Abstract
Growth hormone receptor-null (GHR−/−) mice are dwarf, insulin sensitive, and long-lived in spite of increased adiposity. However, their adiposity is not uniform, with select white adipose tissue (WAT) depots enlarged. To study WAT depot–specific effects on insulin sensitivity and life span, we analyzed individual WAT depots of 12- and 24-month-old GHR− /− and wild-type (WT) mice, as well as their plasma levels of selected hormones. Adipocyte sizes and plasma insulin, leptin, and adiponectin levels decreased with age in both GHR− /− and WT mice. Two-dimensional gel electrophoresis proteomes of WAT depots were similar among groups, but several proteins involved in endocytosis and/or cytoskeletal organization (Ehd2, S100A10, actin), anticoagulation (S100A10, annexin A5), and age-related conditions (alpha2-macroglobulin, apolipoprotein A-I, transthyretin) showed significant differences between genotypes. Because Ehd2 may regulate endocytosis of Glut4, we measured Glut4 levels in the WAT depots of GHR− /− and WT mice. Inguinal WAT of 12-month-old GHR− /− mice displayed lower levels of Glut4 than WT. Overall, the protein changes detected in this study offer new insights into possible mechanisms contributing to enhanced insulin sensitivity and extended life span in GHR− /− mice.
Key Words: Aging, Growth hormone receptor, Adipose tissue depots, Endocytosis, Glut4.
White adipose tissue (WAT) is an endocrine organ with a myriad of functions related to the regulation of metabolism, inflammation and immunity, hemostasis, blood pressure, and glucocorticoid and steroid hormone synthesis (1). Moreover, substantial distinctions have been described for individual WAT depots regarding their contributions to these functions and their cellular characteristics. For example, there are well-described depot-specific differences in their influence on metabolism, endocrine function, and preadipocyte characteristics (2–4).
Growth hormone receptor-null (GHR− /−) mice are dwarf with increased adiposity but remain insulin sensitive and have extended longevity (5). Interestingly, the accumulation of WAT is not uniform and has been shown to localize mainly to the subcutaneous region, whereas intraabdominal depots (eg, epididymal) are proportional in size to those of wild-type (WT) littermates (6). The disconnect between obesity and insulin resistance in these mice makes them an appealing model to study the influence of particular fat depots on insulin sensitivity and aging. The fact that the excess of WAT in these mice is specific to select depots suggests that WAT might play a key role in their beneficial phenotype related to aging. For instance, intraabdominal fat in GHR− /− mice was recently suggested to play a key role in the regulation of insulin sensitivity and longevity (7). However, no studies have directly compared the WAT of GHR− /− mice at adult versus old ages. Therefore, in the current study, we performed a proteomic analysis of WAT that included four depots (one subcutaneous and three intraabdominal) in 12- (adult) and 24-month-old (aged) mice. Numerous similarities in protein expression profiles between GHR− /− and WT mice were found in all WAT depots. However, between genotypes, we observed differences in proteins that function in cytoskeletal reorganization/endocytosis, anticoagulation, and age-related conditions. The variation in the levels of these proteins might contribute to the beneficial attributes of WAT in GHR− /− mice and their extended life span.
Methods
Mice
Male GHR− /− and WT C57BL/6J mice were used in this study. The 12- and 24-month-old groups of WT mice have been described previously (8,9). All mice were kept under specific pathogen-free conditions on a 14-/10-hour light/dark cycle, with normal chow (Prolab RMH 3000 LabDiet; PMI Richmond, Richmond, IN) and water provided ad libitum. Procedures were approved by the Ohio University Animal Care and Use Committee.
Body Composition
Body composition was analyzed using a quantitative NMR apparatus (Minispec, Bruker Optics, Billerica, MA).
Plasma Insulin, Leptin, and Adiponectin
Insulin, leptin, high-molecular-weight (HMW) and total adiponectin levels were measured on fasted plasma samples using ELISA kits as detailed in Supplementary Methods.
WAT Depot Samples
All mice were sacrificed by cervical dislocation, and inguinal (subcutaneous), retroperitoneal, mesenteric, and epididymal (all intraabdominal) WAT were collected and weighed. Percent weight of each depot was calculated as (depot weight/total body weight ×100). Samples for protein analysis were snap-frozen in liquid nitrogen and stored at −80oC until processing. Samples for histology were fixed in 10% formalin, embedded in paraffin, and processed as described below.
Proteomic Analysis and Mass Spectrometry
Two-dimensional gel electrophoresis (2DE) and mass spectrometry procedures have been described previously (8,9). Protein concentration of WAT samples was measured using Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA). Protein content per gram of tissue was estimated based on the concentration and volume of homogenate and the initial weight of the sample.
Western Blotting
Protein (10 µg) isolated from WAT was loaded on 12% acryl-bis sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred to Hybond LFP PVDF membranes (RPN303LFP, GE Healthcare, Waukesha, WI), and blotted with anti-Glut4 (ab654, Abcam, Cambridge, MA) or anti-Ehd2 (C-terminal Eps15 homology domain protein 2; ab23935, Abcam) antibodies. Secondary antibodies coupled to horseradish peroxidase (donkey-anti-goat IgG-HRP, sc2020, Santa Cruz Biotechnologies, Dallas, TX) or Cy5 (ECLPlex goat-anti-rabbit IgG-Cy5, PA45012V, GE Healthcare) were applied and signals detected by addition of Pierce ECL Western Blotting Substrate (32209, Thermo Scientific, Waltham, MA) or with the PharosFX Plus Molecular Imager and External Laser (Bio-Rad). Ponceau S staining of whole lanes was used as loading control. Densitometry was performed with the software ImageJ (NIH, Bethesda, MD: http://imagej.nih.gov/ij).
Histology and Adipocyte Sizing
Hematoxylin and eosin-stained sections of paraffin-embedded WAT samples were examined as described previously; details are provided in Supplementary Methods.
