting and was reduced to less than 2.0 m−2 m−2 due to harvesting (Figure 1). After the 3rd cut GAI first increased and later decreased.
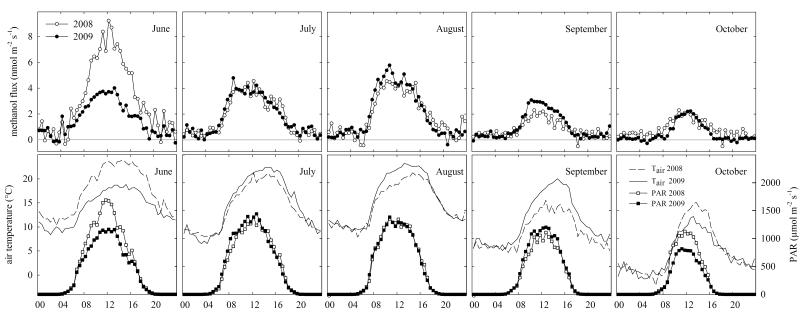
The months from June to October were captured in full in both years and monthly diurnal cycles of Tair and PAR are compared in Figure 2. June 2008 was considerably warmer than 2009, with mean Tair of up to 23.9 °C as compared to 18.8 °C in 2008, while also mean PAR was significantly higher.
Figure 2. Average diurnal cycles of methanol fluxes, air temperature and photosynthetically active radiation (PAR) in June, July, August, September and October 2008 and 2009.
The calculation of Tair and PAR is based on half-hourly values when the methanol flux was measured. Management events were excluded from the calculation.
3.2 Seasonal and inter-annual variability of methanol fluxes
Methanol emissions were close to zero as long as snow covered the ground and for about one week thereafter (spring 2009; Figure 1). The earliest distinct methanol emissions after snowmelt were measured on 8 April 2009 with a daily average of 0.6 nmol m−2 s−1. Afterwards, emissions continued to increase as the weather became warmer. In absence of management actions highest fluxes were recorded during warm periods with daily average temperatures > 20 °C, when average methanol emissions reached up to 5.9 nmol m−2 s−1 (Figure 1). The grass cut increased the daily average fluxes of methanol to values ranging from 9.6 to 21.5 nmol m−2 s−1 (Figure 1). These emissions decreased rapidly on the days following the cut, reaching pre-cut values of around 2 nmol m−2 s−1 after about three days (Figure 1). Another emission peak was found during manure spreading on 20 October 2009 (5.4 nmol m−2 s−1; Figure 1).
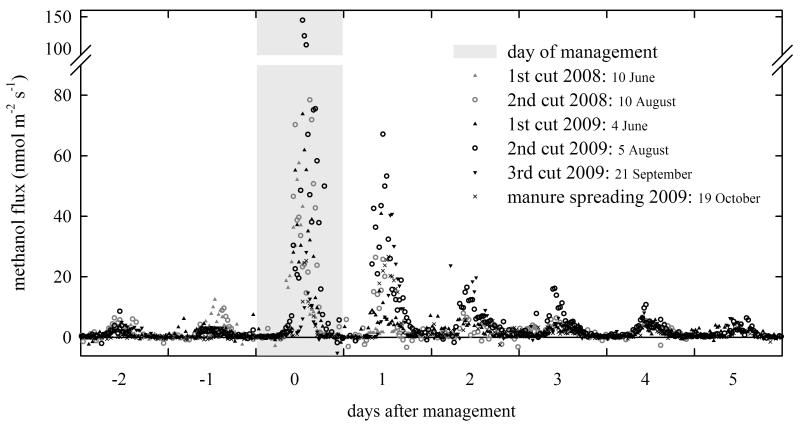
The influence of management activities on half-hourly emissions of methanol is shown in detail in Figure 3 for both years, while corresponding numbers are given in Table 1. On cutting days, maximum fluxes between 40.7 - 144.5 nmol m−2 s−1 were measured. Emissions were far lower one day later, with maximum values ranging from 2.8 nmol m−2 s−1 in June 2008 – a day with a relatively low maximum Tair of 18.8 C° - to 67.1 nmol m−2 s−1 in August 2009. This was similar on the days thereafter, with methanol emissions further decreasing before reaching pre-cut peak values of about 5 nmol m−2 s−1 by the fourth day after the cut (Figure 3).
Figure 3. Half-hourly methanol fluxes before, during and after different management events during the measurement campaigns.
Table 1. Maximum methanol fluxes before, during and after management events a.
| Days after management |
1st cut |
2nd cut |
1st cut |
2nd cut |
3rd cut |
Fertilization |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June 2008 | August 2008 | June 2009 | August 2009 | September 2009 | October 2009 | |||||||
| −2 | 4.1 | (17.6) | 6.4 | (19.4) | 3.9 | (16.6) | 8.6 | (17.0) | 5.0 | (23.7) | 1.2 | (4.3) |
| −1 | 12.5 | (23.4) | 9.6 | (17.4) | 7.5 | (18.6) | 2.4 | (14.2) | 3.3 | (21.5) | 1.1 | (3.8) |
| 0 | 57.6 | (24.5) | 78.4 b | (24.6) | 73.8 | (18.6) | 144.5 b | (22.2) | 28.1 b | (21.9) | 25.3 | (4.9) |
| 1 | 3.8c | (18.6) | 26.4b | (26.5) | 40.9 | (21.3) | 67.1b | (25.4) | 40.7b | (22.0) | 26.7 | (9.9) |
| 2 | -- | 7.9 | (23.9) | 6.9 | (20.4) | 16.5 | (26.4) | 23.7b | (21.9) | 7.5 | (14.1) | |
| 3 | -- | 6.4 | (16.9) | 5.2 | (17.5) | 16.2 | (24.2) | 6.4 | (20.8) | 3.4 | (12.9) | |
| 4 | -- | 6.6 | (23.3) | 5.8 | (19.9) | 10.8 | (21.9) | 8.2 | (19.8) | 5.1 | (10.6) | |
| 5 | -- | -- | 3.9 | (19.3) | 6.4 | (20.9) | 6.3 | (20.8) | 4.1 | (9.7) | ||
Flux rates are reported in nmol m−2 s−1. Numbers in brackets give the maximum air temperature in °C for the respective day
…grass left on field for drying
…day not fully captured by measurements
The effect of fertilization could be captured in October 2009, when the spreading of manure resulted in peak emissions of 25.3 nmol m−2 s−1 on the day of the spreading and even higher emissions of up to 26.7 nmol m−2 s−1 one day later (Figure 3). Those numbers are nearly nine times higher than observed emissions during the days before the manure was applied, when peak values of around 3 nmol m−2 s−1 were found (data not shown). However, the two days before manure spreading - as shown in Figure 3 and Table 1 - were characterized by cold weather with maximum Tair of 5 °C and showed the lowest methanol emissions in October. The immediate influence of fertilization on methanol emissions can be seen in form of elevated peak emissions for four days after the spreading (Figure 3).
Average diurnal cycles of methanol fluxes not related to management actions are shown for both years in Figure 2. Methanol fluxes exhibited a clear diurnal cycle with close-to-zero fluxes during nighttime and emissions which followed the diurnal course of incident PAR and Tair during daytime (Figure 2). Tair corresponded well with the methanol flux on a monthly basis, especially when comparing the two years. June 2008 was the warmest month during the measurement campaigns and showed the highest average methanol emissions with up to 9.2 nmol m−2 s−1 as compared to 4.0 nmol m−2 s−1 in June 2009 when Tair was considerably lower (Figure 2). A similar pattern can be seen in August and September 2009, where air temperatures were warmer and methanol emissions higher than the year before, and to a lesser extent in July. However, in October 2009, when the growing season was coming to an end, warmer conditions did not result in higher methanol emissions (Figure 2).
