Abstract
Hepatic progenitor cells (HPCs) are a potential cell source for liver cell transplantation but do not function like mature liver cells. We sought an effective and reliable method to induce HPC maturation. An immortalized HP14.5 albumin promoter-driven Gaussian luciferase (ALB-GLuc) cell line was established from HPCs isolated from fetal mouse liver of post coitus day 14.5 mice to investigate the effect of induction factors on ALB promoter. HP14.5 parental cells were cultured in DMEM with different combinations of 2% horse serum (HS), 0.1 µM dexamethasone (DEX), 10 ng/mL hepatic growth factor (HGF), and/or 20 ng/mL fibroblast growth factor 4 (FGF4). Trypan blue and crystal violet staining were used to assess cell proliferation with different induction conditions. Expression of hepatic markers was measured by semi-quantitative RT-PCR, Western blot, and immunofluorescence. Glycogen storage and metabolism were detected by periodic acid-Schiff and indocyanine green (ICG) staining. GLuc activity indicated ALB expression. The combination of 2% HS+0.1 µM Dex+10 ng/mL HGF+20 ng/mL FGF4 induced the highest ALB-GLuc activity. Cell proliferation decreased in 2% HS but increased by adding FGF4. Upon induction, and consistent with hepatocyte development, DLK, AFP, and CK19 expression decreased, while ALB, CK18, and UGT1A expression increased. The maturity markers tyrosine aminotransferase and apolipoprotein B were detected at days 3 and 6 post-induction, respectively. ICG uptake and glycogen synthesis were detectable at day 6 and increased over time. Therefore, we demonstrated that HPCs were induced to differentiate into functional mature hepatocytes in vitro, suggesting that factor-treated HPCs may be further explored as a means of liver cell transplantation.
Keywords: Hepatic progenitor cells, Induction, Maturation, Dexamethasone, Hepatic growth factor, Fibroblast growth factor 4
Introduction
Hepatic stem cells (HSCs) are capable of self-renewal and multi-potential differentiation into hepatocytes, biliary epithelial cells, and other cells. HSCs may be involved in the repair and regeneration of liver, and may also serve as an important cell source for liver cell transplantation and generation of bioartificial livers. It has been found that HSCs transplantation for acute and chronic liver diseases has a promising therapeutic effect (1, 2). HSCs may include extrahepatic and intrahepatic sources of stem cells, such as embryonic stem cells, hematopoietic cells, bone marrow mesenchymal stem cells, hepatic oval cells, and small hepatic cells (3). Liver stem cells from different sources have been shown to differentiate into functional hepatocytes in vitro and in vivo. However, the induction efficiency of hepatocyte maturation varies significantly. In vitro studies have shown that lineage-specific hepatic differentiation from embryonic stem cells and bone marrow mesenchymal stem cells into hepatic functional cells is difficult to achieve. The induced cells expressed surface markers with limited hepatocyte function, the differentiation efficiency was relatively low, and terminal differentiation into completely functional hepatocytes has not been realized (4, 5).
Hepatic progenitor cells (HPCs) are the major component of the hepatic parenchyma in early liver development, exhibiting the bio-potential characteristics to directly differentiate into hepatocytes and cholangiocytes. This intermediate state is an essential process of hepatic maturation, not only in liver organogenesis in vivo, but also in hepatic differentiation from various stem cells into mature hepatocytes in vitro (6, 7). HPCs derived from embryonic liver retain the capability of self-renewal and differentiation potential, and have low immunogenicity, indicating potential significant value in clinical applications (8). Thus, HPCs are very useful cell sources for studying the mechanisms behind liver development and for developing novel cell-based therapies for liver diseases. Nonetheless, HPCs have to undergo maturation to become functional liver cells. Most studies thus far have shown that the differentiation efficiency of HPCs is too low to generate sufficient numbers of functional mature hepatocytes (4, 9- 10).
In this study, we investigated the effect of different induction factors on maturation of HPCs in order to identify an effective and reliable method to induce maturation of HPCs in vitro. We found that HPCs can be effectively induced to differentiate into functional mature hepatocytes in vitro by the combination of 2% horse serum (HS)+0.1 μM dexamethasone (Dex)+10 ng/mL hepatocyte growth factor (HGF)+20 ng/mL fibroblast growth factor 4 (FGF4). This in vitro model is useful for elucidating the mechanism of liver development and the directed differentiation of liver stem cells into mature liver cells, which would improve the efficiency and biosafety profile of possible clinical applications for liver stem cell transplantation (11).
