Abstract
Background.
Metabolic syndrome (MetS) is a potentially reversible cause of disability in the elderly people. The published literature suggests that the MetS–disability association is likely to be complex, depending on co-existing risk factors and with possible variation for each of the specific MetS components. Further evidence is needed to understand the specific consequences of the MetS as a whole and as a function of its components.
Methods.
Prospective analyses included data from 6,141 participants (60.9% women) aged 65 and older from the Three-City cohort. Mixed logistic models were used to determine associations between MetS (National Cholesterol Education Program Adult Treatment Panel III criteria) and 7-year incident disability measured as social restriction, mobility limitations (Rosow and Breslau scale), and limitations in instrumental and basic activities of daily living.
Results.
MetS was associated with incident social restriction (odds ratio = 1.55, 95% CI: 1.14–2.09), limited mobility (odds ratio = 1.52, 95% CI: 1.21–1.90), and instrumental activities of daily living limitations (odds ratio = 1.62, 95% CI: 1.24–2.10) after adjustment for a range of potential sociodemographic, health behavior, and health status confounders at baseline. These associations were independent of chronic conditions, including cardiovascular disease and dementia. There was evidence of associations between MetS components: central obesity, high triglycerides, and elevated fasting glucose and incidence of limitations in mobility and instrumental activities of daily living.
Conclusions.
Our results suggest that the increased risk of mobility and instrumental activities of daily living limitations in the elderly people associated with MetS is over and above that associated with its components.
Key Words: Metabolism, Frailty, Epidemiology.
A worldwide increase in the number of disabled elderly people is expected in the coming decades (1). This has led to considerable public health concern about the predicted social and economic burden associated with the growing disabled population and a need for improved prevention. With chronic disease being the leading cause of disability, current research is seeking to identify early stage markers and potentially reversible risk factors for these disorders to inform implementation of prevention programer prior to onset of the disability. In this context, metabolic syndrome (MetS) is of particular interest (2) as it represents a cluster of metabolic and vascular abnormalities, which increase the risk of a range of common chronic cerebrovascular, cardiovascular, and neurological disorders known to be associated with disability. MetS may thus constitute an “early warning signal” for potentially disabling disorders. Importantly, all its components (central obesity, hyperglycemia, dyslipidemia, and hypertension) are highly amenable to therapeutic intervention.
To date, research in this area is very limited. To our knowledge, only five studies have assessed the association between MetS and onset of disability (3–7). Of these, three focused on the association between MetS and mobility limitation only (4,5,7). The other two studies investigated the association of MetS with limitations in instrumental activities of daily living (IADLs) and basic activities of daily living (ADLs). Although the first study showed that MetS was associated with a higher rate of ADL limitations and poorer health-related quality of life (6), the second reported that participants with MetS (but without type 2 diabetes) showed a 3-year decline in activities requiring strength and mobility but not ordinary daily activities (3). The association between MetS and disability is likely to be complex and dependent on factors such as MetS severity and coexisting risk factors, and may vary for each of the specific MetS components. Furthermore, any study of disability needs to be prospective and take into account multiple potential confounding factors, most notably comorbidity, if robust associations that can indicate causal processes are to be identified.
Accordingly, the aim of this study was to examine longitudinally the association between MetS and incident disability using multiple measures of limitation within a large elderly population, the Three-City (3C) Study. Our secondary aim was to examine whether associations observed between MetS and limitations were attributable to MetS overall or driven by a specific component. An advantage of the 3C Study data set is the opportunity to take into account a large range of potential confounders and a sufficiently long prospective follow-up.
Methods
Study Design and Participants
The 3C Study is an ongoing multisite cohort study of 9,294 community-dwelling persons aged 65 years or older and recruited from the electoral rolls of three French cities (Bordeaux, Dijon, and Montpellier) from 1999 to 2001 (8). The ethics committee of the University Hospital of Bicêtre, France, approved the study protocol and written informed consent was obtained from each participant. Participants underwent a standardized evaluation with face-to-face interviews and clinical examinations were undertaken at baseline and at 2, 4, and 7 years in a study center or during home visits.