Between June and October, methanol emissions resulted in the meadow losing a total of 196 mg C m−2 over 103 days in 2008 and 255 mg C m−2 over 134 days in 2009 - the average daily loss (1.9 mg C m−2 d−1) being exactly the same in both years.
3.3 Controls on methanol exchange
In order to explore the controls on methanol exchange we conducted simple and multiple linear regression analyses based on the half-hourly and daily average data (Table 2).
Simple linear regression resulted in equally high positive correlations with the latent heat flux, Tair and PAR, while negative correlations were found for the net ecosystem CO2 exchange (NEE), soil water content at 0.05 m soil depth (SWC) and the fraction of diffuse radiation (fdif). All regressors were highly significant at the significance level p < 0.001 (Table 2). When using daily averages of the same variables, correlations increased for the latent heat flux, Tair and PAR. On a daily timescale the vapor pressure deficit (VPD), Tsoil in 5 cm depth and dGAI became important factors in describing the methanol exchange (Table 2). However, the largest difference was found for the methanol volume mixing ratio (VMR), which was positively correlated with the methanol flux and doubled its influence on a diurnal timescale. To a lesser extent, this was also the case for SWC, the negative correlation of which increased considerably. The longer timescale resulted in close-to-zero correlations for NEE and sensible heat, which were the only non-significant regressors (Table 2).
The multiple linear regression analysis confirmed the dominating influence of Tair and PAR on both timescales. When the methanol concentration was not factored in and based on half-hourly values, Tair and PAR resulted in similarly high partial correlations. When compared to the simple linear regression, the partial correlations of VPD changed in sign and became negative (Table 2). The analysis resulted in gsurf having the highest tolerance value, underlining its unique contribution to the chosen set of variables, followed by SWC. On the half-hourly timescale all regressors were highly significant.
Using daily averages, Tair and PAR remained the most important drivers for methanol exchange and were also the only highly significant variables. Along with these two variables, only fdif resulted in a significance of p < 0.05 and a high partial correlation, the latter being positive as opposed to the negative correlation found in the simple linear regression analysis. Generally, tolerance values hardly changed on the daily timescale as compared to the half-hourly values (Table 2).
In a second step, the methanol VMR was included in the multiple linear regression equation. Compared to the analysis without methanol VMR factored in and using half-hourly values, the influence of Tair decreased considerably (Table 2). Methanol concentration had the second highest partial correlation after PAR and its tolerance value was one of the highest observed. Except for SWC and Tsoil, all variables were highly significant (Table 2). When methanol VMR was factored in on a daily timescale, partial correlations changed considerably. The highest influence on the measured methanol flux was found for the methanol VMR, also the only regressor that was highly significant. In comparison to the same analysis without the methanol VMR, the importance of Tair and PAR diminished clearly, with the former becoming statistically insignificant (Table 2). In contrast, dGAI and SWC became significant, while for fdif the opposite was observed. Tolerance values remained similar for all variables, with dGAI and SWC providing the least redundant contribution to the regression. However, for the methanol concentration the tolerance value on a daily timescale was clearly lower than when using half-hourly values.
The multiple linear regression analysis in Table 2 resulted in all variables combined being able to explain 52 % and 74 % of the observed variance of the measured methanol flux at the half-hourly and daily timescale. When methanol concentration was factored in, the corresponding numbers were somewhat higher (56 % and 80 %, respectively).
With PAR and Tair identified as the main drivers for methanol emissions, Figures 4a and 4b further examine their joint influence using half-hourly values. The measured methanol flux increased linearly with PAR and showed higher sensitivity to PAR as air temperatures increased (Figure 4a). Methanol emissions increased exponentially with Tair and at a given air temperature the measured flux was higher during periods with high PAR values (Figure 4b). Figure 5a shows the seasonal and inter-annual variation of the temperature sensitivity of the methanol flux for 7-day moving time windows. Throughout the measurement campaigns, the methanol flux normalized to 15 °C changed continuously (Figure 5a). In 2008, maximum values were found in June and July. Efflux was minimal right after snowmelt in 2009, but increased over the following weeks until it peaked in mid-May. Generally, the methanol flux normalized to 15 °C decreased towards the end of the growing season in both years, a pattern which was more pronounced in 2008 than in 2009. Figure 5a also shows the slope k of a linear regression between air temperature and the (log-transformed) methanol flux as a measure of its temperature sensitivity. In 2008, highest sensitivities were found directly after the 1st cut and decreased afterwards. Although the temperature sensitivity showed distinct peaks in certain 7-day segments, the general pattern was less pronounced in 2009 than in 2008. Generally, the methanol flux normalized to 15 °C and the temperature sensitivity were in phase and showed a positive correlation, although there were certain time windows throughout the measurement campaigns when this was not the case. One example is the time period directly after the 2nd cut in August 2009, when the methanol flux normalized to 15 °C and the temperature sensitivity were negatively correlated. The r2 given in Figure 5a shows the variance of the observed methanol flux that can be explained by Tair, which was found to be at least 25 % for more than half of the 7-day periods, with a maximum of 80 % in June 2009.
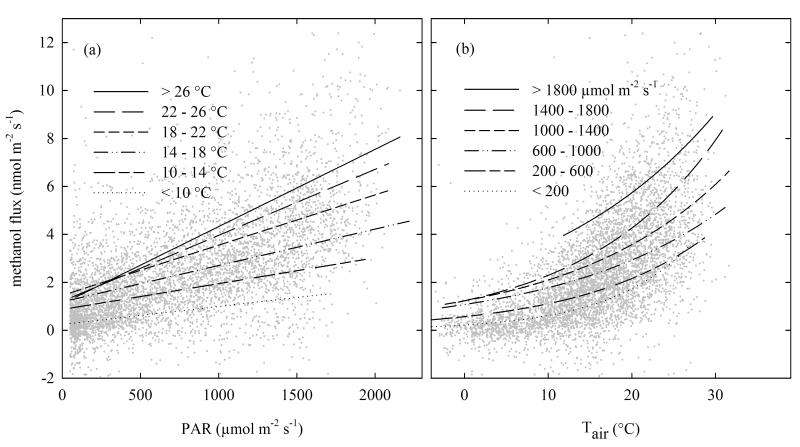
Figure 4. Methanol flux as a function of photosynthetically active radiation (PAR, (a)) for different classes of air temperature (Tair) and as a function of Tair (b) for different classes of PAR on a half-hourly timescale.
Management events, wet canopy conditions and half-hours with PAR below 50 μmol m−2 s−1 were excluded from the analysis. Numbers for the regression lines are given in Table A1.
The residuals in Figure 5b show the difference between the observed and the predicted (based on temperature) methanol flux, where positive residuals show time periods during which the flux has been underestimated. The two lower panels show the resulting slope and r2 when the residuals are linearly related to PAR and fdif. During certain time periods the slopes of PAR and fdif were negatively correlated, while r2 showed a positive correlation of the two variables with the observed residuals, e.g. in June and July 2008 (Figure 5b). The median value for the explained residual variance for PAR and fdif was found to be 22 % and 13 %, respectively, with maximum values of 65 % and 48 %.