Material and Methods
Cell culture and chemicals
Primary HPCs, designated as HP14.5, were isolated from embryonic liver of post coitus day 14.5 mice as previously described (12). Reversibly immortalized HP14.5 containing a simian virus 40 large T (SV40T) antigen flanked by Cre/loxP sites were established by infecting HP14.5 with the retroviral vector SSR#69 and selecting the cells in hygromycin B at a concentration of 0.3 mg/mL (Invitrogen, USA) for 7-10 days. Two-week hepatocytes, designated as LC14d, were isolated from the liver of 14-day old mice in a similar fashion. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS, Hyclone, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in 5% CO2. Cells at a confluency of 90% were passaged every 3-4 days. Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich (USA).
An HP14.5 albumin promoter-driven Gaussian (ALB-GLuc) cell line was established as follows. A 2.5-kb genomic fragment containing mouse ALB promoter was amplified by PCR and cloned into the luciferase reporter plasmid pSEB-GLuc to construct a pSEB-ALB-GLuc plasmid in which the expression of GLuc is driven by the ALB promoter. ALB-GLuc retrovirus was packaged by co-transfecting pSEB-ALB-GLuc and a pCL-Ampho plasmid into HEK293 cells, and then infecting HP14.5 cells to establish a stable cell line, designated as HP14.5 ALB-GLuc.
Gaussia luciferase reporter assay
HP14.5 ALB-GLuc cells were seeded in 24-well culture plates at an initial confluence of 20% and then treated with different induction factors including 0.1 μM Dex, HGF or FGF4 at concentrations of 0, 5, 10, 20, 40, and 80 ng/mL, 10% FBS or 2% HS (Hyclone). Relative ALB promoter-driven GLuc activity can indirectly measure the ALB expression and maturation of hepatocytes. Therefore, the effects of single factors and different combinations of culture conditions on induced maturation of HP14.5 in vitro were detected by GLuc assay. Culture medium was collected from HP14.5 ALB-GLuc cells exposed to different treatments at each of the indicated times. GLuc activity was assayed by using the Gaussian Luciferase Assay Kit (New England Biolabs, USA). All measurements were performed in triplicate.
Cell proliferation assessed by trypan blue staining and crystal violet staining
Trypan blue staining was carried out at D3, D6, D9, D12, and D15 after treatments. Both adherent and floating cells were collected in 2X trypan blue buffer (Beyotime, China) to make suspensions of approximately 106 cells/mL. A 10 μL volume of cell suspension was placed in a hemocytometer counting chamber and the cells in each large square of the grid were counted by light microscopy (TS100, Nikon, Japan). Blue-stained cells were recorded as dead cells. Crystal violet staining was performed at D12 after treatments. Briefly, 20,000 HP14.5 cells per well were seeded in 24-well culture plates and treated with different induction factors. After 12 days, cells were fixed in 4% paraformaldehyde for 10 min and stained with 0.05% crystal violet for 30 min. The plates were washed twice with tap water, drained upside down on paper towels, and photographed. Five hundred microliters of 100% methanol was added to each well to dissolve the dye, which was measured for absorbance at 540 nm. Three independent experiments were performed in duplicate, and representative results are shown.
RT-PCR analysis
At the indicated times, total RNA was extracted from treated cells, and 10 µg of RNA was reverse-transcribed into cDNA with hexamer primers using Superscript II reverse transcriptase (Invitrogen, USA). Five- to 10-fold diluted first strand cDNA was used as PCR templates. PCR primers were 18-20mers, designed by using the Primer 3 program to amplify the 3′-end (approximately 120-150 bp) of the gene of interest (Supplementary Table S1). A touchdown protocol was performed as follows: 94°C, 65°C, and 72°C, 20 s at each temperature, for 10 cycles with a 1°C decrease per cycle. This was followed by 25-30 cycles at 94°C, 55°C, and 72°C, 20 s at each temperature. All PCR products were resolved on 1.5% agarose gels with normalization relative to GAPDH expression in each sample. Real-time PCR reactions were carried out using a Bio-Rad protocol: 94°C, 55°C, and 70°C, 20 s at each temperature, reading plates after 40 cycles. Data are reported as the fold-change with GAPDH normalization.