Outcome of Interest
Three complementary activity limitation indicators were measured at baseline and repeated at each follow-up; mobility, IADLs, and ADLs. Mobility was assessed using the Rosow and Breslau scale (9), which assesses ability to do heavy housework, walk half a mile, and climb stairs. The Lawton–Brody IADL scale was used to assess an individual’s ability to use the telephone, manage medication and money, use public or private transport, shop, and, for women only, prepare meals and do housework and laundry (10). For ADLs, participants were asked whether they needed help for any task included in the Katz ADL scale: bathing, dressing, transferring from bed to chair, toileting, and eating (11). For each domain of disability, participants indicating inability to perform one or more activities without help were considered as having limitations: moderate limitation for mobility limitation and more severe limitation for IADL or ADL limitations.
Social restriction: confinement to bed, home, or outings restricted to the local neighborhood assessed using the International Classification of Functioning, Disability and Health (12) was considered as the fourth outcome.
Explanatory Variables
MetS was defined using the National Cholesterol Education program Adult Treatment Panel III criteria (13), based on the presence of three or more of the following—waist circumference: men greater than 102cm, women greater than 88cm; serum triglycerides greater than or equal to 1.7 mmol/L; high-density lipoprotein cholesterol: men less than 1.04 mmol/L, women less than 1.29 mmol/L; systolic blood pressure greater than or equal to 130 mmHg, diastolic blood pressure greater than or equal to 85 mmHg, or treatment of hypertension; fasting blood glucose greater than or equal to 5.6 mmol/L or presence of type 2 diabetes. As data on the established diagnosis of type 2 diabetes by a practitioner were not available, the use of antidiabetes treatment was considered as a proxy. Waist circumference was measured between the lower rib margin and the iliac crest following normal expiration. Details of procedures for measuring blood pressure, fasting blood glucose, high-density lipoprotein cholesterol, and triglycerides have been previously described (14).
Covariates at Baseline
Socio demographic variables were sex, age, study center, marital status, educational attainment, and household income. Health behaviors included smoking status and alcohol consumption. Health status was ascertained by self-reported history of cardiovascular disease (CVD; including stroke, angina pectoris, myocardial infarction, and cardiac and vascular surgery), self-reported history of respiratory disease (asthma attacks during the last year or chronic bronchitis), use of lipid-lowering drugs, body mass index (BMI) categories (normal: BMI < 25kg/m2/overweight: 25 ≤ BMI < 30kg/m2/obesity: BMI ≥ 30kg/m2), and current depressive symptoms assessed by the Center for Epidemiologic Studies-Depression scale with a cutoff point of greater than or equal to 16 or a current major depressive episode diagnosed by the Mini International Neuropsychiatric Interview algorithms (15). Aging-related impairments also taken into account were visual impairment, defined as having a corrected near visual acuity (Parinaud scale) of more than 4, or difficulties recognizing a familiar face at 4 m; hearing impairment defined as deafness or only able to hear a conversation when a single person speaks loudly; and cognitive impairment defined as having a score below 24 on the Mini-Mental State Examination. Finally, presence of allele ε4 of the apolipoprotein E genotype was included as covariate as it has been found to be associated with moderate activity limitation (16).
Statistical Analyses
The chi-square test was used to compare the categorical characteristics of participants according to the presence of MetS at baseline. For each indicator of activity limitation, longitudinal associations according to MetS status, each of its components and the severity of MetS (assessed as the sum of MetS components at baseline and analyzed as a continuous variable, range 0–5) at baseline were examined in participants free of that limitation at baseline. Thus, the sample size was 3,497 for mobility, 5,764 for IADL, 6,125 for ADL, and 5,893 for social restriction. In longitudinal studies, the within-person responses (ie, the repeated evaluations of the limitations) are correlated. This correlation was accounted for by using a mixed logistic model (17). In brief, this model has four basic advantages: (i) the evolution of limitations within the individual over time are taken into account, including possible reversion to the normal (nondisabled) state; (ii) participants with incomplete responses across time are included in the analysis; (iii) participants do not have to be evaluated at the same time points; and (iv) the model allows within-participant dependency to vary from one participant to another, via random effects. The SAS procedure NLMIXED was used to estimate the parameters (version 9.2).
The association of MetS and its components with incident activity limitations and social restriction during follow-up was first minimally adjusted for sex, center, age, time, and interaction time × age (Model 1). Multivariate odds ratios and their 95% confidence interval (95% CI) were additionally adjusted for income, education level, marital status, alcohol intake, smoking status, BMI, CVD, use of lipid-lowering drugs, respiratory disease, depressive symptoms, and cognitive, visual, and hearing impairments and allele ε4 of the apolipoprotein E genotype (Model 2). Models did not account for time-varying covariates as most covariates were only assessed at baseline. Interactions between MetS and the covariates on regarding the disability outcomes were not found to be statistically significant at p < .05.
