Abstract
Background.
Knowledge of adipose composition in relation to mortality may help delineate inconsistent relationships between obesity and mortality in old age. We evaluated relationships between abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) density, mortality, biomarkers, and characteristics.
Methods.
VAT and SAT density were determined from computed tomography scans in persons aged 65 and older, Health ABC (n = 2,735) and AGES-Reykjavik (n = 5,131), and 24 nonhuman primates (NHPs). Associations between adipose density and mortality (4–13 years follow-up) were assessed with Cox proportional hazards models. In NHPs, adipose density was related to serum markers and tissue characteristics.
Results.
Higher density adipose tissue was associated with mortality in both studies with adjustment for risk factors including adipose area, total fat, and body mass index. In women, hazard ratio and 95% CI for the densest quintile (Q5) versus least dense (Q1) for VAT density were 1.95 (1.36–2.80; Health ABC) and 1.88 (1.31–2.69; AGES-Reykjavik) and for SAT density, 1.76 (1.35–2.28; Health ABC) and 1.56 (1.15–2.11; AGES-Reykjavik). In men, VAT density was associated with mortality in Health ABC, 1.52 (1.12–2.08), whereas SAT density was associated with mortality in both Health ABC, 1.58 (1.21–2.07), and AGES-Reykjavik, 1.43 (1.07–1.91). Higher density adipose tissue was associated with smaller adipocytes in NHPs. There were no consistent associations with inflammation in any group. Higher density adipose tissue was associated with lower serum leptin in Health ABC and NHPs, lower leptin mRNA expression in NHPs, and higher serum adiponectin in Health ABC and NHPs.
Conclusion.
VAT and SAT density provide a unique marker of mortality risk that does not appear to be inflammation related.
Key Words: Obesity, Aging, Leptin, Adiponectin.
Obesity in old age has been inconsistently associated with mortality (1–3), and the clinical utility of body mass index (BMI) as a measure of obesity is controversial (4). Adipose tissue area and particularly visceral adipose tissue (VAT) has been associated with mortality (5,6) although not always independently of BMI (6). Studies using computed tomography (CT) have shown that the amount of adipose in abnormal or ectopic locations, such as surrounding visceral organs and within muscle, has adverse consequences on health (7,8). Thus, adipose tissue distribution and characteristics may be important for assessing mortality risk.
CT imaging allows for measuring tissue radiographic density, a reflection of the physical and biochemical composition of tissue. Lower CT density is associated with greater fatty infiltration of tissue because adipose has a lower density than other tissues on CT. For example, lower CT density of liver is an index of fat accumulation in the liver, which is associated with insulin resistance and diabetes (9,10). Lower CT density of skeletal muscle is indicative of fatty infiltration of muscle and is associated with increased risk of fracture (11) and mobility limitation in old age (8).
Little is known regarding radiographic characteristics of adipose tissue and health states independent of adipose volume. The purpose of this study was to evaluate associations between VAT and subcutaneous adipose tissue (SAT) density and mortality risk in two large epidemiologic cohorts with imaging and outcome data, and to explore mechanisms relevant to adipose tissue density including circulating biomarkers. In order to better understand the potential molecular and cellular basis underlying relationships between adipose tissue density and phenotypes, we directly evaluated VAT and SAT samples from a cohort of nonhuman primates (NHPs) with CT imaging data. We hypothesized that lower adipose tissue density would be associated with increased adipose tissue inflammation, thereby providing a mechanistic link which might lead to increased mortality risk.
Methods
Populations
We utilized data from two independent cohort studies: the Health, Aging, and Body Composition (Health ABC) study and the Age, Gene and/or Environment Susceptibility-Reykjavik (AGES-Reykjavik) study. A sample of middle-aged NHPs was used to explore relationships between adipose tissue density, phenotypes, and mortality.
Health ABC is a prospective longitudinal study of 3,075 community-dwelling initially well-functioning men and women all 70–79 years recruited from a random sample of white Medicare beneficiaries and all black Medicare-eligible residents in Memphis, Tennessee, and Pittsburgh, Pennsylvania. AGES-Reykjavik is a single center population study of 5,764 Icelandic men and women aged 66–96 years. AGES-Reykjavik is a random sample of participants in the Reykjavik study, a cardiovascular cohort begun in 1967 to study heart disease. Both studies required written informed consent and institutional review board approval. Recruitment and study design of Health ABC and AGES-Reykjavik have been published (8,12).
Adipose imaging characteristics, biomarkers, and adipose phenotypes were assessed in 24 female cynomolgus monkeys (Macaca fascicularis, mean age 15 years, range 10–20 years) from Indonesia (Institut Pertanian Bogor, Bogor). Animals consumed an atherogenic diet (42.4% of calories as fat) for 53 months (13–15). All procedures involving NHPs were conducted in compliance with institutional, state, and federal law for the use of NHPs in laboratory settings.
Measures
Health ABC.
We used body composition, clinical, and subclinical disease at baseline (April 1997–June 1998). Percent weight change from midlife to baseline was calculated from recalled weight at age 50. CT imaging at the L4/L5 vertebrae was performed with a Somatom Plus 4 scanner (Siemens, Erlangen, Germany), PQ 200S (Marconi Medical Systems, Cleveland, OH) at the Memphis clinic, and a 9800 Advantage (General Electric, Milwaukee, WI) at the Pittsburgh clinic. Total body adipose was determined from dual-energy x-ray absorptiometry (DXA; Hologic QDR4500A, Waltham, NY). Mortality (representing 14 years of follow-up) was determined from death certificates, hospital records, and interview with next of kin through August 10, 2011. Causes of death were adjudicated by a central committee through March 1, 2011.
AGES-Reykjavik.
Percent weight change from midlife was calculated from midlife measured weight in the Reykjavik study. CT imaging at the L4/L5 vertebrae was measured using a 4 detector system (Sensation; Siemens Medical Systems). Mortality (representing 4–9 years of follow-up) was ascertained from the Icelandic National Roster (http://www.statice.is/Statistics/Population/Births-and-death), an adjudicated registry of deaths through May 31, 2011. Cause of death was ascertained from National Health System Records and was available through December 31, 2009.