Immunofluorescence staining
As previously described (13), methanol was used to fix the treated cells at -20°C for 15 min, 5% goat serum was used to block cells at room temperature (RT) for 1 h. Then, cells were incubated with primary antibodies against delta-like protein (DLK), ALB, α-fetoprotein (AFP), or glucuronosyltransferase 1 A (UGT1A) (Santa Cruz Biotechnology, USA) at RT for 1 h, followed by incubation with DyLight 488-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, USA) at RT for 30 min. The presence of those proteins was recorded under a fluorescence microscope (TE2000-S, Nikon). Untreated cells stained with nonspecific IgG (Santa Cruz Biotechnology, USA) were used as negative controls.
Periodic acid-Schiff (PAS) staining
Cells were seeded in 24-well plates, treated for 12 days and fixed with 4% paraformaldehyde for 10 min, followed by washing with water. Cells were oxidized by staining with 0.5% periodic acid solution for 5 min, and then incubated with Schiff's reagent for 15 min with tap water rinses between treatments. The stained cells were counterstained with hematoxylin solution for 2 min, and thoroughly washed in tap water. In each group, more than 10 non-overlapping fields with positive purple-red cells were recorded under a microscope.
Indocyanine green (ICG) uptake and release
Cells were treated as described previously for 12 days (14). ICG was prepared in DMSO (25 mg/mL stock) and freshly diluted in complete DMEM medium at a final concentration of 1 mg/mL. Cells were washed with PBS and incubated in ICG working solution at 37°C for 1 h. Positive stained cells (green color in the nucleus) were photographed under a microscope after careful washing with several changes of PBS. Complete medium was added to the same cells, which were cultured at 37°C for an additional 6 h, and then observed again to document ICG release. At least 10 non-overlapping vision fields were recorded.
Statistical analysis
Data are reported as means±SD and analyzed using the SPSS 15.0 software (USA). Significant differences among more than three groups were evaluated by analysis of variance, while differences between two groups were evaluated by two-tailed Student t-tests. A P value <0.05 was considered to be statistically significant.
Results
Relative ALB-GLuc activity with treatment by different factors
First, we detected an effect of different induction factors on maturation of HP14.5. ALB is the most abundant protein produced by the liver and its expression is correlated with the maturation of hepatocytes. In HP14.5 cells, an ALB promoter was used to drive the expression of GLuc, which is an indirect indicator of the level of ALB in cells (Figure 1A). Treatment with 2% HS induced higher ALB-GLuc activity than 10% FBS. The activity of ALB-GLuc in 0.1 μM Dex-treated HP14.5 was higher at 4 days than in controls (P<0.05). Both HGF and FGF4 induced ALB-GLuc activity at the beginning of induction day 4 and day 6, respectively. The ALB-GLuc activity plateaued with HGF at concentrations ranging from 10 to 40 ng/mL (P>0.05 among HGF groups, P<0.05 vs control), but decreased with 80 ng/mL. The effect of FGF4 was dose dependent at concentrations between 5 and 20 ng/mL (P<0.05 among FGF 4 groups, P<0.05 vs control; Figure 1B). Thus, the concentrations of HGF and FGF4 chosen to test the optimal induction condition in the following experiment were 10 and 20 ng/mL, respectively.
Figure 1. Effect of different induction factors on albumin promoter-driven Gaussian luciferase (ALB-GLuc) activity. HP14.5 ALB-Gluc cells were treated with induction factors. ALB-GLuc activity was assayed on the indicated day (D). A, Schematic representation of the ALB-GLuc reporter. B, ALB-GLuc activity of HP14.5 cells at D2, D4, and D6 treated with (1) different complete DMEM medium with 10% fetal bovine serum (FBS) and 2% horse serum (HS) (*P<0.05, two-tailed Student t-test); (2) 0.1 μM dexamethasone (Dex) in 10% FBS medium (*P<0.05, two-tailed Student t-test); (3) different concentrations of hepatic growth factor (HGF) (*P<0.05, 10 ng/mL vs 0 ng/mL control, two-tailed Student t-test); (4) different concentrations of fibroblast growth factor 4 (FGF4) (*P<0.05, 20 ng/mL vs 0 ng/mL control, two-tailed Student t-test). C, ALB-GLuc activity of HP14.5 cells at D3, D6, D9, D12, and D15 with single factors and different combinations of culture conditions. Three independent assays were carried out for each group. *P<0.05, Dex+HGF+FGF4 vs control with 2% HS; **P<0.05, Dex+HGF+FGF4 with 2% HS compared to the same treatment with 10% FBS (two-tailed Student t-test).