In supplementary analyses, we further explored the extent to which any associations between MetS and indicators of disability are driven by specific MetS components. In these analyses, associations between MetS and indicators of the disability were adjusted successively for each of the MetS components. To assess the weight of individual MetS components in the MetS–disability indicator association, we calculated the percent attenuation of this association after adding each individual component separately to a model already including MetS. The percent attenuation in the association was determined using the formula [(βMetS − βMetS adjusted for component)/βMetS] ×100, where βs are the coefficients estimated from the mixed logistic model.
Results
Of the 9,080 dementia-free participants included at baseline, 877 missed all follow-up evaluations of disability outcomes (of whom 410 died), 1,029 had missing data for at least one component of MetS, and a further 1,033 had missing data for covariates assessed at baseline. These participants were excluded from the present analyses that were thus conducted on 6,141 participants (2,404 men and 3,737 women). Compared with participants included, those excluded were more likely to be older, have lower education and existing limitations in mobility and daily activities. They were also more likely to have depressive symptoms, have a history of CVD or cognitive, visual, or hearing impairment (p < .0001 for each comparison), and have MetS (p = .007).
In the 6,141 participants included in this study, the prevalence of MetS at baseline was 17.3%. The characteristics of these participants, as a function of MetS status, are presented in Table 1. Compared with participants without MetS, those with MetS were more likely to be male, older, have low educational attainment, lower alcohol intake, and be ex-smokers. MetS was also associated with a higher prevalence of depressive symptoms, cognitive impairment, respiratory disease, and CVD, as well as higher BMI and use of lipid-lowering agents. With the exception of ADL, participants with MetS were more likely to report limitations at baseline.
Table 1.
Characteristics of the 6,141 Participants According to MetS* Status at Baseline
| No MetS | MetS | Chi-square p Value | |||
|---|---|---|---|---|---|
| N = 5,078 | N = 1,063 | ||||
| N | % | N | % | ||
| Sex: women | 3,128 | 61.6 | 609 | 57.3 | .009 |
| Age | |||||
| 65–69 | 1,354 | 26.7 | 228 | 21.5 | .004 |
| 70–74 | 1,739 | 34.2 | 399 | 37.5 | |
| 75–80 | 1,383 | 27.2 | 297 | 27.9 | |
| 80+ | 602 | 11.9 | 139 | 13.1 | |
| Center | |||||
| Bordeaux | 1,087 | 21.4 | 273 | 25.7 | .003 |
| Dijon | 2,775 | 54.7 | 570 | 53.6 | |
| Montpellier | 1,216 | 23.9 | 220 | 20.7 | |
| MetS components | |||||
| Central obesity | 944 | 18.6 | 831 | 78.2 | <.0001 |
| High TG | 389 | 7.7 | 630 | 59.3 | <.0001 |
| Low HDL cholesterol | 196 | 3.9 | 471 | 44.3 | <.0001 |
| High BP | 3,996 | 78.7 | 1,024 | 96.3 | <.0001 |
| Elevated FBG | 468 | 9.2 | 670 | 63.0 | <.0001 |
| Indicators of social restriction and activity limitations | |||||
| Social restriction | 171 | 3.4 | 76 | 7.2 | <.0001 |
| Mobility limitations | 2,047 | 40.8 | 564 | 53.1 | <.0001 |
| IADL limitations | 276 | 5.4 | 97 | 9.1 | <.0001 |
| ADL limitations | 11 | 0.2 | 3 | 0.3 | .72† |
| Education: ≤5 y | 1,145 | 22.6 | 291 | 27.4 | .0007 |
| High income (≥€1,525/mo) | 3,500 | 68.9 | 648 | 60.9 | <.0001 |
| Living alone | 1,713 | 33.7 | 379 | 35.7 | .23 |
| Alcohol | |||||
| 0 | 944 | 18.6 | 239 | 22.5 | .001 |
| 1–36g/d | 3,717 | 73.2 | 719 | 67.6 | |
| >36 g/d | 417 | 8.2 | 105 | 9.9 | |
| Smoking | |||||
| Never | 3,145 | 61.9 | 604 | 56.8 | .002 |
| Former | 1,653 | 32.6 | 405 | 38.1 | |
| Current | 280 | 5.5 | 54 | 5.1 | |
| BMI | |||||
| Normal | 2,739 | 53.9 | 140 | 13.2 | <.0001 |
| Overweight | 1,941 | 38.2 | 503 | 47.3 | |
| Obese | 398 | 7.8 | 420 | 39.5 | |
| Cognitive impairment | 186 | 3.7 | 64 | 6.0 | .0004 |
| Visual impairment | 358 | 7.0 | 91 | 8.5 | .09 |
| Hearing impairment | 303 | 6.0 | 79 | 7.4 | .07 |
| Respiratory disease | 266 | 5.2 | 79 | 7.4 | .005 |
| CVD | 727 | 14.3 | 216 | 20.3 | <.0001 |
| Depressive symptoms | 1,099 | 21.6 | 283 | 26.6 | .0004 |
| Lipid-lowering treatment | 1,560 | 30.7 | 414 | 39.0 | <.0001 |
| APOEε4 | 1,021 | 20.1 | 211 | 19.9 | .85 |
Notes: ADL = basic activities of daily living; APOEε4 = allele ε4 of the apolipoprotein E (having at least one ε4 allele); BMI = body mass index; BP = blood pressure; CVD = cardiovascular diseases (including stroke); FBG = fasting blood glucose; HDL = high-density lipoprotein; IADL = instrumental activities of daily living; MetS = metabolic syndrome; TG = triglycerides.