Statistical Analysis
Most data were compared among genotypes and age groups using a two-way analysis of variance and Tukey’s honestly significant difference post hoc test. Spot intensity data were log-transformed and compared among genotypes, age groups, and depots using a three-way analysis of variance with one within-subjects factor (depot) and two between-subjects factors (genotype and age). Sphericity was tested using Mauchly’s method, and a Greenhouse–Geisser correction was applied when the assumption of sphericity was not met (p < .05). Correlations were evaluated using the Pearson test. Western blot results were compared between genotypes using two-tailed t tests.
Statistical significance cutoffs were p < .01 for spot intensity differences (a more stringent level given the intrinsic variability of the 2DE technique) and p < .05 for all other comparisons and correlations. The softwares used were SPSS v14.0 (Chicago, IL) and SigmaPlot v11 (Systat Software, Inc., San Jose, CA).
Results
Body Weight, Body Composition, and Plasma Hormone Levels
Mean values for body weight, body composition, and plasma hormone levels of the four mouse groups are listed in Table 1. Differences observed between genotypes were as expected based on previous reports (5). Although total and HMW adiponectin were increased at both ages in GHR− /− mice, there was no significant difference between genotypes in the HMW/total adiponectin ratio. Likewise, there was no significant difference in the total adiponectin/leptin ratio. Regarding changes with advancing age, there were decreases for both genotypes in fat mass, insulin, leptin, total and HMW adiponectin, the HMW/total adiponectin ratio, and the total adiponectin/leptin ratio. These decreases were more marked for insulin (~2 fold) and leptin levels (~2–3 fold) than for total and HMW adiponectin (~1–2 fold for total and HMW).
Table 1.
Body Weight, Body Composition, and Hormone Levels
| Wild Type | GHR− /− | Wild Type | GHR− /− | p Value | |||
| Genotype | Age | Genotype × Age | |||||
| Age (mo) | 12 | 12 | 24 | 24 | |||
| N | 10–18 | 13–15 | 13–23 | 8–13 | |||
| Body weight (g) | 36.5±1.0 | 21.4±1.7 | 37.8±1.4 | 17.1±0.8 | <.001* | .271 | .046* |
| Fat mass (g) | 8.4±0.7 | 9.5±1.3 | 5.5±1.1 | 6.0±0.6 | .404 | .002* | .728 |
| Percent fat mass | 21.8±1.6 | 41.7±2.5 | 13.8±2.0 | 34.4±2.1 | <.001* | .001* | .882 |
| Lean mass (g) | 24.9±0.6 | 9.8±0.4 | 26.3±0.6 | 9.3±0.3 | <.001* | .447 | .064 |
| Percent lean mass | 67.0±1.2 | 48.1±2.1 | 72.6±1.8 | 54.8±2.2 | <.001* | .001* | .770 |
| Insulin (ng/mL) | 3.16±0.58 | 0.87±0.19 | 1.53±0.29 | 0.47±0.03 | <.001* | .005* | .079 |
| Leptin (ng/mL) | 31.4±5.1 | 48.6±12.7 | 14.4±6.7 | 15.4±3.1 | .312 | .007* | .366 |
| Total adiponectin (μg/mL) | 29.1±2.0 | 38.5±1.5 | 20.4±1.7 | 37.7±2.5 | <.001* | .009* | .076 |
| HMW adiponectin (μg/mL) | 10.6±1.4 | 15.0±1.3 | 6.2±0.8 | 12.6±1.8 | <.001* | .013* | .424 |
| HMW/total adiponectin | 0.35±0.03 | 0.38±0.02 | 0.29±0.02 | 0.33±0.02 | .114 | .016* | .833 |
| Total adiponectin/leptin (µg/ng) | 1.1±0.1 | 2.1±0.6 | 7.1±2.8 | 4.2±1.3 | .603 | .029* | .276 |
Notes: HMW = high-molecular-weight. Values are mean ± SE.
*p < .05 in a two-way analysis of variance.
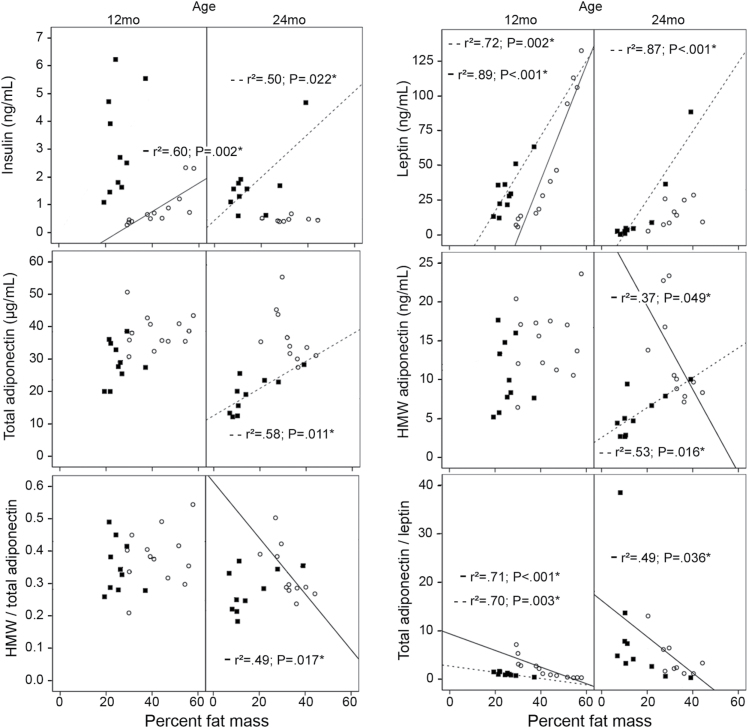
Impact of Age on the Correlations Between Hormone Levels and Fat Mass
Several differences were observed in the correlations between fat mass and hormone levels for the different age groups of GHR− /− mice (Figure 1). Results for 12-month-old GHR− /− mice were similar to those seen in WT controls, for example, fat mass showed a positive correlation to insulin and leptin and no correlation to total or HMW adiponectin. However, striking differences were observed for the 24-month-old group. There were no correlations of fat mass to insulin, leptin, or total adiponectin; only HMW adiponectin correlated to fat mass and this association was negative in GHR− /−, whereas WT mice showed a positive correlation between these two variables. The ratios of HMW/total adiponectin and total adiponectin/leptin also revealed differences in their correlations with percent fat mass in the four groups (Figure 1). It should be mentioned that HMW adiponectin levels correlated closely with total adiponectin levels in all groups, regardless of genotype or age (data not shown).