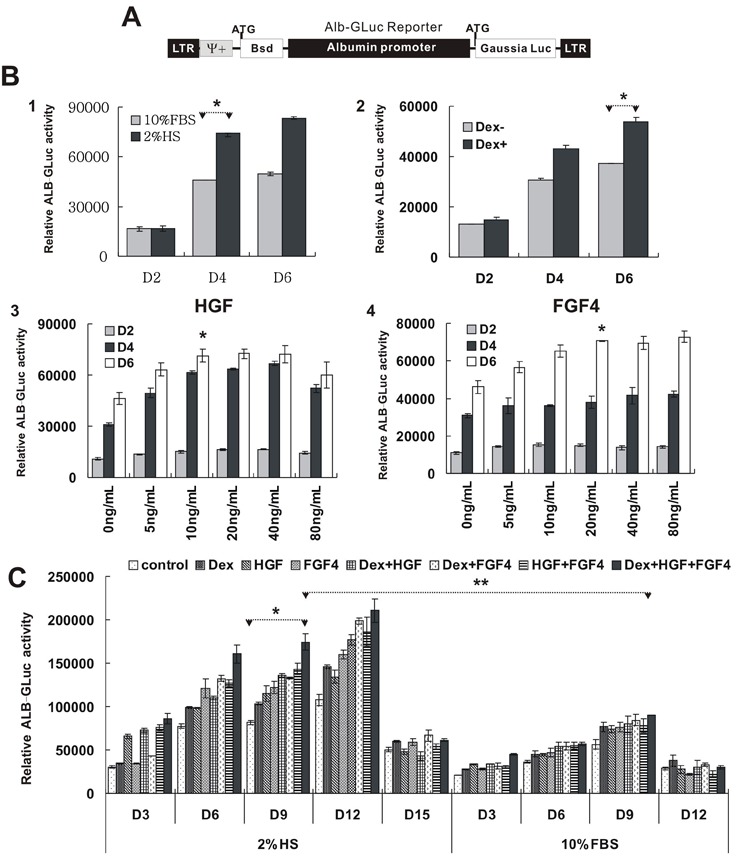
Next, we sought to determine the effect of combinations of different induction factors on maturation of HP14.5. While single factors or combinations of two factors could improve the expression of ALB-GLuc, a combination of 0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4 was the best induction combination in both 10% FBS and 2% HS (P<0.05 vs control). Meanwhile, HP14.5 cells were more sensitive to induction factors in 2% HS than in 10% FBS (P<0.05, 2% HS vs 10% FBS in same treatment of 0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4). When the initial cell density was 20-30% in culture medium with 2% HS, the expression of ALB-GLuc increased with induction time, peaked at day 12, and sharply decreased at day 15. However, in culture medium with 10% FBS, the ALB-GLuc activity increase was apparent and peaked at day 9 (Figure 1C). Therefore, our results indicated that the combination of 2% HS+0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4 may be the best induction condition for ALB activity. We thus chose this condition for the following experiments.
Proliferation of cells treated with different single factors
Why were the peak times of ALB-GLuc activity different in 10% FBS and 2% HS? Is there any single factor that affects cell proliferation? To answer these questions, we further analyzed cell growth with single factor treatments. Figure 2A shows that cells proliferated more slowly in 2% HS than in 10% FBS (P<0.05). Cell numbers reached a maximum at day 9 in 10% FBS but continued to increase after that time in 2% HS. HGF and Dex did not affect cell growth, while FGF4 promoted cell proliferation (P<0.05). The cells in the 10% FBS group were not available for counting at day 15 post-induction because of limited space. We also performed crystal violet staining at day 12 to examine the cell populations in each group. As presented in Figure 2B, the staining intensity was higher in 2% HS than that in 10% FBS, while FGF4 treatment had the highest staining intensity. The result from the colorimetric assays was correlated with the growth curve at day 12 (Figure 2C). Thus, our results indicated that cell proliferation was inhibited in 2% HS but enhanced by FGF4.