*MetS defined according the National Cholesterol Education program Adult Treatment Panel III criteria (13).
†Fisher’s exact test.
The association between MetS status at baseline and incidence of disability over the 7 years of follow-up for each disability indicator was assessed by performing analyses in participants without limitations on the individual scales at baseline. Overall, the percentage of participants with incident limitations (at least one follow-up visit with limitations) was 14.9%, 63.5%, 21.3%, and 2.6% for social restrictions, mobility, IADL, and ADL, respectively, and the reversibility rate (percentage of participants with incident limitations who reversed to normal state at a subsequent visit) was 16.3%, 29.1%, 16.4%, and 12.6%, respectively. Table 2 shows the crude rates of social restriction and mobility and activity limitations by follow-up visit as a function of baseline MetS. After adjustment for sex, center, age, time, and the interaction between age and time, participants with MetS at baseline had double the odds of developing limitations in mobility, IADL, and ADL and nearly triple the odds of developing social restrictions (Model 1) compared with participants without MetS. Further adjustment for socioeconomic factors (income, education, living alone), health behaviors (alcohol, smoking), and health factors (BMI, cognitive, hearing, and visual impairment, depressive symptoms, lipid-lowering treatment, respiratory disease, CVD, and allele ε4 of the apolipoprotein E genotype) substantially attenuated these associations (Model 2) although they remained statistically significant except for the ADL scale (social restrictions: odds ratio = 1.55, 95% CI: 1.14–2.09; mobility limitations: odds ratio = 1.52, 95% CI: 1.21–1.90; and limitations in IADL: odds ratio = 1.62, 95% CI: 1.24–2.10; Table 2). Similar results were obtained when considering the severity of MetS—assessed by the number of MetS components at baseline—as a predictor of future disability (Table 3).
Table 2.
Incident Cases of Limitations by Baseline MetS and Adjusted Risk of Limitations
| No. at Baseline | Follow-up | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 y | 4 y | 7 y | ||||||||
| % | % | % | OR* | 95% CI | p Value | OR† | 95% CI | p Value | ||
| Social restriction | ||||||||||
| Baseline MetS | N = 5,893 | N = 5,713 | N = 5,179 | N = 4,243 | ||||||
| No | 3.5 | 5.3 | 12.0 | 1 | 1 | |||||
| Yes | 6.5 | 8.5 | 22.0 | 2.83 | 2.13–3.75 | <.0001 | 1.55 | 1.14–2.09 | .006 | |
| Mobility | ||||||||||
| Baseline MetS | N = 3,497 | N = 3,344 | N = 3,112 | N = 2,624 | ||||||
| No | 33.1 | 37.9 | 49.5 | 1 | 1 | |||||
| Yes | 44.0 | 45.5 | 60.2 | 1.90 | 1.54–2.35 | <.0001 | 1.52 | 1.21–1.90 | .0003 | |
| IADL | ||||||||||
| Baseline MetS | N = 5,764 | N = 5,575 | N = 5,063 | N = 4,150 | ||||||
| No | 4.7 | 7.6 | 18.5 | 1 | 1 | |||||
| Yes | 9.0 | 10.5 | 30.5 | 2.55 | 1.98–3.27 | <.0001 | 1.62 | 1.24–2.10 | .0003 | |
| ADL | ||||||||||
| Baseline MetS | N = 6,125 | N = 5,927 | N = 5,367 | N = 4,349 | ||||||
| No | 0.6 | 1.0 | 1.8 | 1 | 1 | |||||
| Yes | 0.8 | 1.4 | 2.9 | 2.00 | 1.07–3.77 | .03 | 0.91 | 0.48–1.74 | .79 | |
Notes: ADL = basic activities of daily living; IADL = instrumental activities of daily living; MetS = metabolic syndrome; OR = odds ratio.