Figure 1.
Correlations between plasma hormone levels and percent fat mass for each group. Wild-type mice (■; dotted lines); GHR− /− mice (○; solid lines). Only significant correlations (p < .05) are shown (*). mo = months.
Depot-Specific Weight and Protein Content
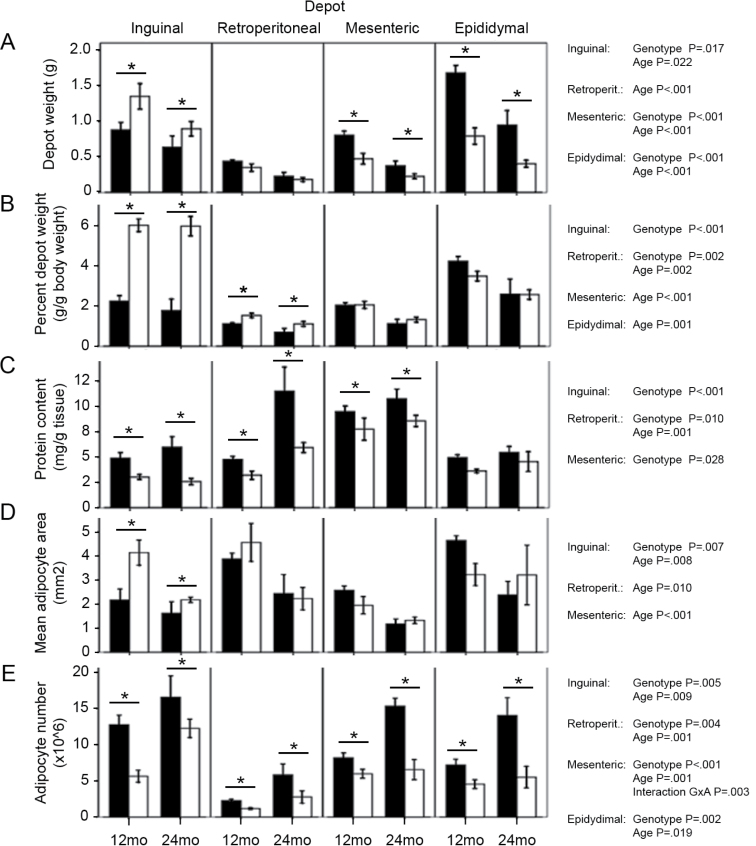
WAT from inguinal, retroperitoneal, mesenteric, and epididymal depots was dissected and weighed (Figure 2A). Each depot weight was then normalized to body weight and expressed as percent depot weight to enable comparisons between genotypes (Figure 2B). As reported previously (6), inguinal and retroperitoneal WAT were significantly enlarged in the GHR− /− mice. Regarding age effects, both genotypes displayed decreases in retroperitoneal, mesenteric, and epididymal depot weights, whereas the size of the inguinal depot remained constant despite advancing age.
Figure 2.
Weight, percent weight, protein content, mean adipocyte size, and adipocyte number of white adipose tissue depots in the four mouse groups (mean ± SE). For depot weights and percent weights, all groups n = 10–15 except for percent weight at 24 months, where n = 6 for both genotypes. For protein content, n = 6 for all groups. For adipocyte cross-sectional area and adipocyte number, n = 6 except for 24-month retroperitoneal (wild type: n = 3; GHR− /−: n = 4) and 24-month epididymal GHR− /− (n = 5). Black bars: wild-type mice; white bars: GHR− /− mice. Significant results for two-way analysis of variance tests performed in each depot are shown on the right of each graph. Where main effects of genotype were detected, differences are marked for both age groups (*). mo = months; retroperit. = retroperitoneal.
Correlations of hormone levels to individual depot weights were evaluated (Supplementary Table 1) and showed differences between genotypes and ages that somewhat followed the trends observed for correlations of percent fat mass (Figure 1). Insulin and leptin levels correlated positively to all depot weights in 24-month-old WT and 12-month-old GHR− /− mice. Only inguinal and mesenteric weight correlated to leptin levels in 12-month-old WT mice. In aged GHR− /− mice, a negative correlation was observed between all depot weights and adiponectin levels (HMW and total).
Protein content (milligram of protein per gram of tissue) was lower in GHR− /− mice than WT in all WAT depots except epididymal (Figure 2C). In addition, retroperitoneal depots showed an increase in protein content in aged mice compared with 12-month-old animals.
Adipocyte Size and Number
The cross-sectional areas of adipocytes were measured in each of the WAT depots and compared with the values previously reported for WT mice (8,9). Differences in adipocyte sizes between genotypes and age groups showed a similar pattern as that observed for percent depot weights (Figure 2D). That is, mean inguinal WAT adipocyte size was larger in GHR− /− than WT mice, whereas mean adipocyte size decreased with age in the four WAT depots of all mice (except in epididymal WAT of GHR− /− mice). Thus, it appears that the marked increase in inguinal fat mass in GHR− /− mice is the result of an increased size of inguinal adipocytes when compared with WT mice. The distributions of adipocyte sizes measured in each WAT depot for the four age and genotype groups are shown in Supplementary Figure 1.
Regarding adipocyte numbers, as previously reported for WT mice (9), the estimated number of adipocytes in GHR− /− depots increased with age (Figure 2E). Interestingly, GHR− /− mice displayed a more marked age-related increase in adipocyte numbers in inguinal WAT than in the other depots. In advanced age, increased cellularity might help maintain the size of the GHR− /− inguinal depot, thus compensating for the age-related decrease in adipocyte sizes.