Figure 2. Cell proliferation of the induced hepatic progenitor cells (HPCs). Cells were plated onto 24-well plates at an initial density of 20,000/well, and treated with 0.1 μM Dex, 10 ng/mL HGF, 20 ng/mL FGF4 in 10% FBS or 2% HS. A, Cell proliferation was determined using trypan cell counting. *P<0.05 FGF4 vs control; P<0.05 2% HS vs 10% FBS; P>0.05 control vs Dex or HGF (two-tailed Student t-tests). B, Crystal violet staining of HP14.5 cells at day 12 of induction. C, Absorbance of dissolved blue dye in each well in the same set of cells as in B. See Figure 1 for explanation of abbreviations. *P<0.05 vs control; **P<0.05 2%HS vs 10%FBS in same FGF4 treatment (two-tailed Student t-tests).

In vitro induced maturation of HPCs
We investigated whether the optimal induction condition for ALB-GLuc activity could also induce maturation of HP14.5 cells. Morphologically, untreated HP14.5 cells were large, flat, had an irregular polygonal shape and some of them had two or more nuclei. By day 3 of induction, the untreated cells were 80% confluent and actively proliferating. Compared with untreated HP14.5 cells, at day 12, the induced cells became tightly arranged and exhibited the typical paving stone morphology of hepatic cells (Figure 3A). RT-PCR was performed to detect the expression of liver cell markers over 12 days of induction. As shown in Figure 3B, DLK and cytokeratin-19 (CK19), two hepatic stem cell markers, began to decline at day 3 and continued to decline until day 12. The expression of AFP initially increased, followed by a decrease from D6 onward. The expression of the liver cell specific markers ALB and cytokeratin-18 (CK18) continuously increased during the whole induction period. Two other liver-specific proteins, tyrosine aminotransferase (TAT) and apolipoprotein B (ApoB) were detectable at day 3 and day 6, respectively, and continued to increase during induction. Immunofluorescence staining results of DLK, AFP, and ALB were consistent with those obtained using RT-PCR. DLK protein was localized on cell membranes while the other proteins were largely distributed in the cytoplasm. The liver microsomal marker UGT1A also appeared in the cytoplasm and its expression increased significantly during induction (Figure 3C). Real-time PCR results further indicated that the expression of AFP, ALB, CK18, and TAT in the treated cells at day 12 was significantly greater than in the untreated cells, and was nearly the same as the expression level seen in LC14d cells, which were used as positive liver cell controls (Figure 3D).
Figure 3. Expression of liver cell markers during the induced maturation of hepatic progenitor cells (HPCs). Cells were treated with the combination condition of 2% HS+0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4. A, The morphology of untreated and treated cells at D3, D6, D9, and D12 days after induction. Panels 1, 2, 3, and 4 are untreated cells; 7, 8, 9, and 10 are induced cells. Scale bar=200 μm. B, RT-PCR analysis of hepatic-related genes DLK, CK19, AFP, ALB, CK18, TAT, and ApoB at D0, D3, D6, D9, and D12 after induction. RT-PCR results were confirmed in at least three independent experiments, and representative results are shown. C, Immunofluorescence staining of DLK, AFP, ALB, and UGT1A markers at D0, D3, D6, D9, and D12 days after induction. Negative controls (NC) are cells stained with nonspecific IgG. Scale bar=200 μm. D, Real-time PCR analysis of hepatic related genes AFP, ALB, CK18, TAT of the induced cells at D12 compared with untreated cells as negative controls and adult liver cells as positive controls. See Figure 1 for explanation of abbreviations. *P<0.05 induced D12 vs control D12 (two-tailed Student t-test); **P<0.05 LC14d vs induced D12 (two-tailed Student t-test).