Nonhuman primates.
BMI was calculated as body weight (kilogram)/trunk length (square meter) (16). Whole-body CT scans were obtained prior to necropsy. Abdominal VAT and SAT area were determined between L4/L5 contiguous DICOM images. Fasting serum for biomarker analyses and VAT and SAT samples sectioned from abdominal adipose were collected at necropsy.
The Supplementary Material provides additional details of CT image analysis and other methods. Briefly, the mean attenuation (Hounsfield Units, HU) of adipose tissue within VAT and SAT were recorded with adipose tissue thresholds set at −190 to −30 HU using specialized software packages.
Mediator analysis.
Details of mediator analyses are provided in Supplementary Material. In Health ABC and AGES-Reykjavik, blood samples were collected following an overnight fast. C-reactive protein (CRP), IL-6, leptin, and adiponectin were measured in Health ABC. Participants missing CRP (N = 29), IL-6 (N = 142), leptin (N = 41), or adiponectin (N = 24) were excluded from analysis of mediators. CRP was measured in AGES-Reykjavik. CRP was missing in two participants who were excluded from analysis of mediators. In NHPs, mediators including leptin and adiponectin were measured in serum and VAT and SAT. Gene expression of leptin, adiponectin, IL-6, TNF-α, MCP-1, CD68, CD3, CD4, and T-helper cells were assessed, and adipose cell size was determined.
Statistical Analysis
Covariates.
Sociodemographic variables included age and education (<high school, high school graduate, or postsecondary), race, and study site within Health ABC. Lifestyle factors included smoking history, alcohol consumption, and physical activity. Smoking was defined as more than 100 cigarettes in a lifetime and characterized as never, former, or current. Alcohol consumption was defined as current, former, or never in Health ABC and as current versus none in AGES-Reykjavik. Physical activity was assessed as activity 7 days prior to baseline in Health ABC (17) and as frequency of moderate to vigorous activity 1 year prior to baseline in AGES-Reykjavik. Overnight hospitalization in the 5 years prior to baseline was determined from self-report and hospital records. Medical conditions (diabetes, hypertension, cancer, congestive heart failure, coronary heart disease, stroke, myocardial infarction, peripheral arterial disease, and osteoporosis) were determined from self-report, medications, and clinical assessments.
Participants with missing CT data (N = 675) or covariables (N = 298) were excluded leaving 2,735 participants from Health ABC and 5,131 from AGES-Reykjavik. Excluded participants in Health ABC were more likely to be older, female, report never consuming alcohol (p < .05), black, have higher BMI, and lower education (p < .001) and, in AGES-Reykjavik, have higher prevalence of hospitalization and less weight gain from midlife (p < .001).
Sex, race, adipose depot, and study site-specific quintiles of adipose tissue density were created as the distribution of adipose tissue density varied within these strata. Analyses are presented by adipose depot, study, and sex. Cox proportional hazards models were used to examine associations between VAT and SAT density and mortality with density as continuous variables and in quintiles; top quintiles were compared with the lowest quintile. p Values for trends are reported. Proportional hazards assumption was met for each covariate. Model 1 was adjusted for age, education, race, and study site, then for sagittal diameter, BMI, and adipose tissue area (Model 2). Model 3 was adjusted for smoking, alcohol consumption, physical activity, and comorbidities. Model 4 was adjusted for weight change from midlife and prior hospitalization. Participants in AGES-Reykjavik did not have DXA images, thus BMI was used as a covariate for adiposity in models. Sensitivity analysis was performed in Health ABC using total body fat from DXA. Generalized linear models adjusted for BMI and adipose area were used to examine associations between adipose density quintiles and adipocytokines with least-squares means and p value for trend reported. Statistical analyses were performed with SPSS version 19.0.
All NHPs were female, so there was no need for stratification. Pearson r correlations were used to examine the relationships between adipose tissue density, molecular, and cellular characteristics and other phenotypes. Partial correlations included correction for the contribution of overall adiposity using BMI as a covariate.
Results
There were 1,337 deaths in Health ABC and 1,207 deaths in AGES-Reykjavik. Cause of death was available for 1,192 participants in Health ABC and 898 participants in AGES-Reykjavik. The main underlying causes of death were cancer (28.7% Health ABC, 31.1% AGES-Reykjavik) and cardiovascular disease (26.6% Health ABC, 43.0% AGES-Reykjavik). Baseline characteristics of participants in Health ABC and AGES-Reykjavik according to quintiles of VAT density are shown in Table 1 and Supplementary Table 1. VAT density was positively associated with older age and higher education (except for men in Health ABC). Participants with denser adipose tissue were likely to report never smoking or current smoking, lower BMI, weight loss from midlife, and, in AGES-Reykjavik, more comorbidities. Adipose tissue density was inversely correlated with tissue area in men and women in both studies (Supplementary Table 2).
Table 1.