WT mice alone showed significant correlations between mean adipocyte size of each WAT depot and hormone levels (Supplementary Table 2). Total adiponectin levels correlated positively with epididymal mean adipocyte size in adult and aged mice. In the aged group, mean cell sizes in all depots correlated positively with HMW adiponectin, but inguinal and mesenteric adipocyte sizes also correlated positively with insulin, leptin, and total adiponectin levels. Adipocyte numbers in each depot showed no correlations with any of the hormones measured (data not shown).
WAT Proteome Differences Between GHR− /− and WT Mice
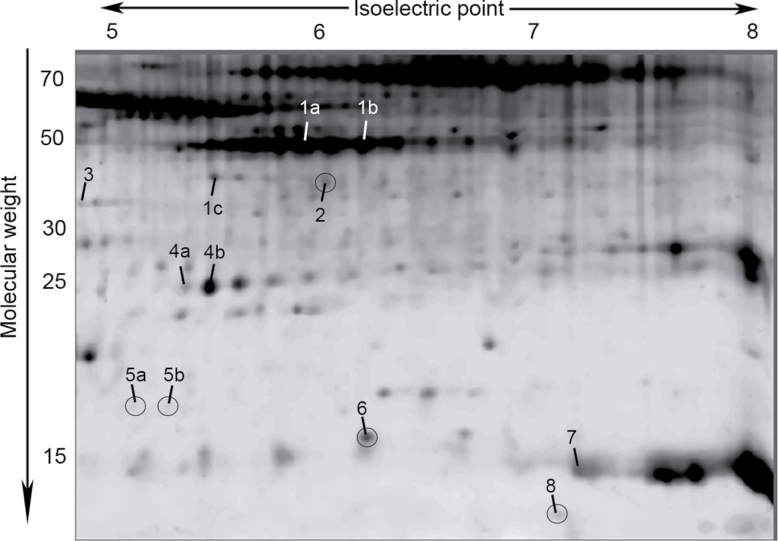
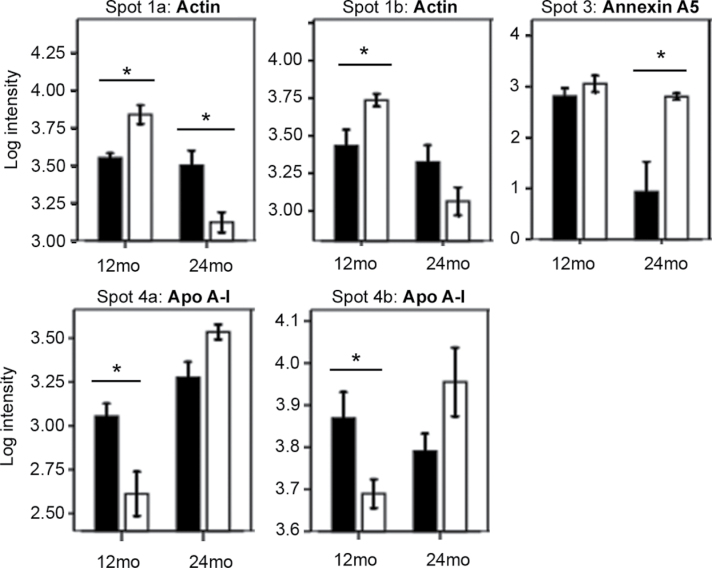
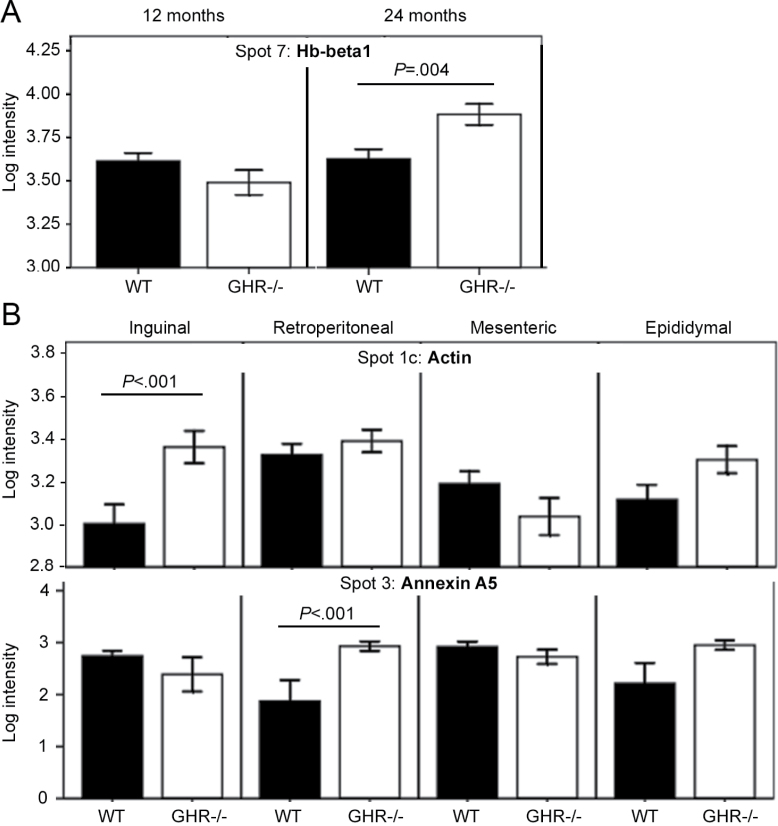
We used a proteomics approach to initiate an exploration of underlying molecular mechanisms that might explain the observed differences between GHR− /− and WT mice. Proteins from WAT depots of adult and aged mice of each genotype were isolated and resolved by 2DE (Figure 3). Of 169 protein spots analyzed, 70 showed significant differences among the studied groups (data not shown). Interestingly, only 12 out of 70 spots showed significant differences between genotypes (main effect or interactions, Figures 4–6 and Supplementary Figures 3–5). The remaining spots showed significant differences among depots or age groups, with no effects of genotype (data not shown). Because the phenotypic differences between GHR− /− and WT mice were the major interest in this study, we focused on the 12 spots that showed intensity differences between genotypes (Figure 3). Three-dimensional views of spot intensities for these 12 spots are shown in Supplementary Figure 2. The 12 spots represented 8 proteins (Table 2 and Supplementary Table 3). Thus, some proteins were detected in more than one spot, indicative of posttranslational modifications. A general theme of endocytosis/cytoskeletal organization, anticoagulation, amyloid production, and other age-related conditions was noted among these proteins (Table 2).