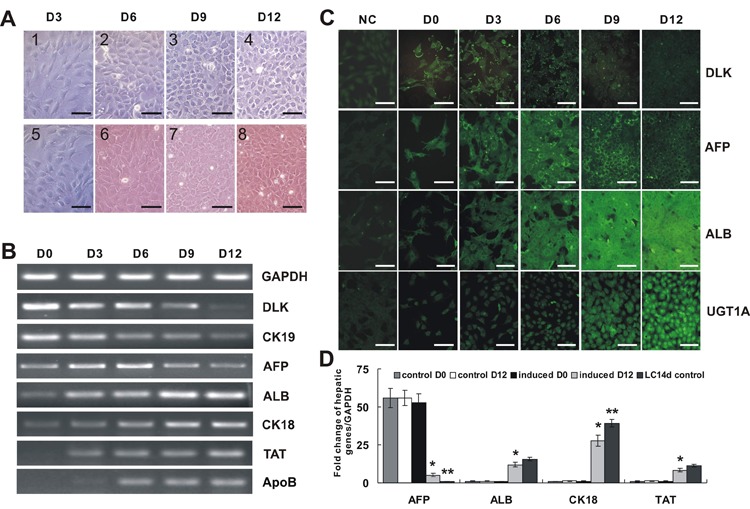
Figure 4. Hepatic functions of induced hepatic progenitor cells (HPCs). Hepatic functions of induced HPCs were analyzed at D0, D3, D6, D9, and D12 following treatment with 2% HS+0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4. A, PAS staining of the glycogen storage and accumulation function in the induced HP14.5 cells. The red granular staining in cytoplasm is indicated with arrows. B, ICG uptake and release assay for the transport and metabolism function of the induced HPCs. Green-stained cells are indicated with arrows. The staining was performed in at least three independent experiments, and representative results are shown. See Figure 1 for explanation of abbreviations. Scale bar=200 μm.

Glycogen storage and ICG uptake/release function of induced HP14.5 cells in vitro
Mature hepatocytes are able to carry out the function of glycogen storage and accumulation in granule form in the cytosol, which can be demonstrated by PAS staining (15). The untreated HP14.5 cells were mostly PAS-negative. After 6 days of induction, PAS-positive granules started to appear in the cytoplasm and significantly increased at day 9 and day 12 of induction. ICG is a cyanine dye used to determine hepatic function (16). No ICG-positive cells were observed in the untreated group. At day 3 of induction, fewer than 5% of the cells took up ICG (green nuclear stain) from the medium and excluded the absorbed ICG 6 h later. The ICG positivity gradually increased along the induction time, and more than 70% of the cells were positive at day 12. The above results demonstrated that the reported induction method could induce not only the expression of liver cell markers but also the function of HP14.5 cells, providing an effective means to induce the maturation of HPCs in vitro.
Discussion
End-stage liver diseases and acute liver failure have been considered treatable only with liver transplantation (17). Liver transplantation is an organ transplantation that involves major surgery and is limited by the scarcity of donor organs, need of life-long immunosuppression, and high cost. Recent interest has focused on cell transplantation as an alternative to organ transplantation. Liver cell transplantation is a simple, safe, and relatively less costly procedure that can take advantage of freshly discarded liver segments, and shows great promise for the treatment of many liver diseases (18). Embryonic stem cells, bone marrow hematopoietic cells, mesenchymal stem cells, and umbilical cord blood cells have all been shown to differentiate into a hepatic linage with hepatocyte-like morphology and cell markers. But few hepatic functions have been demonstrated in vivo (4,19). HPCs are progenitor cells originated from liver and capable of differentiating into hepatocytes and biliary cells. HPCs also express the stem cell-related markers with self-renewal capacity, serving as a continual and readily available source of cells for liver cell transplantation (20). However, HPCs are not mature enough to recover liver function in vivo. In this study, we tested various induction conditions of in vitro culture of HP14.5 cells in order to identify the best method to induce maturation of HPCs.