*Adjusted for center, baseline age, time, baseline interaction age × time, and sex.
†Adjusted for center, baseline age, time, baseline interaction age × time, sex, education, income, living alone, alcohol, body mass index, smoking, cognitive impairment, visual impairment, hearing impairment, depressive symptoms, respiratory disease, cardiovascular disease, lipid-lowering treatment, and allele ε4 of the apolipoprotein E genotype.
Table 3.
Associations Between Baseline Number of MetS Components and Risk of Limitation Incidence
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | p Value | OR† | 95% CI | p Value | |
| Social restriction | ||||||
| No. of MetS components | 1.62 | 1.45–1.81 | <.0001 | 1.24 | 1.10–1.40 | .0005 |
| Mobility | ||||||
| No. of MetS components | 1.29 | 1.20–1.39 | <.0001 | 1.18 | 1.09–1.29 | <.0001 |
| IADL | ||||||
| No. of MetS components | 1.52 | 1.38–1.67 | <.0001 | 1.27 | 1.14–1.41 | <.0001 |
| ADL | ||||||
| No. of MetS components | 1.39 | 1.09–1.77 | .007 | 0.97 | 0.74–1.26 | .80 |
Notes: ADL = basic activities of daily living; IADL = instrumental activities of daily living; MetS = metabolic syndrome; OR = odds ratio.
*Adjusted for center, baseline age, time, baseline interaction age × time, and sex.
†Adjusted for center, baseline age, time, baseline interaction age × time, sex, education, income, living alone, alcohol, BMI, smoking, cognitive impairment, visual impairment, hearing impairment, depressive symptoms, respiratory disease, cardiovascular disease, lipid-lowering treatment, and allele ε4 of the apolipoprotein E genotype.
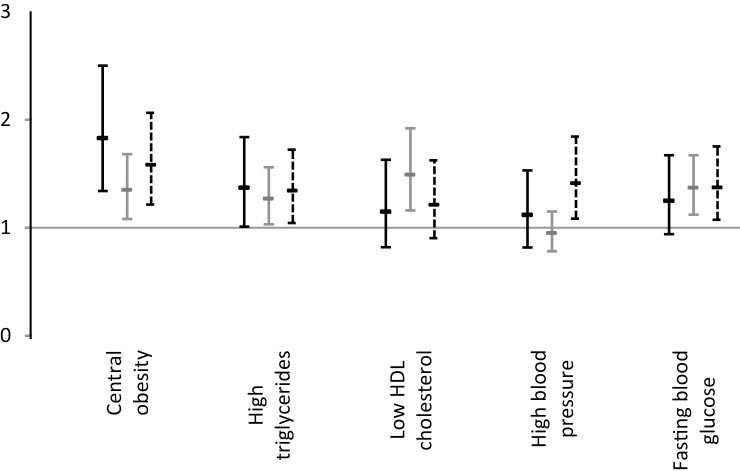
Multivariate analyses of associations between each component of MetS and onset of limitations in IADL, mobility and social restriction showed that of the five criteria, central obesity and high triglyceride levels were associated with all three disability outcomes (Figure 1). The elevated blood glucose component was only associated with the onset of mobility and IADL limitations; the low high-density lipoprotein cholesterol component was only associated with onset of mobility limitations; and the high blood pressure component with the onset of limitations in IADL.
Figure 1.
Fully adjusted odds ratio and 95% CI of limitations by metabolic syndrome component. Black bar: social restriction, grey bar: Rosow and Breslau mobility scale, dotted bar: IADL limitations. Adjusted for sex, center, age, time, interaction time × age, income, education level, marital status, alcohol intake, smoking status, body mass index, cardiovascular disease, respiratory diseases, depressive symptoms, use of lipid-lowering drugs, and cognitive, visual, and hearing impairment and allele ε4 of the apolipoprotein E genotype.