Select Health ABC and AGES-Reykjavik Participant Characteristics in Quintiles of VAT Density
| Q1 (lowest density) | Q2 | Q3 | Q4 | Q5 (highest density) | p Value for Trend | |
|---|---|---|---|---|---|---|
| Women, Health ABC | ||||||
| No. of participants | 277 | 279 | 279 | 280 | 275 | |
| Age in years, mean (SD) | 73.2 (2.9) | 73.3 (2.8) | 73.3 (2.7) | 73.8 (2.8) | 74.0 (3.1) | .002 |
| Education, n (%) | .02 | |||||
| <High school | 66 (23.8) | 61 (21.9) | 57 (20.4) | 59 (21.1) | 57 (20.7) | |
| High school graduate | 109 (39.4) | 100 (35.8) | 83 (29.7) | 97 (34.6) | 75 (27.3) | |
| Postsecondary | 102 (36.8) | 118 (42.3) | 139 (49.8) | 124 (44.3) | 143 (52.0) | |
| BMI in kg/m2, mean (SD) | 30.1 (4.5) | 28.9 (4.4) | 28.2 (5.1) | 26.6 (4.7) | 23.2 (4.1) | <.001 |
| Weight change from age 50 in %, mean (SD) | 15.8 (14.1) | 13.8 (14.9) | 9.67 (13.2) | 6.31 (11.6) | −2.93 (12.3) | <.001 |
| Comorbid conditions, n (%) | .23 | |||||
| 0 | 35 (12.6) | 45 (16.1) | 48 (17.2) | 43 (15.4) | 45 (16.4) | |
| 1 | 71 (25.6) | 72 (25.8) | 61 (21.9) | 78 (27.9) | 87 (31.6) | |
| 2 | 74 (26.7) | 79 (28.3) | 89 (31.9) | 67 (23.9) | 69 (25.1) | |
| ≥3 | 97 (35.0) | 83 (29.7) | 81 (29.0) | 92 (32.9) | 74 (26.9) | |
| Hospitalization in previous 5 y, n (%) | 98 (35.4) | 90 (32.3) | 96 (34.4) | 96 (34.3) | 106 (38.5) | .63 |
| VAT area in cm2, mean (SD) | 193 (58.9) | 150 (45.5) | 132 (42.2) | 105 (34.0) | 68.2 (30.4) | <.001 |
| SAT area in cm2, mean (SD) | 393 (105) | 374 (109) | 361 (109) | 326 (116) | 231 (105) | <.001 |
| Men, Health ABC | ||||||
| No. of participants | 268 | 269 | 271 | 269 | 268 | |
| Age in years, mean (SD) | 73.6 (2.9) | 73.6 (2.9) | 73.4 (2.9) | 74.0 (2.8) | 73.9 (2.9) | .07 |
| Education, n (%) | .19 | |||||
| <High school | 74 (27.6) | 63 (23.4) | 79 (29.2) | 68 (25.3) | 70 (26.1) | |
| High school graduate | 64 (23.9) | 68 (25.3) | 58 (21.4) | 53 (19.7) | 44 (16.4) | |
| Postsecondary | 130 (48.5) | 138 (51.3) | 134 (49.4) | 148 (55.0) | 154 (57.5) | |
| BMI in kg/m2, mean (SD) | 29.3 (3.7) | 28.3 (3.4) | 27.5 (3.4) | 26.4 (3.4) | 23.9 (3.0) | <.001 |
| Weight change from age 50 in %, mean (SD) | 10.2 (12.0) | 6.44 (12.4) | 5.19 (10.4) | 1.11 (11.2) | −5.35 (10.3) | <.001 |
| Comorbid conditions, n (%) | .85 | |||||
| 0 | 46 (17.2) | 45 (16.7) | 44 (16.2) | 46 (17.1) | 55 (20.5) | |
| 1 | 85 (31.7) | 81 (30.1) | 78 (28.8) | 87 (32.3) | 74 (27.6) | |
| 2 | 75 (28.0) | 74 (27.5) | 70 (25.8) | 64 (23.8) | 76 (28.4) | |
| ≥3 | 62 (23.1) | 69 (25.7) | 79 (29.2) | 72 (26.8) | 63 (23.5) | |
| Hospitalization in previous 5 y, n (%) | 106 (39.6) | 120 (44.6) | 111 (41.0) | 112 (41.6) | 111 (41.4) | .82 |
| VAT area, mean (SD) | 225 (72.8) | 178 (57.4) | 155 (50.7) | 129 (49.5) | 88.3 (38.3) | <.001 |
| SAT area, mean (SD) | 275 (84.3) | 456 (79.8) | 242 (78.3) | 213 (74.2) | 155 (64.1) | <.001 |
| Women, AGES-Reykjavik | ||||||
| No. of participants | 585 | 585 | 585 | 585 | 584 | |
| Age in years, mean (SD) | 75.5 (5.0) | 76.2 (5.4) | 76.3 (5.5) | 76.8 (5.7) | 77.1 (6.2) | <.001 |
| Education, n (%) | <.001 | |||||
| <High school | 179 (30.6) | 197 (33.7) | 181 (30.9) | 153 (26.2) | 146 (25.0) | |
| High school graduate | 303 (51.8) | 264 (45.1) | 271 (46.3) | 284 (48.5) | 263 (45.0) | |
| Postsecondary | 103 (17.6) | 124 (21.2) | 133 (22.7) | 148 (25.3) | 175 (30.0) | |
| BMI in kg/m2, mean (SD) | 30.5 (4.7) | 29.0 (4.3) | 27.6 (4.1) | 25.7 (3.5) | 23.1 (3.4) | <.001 |
| Weight change from midlife in %, mean (SD) | 13.8 (12.8) | 9.18 (12.6) | 5.48 (12.6) | 3.23 (11.9) | −5.21 (12.2) | <.001 |
| Comorbid conditions, n (%) | .02 | |||||
| 0 | 49 (8.4) | 61 (10.4) | 61 (10.4) | 84 (14.4) | 77 (13.2) | |
| 1 | 251 (42.9) | 223 (38.1) | 224 (38.3) | 238 (40.7) | 221 (37.8) | |
| 2 | 143 (24.4) | 160 (27.4) | 169 (28.9) | 143 (24.4) | 172 (29.5) | |
| ≥3 | 142 (24.3) | 141 (24.1) | 131 (22.4) | 120 (20.5) | 114 (19.5) | |
| Hospitalization in previous 5 y, n (%) | 288 (49.2) | 270 (46.2) | 286 (48.9) | 260 (44.4) | 273 (46.7) | .45 |
| VAT area, mean (SD) | 220 (64.2) | 179 (51.1) | 150 (42.7) | 121 (38.5) | 82.3 (33.8) | <.001 |
| SAT area, mean (SD) | 350 (111) | 334 (105) | 311 (101) | 275 (91.5) | 203 (83.0) | <.001 |
| Men, AGES-Reykjavik | ||||||
| No. of participants | 441 | 442 | 441 | 442 | 441 | |
| Age in years, mean (SD) | 76.1 (5.0) | 76.0 (5.2) | 76.5 (5.4) | 76.8 (5.6) | 77.5 (5.5) | <.001 |
| Education, n (%) | .25 | |||||
| <High school | 74 (16.8) | 67 (15.2) | 61 (13.8) | 74 (16.7) | 81 (18.4) | |
| High school graduate | 249 (56.5) | 241 (54.5) | 241 (54.6) | 224 (50.7) | 215 (48.8) | |
| Postsecondary | 118 (26.8) | 134 (30.3) | 139 (31.5) | 144 (32.6) | 145 (32.9) | |
| BMI in kg/m2, mean (SD) | 29.5 (3.6) | 28.3 (3.4) | 27.0 (3.0) | 25.8 (3.0) | 23.6 (3.1) | <.001 |