Figure 3.
Representative 2D gel of white adipose tissue showing 12 spots that displayed significant main effects of genotype or significant interactions of genotype and age and/or depot (p < .01). Numbered labels correspond to protein identities shown in Table 2 and Supplementary Table 3. Spots showing main effects of genotype are circled. Three-dimensional views of spot intensities are shown in Supplementary Figure 2.
Figure 4.
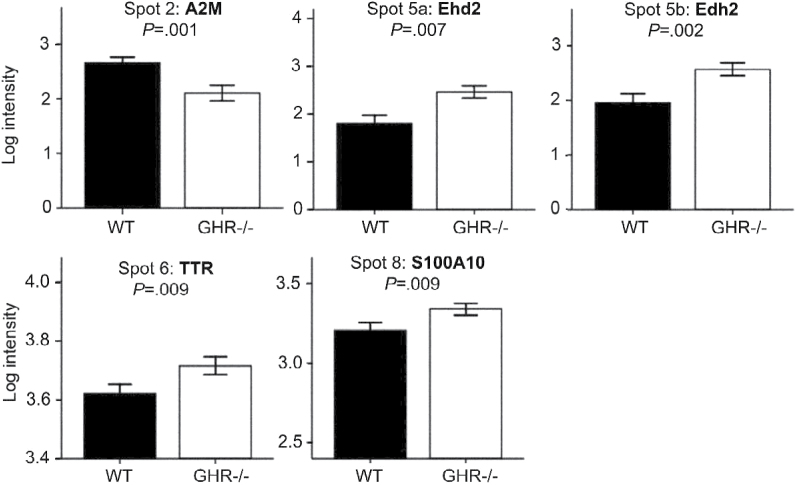
Log intensity (mean ± SE) of protein spots that were significantly different between genotypes (main genotype effect p < .01 in a three-way analysis of variance, n = 6 mice per group). Intensity values of all white adipose tissue depots at 12 and 24 months are grouped in each graph. Intensities in separate depot and age groups are shown in Supplementary Figure 3. A2M = alpha2-macroglobulin; Ehd2 = EH domain–containing protein 2; TTR = transthyretin.
Figure 6.
Retroperitoneal white adipose tissue (WAT) log intensity values (mean ± SE) of protein spots that showed significant interactions of Genotype × Depot × Age (p < .01 in a three-way analysis of variance, n = 6 mice per group). Intensities for all WAT depots and age groups are shown in Supplementary Figure 5. Black bars: wild-type mice; white bars: GHR− /− mice; (*) significant differences between genotypes. ApoA-I = apolipoprotein A-I; mo = months.
Table 2.
Summary of Protein Identities and Functions for the Spots Analyzed
| Spot | Protein Identity | Function |
|---|---|---|
| Cytoskeleton related | ||
| 1a, 1b, 1c | Actin | Microfilaments; muscle contraction |
| 5a, 5b | EH domain–containing protein 2 | Regulation of endocytosis |
| 8 | S100A10 | Plasminogen receptor, binds annexin A2 and may be involved in exocytosis |
| Amyloid producing | ||
| 4a, 4b | Apolipoprotein A-I | Cholesterol transport in HDL, anti-inflammatory functions |
| 6 | Transthyretin | Binds thyroid hormones and RBP4 |
| Other | ||
| 2 | Alpha2-macroglobulin | Proteinase inhibitor |
| 3 | Annexin A5 | Anticoagulant protein |
| 7 | Hemoglobin subunit β-1 | Oxygen transport |
Notes: HDL = high-density lipoprotein; RBP4 = retinol binding protein 4. Spot labels as shown in Figure 3; protein identities as in Supplementary Table 3.
To evaluate whether the circulating levels of insulin, leptin, and total or HMW adiponectin were associated with the proteomic differences, we analyzed the correlations between these hormones and the intensities of the 12 spots that showed differences between genotypes. We found several significant associations (Supplementary Table 4), with some similarities between the results obtained for total and HMW adiponectin.
Ehd2 and Glut4 Protein Levels
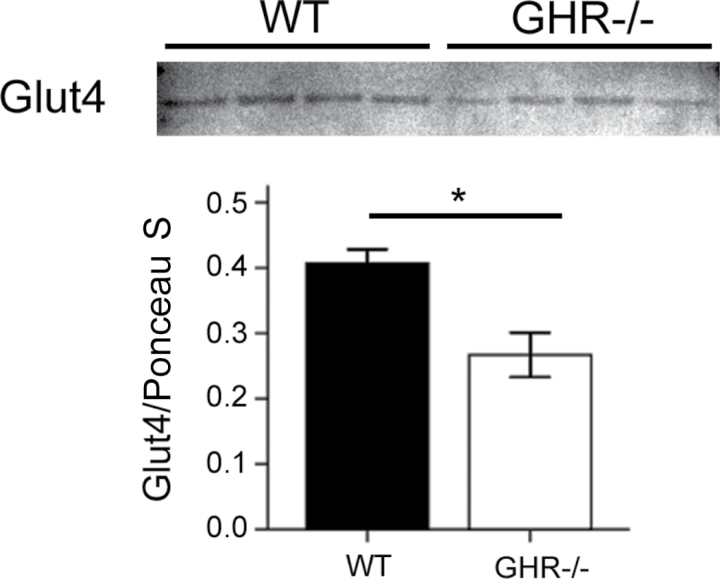
Results of 2D gels revealed increased levels of two low-MW fragments of Ehd2 in GHR− /− depots compared with WT (spots 5a and 5b, Figures 3 and 4). Ehd2 associates to the insulin-regulated glucose transporter Glut4 in adipocytes and has been shown to influence the presence of Glut4 on the plasma membrane (10). Thus, we explored the possible connection between adipose tissue Ehd2 levels and the enhanced insulin sensitivity of GHR− /− mice. We measured Glut4 and Ehd2 levels (full-length protein) by Western blotting. The results for Glut4 showed a significant decrease (p = .013) at 12 months in GHR− /− inguinal WAT compared with WT (Figure 7). However, no differences between genotypes were observed for Glut4 or full-length Ehd2 in any of the other 12- and 24-month-old inguinal and epididymal WAT samples or in a separate cohort of 6-month-old samples of the same genotypes (data not shown).