Dex, HGF, and/or FGF4 have been shown to induce bone marrow mesenchymal stem cells, hematopoietic cells, or mouse embryonic stem cell differentiation to hepatic cell lines (21,22). However, there are still no corresponding reports on the optimal concentration and combinations of induction factors in the differentiation of HPCs to mature hepatocytes. ALB is a marker of mature liver cells and is widely used to detect the maturation of hepatic cells (23). Here, we constructed a stable cell line expressing an ALB-promoter-driven GLuc reporter gene. During the liver cell differentiation process, many transcriptional factors regulate the expression levels of ALB by activating or inhibiting the ALB promoter. Moreover, GLuc is a natural secretary luciferase isolated from the marine copepod Gaussia princeps that can be released into the culture medium (24). Here, we observed that the combination of 2% HS+0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4 induced the highest ALB-GLuc activity. Our results differed from those reported by Oh et al. (25) in which high, 0.5-5.0 mg/mL, concentrations of HGF were used to induce the differentiation of bone marrow mesenchymal stem cells into liver cells. The time point of ALB increase in this study was much earlier than that for the induced extrahepatic stem cells (26), probably because HP14.5 cells are more committed fetal HPCs and hence more responsive to the exogenous induction factors.
ALB-GLuc activity was only an indicator. We further confirmed the effect of this combination induction method. Compared with untreated cells, induced cells became neatly arranged and exhibited the typical liver cell morphology of polyhedron-shape. As previously reported (10), HP14.5 highly expressed the hepatic stem/progenitor cell markers DLK, AFP, and CK19. Upon induction, the expression of DLK and CK19 gradually decreased. CK19 is not a unique marker for liver stem cells, as it is also a marker of biliary differentiation (27), its marked reduction reflects HP14.5 cell differentiation into mature hepatic cells, but not bile duct cells. AFP is a major plasma protein produced by the liver during fetal development and is thought to be the fetal form of serum albumin. The highest AFP levels are present in the fetus and decrease at the end of the first trimester (28). HP14.5 cells were isolated from post-coitus day 14.5 embryonic liver, which may not represent the high point of the APF level. Thus, APF expression firstly increased and then decreased by day 6 during the induction process. Furthermore, other hepatic-specific proteins ALB, CK18, UGT1A produced by mature liver cells continued to rise. TAT became detectable by day 3, and ApoB, by day 6 after induction. On day 12, the induced cells exhibited expression of liver markers comparable to adult mouse liver cells. Therefore, our result revealed that HP14.5 cells could be effectively differentiated into mature hepatocytes.
To become a reliable cell source for liver cell transplantation, the induced HPCs should have good liver function. PAS staining is primarily used to identify glycogen in tissues. Functional hepatocytes are capable of glycogen synthesis and accumulation (15). ICG is a nontoxic cyanine dye used in hepatic function diagnostics. ICG is metabolized microsomally in liver cells and removed from the liver exclusively in bile juice (16). Microsomes were present in the nucleus, thus the green nuclear staining reflected the function of ICG metabolism. With the induction conditions used here, ICG uptake/release and PAS-positive cells were observed at day 6, and gradually increased to more than 70% by day 12, suggesting the induced HP14.5 cells had hepatic function.
Taken together, the results demonstrate that the combination of 2% HS+0.1 μM Dex+10 ng/mL HGF+20 ng/mL FGF4 effectively induced the maturation and function of HPCs. It is conceivable that more effective methods need to be further explored because fewer than 100% of the induced cells had hepatic functions. Nevertheless, we report a relatively effective method to induce hepatic differentiation and maturation of HPCs.
Supplementary Material
Acknowledgments
This research was supported in part by grants from the National Natural Science Foundation of China (No. 81100309 to Y. Bi and No. 81071770 to T. Feng).
Footnotes
First published online July 16, 2013.