The methanol exchange over both growing seasons was simulated by fitting observed fluxes to (log-transformed) Tair in subsequent 7-day segments, resulting in varying values for sensitivity k and offset d for each segment, which were then used to simulate half-hourly fluxes based on measured air temperatures. The simulated cumulative carbon emission of 0.277 g C m−2 was slightly lower than the observed emission of 0.290 g C m−2. When k and d were derived from the pooled data and therefore constant for both years, the carbon loss amounted to 0.274 g C m−2, i.e. only 1 % below the value calculated based on temporally varying parameters. Note that days influenced by management events were not included in these calculations.
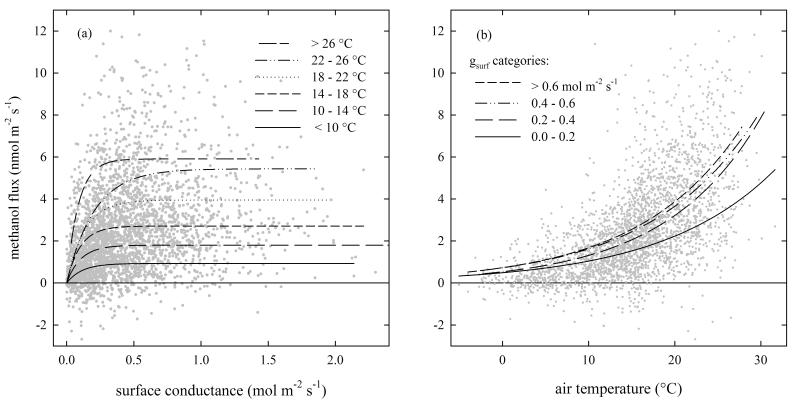
In the literature, Tair and gs have been identified as important drivers for methanol emissions at the leaf level [e.g. Hüve et al., 2007; Niinemets et al., 2004; Harley et al., 2007]. Figure 6a thus explores ecosystem-scale methanol emissions as a function of gsurf stratified according to Tair. The methanol flux was approximately linearly related to gsurf at low gsurf values and became independent of gsurf at higher values. The gsurf value up to which a linear relationship was observed rose with air temperature and was found at 0.2 mol m−2 s−1 for Tair < 10 °C and at 0.5 mol m−2 s−1 during warm periods with Tair between 22 – 26 °C (Figure 6a). Figure 6b shows the methanol flux as a function of Tair stratified into classes of surface conductance. Except for very low gsurf values, the temperature response is largely independent of gsurf.
Figure 6. (a) The influence of surface conductance (gsurf) on the observed methanol emissions in different classes of air temperature (Tair). (b) Methanol flux as a function of Tair, lines illustrate different classes of gsurf.
Management events, wet canopy conditions and half-hours with PAR below 50 μmol m−2 s−1 were excluded from the analysis. Numbers for the regression lines are given in Table A1.
4 Discussion
4.1 Magnitude of methanol exchange
Daily average fluxes depicted in Figure 1 and diurnal cycles of methanol fluxes in Figure 2 showed a general emission from the canopy into the atmosphere, which is in accordance with Brunner et al. [2007], who measured methanol fluxes over an intensively and extensively managed grassland in Switzerland. In the same study, maximum fluxes not related to management events reached up to 18.4 nmol m−2 s−1 over the extensively managed field, which is similar to the 17.9 nmol m−2 s−1 found in the present study (data not shown). Diurnal cycles of methanol as reported by Brunner et al. [2007] for the mature phase during August show peak emissions of 7 nmol m−2 s−1 around noon and confirm the findings in the present study, which showed average peak emissions of up to 6 nmol m−2 s−1 in August 2008 (Figure 2).
Brunner et al. [2007] calculated the cumulative methanol emissions during the mature phase of the extensive field about three weeks before cutting, which resulted in a total emission of 6.3 mg C m−2 over 6 days. This compares well with our findings of 8.5 and 7.7 mg C m−2 during similar time periods in July (2008 and 2009, respectively). However, the field site in Neustift still showed strong growth rates during this phase, whereas the LAI reported by Brunner et al. [2007] increased only slightly during the 6-day period.
The highest methanol emissions over the course of the whole measurement campaign were consistently measured on the day of cutting and the days thereafter, when methanol stored inside severed plant cells escaped to the atmosphere [Niinemets et al., 2004; Loreto et al., 2006]. The observed maximum fluxes shown in Table 1 compare very well to numbers reported in the literature, where numerous studies reported maximum methanol fluxes in the range of 69.4 – 110.9 nmol m−2 s−1 on the day of cutting [Davison et al., 2008; Brunner et al., 2007; Warneke et al., 2002; Karl et al., 2001b]. Methanol emissions were still elevated one day after the cut, for which the same studies reported maximum fluxes of 6.9 – 44.2 nmol m−2 s−1 as compared to 3.8 – 67.1 nmol m−2 s−1 in the present study (Table 1). A secondary methanol emission pulse was occasionally observed when the drying hay was turned, as occurred after the 2nd cut in August 2009 or the 3rd cut in September 2009, which presumably allowed residual liquid phase methanol to evaporate. Davison et al. [2008] reported average daytime fluxes of methanol between 7:00 – 17:00 local time for 3 days in June following the cutting of agricultural grassland. Numbers given in their study range from 8.7 – 15.5 nmol m−2 s−1, while the five cutting dates observed in the present study showed average methanol fluxes during the same time of day in the range of 2.0 – 27.9 nmol m−2 s−1.
To our knowledge this study is the first to report elevated methanol emissions after the field-scale application of organic fertilizer. The spreading of organic manure in October 2009 resulted in strong emissions of methanol on the day of fertilization and the following days, with peak emissions of the same order of magnitude as observed during the 3rd cut in 2009 (Table 1). Due to the rapid emission response it is unlikely that the organic fertilizer had an immediate stimulating effect on canopy growth causing methanol emissions to rise because of increased pectin demethylation. Instead, the observed peaks of methanol efflux were probably due to increased microbial activity during the decomposition of organic matter brought in by fertilization, which can be the cause for the release of substantial amounts of VOCs at the soil-litter interface [Ramirez et al., 2010] and may have been fueled by increased nutrient availability and, during this specific fertilization event, rising air temperatures. Fresh manure has been reported to contain large amounts of alcohols, mainly methanol and ethanol [Sun et al., 2008; Ngwabie et al., 2008]. Although the mature manure used for fertilization in Neustift was stored for several weeks prior to application, there might still have been considerable amounts of alcohols in the liquid parts of the manure from within the manure heap. Therefore, the spreading of the fertilizer and the accompanying expansion of its surface area in combination with relatively high air temperatures may have lead to increased volatilization of methanol to the atmosphere. However, as soil VOC production has not received much attention in past studies, little is known about the emission of microbially-produced VOCs and their emission patterns among different soil and litter types [Leff and Fierer, 2008].
4.2 Drivers of methanol flux
The highly positive (partial) correlations of the methanol VMR with the observed methanol flux (Table 2), i.e. methanol emission increased with ambient concentrations, is counterintuitive as from Fick’s first law of diffusion one would expect a negative correlation, i.e. methanol emissions to increase as ambient concentrations decrease due to an increase in the diffusion gradient. We thus interpret the observed positive correlation between the methanol flux and ambient VMR to reflect increases in ambient VMR during periods of high emissions. However, it is difficult to substantiate to what extend the local methanol emissions contributed to the observed methanol VMR. For the reasons stated above, methanol VMR was not included in the discussion of the other dominant drivers.