Figure 7.
Western blotting results for Glut4 in 12-month-old inguinal white adipose tissue (n = 4 for each group). Top panel shows blot image, and bottom panel displays quantification results. The intensity of Glut4 bands was normalized to total lane intensity of Ponceau S staining as loading control. (*) indicates significant differences in a two-tailed t test (p < .05).
Discussion
The goal of this study was to compare WAT depots and their age-related changes between GHR− /− and WT mice. Despite their extended longevity, very few studies have focused on older GHR− /− mice. We used 12- and 24-month-old mice, representing “adult” and “aged” states. The “aged” time point selected represents a time where both genotypes are losing fat mass (11), but although WT mice are approaching the end of their lives, GHR− /− mice usually live for another 6 months or longer (12). Differences among WAT depots and their response to age have been described previously for WT mice (8,9). We chose to compare them with GHR− /− mice given their unique combination of obesity (mainly subcutaneous), with high insulin sensitivity and extended longevity (5). These characteristics make GHR− /− mice a convenient tool for the study of depot-specific influences of obesity on insulin resistance and aging.
Several reports have shown that glucose is low to normal in GHR− /− mice throughout life and that their insulin sensitivity is markedly enhanced when compared with controls (5). We observed body weight, body composition, and hormone level differences between GHR− /− and control mice, which were as expected based on previous studies (6,13).
The impact of age on fat mass and plasma levels of insulin, leptin, and adiponectin in GHR− /− mice was similar to that observed for WT controls (all variables decrease in aged animals). However, aged GHR− /− mice lacked an association of insulin and leptin levels to fat mass. In contrast, these mice showed a negative association between fat mass and adiponectin (total and HMW) levels, as normally observed for obese individuals (14). The differences between GHR− /− and WT mice in fat mass and hormone correlations possibly reflect distinctions in the physiology of WAT in each genotype. For instance, differences in WAT adiponectin protein content were recently found in 6-month-old GHR− /− compared with WT mice (13). Regarding adipocyte size and protein content, both genotypes showed changes with age that were consistent with the observed decrease in depot weights, that is, a decrease in mean adipocyte size and a slight increase in protein content in most WAT depots. Also, the overall increase in adipocyte sizes in GHR− /− mice suggests a possible link between GHR deficiency and altered lipid storage proteins such as perilipin 1. Future studies should analyze the levels of lipid storage markers in GHR− /− and WT mouse WAT, especially in the inguinal depot.
We have initiated studies to help elucidate the mechanism(s) responsible for the physiological distinctions between GHR− /− and WT mice. In this regard, we analyzed the proteomes of four WAT depots in 12- and 24-month-old GHR− /− mice and compared them with those of WT mice. Surprisingly, we found more protein spot intensities to be different among WAT depots and age groups than between genotypes. Several of the proteins identified to be different between genotypes are associated with cytoskeletal rearrangements during endocytosis (Ehd2, S100A10, annexin A5, actin) and anticoagulation (S100A10, annexin A5). Interestingly, altered endocytosis has been reported for several aging tissues (15,16), and an increased cardiovascular disease risk with normal aging is well documented. Because the focus of this study was to evaluate genotype differences, the 58 spots found by 2DE that did not show genotype effects were not described here. Most of these proteins have been included in our previous reports regarding depot and age effects in WAT of WT mice (8,9).
We chose to focus on Ehd2 because it has been shown to regulate Glut4 internalization (10,17), which could be important in establishing higher insulin sensitivity in GHR− /− than WT mice. Ehd2 is a 61-kDa protein whose over- expression inhibits the internalization of endocytic vesicles, causing them to remain at the plasma membrane (18,19). In our study, two Ehd2 spots were increased in WAT depots of GHR− /− mice compared with WT. It is tempting to speculate that increased Ehd2 expression in GHR− /− WAT could lead to prolonged presence of Glut4 on the plasma membrane and enhanced glucose uptake. Inhibition of GH action in vivo has been shown to increase Glut4 at the plasma membrane in rat adipocytes (20), but no mechanism has been reported. However, levels of the full-length Ehd2 protein on Western blots did not show any differences between GHR− /− and WT mice. Considering that the Ehd2 spots found by 2DE did not contain the full-length protein but fragments of approximately 18 kDa of its C-terminal end (Figure 3 and Supplementary Table 3), the possibility remains that the levels of full-length protein are regulated differently from the levels of the two fragments. Unfortunately, the low-MW fragments of Ehd2 were not detected by the antibody (data not shown), preventing us from validating the observations from our 2D gels. The question of whether increased levels of these fragments could prolong the presence of Glut4 on the cell membrane and lead to increased glucose uptake in GHR− /− WAT also remains unanswered but is an intriguing possibility. These C-terminal fragments contain regions of the protein that allow for its proper oligomerization and function (19) and could therefore exert an important role in the regulation of endocytosis. However, the activity of Ehd2 is not specific for Glut4 and might also affect endocytosis of other membrane transporters or receptors (17).
In terms of Glut4, to our knowledge, there are no previous reports of this protein’s levels in WAT depots of GHR− /− mice. Our data showed a significant decrease of Glut4 levels in 12-month-old inguinal fat of GHR− /− compared with WT mice, but no differences in epididymal WAT or other ages studied. A decrease in Glut4 levels has also been reported for cardiac muscle of GHR− /− mice (21), whereas no difference was found between GHR− /− and WT Glut4 levels in skeletal muscle (22). Given the proposed effects of GH and Ehd2 in Glut4 availability on the plasma membrane (see above), further studies are needed to determine the location of Glut4 (plasma membrane vs internal vesicles) before conclusions regarding WAT insulin sensitivity can be drawn.