| Weight change from midlife in %, mean (SD) | 10.1 (10.8) | 5.85 (10.4) | 2.17 (9.3) | −0.51 (9.6) | −5.89 (9.2) | <.001 |
| Comorbid conditions, n (%) | <.001 | |||||
| 0 | 37 (8.4) | 42 (9.5) | 48 (10.9) | 58 (13.1) | 81 (18.4) | |
| 1 | 126 (28.6) | 153 (34.6) | 148 (33.6) | 162 (36.7) | 144 (32.7) | |
| 2 | 108 (24.5) | 97 (21.9) | 107 (24.3) | 106 (24.0) | 95 (21.5) | |
| ≥3 | 170 (38.5) | 150 (33.9) | 138 (31.3) | 116 (26.2) | 121 (27.4) | |
| Hospitalization in previous 5 y, n (%) | 232 (52.6) | 223 (50.5) | 237 (53.7) | 238 (53.8) | 247 (56.0) | .57 |
| VAT area, mean (SD) | 287 (82.6) | 241 (67.1) | 205 (58.6) | 169 (51.7) | 112 (44.4) | <.001 |
| SAT area, mean (SD) | 255 (87.1) | 233 (84.8) | 211 (72.9) | 185 (71.2) | 134 (63.9) | <.001 |
Notes: AGES-Reykjavik = Age, Gene/Environment Susceptibility-Reykjavik; BMI = body mass index; Health ABC = Health, Aging, and Body Composition; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
Table 2 shows associations between quintiles of VAT, SAT density and mortality in Health ABC. Increasing density was associated with mortality in Model 1 with the exception of VAT density in men. Adjustment for BMI and adipose area (Model 2) increased the risk of mortality; in women, hazard ratio (95% CI): VAT Q4 1.56 (1.14–2.14) and Q5 2.00 (1.40–2.86) and SAT Q5 1.76 (1.35–2.28); in men, VAT Q5 1.51 (1.11–2.06) and SAT Q4 1.37 (1.08–1.73) and Q5 1.56 (1.22–2.00). Adjustment for hospitalization and weight change from midlife (Model 4) attenuated the trend for VAT density in men (p = .09), but Q5 remained significantly associated with mortality, 1.41 (1.03–1.92). Analysis of VAT and SAT density as continuous variables (Model 4) showed similar results: increased risk of mortality in women and men with the densest adipose. Adjustment for VAT and SAT area in quintiles (category boundaries reported in Supplementary Table 3) rather than as continuous variables produced similar results. Adjustment for total body fat from DXA (N = 2,726) instead of BMI did not alter associations. Excluding deaths within 2 years of baseline to control for preexisting conditions did not alter associations.
Table 2.
Association of Adipose Tissue Density at Baseline in Quintiles With Mortality, Health ABC
| No. at Risk | No. of Events | Rate Per 1,000 person-y | Model 1*, HR (95% CI) | Model 2†, HR (95% CI) | Model 3‡, HR (95% CI) | Model 4§, HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| VAT | |||||||
| Women | |||||||
| Q1 | 277 | 107 | 33.5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 279 | 105 | 32.1 | 0.96 (0.74–1.26) | 1.10 (0.83–1.46) | 1.13 (0.85–1.51) | 1.14 (0.86–1.51) |
| Q3 | 279 | 112 | 34.7 | 1.06 (0.81–1.38) | 1.29 (0.96–1.73) | 1.26 (0.94–1.70) | 1.20 (0.89–1.62) |
| Q4 | 280 | 127 | 40.2 | 1.20 (0.93–1.55) | 1.56 (1.14–2.14) | 1.52 (1.11–2.09) | 1.46 (1.06–2.00) |
| Q5 | 275 | 140 | 46.5 | 1.43 (1.11–1.84) | 2.00 (1.40–2.86) | 1.95 (1.36–2.80) | 1.72 (1.19–2.48) |
| p value for trend | .01 | .01 | .003 | .04 | |||
| Men | |||||||
| Q1 | 268 | 147 | 52.2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 269 | 138 | 48.0 | 0.89 (0.70–1.12) | 0.99 (0.78–1.27) | 1.03 (0.80–1.32) | 0.96 (0.75–1.24) |
| Q3 | 271 | 148 | 51.5 | 0.96 (0.77–1.21) | 1.11 (0.86–1.43) | 1.14 (0.89–1.48) | 1.10 (0.85–1.42) |
| Q4 | 269 | 150 | 52.9 | 0.97 (0.77–1.23) | 1.17 (0.89–1.54) | 1.21 (0.92–1.60) | 1.15 (0.87–1.51) |
| Q5 | 268 | 163 | 63.8 | 1.20 (0.96–1.50) | 1.51 (1.11–2.06) | 1.52 (1.12–2.08) | 1.41 (1.03–1.92) |
| p value for trend | .11 | .03 | .05 | .09 | |||
| SAT | |||||||
| Women | |||||||
| Q1 | 277 | 100 | 30.9 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 278 | 102 | 31.5 | 1.01 (0.77–1.34) | 1.02 (0.78–1.35) | 1.05 (0.79–1.38) | 1.06 (0.81–1.40) |
| Q3 | 280 | 111 | 34.1 | 1.06 (0.81–1.39) | 1.06 (0.80–1.39) | 1.07 (0.81–1.40) | 1.10 (0.84–1.52) |
| Q4 | 279 | 123 | 38.5 | 1.20 (0.92–1.57) | 1.23 (0.94–1.61) | 1.21 (0.92–1.59) | 1.19 (0.91–1.56) |
| Q5 | 276 | 155 | 52.7 | 1.70 (1.32–2.20) | 1.76 (1.35–2.28) | 1.58 (1.25–2.19) | 1.58 (1.21–2.07) |
| p value for trend | <.001 | <.001 | .002 | .007 | |||
| Men | |||||||
| Q1 | 269 | 134 | 45.5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 268 | 140 | 48.5 | 1.05 (0.82–1.33) | 1.03 (0.81–1.30) | 1.09 (0.85–1.39) | 0.99 (0.78–1.26) |
| Q3 | 271 | 134 | 45.5 | 0.98 (0.77–1.25) | 0.97 (0.76–1.23) | 1.06 (0.82–1.37) | 0.95 (0.74–1.21) |
| Q4 | 269 | 167 | 62.6 | 1.39 (1.10–1.74) | 1.37 (1.08–1.73) | 1.55 (1.20–2.01) | 1.35 (1.06–1.71) |
| Q5 | 268 | 171 | 68.2 | 1.55 (1.23–1.94) | 1.56 (1.22–2.00) | 1.74 (1.31–2.31) | 1.49 (1.16–1.91) |
| p value for trend | <.001 | <.001 | <.001 | <.001 | |||
Notes: Health ABC = Health, Aging, and Body Composition; HR = hazard ratio; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
*Adjusted for age, race, study site, and education.