Further analyses in which MetS as a whole was adjusted successively for each of its components were performed to assess the extent to which specific MetS components may drive the overall MetS–activity limitation association (Supplementary Appendix Table A). Except for the MetS–social restriction association, which was no longer statistically significant after adjustment for central obesity, these analyses showed that MetS remained significantly associated with social restriction, mobility, and IADL limitations regardless of the component adjusted for in the analyses. These models also showed an independent effect of central obesity (p = .03) and high blood pressure (p = .05) on limitations in IADL. The central obesity component was also found to be independently associated with social restriction (p = .002). The percentage by which the MetS–limitation associations were attenuated by adjustment for the individual MetS components was calculated in additional analyses (Supplementary Appendix Table B). On all scales, the percent attenuation achieved when including central obesity and elevated blood glucose together was slightly higher than the sum of the percent attenuation achieved by each component alone, suggesting that central obesity may potentiate the impact of elevated blood glucose on limitations.
To explore whether associations between MetS and incident limitations could be driven by CVD, dementia, or type 2 diabetes, we reran the analyses after excluding (i) participants with prevalent CVD at baseline and those who developed CVD over the follow-up (Supplementary Appendix Table C), (ii) incident cases of dementia (Supplementary Appendix Table D), and (iii) participants with type 2 diabetes (assessed by using treatment for type 2 diabetes; Supplementary Appendix Table E). This had little effect on the associations.
Discussion
This study of a large cohort of community-dwelling elderly participants shows that MetS increased the odds of developing social restrictions and limitations in mobility and IADL over a 7-year follow-up by at least 50%. Our analysis took into account a range of sociodemographic variables, health behaviors, and health status factors such as depression and cognitive, visual, and hearing impairment; we confirmed that the association was not mediated by CVD and dementia, which also increase with aging and are associated with disability. Of the five MetS components, central obesity and high triglycerides were significantly associated with the incidence of limitations assessed by all three scales. In addition, elevated fasting glucose was associated with mobility and IADL limitations, whereas low high-density lipoprotein cholesterol was associated with mobility and high blood pressure with IADL. However, MetS overall increased the odds of moderate mobility limitations and more severe limitations measured by IADL.
Our results on the association between MetS and mobility restriction are in line with previous longitudinal studies carried out in the elderly people (3–5,7). Although the definition of mobility limitation and the prevalence of MetS varies between studies (from 29% to more than 45%), an increase in the rate of mobility limitations associated with MetS is reported by all. The study that failed to observe this association was limited to nonobese men (7). Our findings, carried out in an elderly cohort with a lower prevalence of MetS (17.3%) and longer follow-up, bring additional evidence to the existing literature by showing that MetS increased the odds of social and mobility restrictions by more than 50%.
Several studies that focus on the determinants of disability evaluated mobility limitations as they are common at advanced age and highly predictive of more severe and rapid progression to disability (4,5,7). Furthermore, mobility difficulties at older ages constitute a stage early enough in the disablement process to be amenable to intervention (18). However, to examine the predictive link between MetS and overall disability in old age, it is important to use scales that not only assess mobility difficulties but also limitations in more complex activities of daily living, usually assessed with IADL and ADL scales. Epidemiological observations are scarce regarding the association between MetS and ADL/IADL limitations. One cross-sectional study has reported in a stroke-free population that MetS was associated with a twofold increased prevalence of dependence as measured by ADL and IADL (6). Only one study has investigated this association prospectively (3). This study, carried out on a cohort of older Mexican Americans, failed to observe an association between MetS and progression of ADL/IADL limitations in participants without type 2 diabetes. Our study is the first to provide evidence of an association between MetS and incidence of limitations in IADL over a 7-year follow-up. This association was found to be independent of elevated fasting blood glucose, antidiabetic drugs, or other MetS components suggesting that MetS may constitute an independent predictor of limitation. Although a significant association was observed between MetS and incidence of limitations in ADL in sex and age-adjusted analyses, this association was lost after controlling for health behavior and health status.
Further studies prospectively assessing associations between MetS and incidence of limitations in ADL are needed, as the present study, with less than 3% incident ADL cases over the 7-year follow-up, may not be sufficiently powered. Future work is needed to establish whether MetS plays a role at all stages of the disability process. This would suggest that detecting and managing metabolic disorders at an early stage might be beneficial to delay disability onset among the high number who would otherwise end up with mobility or activity limitations.