Variations in actin levels might also be related to endocytosis and cytoskeletal reorganization in WAT of GHR− /− mice. However, the large number of actin isoforms present in WAT (Figure 3, long train of spots at ~48 kDa) suggests that the differences detected might be negligible when compared with total levels. Regarding S100A10, this protein interacts with annexin A2, which regulates actin reorganization in stress-induced premature cellular senescence (23). In addition to cytoskeletal reorganization functions, these two proteins can bind plasminogen and stimulate plasmin production, which favors angiogenesis (24) and migration of macrophages from the bloodstream to tissues (25). Both of these processes have been linked to obesity and are thus consistent with the increase in S100A10 observed in WAT depots from obese GHR− /− mice. Also, the increase in S100A10 levels is in agreement with the regulation by GH observed for other S100 proteins (26). Furthermore, we also detected changes in annexin A5, which is closely related to the inhibition of blood coagulation (27). Anticoagulant properties of blood from GHR− /− mice have not been previously assessed and warrant further attention as they might contribute to the extended life span of these mice.
Other proteins identified in our proteomic study are normally found in the circulation and have been suggested as markers of aging and linked to age-related diseases such as amyloidosis, Alzheimer’s disease, and atherosclerosis (alpha2-macroglobulin, transthyretin, and apolipoprotein A-I) (28–34). Previous reports have shown that GH can regulate levels of alpha2-macroglobulin (35) and transthyretin (36). In addition, an aging study of plasma proteomes in GHR− /− versus WT mice found variations in apolipoprotein A-I and hemoglobin-β levels (37).
The retroperitoneal depot was particularly affected by GHR deletion and age. Protein content was significantly increased with age in both genotypes, and differences in the levels of specific protein species were found between GHR− /− and WT mice only in this depot. It is possible that age-related changes might be more drastic in retroperitoneal WAT, which might render the effects of GHR deletion more evident than in the other depots. This is consistent with the greater age-related changes in preadipocyte gene expression found in retroperitoneal than epididymal WAT (38).
In all, our proteomics data show that WT and GHR− /− mice display substantial similarities in their WAT depot protein profiles despite the marked differences observed in fat mass, insulin sensitivity, and life span between genotypes. However, it should be noted that undetected differences could also exist. For example, membrane associated and low abundance proteins are usually underrepresented in proteomic analyses. Still, the physiological differences displayed between genotypes might depend not only on distinctive protein profiles but also on the sizes of each WAT depot, especially considering the vast increase in percent inguinal WAT mass in the GHR− /− mice.
Our previous data suggest that the inguinal WAT depot normally displays lower metabolic rate and oxidative stress than other depots (8). Therefore, the possibility exists that the enlarged mass of this depot in GHR− /− mice accounts for some of the beneficial effects observed in these mice, leading to enhanced insulin sensitivity and prolonged longevity. This is consistent with transplantation studies where subcutaneous fat transplanted into the intraabdominal area improved insulin sensitivity in WT mice (39). Also in agreement is the recently reported phenotype of fat-specific GHR gene–disrupted mice (FaGHRKO mice), which not only display markedly enlarged inguinal mass but also significantly enlarged retroperitoneal and mesenteric fat mass (40). The enlargement of both subcutaneous and intraabdominal depots (as opposed to the mainly subcutaneous obesity seen in GHR− /− mice) seems to not be as beneficial in terms of glucose homeostasis given that these mice have normal plasma insulin and glucose levels and normal glucose and insulin tolerance. Moreover, the differences between phenotypes of GHR− /− and FaGHRKO mice suggest that tissue cross talk might be important in establishing the “healthy” obese phenotype observed in GHR− /− mice as opposed to a direct role of GHR disruption in WAT.
Finally, it is important to note that this study used whole tissue for proteomic analysis. Despite the varied cell types present in WAT (adipocytes, preadipocytes, immune cells, fibroblasts, neural cells, endothelial cells, etc.), none of the proteins identified were indicative of variations in specific cell populations in the WAT depots of GHR− /− and WT mice. It would be interesting to establish whether the changes detected, such as in endo/exocytosis, affect all WAT cells or only certain cell types. It should also be noted that the mice used in this study were not checked for pathologies. GHR− /− mice are protected from cancer and age-related conditions, but WT mice can develop cancer and glomerulonephritis (41). Although most 24-month-old mice are healthy, the presence of diseases among our mice cannot be excluded and might have affected our results.
In summary, whereas some age-related changes were similar in WT and GHR− /− mice, some striking differences were observed between genotypes. For example, the opposite correlations in each genotype between adiponectin levels and fat mass at old age warrant further study. In addition, important variations between GHR− /− and WT mice were found in proteins involved in cytoskeletal reorganization and endocytosis, possibly influencing the presence of receptors and transporters (such as Glut4) on cell membranes, which could affect insulin sensitivity. Changes in cytoskeletal architecture might reflect delayed cellular senescence and be related to the prolonged longevity of GHR− /− mice. In fact, cellular senescence was recently proposed to be at the root of adipose tissue and systemic dysfunction in aging (42,43). Thus, the role of the detected proteins in relation to insulin sensitivity and extended longevity of GHR−/− mice will be the subject of future studies.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Figure 5.
Log intensity (mean ± SE) of protein spots that showed significant interactions of Genotype × Age (A) or Genotype × Depot (B); p < .01 in a three-way analysis of variance, n = 6 mice per group). Spot 3 also showed a significant interaction of Genotype × Depot × Age. Genotype differences in each age group (A) or depot (B) are highlighted. Intensity values of all white adipose tissue depots (A) or 12 and 24 months (B) are grouped in each graph. Intensities in each depot and age group are shown in Supplementary Figure 4. Hb-beta1 = hemoglobin β-1; WT = wild type.