†Adjusted for Model 1 covariates plus body mass index, fat area of respective fat depot, and sagittal diameter.
‡Adjusted for Model 2 covariates plus smoking status, drinking status, physical activity, and comorbid conditions.
§Adjusted for Model 3 covariates plus weight history (% change from midlife) and prior hospitalization.
Table 3 shows associations between quintiles of VAT and SAT density and mortality in AGES-Reykjavik. Adjustment for adiposity in Model 2 increased the risk of mortality with higher VAT and SAT density in women, hazard ratio (95% CI): VAT Q5 1.88 (1.31–2.69) and SAT Q5 1.56 (1.15–2.11); in men, SAT Q4 1.43 (1.07–1.91) and Q5 1.70 (1.24–2.35). Adjustment for hospitalization and weight change from midlife (Model 4) attenuated the association between density and mortality, and only VAT density in women remained significant. VAT density as a continuous variable showed increased risk of mortality (Model 4) in women, 1.03 (1.02–1.05), but not in men, 1.00 (0.99–1.02). SAT density as a continuous variable also showed increased risk of mortality in women only (Model 4), 1.03 (1.01–1.04) and 1.01 (1.00–1.03). Results were similar with adjustment for adipose tissue area in quintiles rather than as continuous variables.
Table 3.
Association of Adipose Tissue Density at Baseline in Quintiles With Mortality, AGES-Reykjavik
| No. at Risk | No. of Events | Rate Per 1,000 Person-y | Model 1*, HR (95% CI) | Model 2†, HR (95% CI) | Model 3‡, HR (95% CI) | Model 4§, HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| VAT | |||||||
| Women | |||||||
| Q1 | 585 | 103 | 27.0 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 585 | 102 | 26.4 | 0.87 (0.66–1.15) | 1.03 (0.78–1.38) | 1.07 (0.80–1.43) | 1.04 (0.78–1.39) |
| Q3 | 585 | 101 | 25.9 | 0.84 (0.64–1.10) | 1.10 (0.81–1.49) | 1.13 (0.83–1.53) | 1.02 (0·75–1.39) |
| Q4 | 585 | 112 | 28.9 | 0.87 (0.67–1.14) | 1.23 (0.88–1.71) | 1.24 (0.89–1.73) | 1.17 (0.84–1.63) |
| Q5 | 584 | 151 | 40.8 | 1.25 (0.97–1.61) | 1.88 (1.31–2.69) | 1.92 (1.34–2.74) | 1.65 (1.15–2.36) |
| p value for trend | .007 | .001 | .001 | .01 | |||
| Men | |||||||
| Q1 | 441 | 112 | 40.7 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 442 | 123 | 44.7 | 1.12 (0.87–1.45) | 1.13 (0.87–1.47) | 1.19 (0.91–1.55) | 1.14 (0.87–1.49) |
| Q3 | 441 | 128 | 46.5 | 1.09 (0.85–1.41) | 1.10 (0.84–1.45) | 1.18 (0.90–1.55) | 1.07 (0.81–1.41) |
| Q4 | 442 | 125 | 46.0 | 1.03 (0.80–1.33) | 1.04 (0.78–1.40) | 1.17 (0.87–1.58) | 1.02 (0.75–1.38) |
| Q5 | 441 | 150 | 56.6 | 1.20 (0.94–1.54) | 1.23 (0.88–1.71) | 1.28 (0.92–1.79) | 1.06 (0.75–1.49) |
| p value for trend | .59 | .67 | .69 | .88 | |||
| SAT | |||||||
| Women | |||||||
| Q1 | 585 | 84 | 20.7 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 584 | 88 | 22.6 | 0.96 (0.71–1.29) | 0.90 (0.67–1.22) | 0.89 (0.66–1.20) | 0.81 (0.60–1.10) |
| Q3 | 585 | 88 | 23.0 | 0.96 (0.71–1.30) | 0.88 (0.65–1.20) | 0.88 (0.65–1.20) | 0.81 (0.59–1.10) |
| Q4 | 585 | 116 | 30.7 | 1.28 (0.96–1.69) | 1.14 (0.85–1.53) | 1.16 (0.87–1.57) | 0.98 (0.72–1.32) |
| Q5 | 585 | 193 | 53.8 | 1.82 (1.40–2.37) | 1.56 (1.15–2.11) | 1.48 (1.09–2.00) | 1.14 (0.82–1.58) |
| p value for trend | <.001 | <.001 | .001 | .08 | |||
| Men | |||||||
| Q1 | 441 | 112 | 40.3 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 442 | 112 | 40.1 | 1.00 (0.77–1.30) | 1.11 (0.84–1.45) | 1.14 (0.87–1.49) | 1.06 (0.80–1.39) |
| Q3 | 441 | 118 | 42.7 | 1.05 (0.81–1.36) | 1.22 (0.92–1.61) | 1.21 (0.91–1.61) | 1.10 (0.82–1.46) |
| Q4 | 442 | 137 | 50.9 | 1.15 (0.90–1.48) | 1.43 (1.07–1.91) | 1.51 (1.12–2.03) | 1.27 (0.93–1.73) |
| Q5 | 441 | 159 | 61.1 | 1.28 (1.01–1.63) | 1.70 (1.24–2.35) | 1.64 (1.18–2.26) | 1.25 (0.88–1.77) |
| p value for trend | .19 | .01 | .02 | .59 | |||
Notes: AGES-Reykjavik = Age, Gene/Environment Susceptibility-Reykjavik; HR = hazard ratio; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
*Adjusted for age and education.