The statistical analysis (Table 2) identified Tair and PAR as the key drivers of methanol emissions which jointly explained 47 % and 70 % of the variability in half-hourly and daily average methanol fluxes, respectively. Some regressors, e.g. the sensible heat flux, were significantly related to the methanol flux at the half-hourly but not at the daily time step, which reflects their diurnal co-variation with Tair and PAR. In contrast, SWC, which exhibits little or no variability at the sub-daily time step, became more significant in the analysis of daily average data.
The dominant control of temperature on methanol emission has two probable reasons: Firstly, warm air temperatures during the growing season often constitute ideal conditions for plant growth. Pectin methylesterases (PMEs) are cell wall enzymes that – among other functions – enable the expansion of cell walls by catalyzing the demethylation of pectin within the plant cells [Körner et al., 2009; Frenkel et al., 1998]. The enzymatic activity of PMEs increases with leaf temperature, resulting in higher rates of methanol production within the leaf. Secondly, the temperature dependent gas/liquid-phase distribution coefficient H (Henry’s law constant) determines the partitioning between the liquid- and gas-phase within the leaf interior [Niinemets and Reichstein, 2003]. Higher temperatures lead to increased partitioning to the gas-phase and to an increased release of methanol. The temporal analysis of the temperature dependency of the methanol flux convincingly shows that both the methanol emission normalized to a reference temperature and the temperature sensitivity change seasonally and differ inter-annually, suggesting that emission algorithms with constant parameters may be inappropriate for simulating methanol flux variability at time scales covering one or more growing seasons [Niinemets et al., 2010].
Based on leaf-level laboratory experiments, Harley et al. [2007] concluded that there is not enough evidence for a direct effect of PAR on methanol production and emission. While any direct effect of PAR on the production and release of methanol is hard to quantify at the canopy scale where PAR is generally correlated with air temperature, this study provides several lines of evidence (Table 2, Figs. 4 and 5) that PAR may be controlling methanol emissions in addition to and independently of air temperature. Further studies at the leaf-level, both in the field and laboratory, are required to shed light on the possible independent role of PAR in controlling methanol emissions and the associated biochemical pathways.
When methanol is produced, it has to partition to the gas phase and diffuse to the intercellular air space before being emitted through the stomata [Folkers et al., 2008]. Niinemets and Reichstein [2003] conclusively showed that the stomatal sensitivity of VOC emissions is mainly affected by the Henry’s law constant. As methanol has a low H value and is highly soluble in water, its emission is directly limited by stomatal conductance. As a consequence, the regulation of water loss in plants by adjusting stomatal conductance in order to avoid dehydration [Jones, 1996] directly affects the efflux of methanol from leaves [MacDonald and Fall, 1993]. During night stomata are largely closed, which can lead to an accumulation of methanol in the leaf internal gaseous pool [Niinemets and Reichstein, 2003]. In the morning the gas phase pool is rapidly emptied upon stomatal opening, leading typically to a transient emission peak [Nemecek-Marshall et al., 1995]. This could not be observed in the present study where the diurnal emission pattern smoothly followed the course of the two major abiotic drivers, air temperature and PAR (Figure 2). Lacking a scale-appropriate metric of canopy (gs), we used the surface conductance (gsurf) as a proxy, recognizing that gsurf includes a generally unknown contribution by soil evaporation which however is deemed negligible once the canopy is reasonably closed [Wieser et al., 2008]. Contrary to our hypothesis, gsurf explained only a minor fraction of the variability in methanol fluxes (Table 2) and limited methanol emissions only at very low values (Figure 5). This apparent contradiction to earlier leaf-level experimental [Nemecek-Marshall et al., 1995; Hüve et al., 2007] and modeling [Niinemets and Reichstein, 2003] studies may be attributed to two main causes: First, a plant canopy under field conditions does not behave like a single leaf under controlled environmental conditions in an enclosure. Rather, leaves in a plant canopy experience vertical gradients in microclimate, environmental conditions in a plant canopy are highly dynamic and may differ clearly from the conditions at screen height. As a consequence, vertical gradients in stomatal conductance are typically found in plant canopies [Sinclair et al., 1976], which may act to smear out any transient methanol emissions in response to changes in stomatal conductance. For example, upon sun rise sunlit leaves in the upper canopy may be expected to open stomata, while leaves in the lower canopy still experience low light intensities with stomata largely closed. Second, the averaging time of our eddy covariance flux measurements (0.5 h) is much longer than typical half-life times of the aqueous pool (< 500 s; Niinemets et al., 2004), which again causes any transient emissions to be smeared out. Finally, it should be acknowledged that ecosystem-atmosphere flux measurements contain an unknown soil component (both uptake/emission reported for methanol; Karl et al., 2004, 2005; Schade et al., 2008) which may act to buffer any dynamic leaf response.
Methanol is produced as a by-product of the demethylation of pectin in plant cell walls by the temperature dependent enzyme pectin methylesterase [Nemecek-Marshall et al., 1995]. Hüve et al. [2007] compared old and young (growing) leaves and found for the latter the peak methanol emission rate to be about four times higher than in mature leaves. In the present study, no direct measurements of plant growth were available, instead the daily change in the amount of photosynthetically active plant area, dGAI, was used as a surrogate for growth. In contrast to our hypothesis, dGAI clearly ranked behind abiotic variables such as air temperature or PAR in determining methanol fluxes. The reasons for this discrepancy to the leaf-level laboratory measurements mentioned above are unclear at present. Possibly, the temporal resolution of our GAI measurements from which dGAI is inferred is too poor to properly resolve daily changes in growth rate. The relationship between dGAI and methanol fluxes may also be obscured by an inverse correlation between GAI and dGAI – dGAI is smallest shortly before the cuts when GAI and thus the amount of methanol-emitting plant area is highest. It should though also be mentioned that we lack a clear understanding of the growth rhythms of the various plant species of this grassland (see also Brunner et al., 2007), which generally follow different spatial and temporal strategies [Bahn et al., 1994] and thus may exhibit contrasting growth rhythms and magnitudes. On another note, most of our laboratory knowledge is based on dicots and grasses possibly behave differently due to a different form of pectin [Galbally and Kirstine, 2002].
5 Summary and conclusion
Two years of methanol flux measurements above a temperate mountain grassland site in Neustift, Stubai valley, revealed that methanol was continuously emitted by the vegetation and exhibited a large inter-annual variability. Management actions at the field site, i.e. cutting and the application of organic fertilizer, were found to represent the largest perturbations of the seasonal methanol exchange at the investigated grassland ecosystem. In addition to the well-known positive relationship with temperature, a direct control of methanol emissions by PAR was proposed which merits further studies. In contrary to expectations based on leaf-level laboratory studies, surface conductance and the diurnal change in GAI, which were used as proxies for the canopy-integrated stomatal conductance and growth respectively, explained only a minor fraction of the temporal variability in methanol fluxes. We suggest this discrepancy to result from differences in spatial and temporal scale of flux as opposed to leaf-level enclosure measurements and controlled laboratory environments as opposed to real-world field conditions. In order to close the apparent gap that exists in transferring leaf-level laboratory knowledge to in situ conditions at the ecosystem scale, which hampers the development and parameterization of ecosystem models, concurrent studies on leaf/soil and ecosystem-scale methanol exchange under field conditions are advocated.