References
- 1.Feldmann G. Liver transplantation of hepatic stem cells: potential use for treating liver diseases. Cell Biol Toxicol. 2001;17:77–85. doi: 10.1023/A:1010954020488. [DOI] [PubMed] [Google Scholar]
- 2.Kakinuma S, Nakauchi H, Watanabe M. Hepatic stem/progenitor cells and stem-cell transplantation for the treatment of liver disease. J Gastroenterol. 2009;44:167–172. doi: 10.1007/s00535-008-2297-z. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Alvarez N, Soto-Gutierrez A, Kobayashi N. Hepatic stem cells and liver development. Methods Mol Biol. 2010;640:181–236. doi: 10.1007/978-1-60761-688-7_10. [DOI] [PubMed] [Google Scholar]
- 4.Sharma AD, Cantz T, Vogel A, Schambach A, Haridass D, Iken M, et al. Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 2008;17:313–323. doi: 10.3727/096368908784153896. [DOI] [PubMed] [Google Scholar]
- 5.Tomiyama K, Miyazaki M, Nukui M, Takaishi M, Nakao A, Shimizu N, et al. Limited contribution of cells of intact extrahepatic tissue origin to hepatocyte regeneration in transplanted rat liver. Transplantation. 2007;83:624–630. doi: 10.1097/01.tp.0000253942.16061.d9. [DOI] [PubMed] [Google Scholar]
- 6.Sangan CB, Tosh D. Hepatic progenitor cells. Cell Tissue Res. 2010;342:131–137. doi: 10.1007/s00441-010-1055-8. [DOI] [PubMed] [Google Scholar]
- 7.Brolen G, Sivertsson L, Bjorquist P, Eriksson G, Ek M, Semb H, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Li W, Liu B, Wang P, Li W, Zhang H. Efficient generation of functional hepatocyte-like cells from human fetal hepatic progenitor cells in vitro . J Cell Physiol. 2012;227:2051–2058. doi: 10.1002/jcp.22934. [DOI] [PubMed] [Google Scholar]
- 9.Ichinohe N, Tanimizu N, Ooe H, Nakamura Y, Mizuguchi T, Kon J, et al. Differentiation capacity of hepatic stem/progenitor cells isolated from D-galactosamine-treated rat livers. Hepatology. 2013;57:1192–1202. doi: 10.1002/hep.26084. [DOI] [PubMed] [Google Scholar]
- 10.Ichinohe N, Kon J, Sasaki K, Nakamura Y, Ooe H, Tanimizu N, et al. Growth ability and repopulation efficiency of transplanted hepatic stem cells, progenitor cells, and mature hepatocytes in retrorsine-treated rat livers. Cell Transplant. 2012;21:11–22. doi: 10.3727/096368911X580626. [DOI] [PubMed] [Google Scholar]
- 11.Gridelli B, Vizzini G, Pietrosi G, Luca A, Spada M, Gruttadauria S, et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl. 2012;18:226–237. doi: 10.1002/lt.22322. [DOI] [PubMed] [Google Scholar]
- 12.Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC, et al. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem. 2009;108:295–303. doi: 10.1002/jcb.22254. [DOI] [PubMed] [Google Scholar]
- 13.Bi Y, Gong M, Zhang X, Zhang X, Jiang W, Zhang Y, et al. Pre-activation of retinoid signaling facilitates neuronal differentiation of mesenchymal stem cells. Dev Growth Differ. 2010;52:419–431. doi: 10.1111/j.1440-169X.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Zhang WY, Gong M, Huang JY, Tang N, Feng T, et al. Low serum concentration facilitates the differentiation of hepatic progenitor cells. Saudi Med J. 2011;32:128–134. [PubMed] [Google Scholar]
- 15.Liu YN, Zhang J, He QH, Dai X, Shen L. Isolation and characterization of epithelial progenitor cells from human fetal liver. Hepatol Res. 2008;38:103–113. doi: 10.1111/j.1872-034X.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. Cell therapies for liver diseases. Liver Transpl. 2012;18:9–21. doi: 10.1002/lt.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passier R, Mummery C. Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovasc Res. 2003;58:324–335. doi: 10.1016/S0008-6363(02)00770-8. [DOI] [PubMed] [Google Scholar]
- 20.Parveen N, Aleem AK, Habeeb MA, Habibullah CM. An update on hepatic stem cells: bench to bedside. Curr Pharm Biotechnol. 2011;12:226–230. doi: 10.2174/138920111794295765. [DOI] [PubMed] [Google Scholar]
- 21.Banas A, Yamamoto Y, Teratani T, Ochiya T. Stem cell plasticity: learning from hepatogenic differentiation strategies. Dev Dyn. 2007;236:3228–3241. doi: 10.1002/dvdy.21330. [DOI] [PubMed] [Google Scholar]
- 22.Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, et al. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461–7465. doi: 10.3748/wjg.v11.i47.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44:1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- 24.Wiles S, Ferguson K, Stefanidou M, Young DB, Robertson BD. Alternative luciferase for monitoring bacterial cells under adverse conditions. Appl Environ Microbiol. 2005;71:3427–3432. doi: 10.1128/AEM.71.7.3427-3432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 27.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci. 2006;63:2922–2938. doi: 10.1007/s00018-006-6258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