†Adjusted for Model 1 covariates plus body mass index, area of respective fat depot, and sagittal diameter.
‡Adjusted for Model 2 covariates plus smoking status, drinking status, physical activity, comorbid conditions, and time of computed tomography scan.
§Adjusted for Model 3 covariates plus weight history (% change from midlife) and prior hospitalization.
Associations between quintiles of VAT and SAT area with mortality in these cohorts were modest. Models were adjusted for Model 4 covariables as in Tables 2 and 3. In Health ABC, VAT and SAT area were significantly associated with mortality risk in men (p = .05, p = .01) and in women, only SAT area was associated with mortality (p = .004). Adjustment for adipose tissue density attenuated the relationship with SAT area in men (p = .35) and women (p = .14). In AGES-Reykjavik, VAT and SAT area were not associated with mortality risk in men (p = .45, p = .75); however, VAT and SAT area were associated with mortality risk in women (p < .001, p < .001). Associations were similar with the addition of VAT density to the model (p < .001) and were reduced but remained significant with the addition of SAT density (p = .03).
Serum adipocytokines within quintiles of VAT and SAT density in Health ABC and AGES-Reykjavik are shown in Table 4. CRP was the only mediator measured in the AGES-Reykjavik study. In Health ABC, denser VAT was associated with lower leptin and higher adiponectin levels. With additional adjustment for total body fat, associations with VAT and adiponectin remained and associations with leptin tended toward significance (women: p = .07, men: p = .09). Denser SAT density was associated with higher adiponectin but was not associated with leptin. Except for an inverse association of CRP with VAT density in men in Health ABC, CRP and IL-6 were not associated with adipose density. Adjustment for adiponectin in Model 4 (Table 2) attenuated the association between VAT density and mortality in men but not in women, there was no effect of leptin in the model. Adjustment for adiponectin and leptin did not attenuate associations between SAT density and mortality.
Table 4.
Association of Adipose Tissue Density at Baseline in Quintiles With Adipocytokines
| Mean (SE) | N | Q1 | Q2 | Q3 | Q4 | Q5 | p Value for Trend |
|---|---|---|---|---|---|---|---|
| Women, Health ABC | |||||||
| VAT | |||||||
| IL-6, pg/mL | 1,313 | 2.32 (0.14) | 2.43 (0.12) | 2.31 (0.12) | 2.14 (0.12) | 2.43 (0.14) | .38 |
| Leptin, ng/mL | 1,369 | 23.1 (0.82) | 23.3 (0.70) | 21.7 (0.68) | 19.7 (0.70) | 17.8 (0.82) | .001 |
| Adiponectin, μg/mL | 1,378 | 12.1 (0.47) | 12.1 (0.40) | 12.7 (0.40) | 13.7 (0.41) | 16.7 (0.48) | .001 |
| CRP, μg/mL | 1,374 | 3.51 (0.34) | 3.33 (0.29) | 3.15 (0.28) | 3.00 (0.29) | 3.32 (0.34) | .81 |
| SAT | |||||||
| IL-6, pg/mL | 1,313 | 2.47 (0.12) | 2.07 (0.12) | 2.33 (0.12) | 2.24 (0.12) | 2.53 (0.13) | .06 |
| Leptin, ng/mL | 1,369 | 22.2 (0.70) | 21.4 (0.69) | 21.6 (0.67) | 20.2 (0.68) | 20.3 (0.74) | .22 |
| Adiponectin, μg/mL | 1,378 | 12.5 (0.42) | 12.8 (0.41) | 12.4 (0.40) | 13.8 (0.41) | 15.8 (0.44) | <.001 |
| CRP, μg/mL | 1,374 | 3.94 (0.29) | 2.96 (0.29) | 2.91 (0.28) | 3.24 (0.28) | 3.26 (0.31) | .08 |
| Men, Health ABC | |||||||
| VAT | |||||||
| IL-6, pg/mL | 1,280 | 2.65 (0.13) | 2.29 (0.12) | 2.29 (0.12) | 2.43 (0.12) | 2.60 (0.14) | .07 |
| Leptin, ng/mL | 1,325 | 9.39 (0.41) | 8.44 (0.37) | 7.90 (0.36) | 7.42 (0.37) | 6.47 (0.42) | <.001 |
| Adiponectin, μg/mL | 1,333 | 7.22 (0.37) | 8.91 (0.33) | 8.07 (0.32) | 10.2 (0.33) | 12.8 (0.37) | <.001 |
| CRP, μg/mL | 1,332 | 3.91 (0.34) | 2.41 (0.30) | 2.33 (0.30) | 2.52 (0.30) | 2.39 (0.34) | .003 |
| SAT | |||||||
| IL-6, pg/mL | 1,280 | 2.26 (0.13) | 2.36 (0.12) | 2.46 (0.17) | 2.53 (0.12) | 2.65 (0.13) | .37 |
| Leptin, ng/mL | 1,325 | 7.92 (0.39) | 8.39 (0.36) | 8.01 (0.35) | 7.59 (0.36) | 7.70 (0.39) | .60 |
| Adiponectin, μg/mL | 1,333 | 8.25 (0.36) | 9.32 (0.33) | 8.57 (0.33) | 9.20 (0.33) | 11.8 (0.37) | <.001 |
| CRP, μg/mL | 1,332 | 2.99 (0.33) | 2.60 (0.30) | 2.78 (0.30) | 2.79 (0.30) | 2.39 (0.33) | .76 |