We assessed whether components of MetS were associated with the onset of disability-related limitations. Our findings are concordant with those previously reported in literature as we observed that central obesity (19,20), high blood pressure (5,21), and elevated fasting glucose (22) increased the odds of new-onset IADL limitations and that central obesity was associated with mobility limitations (23,24) independently of having MetS. In terms of designing efficient intervention strategies, it is important to determine whether the MetS–health outcome association is driven by all or only some of its components. Our findings show that MetS remains significantly associated with new onset limitations after adjustment for each of its components, making it less probable that the observed predictive effect of MetS on the disability process is completely driven by a single component. Furthermore, we show that the percent attenuation of the MetS–limitation association achieved when including its two leading components—central obesity and elevated fasting glucose—was rather higher than the sum of the percent attenuation achieved by each component alone. This suggests that central obesity may potentiate the effect of elevated fasting blood glucose, a finding that reinforces the clinical utility of the MetS concept (2).
The MetS–limitation association is biologically plausible with possible underlying pathophysiological mechanisms including chronic inflammatory processes (and their catabolic consequences on muscle) (25), hyperglycemia-related mechanisms (5) (fatigue, headache, oxidative stress), and obesity-related mechanism (26) (pain, osteoarthritis, reduced physical activity). All these mechanisms contribute to muscle mass decrease and impaired muscle strength predisposing individuals to limitations involving physical activity (27).
Our study was subject to a number of limitations. First, disability outcomes were self-reported, which may have led to overreporting in participants who suffered from metabolic (eg, obesity) or vascular (eg, hypertension) disorders. However, this may be counterbalanced by the fact that participants included in the present study were less likely to report limitations compared with those excluded. Second, in spite of adjustments for a large number of potential confounding factors—measures of physical health (comorbidity, sensory impairments, and health behaviors), cognitive impairment, and depression—the possibility remains that unmeasured confounders, such as physical activity at baseline, may still explain part of the associations between MetS or its components and the incidence of limitations. On the other hand, possible overadjustment may have occurred when several factors were added to the models, such as BMI, as these would dilute the influence of MetS components. A third drawback concerns the National Cholesterol Education program (Adult Treatment Panel III) (13) definition of MetS as other definitions have also been proposed (2,28). However, the National Cholesterol Education program Adult Treatment Panel III criteria remain the most widely used, allowing comparison of our results with other studies. The prevalence of MetS in our study (17.3%) is about half that commonly observed in the U.S. population (29,30). The 3C Study participants—all volunteers—have been shown to be healthier, with higher socioeconomic status compared with the general population of French elderly people; a factor that may limit the generalizability of our findings. Finally, a large number of participants were excluded from the present study due to existing high rates of both MetS and disability. However, this is more likely to have weakened the associations presented rather than negate the results.
Despite these limitations, the present prospective study with a multicenter design based on a large community sample permitted a detailed evaluation of the onset of limitations over 7 years. The use of a number of validated scales exploring the major domains of dependency corresponding to levels of disability severity allowed us to show that MetS is associated with a 50% increase in the odds of developing social restriction and limitations in mobility and a 62% increase in the odds of difficulties with IADLs. Our findings suggest that MetS increased both the onset and severity of disability at older ages. Additional analyses are needed to assess whether the screening of MetS in clinical practice and the optimal management of obesity and hyperglycemia in older participants by the medical practitioner may help to reduce age-related disability and delay the loss of autonomy with its subsequent risk of institutionalization.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
This work was supported by the Agence Nationale de la Recherche— The French National Research Agency under the Programme National de Recherche en Alimentation et nutrition humaine project COGINUT (ANR-06-PNRA-005) and under the Programme Longévité et vieillissement project (07-LVIE-004 and 07-LVIE 003 01) and by The Institut de Recherche en Santé Publique, Paris, France. M.K. was supported by the National Institutes of Health National Heart Lung and Blood Institute (R01HL36310), the Medical Research Council (K013351), and a professorial fellowship from the Economic and Social Research Council. T.N.A. was supported by the National Institutes of Health National Heart Lung and Blood Institute (R01HL36310) and by the Languedoc-Roussillon Region (Chercheur d’avenir 2011). The 3C Study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (Inserm), Victor-Segalen Bordeaux2 University, and Sanofi-Aventis. The 3C Study was also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN Mutuelle Générale de l'Education Nationale, the Institut de la Longévité, Agence Française de Sécurité Sanitaire des Produits de Santé, the Regional Governments of Aquitaine, Bourgogne and Languedoc-Roussillon, the Fondation de France, the Ministry of Research-Inserm Programme (Cohorts and collection of biological material), and Novartis. The Lille Genopole was supported by an unconditional grant from Eisai.