Funding
This work was supported in part by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll (J.K.); National Institutes of Health grants DK075436 (J.K.), AG019899 (J.K.), and 1P01AG031736 (J.K., D.B., E.L.); the Diabetes Institute (L.S., D.B., E.L.); the BioMolecular Innovation and Technology Partnership (L.S.) at Ohio University; and American Veterans (J.K., C.V., E.L.).
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1. Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293 [DOI] [PubMed] [Google Scholar]
- 2. Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes. 2011;6(suppl 1):13–20 [DOI] [PubMed] [Google Scholar]
- 3. Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf). 2012;205:194–208 [DOI] [PubMed] [Google Scholar]
- 5. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32:356–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masternak MM, Bartke A, Wang F, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring). 2012;20:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sackmann-Sala L, Berryman DE, Lubbers ER, et al. Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice. Age (Dordr). 2012;34:1225–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park SY, Ha BG, Choi GH, et al. EHD2 interacts with the insulin-responsive glucose transporter (GLUT4) in rat adipocytes and may participate in insulin-induced GLUT4 recruitment. Biochemistry. 2004;43:7552–7562 [DOI] [PubMed] [Google Scholar]
- 11. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810 [DOI] [PubMed] [Google Scholar]
- 13. Lubbers ER, List EO, Jara A, et al. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaser S, Tatarczyk T, Stadlmayr A, et al. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur J Clin Invest. 2008;38:827–834 [DOI] [PubMed] [Google Scholar]
- 15. Blanpied TA, Scott DB, Ehlers MD. Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Aging. 2003;24:1095–1104 [DOI] [PubMed] [Google Scholar]
- 16. Simon-Santamaria J, Malovic I, Warren A, et al. Age-related changes in scavenger receptor-mediated endocytosis in rat liver sinusoidal endothelial cells. J Gerontol A Biol Sci Med Sci. 2010;65:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guilherme A, Soriano NA, Bose S, et al. EHD2 and the novel EH domain binding protein EHBP1 couple endocytosis to the actin cytoskeleton. J Biol Chem. 2004;279:10593–10605 [DOI] [PubMed] [Google Scholar]
- 18. Morén B, Shah C, Howes MT, et al. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol Biol Cell. 2012;23:1316–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoeber M, Stoeck IK, Hänni C, Bleck CK, Balistreri G, Helenius A. Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J. 2012;31:2350–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilgour E, Baldwin SA, Flint DJ. Divergent regulation of rat adipocyte GLUT1 and GLUT4 glucose transporters by GH. J Endocrinol. 1995;145:27–33 [DOI] [PubMed] [Google Scholar]
- 21. Giani JF, Bonkowski MS, Muñoz MC, et al. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol A Biol Sci Med Sci. 2008;63:788–797 [DOI] [PubMed] [Google Scholar]
- 22. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–684 [DOI] [PubMed] [Google Scholar]
- 23. Chrétien A, Delaive E, Dieu M, et al. Upregulation of annexin A2 in H(2)O(2)-induced premature senescence as evidenced by 2D-DIGE proteome analysis. Exp Gerontol. 2008;43:353–359 [DOI] [PubMed] [Google Scholar]
- 24. Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118:3172–3181 [DOI] [PubMed] [Google Scholar]
- 25. Phipps KD, Surette AP, O’Connell PA, Waisman DM. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Cancer Res. 2011;71:6676–6683 [DOI] [PubMed] [Google Scholar]
- 26. Chung L, Nelson AE, Ho KK, Baxter RC. Proteomic profiling of growth hormone-responsive proteins in human peripheral blood leukocytes. J Clin Endocrinol Metab. 2009;94:3038–3043 [DOI] [PubMed] [Google Scholar]
- 27. Andree HA, Stuart MC, Hermens WT, et al. Clustering of lipid-bound annexin V may explain its anticoagulant effect. J Biol Chem. 1992;267:17907–17912 [PubMed] [Google Scholar]
- 28. Kondo T, Sakaguchi M, Namba M. Two-dimensional gel electrophoretic studies on the cellular aging: accumulation of alpha-2-macroglobulin in human fibroblasts with aging. Exp Gerontol. 2001;36:487–495 [DOI] [PubMed] [Google Scholar]
- 29. Mayot G, Vidal K, Martin JF, et al. Prognostic values of alpha2-macroglobulin, fibrinogen and albumin in regards to mortality and frailty in old rats. Exp Gerontol. 2007;42:498–505 [DOI] [PubMed] [Google Scholar]
- 30. Wagner V, Wagnerová M, Zvára K. Age correlation of alpha-2-macroglobulin levels in healthy subjects. Physiol Bohemoslov. 1982;31:359–364 [PubMed] [Google Scholar]
- 31. Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine Alzheimer’s disease: is it protective? J Neurosci. 2011;31:12483–12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reynolds CA, Prince JA, Feuk L, Brookes AJ, Gatz M, Pedersen NL. Longitudinal memory performance during normal aging: twin association models of APOE and other Alzheimer candidate genes. Behav Genet. 2006;36:185–194 [DOI] [PubMed] [Google Scholar]
- 33. Teoh CL, Griffin MD, Howlett GJ. Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein Cell. 2011;2:116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramella NA, Rimoldi OJ, Prieto ED, et al. Human apolipoprotein A-I-derived amyloid: its association with atherosclerosis. PLoS One. 2011;6:e22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campbell GS, Meyer DJ, Raz R, Levy DE, Schwartz J, Carter-Su C. Activation of acute phase response factor (APRF)/Stat3 transcription factor by growth hormone. J Biol Chem. 1995;270:3974–3979 [DOI] [PubMed] [Google Scholar]
- 36. Samadani U, Costa RH. The transcriptional activator hepatocyte nuclear factor 6 regulates liver gene expression. Mol Cell Biol. 1996;16:6273–6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ding J, Berryman DE, Jara A, Kopchick JJ. Age- and sex-associated plasma proteomic changes in growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2012;67:830–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27:524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]