| Women, AGES-Reykjavik | |||||||
| VAT | |||||||
| CRP, μg/mL | 2,923 | 4.01 (0.30) | 3.59 (0.26) | 3.73 (0.25) | 3.53 (0.26) | 3.62 (0.30) | .79 |
| SAT | |||||||
| CRP, μg/mL | 2,923 | 3.68 (0.27) | 4.11 (0.26) | 3.47 (0.26) | 3.73 (0.26) | 3.49 (0.28) | .41 |
| Men, AGES-Reykjavik | |||||||
| VAT | |||||||
| CRP, μg/mL | 2,206 | 3.32 (0.36) | 3.76 (0.32) | 3.63 (0.31) | 3.49 (0.32) | 4.05 (0.37) | .68 |
| SAT | |||||||
| CRP, μg/mL | 2,206 | 3.44 (0.37) | 3.34 (0.32) | 4.01 (0.31) | 3.56 (0.32) | 3.90 (0.36) | .57 |
Notes: Least-square means adjusted for body mass index and area of respective fat depot. AGES-Reykjavik = Age, Gene/Environment Susceptibility-Reykjavik; CRP = C-reactive protein; Health ABC = Health, Aging, and Body Composition; NHPs = nonhuman primates; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
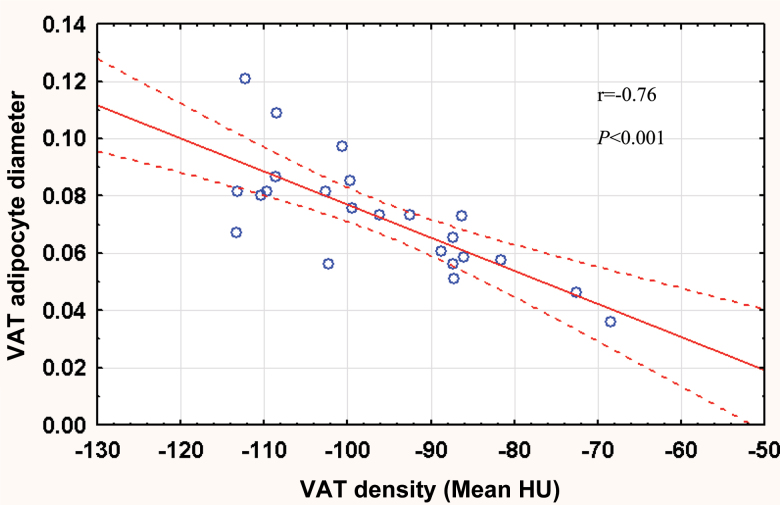
In NHPs, denser VAT and SAT were both associated with smaller adipocytes (r = −.76, p < .001, Figure 1 and r = −.59, p = .003, respectively). IL-6, CRP, and MCP-1 were not associated with adipose tissue density (Table 5). Lower serum and mRNA expression of leptin were associated with denser VAT and SAT. Denser VAT, but not denser SAT, was associated with higher serum adiponectin.
Figure 1.
Plot of visceral adipose tissue (VAT) density (Hounsfield Units [HU]) vs mean VAT cell diameter in nonhuman primates.
Table 5.
Relationships Between Adipose Tissue Density, Biomarkers, and Adipose Gene Expression in NHPs
| Variable | VAT Density | SAT Density | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Serum biomarkers | ||||
| sICAM-1 | .22 | .31 | −.08 | .71 |
| TNF-α | .01 | .98 | −.17 | .42 |
| MCP-1 | .36 | .08 | .29 | .17 |
| IL-6 | −.06 | .77 | −.02 | .93 |
| CRP | −.01 | .96 | .18 | .40 |
| Leptin | −.81 | <.001 | −.76 | <.001 |
| Adiponectin | .45 | .03 | .23 | .28 |
| Glucose | −.25 | .23 | −.19 | .39 |
| Insulin | −.09 | .67 | −.12 | .58 |
| Gene expression | ||||
| SAT-CD68 | −.19 | .36 | −.03 | .89 |
| SAT-CD3 | .03 | .89 | .04 | .87 |
| SAT-CD4 | −.21 | .32 | −.04 | .84 |
| SAT-IL-6 | −.21 | .32 | −.25 | .24 |
| SAT-TNF-α | .01 | .96 | .00 | .99 |
| SAT-MCP-1 | −.25 | .24 | −.10 | .63 |
| SAT-leptin | −.38 | .07 | −.47 | .02 |
| SAT-adiponectin | .14 | .51 | .03 | .89 |
| VAT-CD68 | −.21 | .32 | −.07 | .73 |
| VAT-CD3 | .02 | .91 | .09 | .66 |
| VAT-CD4 | −.02 | .92 | .13 | .54 |
| VAT-IL-6 | −.25 | .24 | −.02 | .93 |
| VAT-TNF-α | −.01 | .97 | .06 | .78 |
| VAT-MCP-1 | .10 | .65 | .17 | .43 |
| VAT-leptin | −.63 | .001 | −.60 | .002 |
| VAT-adiponectin | .19 | .37 | .18 | .41 |
Notes: CRP = C-reactive protein; NHPs = nonhuman primates; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue.
Discussion
To our knowledge, this is the first study to examine adipose tissue density in relation to health correlates and outcomes. Our findings show increased risk of death in men and women with the highest VAT and SAT density even after controlling for BMI, fat mass, VAT and SAT area. This relationship appears to be influenced by illness as weight change from midlife and prior hospitalizations attenuated the effect of adipose tissue density on mortality. In humans and NHPs, denser adipose was associated with higher adiponectin and lower leptin. Adipose tissue density was not consistently associated with circulating inflammation markers in humans, or with adipose expression of inflammation markers (IL-6, TNF-α, MCP-1) or inflammatory cells (macrophages, T cells) in NHPs.
We originally hypothesized that more lipid-filled adipose tissue associated with inflammation would increase risk of death, but our results did not support this. In the human populations, denser VAT and SAT were associated with mortality but were not associated with circulating markers of inflammation consistent with systemic inflammation. Similarly, in NHPs, there was no evidence for associations between serum or inflammatory cell markers and adipose density. Instead, there were robust associations between adipose tissue density, leptin, and adiponectin.