Acknowledgements
This study was financially supported by the Austrian National Science Fund under contract P19849-B16, the Tyrolean Science Fund under contract Uni-404/486. Family Hofer (Neustift, Austria) is acknowledged for granting us access to the study site. Additional support was obtained by the Translational-Research-Programm (L518-N20) of the Austrian National Science Fund and the Industry-Academia Partnerships and Pathways (IAPP; 218065) funded by the European Commission.
6 Appendix.
Table A1 gives details for the regressions shown in Figures 4 and 6.
Table A1. Regression lines for Figures 4 and 6 .
| Regression lines for Figure 4 (a) | Equation: f = k+a*x | |||||
|---|---|---|---|---|---|---|
| air temperature (°C) | < 10 | 10 – 14 | 14 – 18 | 18 – 22 | 22 – 26 | > 26 |
|
|
||||||
| r | 0.24 *** | 0.33 *** | 0.42 *** | 0.49 *** | 0.56 *** | 0.48 *** |
| Standard Error of Estimate | 1.05 | 1.40 | 1.64 | 1.95 | 2.03 | 2.61 |
| k | 0.24 | 0.87 | 1.18 | 1.45 | 1.21 | 1.10 |
| a | 7.5 * 10−4 | 1 * 10−3 | 2 * 10−3 | 2 * 10−3 | 3 * 10−3 | 3 * 10−3 |
| Regression lines for Figure 4 (b) | Equation: f=a*exp(b*x) | |||||
| PAR (μmol m−2 s−1) | < 200 | 200 - 600 | 600 - 1000 | 1000 - 1400 | 1400 - 1800 | > 1800 |
| r | 0.37 *** | 0.44 *** | 0.43 *** | 0.44 *** | 0.55 *** | 0.37 *** |
| Standard Error of Estimate | 1.51 | 1.34 | 1.59 | 1.97 | 2.01 | 2.87 |
| a | 0.22 | 0.57 | 1.07 | 1.23 | 1.23 | 2.30 |
| b | 0.10 | 0.07 | 0.05 | 0.05 | 0.06 | 0.05 |
| Regression lines for Figure 6 (a) | Equation: f=a*(1-exp(-b*x)) | |||||
| air temperature (°C) | < 10 | 10 – 14 | 14 – 18 | 18 – 22 | 22 – 26 | > 26 |
| r | 0.24 *** | 0.24 *** | 0.10 * | 0.20 *** | 0.34 *** | 0.20 (ns) |
| Standard Error of Estimate | 0.90 | 1.27 | 1.75 | 2.20 | 2.50 | 3.19 |
| a | 0.93 | 1.80 | 2.71 | 3.95 | 5.44 | 5.91 |
| b | 8.88 | 9.54 | 9.97 | 7.6 | 4.84 | 11.37 |
| Regression lines for Figure 6 (b) | Equation: f=a*exp(b*x) | |||||
|
| ||||||
| surface conductance (mol m−2 s−1) | 0 – 0.2 | 0.2 – 0.4 | 0.4 – 0.6 | > 0.6 | ||
| r | 0.47 *** | 0.57 *** | 0.60 *** | 0.58 *** | ||
| Standard Error of Estimate | 1.44 | 1.83 | 1.85 | 1.88 | ||
| a | 0.49 | 0.54 | 0.72 | 0.73 | ||
| b | 0.08 | 0.09 | 0.08 | 0.08 | ||
|
| ||||||
correlation coefficient
equation coefficients
slope
photosynthetic active radiation
significant with p < 0.05
highly significant with p < 0.001
not significant
Significance levels are corrected for the mean of the observations (SigmaPlot 11.0, Systat Software, Inc.)
Reference list
- Arey J, Winter AM, Atkinson R, Aschmann SM, Long WD, Morrison CL. The emission of (Z)-3-hexen-1-ol, (Z)-3-hexenylacetate and other oxygenated hydrocarbons from agricultural plant species. Atmospheric Environment. Part A. General Topics. 1991;25(5-6):1063–1075. doi:10.1016/0960-1686(91)90148-Z. [Google Scholar]
- Atkinson R. Atmospheric chemistry of VOCs and NOx. Atmospheric Environment. 2000;34(12-14):2063–2101. doi:10.1016/S1352-2310(99)00460-4. [Google Scholar]
- Aubinet M, Grelle A, Ibrom A, Rannik Ü, Moncrieff J, Foken T, Kowalski AS, Martin PH, Berbigier P, Bernhofer C, Clement R, Elbers J, Granier A, Grünwald T, Morgenstern K, Pilegaard K, Rebmann C, Snijders W, Valentini R, Vesala T. Estimates of the Annual Net Carbon and Water Exchange of Forests: The EUROFLUX Methodology. Advances in Ecological Research. 2000;30:113–175. doi:10.1016/S0065-2504(08)60018-5. [Google Scholar]
- Bahn M, Cernusca A, Tappeiner U, Tasser E. Wachstum krautiger Arten auf einer Mähwiese und einer Almbrache. Verhandlungen der Gesellschaft für Ökologie. 1994;23:23–30. [Google Scholar]
- Baker B, Guenther A, Greenberg J, Fall R. Canopy level fluxes of 2-methyl-3-buten-2-ol, acetone, and methanol by a portable relaxed eddy accumulation system. Environmental Science & Technology. 2001;35(9):1701–1708. doi: 10.1021/es001007j. doi:10.1021/es001007j. [DOI] [PubMed] [Google Scholar]
- Baldocchi DD, Hicks BB, Meyers TP. Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology. 1988;69(5):1331–1340. doi:10.2307/1941631. [Google Scholar]
- Bamberger I, Hörtnagl L, Schnitzhofer R, Graus M, Ruuskanen TM, Müller M, Dunkl J, Wohlfahrt G, Hansel A. BVOC fluxes above mountain grassland. Biogeosciences. 2010;7(5):1413–1424. doi: 10.5194/bg-7-1413-2010. doi:10.5194/bg-7-1413-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo V, Gimeno BS, Sanz J, de La Torre D, Gil JM. Assessment of the ozone sensitivity of 22 native plant species from Mediterranean annual pastures based on visible injury. Atmospheric Environment. 2003;37(33):4667–4677. doi:10.1016/j.atmosenv.2003.07.002. [Google Scholar]
- Bernard SM, Samet JM, Grambsch A, Ebi KL, Romieu I. The potential impacts of climate variability and change on air pollution-related health effects in the United States. Environmental health perspectives. 2001;109(Suppl 2):199. doi: 10.1289/ehp.109-1240667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A, Ammann C, Neftel A, Spirig C. Methanol exchange between grassland and the atmosphere. Biogeosciences. 2007:395–410. [Google Scholar]
- Chameides W, Lindsay R, Richardson J, Kiang C. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science. 1988;241(4872):1473–1475. doi: 10.1126/science.3420404. doi:10.1126/science.3420404. [DOI] [PubMed] [Google Scholar]
- Cojocariu C, Escher P, Haberle K-H, Matyssek R, Rennenberg H, Kreuzwieser J. The effect of ozone on the emission of carbonyls from leaves of adult Fagus sylvatica. Plant, Cell and Environment. 2005;28(5):603–611. doi:10.1111/j.1365-3040.2005.01305.x. [Google Scholar]
- Cojocariu C, Kreuzwieser J, Rennenberg H. Correlation of short-chained carbonyls emitted from Picea abies with physiological and environmental parameters. New Phytologist. 2004;162(3):717–727. doi: 10.1111/j.1469-8137.2004.01061.x. doi:10.1111/j.1469-8137.2004.01061.x. [DOI] [PubMed] [Google Scholar]
- Custer TG, Schade GW. Methanol and acetaldehyde fluxes over ryegrass. Tellus B. 2007;59(4):673–684. doi:10.1111/j.1600-0889.2007.00294.x. [Google Scholar]
- Davison B, Brunner A, Ammann C, Spirig C, Jocher M, Neftel A. Cut-induced VOC emissions from agricultural grasslands. Plant biology (Stuttgart, Germany) 2008;10(1):76–85. doi: 10.1055/s-2007-965043. doi:10.1055/s-2007-965043. [DOI] [PubMed] [Google Scholar]