Conflict of Interest
Authors report no conflict of interest.
Acknowledgments
We thank Dr. Jane Ferrie for proof reading our manuscript. I.C. researched data, conducted the statistical analyses, and cowrote the final drafts and is the guarantor. K.P. researched data and contributed to the discussion and reviewed/edited the manuscript. M.L.A. researched data and contributed to the discussion and reviewed/edited the manuscript. V.G. researched data. C.B. researched data and reviewed/edited the manuscript. P.B.G. researched data and reviewed/edited the manuscript. K.B. contributed to the discussion and reviewed/edited the manuscript. M.K. contributed to the discussion and reviewed/edited the manuscript. K.R. researched data and contributed to the discussion and reviewed/edited the manuscript. T.N.A. researched data and wrote the first draft and the final draft. I.C. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lafortune G, Balestat G. Trends in Severe Disability Among Elderly People: Assessing the Evidence in 12 OECD Countries and the Future Implications. Paris, France: OECD; 2007.10.1787/217072070078 [Google Scholar]
- 2. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 3. Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. 2007;62:766–773 [DOI] [PubMed] [Google Scholar]
- 4. Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54:502–506 [DOI] [PubMed] [Google Scholar]
- 5. Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roriz-Cruz M, Rosset I, Wada T, et al. Stroke-independent association between metabolic syndrome and functional dependence, depression, and low quality of life in elderly community-dwelling Brazilian people. J Am Geriatr Soc. 2007;55:374–382 [DOI] [PubMed] [Google Scholar]
- 7. Stenholm S, Koster A, Alley DE, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women–results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. 3C Study Group Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–325 [DOI] [PubMed] [Google Scholar]
- 9. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol B Psychol Sci Soc Sci. 1966;21:556–559 [DOI] [PubMed] [Google Scholar]
- 10. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186 [PubMed] [Google Scholar]
- 11. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30 [DOI] [PubMed] [Google Scholar]
- 12. ICF International Classification of Functioning, Disability and Health. Geneva, Switzerland: World Health Organization; 2001 [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 14. Akbaraly TN, Ancelin ML, Jaussent I, et al. Metabolic syndrome and onset of depressive symptoms in the elderly: findings from the three-city study. Diabetes Care. 2011;34:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 4–57. [PubMed] [Google Scholar]
- 16. Kulminski A, Ukraintseva SV, Arbeev KG, et al. Association between APOE epsilon 2/epsilon 3/epsilon 4 polymorphism and disability severity in a national long-term care survey sample. Age Ageing. 2008;37:288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrière I, Bouyer J. Choosing marginal or random-effects models for longitudinal binary responses: application to self-reported disability among older persons. BMC Med Res Methodol. 2002;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guralnik JM. Assessment of physical performance and disability in older persons. Muscle Nerve Suppl. 1997;5:S14–S16 [PubMed] [Google Scholar]
- 19. Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. JAMA. 2007;298:2020–2027 [DOI] [PubMed] [Google Scholar]
- 20. Larrieu S, Pérès K, Letenneur L, et al. Relationship between body mass index and different domains of disability in older persons: the 3C study. Int J Obes Relat Metab Disord. 2004;28:1555–1560 [DOI] [PubMed] [Google Scholar]
- 21. Caskie GI, Sutton MC, Margrett JA. The relation of hypertension to changes in ADL/IADL limitations of Mexican american older adults. J Gerontol B Psychol Sci Soc Sci. 2010;65:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Diabetes as a predictor of change in functional status among older Mexican Americans: a population-based cohort study. Diabetes Care. 2003;26:314–319 [DOI] [PubMed] [Google Scholar]
- 23. Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes (Lond). 2006;30:364–373 [DOI] [PubMed] [Google Scholar]
- 24. Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring). 2007;15:1886–1894 [DOI] [PubMed] [Google Scholar]
- 25. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17 [DOI] [PubMed] [Google Scholar]
- 26. Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934 [DOI] [PubMed] [Google Scholar]
- 27. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 28. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 29. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359 [DOI] [PubMed] [Google Scholar]
- 30. Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783 [DOI] [PubMed] [Google Scholar]