The association between less dense adipose tissue and higher leptin likely reflects the positive correlation between leptin and body fat (18) as adjustment for total body fat attenuated associations in Health ABC. Associations may also be related to weight change as leptin levels decrease following weight loss (19) and in our study, participants with denser adipose tissue and the lowest leptin levels had experienced weight loss from midlife. Despite the association of high serum adiponectin with a favorable cardiovascular risk profile, there is a growing body of evidence including a study in Health ABC (20) that suggests high adiponectin is predictive of mortality from all causes and from cardiovascular disease (21–23). This paradoxical relationship may result from functional adiponectin resistance: increased adiponectin expression in tissue but downregulation of adiponectin receptors (24). The notion of adiponectin resistance is supported by the mortality relationships in our study although the mechanistic underpinnings of adiponectin and adipose tissue density are not clear.
Observations from feeding studies and disease-associated wasting may help explain the basis of adipose tissue density. Overfeeding studies have shown that weight gain is accompanied by increased collagen (25) and connective tissue deposition (26) in adipose as well as increased adipocyte lipid and adipocyte cell size. This is in line with the data from NHPs showing larger adipocyte size with lower density adipose tissue and data from the human populations showing lower density adipose tissue in participants with heavy body weights. Studies in cancer-associated wasting, which has overlapping features with age-related body composition changes (27), report that weight loss is associated with adipose tissue remodeling including shrunken adipocytes, increased fibrosis (28), decreased fat cell volume, and altered gene expression related to cell and tissue structure (29). In our analysis, weight loss from midlife and prior hospitalization when weight loss is likely were associated with denser adipose tissue, suggesting that although adipose tissue fat content decreased, accumulated connective tissue remained. Together, this suggests that adipose tissue characteristics reflect deteriorating health in old age and serve as a marker of former obesity.
In our analysis, the greatest mortality risk was for the group with average BMI in the normal range. In old age, the mortality risk associated with BMI categories is confounded by several factors including fat redistribution and weight history (4). We suggest that adipose tissue characteristics may also be a confounder because our results show higher mortality risk for denser adipose tissue despite normal BMI, smaller adipocyte size, and a seemingly improved metabolic profile compared with individuals with less dense adipose. Thus, knowledge of adipose tissue characteristics may help disentangle the relationship between adiposity and mortality in older adults.
A strength of this study is the replication of the relationship between adipose tissue density and mortality in diverse populations of older people. The Health ABC and AGES-Reykjavik participants have different ethnic backgrounds, different age ranges (66–96 years in AGES-Reykjavik and 70–79 years in Health ABC), and Health ABC participants were selected to be initially well functioning, whereas there was no similar criterion in AGES-Reykjavik. There are also limitations of this study. These results need to be examined in younger populations to assess generalizability. Adipose tissue density was estimated from CT scans, and although we have biopsies from NHPs, no adipose tissue was available from the human populations.
The data presented here provide a first analysis of the predictive value of adipose tissue density on survival in older adults even when the adipose depot itself is not associated with risk of death. The association between weight loss and adipose density may help to explain the association between weight cycling and mortality but whether adipose tissue density increases in both voluntary and involuntary weight loss is unclear. Although these questions remain, the consistency of the results across studies suggests the need for further evaluation of CT-based adipose tissue density that may have an impact on estimation of obesity risk.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org
Funding
This work was supported by National Institutes of Health (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106; R01-AG28050, R01-AG28641, R01-HL39789, R01-NR-12459) and the Pepper Older Americans for Independence Center (P30 AG21332). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. R.A.M. is supported by a Banting Postdoctoral Fellowship.
References
- 1. Diehr P, Bild DE, Harris TB, Duxbury A, Siscovick D, Rossi M. Body mass index and mortality in nonsmoking older adults: the Cardiovascular Health Study. Am J Public Health. 1998;88:623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allison DB, Gallagher D, Heo M, Pi-Sunyer FX, Heymsfield SB. Body mass index and all-cause mortality among people age 70 and over: the Longitudinal Study of Aging. Int J Obes Relat Metab Disord. 1997;21:424–431 [DOI] [PubMed] [Google Scholar]
- 3. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105 [DOI] [PubMed] [Google Scholar]
- 4. Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond). 2005;29:1011–1029 [DOI] [PubMed] [Google Scholar]
- 5. Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–341 [DOI] [PubMed] [Google Scholar]
- 6. McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes Care. 2012;35:296–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420 [DOI] [PubMed] [Google Scholar]
- 8. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 9. Stone BG, Van Thiel DH. Diabetes mellitus and the liver. Semin Liver Dis. 1985;5:8–28 [DOI] [PubMed] [Google Scholar]
- 10. Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916 [DOI] [PubMed] [Google Scholar]
- 11. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol Psychol. 2005;69:67–84 [DOI] [PubMed] [Google Scholar]
- 14. Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shively CA, Clarkson TB. The unique value of primate models in translational research. Nonhuman primate models of women’s health: introduction and overview. Am J Primatol. 2009;71:715–721 [DOI] [PubMed] [Google Scholar]
- 16. Register TC, Jayo MJ, Jerome CP. Oral contraceptive treatment inhibits the normal acquisition of bone mineral in skeletally immature young adult female monkeys. Osteoporos Int. 1997;7:348–353 [DOI] [PubMed] [Google Scholar]
- 17. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509 [DOI] [PubMed] [Google Scholar]
- 18. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295 [DOI] [PubMed] [Google Scholar]
- 19. Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413 [DOI] [PubMed] [Google Scholar]
- 20. Poehls J, Wassel CL, Harris TB, et al. Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517 [DOI] [PubMed] [Google Scholar]
- 22. Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hascoet S, Elbaz M, Bongard V, et al. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler Thromb Vasc Biol. 2013;33:e19–e29 [DOI] [PubMed] [Google Scholar]
- 24. Van Berendoncks AM, Garnier A, Beckers P, et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3:185–194 [DOI] [PubMed] [Google Scholar]
- 25. Pasarica M, Gowronska-Kozak B, Burk D, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alligier M, Meugnier E, Debard C, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97:E183–E192 [DOI] [PubMed] [Google Scholar]
- 27. Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21 [DOI] [PubMed] [Google Scholar]
- 28. Mracek T, Stephens NA, Gao D, et al. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer. 2011;104:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dahlman I, Mejhert N, Linder K, et al. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer. 2010;102:1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]