- Duncan BN, Logan JA, Bey I, Megretskaia IA, Yantosca RM, Novelli PC, Jones NB, Rinsland CP. Global budget of CO, 1988–1997: Source estimates and validation with a global model. Journal of Geophysical Research. 2007;112(D22301):1–29. doi:10.1029/2007JD008459. [Google Scholar]
- Fall R, Benson AA. Leaf methanol - the simplest natural product from plants. Trends in Plant Science. 1996;1(9):296–301. doi:10.1016/S1360-1385(96)88175-0. [Google Scholar]
- Foken T, Wichura B. Tools for quality assessment of surface-based flux measurements. Agricultural and Forest Meteorology. 1996;78:83–105. doi:10.1016/0168-1923(95)02248-1. [Google Scholar]
- Folberth GA, Hauglustaine DA, Lathière J, Brocheton F. Interactive chemistry in the Laboratoire de Météorologie Dynamique general circulation model: model description and impact analysis of biogenic hydrocarbons on tropospheric chemistry. Atmospheric Chemistry and Physics. 2006;6(8):2273–2319. doi:10.5194/acp-6-2273-2006. [Google Scholar]
- Folkers A, Hüve K, Ammann C, Dindorf T, Kesselmeier J, Kleist E, Kuhn U, Uerlings R, Wildt J. Methanol emissions from deciduous tree species: dependence on temperature and light intensity. Plant biology. 2008;10(1):65–75. doi: 10.1111/j.1438-8677.2007.00012.x. doi:10.1111/j.1438-8677.2007.00012.x. [DOI] [PubMed] [Google Scholar]
- Folkins I, Chatfield RB. Impact of acetone on ozone production and OH in the upper troposphere at high NOx. Journal of Geophysical Research. 2000;105(D9):11–585. [Google Scholar]
- Frenkel C, Peters JS, Tieman DM, Tiznado ME, Handa AK. Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (Lycopersicon esculentum) fruit. Journal of Biological Chemistry. 1998;273(8):4293–4295. doi: 10.1074/jbc.273.8.4293. doi:10.1074/jbc.273.8.4293. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Doskey PV. Air-surface exchange of nonmethane organic compounds at a grassland site: seasonal variations and stressed emissions. Journal of Geophysical Research. 1998;103(D11):13153–13168. doi:10.1029/98JD00924. [Google Scholar]
- Galbally IE, Kirstine W. The production of methanol by flowering plants and the global cycle of methanol. Journal of Atmospheric Chemistry. 2002;43:195–229. doi:10.1023/A:1020684815474. [Google Scholar]
- Gouw J. A. de, Howard CJ, Custer TG, Baker B, Fall R. Proton-transfer chemical-ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environmental Science & Technology. 2000;34(12):2640–2648. doi:10.1021/es991219k. [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. A global model of natural volatile organic compound emissions. Journal of Geophysical Research. 1995;100(D5):8873–8892. doi:10.1029/94JD02950. [Google Scholar]
- Hammerle A, Haslwanter A, Tappeiner U, Cernusca A, Wohlfahrt G. Leaf area controls on energy partitioning of a temperate mountain grassland. Biogeosciences. 2008;5(2):421–431. doi: 10.5194/bg-5-421-2008. doi:10.5194/bg-5-421-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, Jordana A, Holzinger R, Prazellera P, Vogel W, Lindinger W. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. International Journal of Mass Spectrometry and Ion Processes. 1995;149-150(95):609–619. doi:10.1016/0168-1176(95)04294-U. [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A. Environmental controls over methanol emission from leaves. Biogeosciences. 2007;4(6):1083–1099. doi:10.5194/bg-4-1083-2007. [Google Scholar]
- Heikes BG, Chang W, Pilson MEQ, Swift E, Singh HB, Guenther A, Jacob DJ, Field BD, Fall R, Riemer D, Brand L. Atmospheric methanol budget and ocean implication. Global Biogeochemical Cycles. 2002;16(4):13. doi:10.1029/2002GB001895. [Google Scholar]
- Hollinger DY, Richardson AD. Uncertainty in eddy covariance measurements and its application to physiological models. Tree physiology. 2005;25(7):873–885. doi: 10.1093/treephys/25.7.873. [DOI] [PubMed] [Google Scholar]
- Hsieh C-I, Katul G, Chi T. An approximate analytical model for footprint estimation of scalar fluxes in thermally stratified atmospheric flows. Advances in Water Resources. 2000;23(7):765–772. doi:10.1016/S0309-1708(99)00042-1. [Google Scholar]
- Hörtnagl L, Clement R, Graus M, Hammerle A, Hansel A, Wohlfahrt G. Dealing with disjunct concentration measurements in eddy covariance applications: A comparison of available approaches. Atmospheric Environment. 2010;44(16):2024–2032. doi: 10.1016/j.atmosenv.2010.02.042. doi:10.1016/j.atmosenv.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüve K, Christ MM, Kleist E, Uerlings R, Niinemets Ü, Walter A, Wildt J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. Journal of experimental botany. 2007;58(7):1783–93. doi: 10.1093/jxb/erm038. doi:10.1093/jxb/erm038. [DOI] [PubMed] [Google Scholar]
- Jacob DJ, Field BD, Li Q, Blake DR, de Gouw JA, Warneke C, Hansel A, Wisthaler A, Singh HB, Guenther A. Global budget of methanol: constraints from atmospheric observations. Journal of Geophysical Research. 2005;110:D08303. doi:10.1029/2004JD005172. [Google Scholar]
- Jones HG. In: Water Deficits: Plant Responses From Cell to Community, Drought tolerance and water-use efficiency. Smith JAC, Griffiths H, editors. BIOS Scientific; Oxford: 1996. [Google Scholar]
- Kaimal JC, Finnigan JJ. Atmospheric boundary layer flows: their structure and measurement. Oxford University Press; 1994. [Google Scholar]
- Kanakidou M, Seinfeld JH, Pandis SN, Barnes I, Dentener FJ, Facchini MC, Dingenen RV. Organic aerosol and global climate modelling: a review. Atmospheric Chemistry and Physics. 2005;5:1053–1123. doi:10.5194/acpd-4-5855-2004. [Google Scholar]
- Karl T, Fall R, Jordan A, Lindinger W. On-line analysis of reactive VOCs from urban lawn mowing. Environmental science & technology. 2001a;35(14):2926–2931. doi: 10.1021/es010637y. [DOI] [PubMed] [Google Scholar]
- Karl T, Guenther A, Jordan A, Fall R, Lindinger W. Eddy covariance measurement of biogenic oxygenated VOC emissions from hay harvesting. Atmospheric Environment. 2001b;35(3):491–495. [Google Scholar]
- Karl T, Guenther A, Spirig C, Hansel A, Fall R. Seasonal variation of biogenic VOC emissions above a mixed hardwood forest in northern Michigan. Geophysical Research Letters. 2003;30(23) doi:10.1029/2003GL018432. [Google Scholar]
- Karl T, Harley P, Guenther A, Rasmussen R, Baker B, Jardine K, Nemitz E. The bi-directional exchange of oxygenated VOCs between a loblolly pine (Pinus taeda) plantation and the atmosphere. Atmospheric Chemistry and Physics. 2005;5(11):3015–3031. doi:10.5194/acp-5-3015-2005. [Google Scholar]
- Karl T, Potosnak M, Guenther A, Clark D, Walker J, Herrick JD, Geron C. Exchange processes of volatile organic compounds above a tropical rain forest: implications for modeling tropospheric chemistry above dense vegetation. Journal of Geophysical Research. 2004;109(D18) doi:10.1029/2004JD004738. [Google Scholar]
- Karl T, Spirig C, Rinne J, Stroud C, Prevost P, Greenberg J, Fall R, Guenther A. Virtual disjunct eddy covariance measurements of organic compound fluxes from a subalpine forest using proton transfer reaction mass spectrometry. Atmospheric Chemistry and Physics. 2002;2(4):279–291. [Google Scholar]
- Keppler F, Hamilton JTG, McRoberts WC, Vigano I, Brass M, Röckmann T. Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytologist. 2008;178:808–814. doi: 10.1111/j.1469-8137.2008.02411.x. [DOI] [PubMed] [Google Scholar]
- Kirstine W, Galbally IE, Ye Y, Hooper M. Emissions of volatile organic compounds (primarily oxygenated species) from pasture. Journal of Geophysical Research. 1998;103(D9):10605–10619. doi:10.1029/97JD03753. [Google Scholar]
- Kulmala M, et al. A new feedback mechanism linking forests, aerosols, and climate. Atmospheric Chemistry and Physics. 2004;4(2):557–562. doi:10.5194/acp-4-557-2004. [Google Scholar]
- Körner E, von Dahl CC, Bonaventure G, Baldwin IT. Pectin methylesterase NaPME1 contributes to the emission of methanol during insect herbivory and to the elicitation of defence responses in Nicotiana attenuata. Journal of experimental botany. 2009;60(9):2631–2640. doi: 10.1093/jxb/erp106. doi:10.1093/jxb/erp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lary DJ, Shallcross DE. Central role of carbonyl compounds in atmospheric chemistry. Journal of Geophysical Research. 2000;105(D15):19771–19778. doi:10.1029/1999JD901184. [Google Scholar]
- Leff JW, Fierer N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biology and Biochemistry. 2008;40(7):1629–1636. doi:10.1016/j.soilbio.2008.01.018. [Google Scholar]
- Lenschow DH, Kristensen L. Uncorrelated noise in turbulence measurements. Journal of Atmospheric and Oceanic Technology. 1985;2:68–81. [Google Scholar]
- Lindinger W, Hansel A, Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR MS) medical applications, food control and environmental research. International Journal of Mass Spectrometry and Ion Processes. 1998;173(3):191–241. doi:10.1016/S0168-1176(97)00281-4. [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, cell & environment. 2006;29(9):1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. doi:10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Lozán JL. Angewandte Statistik für Naturwissenschafter. Pareys Stu.; Paul Parey, Hamburg: 1992. [Google Scholar]
- MacDonald RC, Fall R. Detection of substantial emissions of methanol from plants to the atmosphere. Atmospheric Environment. Part A. General Topics. 1993;27(11):1709–1713. doi:10.1016/0960-1686(93)90233-O. [Google Scholar]
- Mao H, Talbot R, Nielsen C, Sive B. Controls on methanol and acetone in marine and continental atmospheres. Geophysical Research Letters. 2006;33(2) doi:10.1029/2005GL024810. [Google Scholar]
- Massman WJ. A simple method for estimating frequency response corrections for eddy covariance systems. Agricultural and Forest Meteorology. 2000;104(3):185–198. doi:10.1016/S0168-1923(00)00164-7. [Google Scholar]
- McMillen RT. An eddy correlation technique with extended applicability to non-simple terrain. Boundary-Layer Meteorology. 1988;43(3):231–245. doi:10.1007/BF00128405. [Google Scholar]
- Millet DB, Jacob DJ, Custer TG, de Gouw JA, Goldstein AH, Karl T, Singh HB, Sive B, Talbot R, Warneke C, Williams J. New constraints on terrestrial and oceanic sources of atmospheric methanol. Atmospheric Chemistry and Physics Discussions. 2008;8(2):7609–7655. doi:10.5194/acpd-8-7609-2008. [Google Scholar]
- Moore CJ. Frequency response corrections for eddy correlation systems. Boundary-Layer Meteorology. 1986;37(1-2):17–35. doi:10.1007/BF00122754. [Google Scholar]
- Nemecek-Marshall M, MacDonald RC, Franzen JJ, Wojciechowski CL, Fall R. Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development) Plant Physiology. 1995;108(4):1359. doi: 10.1104/pp.108.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwabie NM, Schade GW, Custer TG, Linke S, Hinz T. Abundances and flux estimates of volatile organic compounds from a dairy cowshed in germany. Journal of Environment Quality. 2008;37(2):565. doi: 10.2134/jeq2006.0417. doi:10.2134/jeq2006.0417. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in plant science. 2004;9(4):180–6. doi: 10.1016/j.tplants.2004.02.006. doi:10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010;7(6):1809–1832. doi:10.5194/bg-7-1809-2010. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: A sensitivity analysis. Journal of Geophysical Research. 2003a;108(D7):4211. doi:10.1029/2002JD002626. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. Journal of Geophysical Research. 2003b;108(D7):17. doi:10.1029/2002JD002620. [Google Scholar]
- Palmer PI, Jacob DJ, Fiore AM, Martin RV. Mapping isoprene emissions over North America using formaldehyde column observations from space. Journal of Geophysical Research. 2003;108(D6):1–47. doi:10.1029/2002JD002153. [Google Scholar]
- Ramanathan V, Crutzen PJ, Kiehl JT, Rosenfeld D. Aerosols, climate, and the hydrological cycle. Science. 2001;294(5549):2119–2124. doi: 10.1126/science.1064034. doi:10.1126/science.1064034. [DOI] [PubMed] [Google Scholar]
- Ramankutty N, Foley JA. Estimating historical changes in global land cover: Croplands from 1700 to 1992. Global Biogeochemical Cycles. 1999;13(4):997. doi:10.1029/1999GB900046. [Google Scholar]
- Ramirez KS, Lauber CL, Fierer N. Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry. 2010;99(1-3):97–107. doi:10.1007/s10533-009-9393-x. [Google Scholar]
- Riemer D, Pos W, Milne P, Farmer C, Zika R, Apel E, Olszyna K, Kliendienst T, Lonneman W, Bertman S, Shepson P, Starn T. Observations of nonmethane hydrocarbons and oxygenated volatile organic compounds at a rural site in the southeastern United States. Journal of Geophysical Research. 1998;103(D21):28111–28128. doi:10.1029/98JD02677. [Google Scholar]
- Rinne J, Ruuskanen TM, Reissell A. On-line PTR-MS measurements of atmospheric concentrations of volatile organic compounds in a European boreal forest ecosystem. Boreal Environment Research. 2005 Oct;:425–436. [Google Scholar]
- Rinne J, Taipale R, Markkanen T. Hydrocarbon fluxes above a Scots pine forest canopy: measurements and modeling. Atmospheric. 2007;7(12):3361–3372. doi:10.5194/acp-7-3361-2007. [Google Scholar]
- Ruuskanen TM, Müller M, Schnitzhofer R, Karl T, Graus M, Bamberger I, Hörtnagl L, Brilli F, Wohlfahrt G, Hansel A. Eddy covariance VOC emission and deposition fluxes above grassland using PTR-TOF. Atmospheric Chemistry and Physics Discussions. 2010;10(9):21077–21108. doi: 10.5194/acp-11-611-2011. doi:10.5194/acpd-10-21077-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade GW, Custer TG. OVOC emissions from agricultural soil in northern Germany during the 2003 European heat wave. Atmospheric Environment. 2004;38(36):6105–6114. doi:10.1016/j.atmosenv.2004.08.017. [Google Scholar]
- Schade GW, Goldstein AH. Fluxes of oxygenated volatile organic compounds from a ponderosa pine plantation. Journal of Geophysical Research. 2001;106(D3):3111–3123. doi:10.1029/2000JD900592. [Google Scholar]
- Schade GW, Solomon SJ, Dellwik E, Pilegaard K, Ladstätter-Weissenmayer A. Methanol and other VOC fluxes from a Danish beech forest during springtime. Biogeosciences Discussions. 2008;5(6):4315–4352. doi:10.5194/bgd-5-4315-2008. [Google Scholar]
- Sinclair TR, Murphy CE, Knoerr KR. Development and evaluation of simplified models for simulating canopy photosynthesis and transpiration. The Journal of Applied Ecology. 1976;13(3):813. doi:10.2307/2402257. [Google Scholar]
- Singh HB, Kanakidou M, Crutzen PJ, Jacob DJ. High concentrations and photochemical fate of oxygenated hydrocarbons in the global troposphere. Nature. 1995;378(6552):50–54. doi:10.1038/378050a0. [Google Scholar]
- Singh HB, Salas LJ, Chatfield RB, Czech E, Fried A, Walega J, Evans MJ, Field BD, Jacob DJ, Blake DR, Heikes BG, Talbot R, Sachse G, Crawford JH, Avery MA, Sandholm S, Fuelberg H. Analysis of the atmospheric distribution, sources, and sinks of oxygenated volatile organic chemicals based on measurements over the Pacific during TRACE-P. Journal of Geophysical Research. 2004;109(D15) doi:10.1029/2003JD003883. [Google Scholar]
- Sinha V, Williams J, Meyerhöfer M, Riebesell U, Paulino AI, Larsen A. Air sea fluxes of methanol, acetone, acetaldehyde, isoprene and DMS from a Norwegian fjord following a phytoplankton bloom in a mesocosm experiment. Atmospheric Chemistry and Physics. 2007;7(3):739–755. doi:10.5194/acp-7-739-2007. [Google Scholar]
- Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. European Journal of Soil Science. 2003;54(4):779–791. doi:10.1046/j.1351-0754.2003.0567.x. [Google Scholar]
- Sommariva R, Bloss WJ, Brough N, Carslaw N, Flynn M, Haggerstone A-L, Heard DE, Hopkins JR, Lee JD, Lewis AC, McFiggans G, Monks PS, Penkett SA, Pilling MJ, Plane JMC, Read KA, Saiz-Lopez A, Rickard AR, Williams PI. OH and HO2 chemistry during NAMBLEX: roles of oxygenates, halogen oxides and heterogeneous uptake. Atmospheric Chemistry and Physics Discussions. 2005;5(6):10947–10996. doi:10.5194/acpd-5-10947-2005. [Google Scholar]
- Spirig C, Neftel A, Ammann C, Dommen J, Grabmer W, Thielmann A, Schaub A, Beauchamp J, Wisthaler A, Hansel A. Eddy covariance flux measurements of biogenic VOCs during ECHO 2003 using proton transfer reaction mass spectrometry. Atmospheric Chemistry and Physics. 2005;5(2):465–481. doi:10.5194/acp-5-465-2005. [Google Scholar]
- Sun H, Trabue SL, Scoggin K, Jackson WA, Pan Y, Zhao Y, Malkina IL, Koziel JA, Mitloehner FM. Alcohol, volatile fatty acid, phenol, and methane emissions from dairy cows and fresh manure. Journal of Environment Quality. 2008;37(2):615. doi: 10.2134/jeq2007.0357. doi:10.2134/jeq2007.0357. [DOI] [PubMed] [Google Scholar]
- Talbot R, Mao H, Sive B. Diurnal characteristics of surface level O 3 and other important trace gases in New England. Journal of Geophysical Research. 2005;110(D9) doi:10.1029/2004JD005449. [Google Scholar]
- Tappeiner U, Borsdorf A, Tasser E. Mapping the Alps. Spektrum; 2008. [Google Scholar]
- Tie X, Guenther A, Holland E. Biogenic methanol and its impacts on tropospheric oxidants. Geophysical Research Letters. 2003;30(17):3–6. doi:10.1029/2003GL017167. [Google Scholar]
- Warneke C, Karl T, Judmaier H, Hansel A, Jordan A, Lindinger W, Crutzen PJ. Acetone, methanol, and other partially oxidized volatile organic emissions from dead plant matter by abiological processes: Significance for atmospheric HOx chemistry. Global Biogeochemical Cycles. 1999;13(1):9. doi:10.1029/98GB02428. [Google Scholar]
- Warneke C, Luxembourg SL, de Gouw JA, Rinne HJI, Guenther A, Fall R. Disjunct eddy covariance measurements of oxygenated volatile organic compounds fluxes from an alfalfa field before and after cutting. Journal of Geophysical Research. 2002;107(D8):4067. doi:10.1029/2001JD000594. [Google Scholar]
- Wieser G, Hammerle A, Wohlfahrt G. The water balance of grassland ecosystems in the Austrian Alps. Arctic, Antarctic, and Alpine. 2008;40(2):439–445. doi: 10.1657/1523-0430(07-039)[WIESER]2.0.CO;2. doi:10.1657/1523-0430(07-039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt G, Anfang C, Haslwanter A, Newesely C, Schmitt M, Drösler M, Pfadenhauer J, Cernusca A. Quantifying nighttime ecosystem respiration of a meadow using eddy covariance, chambers and modelling. Agricultural and Forest Meteorology. 2005;128(3-4):141–162. doi:10.1016/j.agrformet.2004.11.003. [Google Scholar]
- Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: Effects of weather and management. Journal of Geophysical Research. 2008;113(D08110) doi: 10.1029/2007jd009286. doi:10.1029/2007JD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt G, Haslwanter A, Hörtnagl L, Jasoni RL, Fenstermaker LF, Arnone JA, III, Hammerle A. On the consequences of the energy imbalance for calculating surface conductance to water vapour. Agricultural and Forest Meteorology. 2009;149(9):1556–1559. doi: 10.1016/j.agrformet.2009.03.015. doi:10.1016/j.agrformet.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Kawashima S, Tsuruta H. Carbon monoxide, hydrogen, and methane uptake by soils in a temperate arable field and a forest. Journal of Geophysical Research. 2000;105(D11):14347–14362. doi:10.1029/1999JD901156. [Google Scholar]