Abstract
Mutations causing decreased somatotrophic signaling are known to increase insulin sensitivity and extend life span in mammals. Caloric restriction and every other day (EOD) dietary regimens are associated with similar improvements to insulin signaling and longevity in normal mice; however, these interventions fail to increase insulin sensitivity or life span in growth hormone receptor knockout (GHRKO) mice. To investigate the interactions of the GHRKO mutation with caloric restriction and EOD dietary interventions, we measured changes in the metabolic parameters oxygen consumption (VO2) and respiratory quotient produced by either long-term caloric restriction or EOD in male GHRKO and normal mice. GHRKO mice had increased VO2, which was unaltered by diet. In normal mice, EOD diet caused a significant reduction in VO2 compared with ad libitum (AL) mice during fed and fasted conditions. In normal mice, caloric restriction increased both the range of VO2 and the difference in minimum VO2 between fed and fasted states, whereas EOD diet caused a relatively static VO2 pattern under fed and fasted states. No diet significantly altered the range of VO2 of GHRKO mice under fed conditions. This provides further evidence that longevity-conferring diets cause major metabolic changes in normal mice, but not in GHRKO mice.
Key Words: Growth hormone receptor knockout mouse, Indirect calorimetry, Every other day diet, Caloric restriction, Metabolism, Intermittent fasting.
Mutations, which reduce somatotrophic (growth hormone [GH] and insulin-like growth factor-1) signaling (1), as well as dietary regimens based on reduction of caloric intake (caloric restriction [CR] and every other day [EOD] diet) (2,3), are known to delay aging and increase life span in mice. Long-lived GH receptor/GH-binding protein gene-disrupted (growth hormone receptor knockout [GHRKO]) mice are resistant to GH action and have severely depressed serum insulin-like growth factor-1 and reduced body size and weight despite increased adiposity, decreased serum glucose and insulin, and increased insulin sensitivity (4). CR and EOD feeding regimens have also been shown to increase insulin sensitivity, decrease serum glucose and insulin levels, and increase life span in rodents (3,5,6).
Decreased caloric intake and the GHRKO mutation may act through overlapping mechanisms to increase life span. Evidence for this comes from experiments where identical CR (7) and EOD (8) regimens produced increases in both insulin sensitivity and life span extension in normal mice, but produced no changes in insulin sensitivity or longevity in GHRKO mice.
Understanding the metabolic alterations, which accompany increased lifespan, may provide some mechanistic clues as to how extension of life span is achieved. We have previously shown that decreased GH signaling is associated with increased oxygen consumption (VO2) per unit body mass and decreased respiratory quotient (RQ) in young adult mice (9); however, it was unknown whether this effect endures into advanced age. Here, we examine VO2 and RQ in old age mice near the end of their life span. There have been many studies examining the impact of CR on metabolism (5); however, the impact of the EOD diet on the VO2 and RQ has not been explored in mice. This study is also one of few (6,10) that directly compare the effects of CR and EOD dietary regimens in animals from the same strain. Importantly, in this study, we also examine the effect of the interaction of decreased GH signaling with CR and EOD diets.
Materials and Methods
Animals
GHRKO (−/−) and normal (+/−) male mice were produced in our breeding colony by mating −/− males to +/− females derived from GHRKO animals (4) kindly provided by Dr. J. J. Kopchick (Ohio University, Athens, Ohio). Animals were housed under temperature- and light-controlled conditions (21°C–23°C and 12-hour light/12-hour dark cycle) and were fed Lab Diet Formula 5001 (23.4% protein, 4.5% fat, and 5.8% fiber; Ralston Purina). All animal protocols for this study were approved by the animal care and use committee of Southern Illinois University.
The mice used in this experiment were 29–33 months old, which for normal AL mice is close to median life span. Mice of this age were selected to investigate the metabolic differences in biologic aging caused by dietary and genotypic interactions in mice of similar advanced chronological age. Also, we have shown that decreased GH signaling is associated with increased VO2 per unit body mass and decreased RQ in young adult mice (9). Here, we have explored whether this effect endures into advanced age.
Diets
All animals (n of 6–9 per genotype per diet group) were started on their respective diets at 8–12 weeks of age and were 29–33 months old at the time of indirect calorimetry (Supplementary Figure 6). Animals were gradually placed on 30% CR by receiving 90% of the amount of food consumed by AL controls in the initial week, 80% the following week, and 70% throughout the rest of the studies. EOD animals were fed in alternating cycles of one 24-hour period of AL access to food, followed by a 24-hour period of fasting, throughout their adult lives (26–31 months). During testing, the diets were administered as they had been throughout the mice’s life span. AL mice received open access to food on the acclimation and fed day, CR mice received 70% of their AL counterpart’s total food consumption on the acclimation and fed days, and EOD mice were fasted on the acclimation day and fed AL on the fed day. All mice were fasted on the fasted day. All mice were administered their respective diets between 4 pm and 5 pm; this time frame was used when food was removed at the beginning of the fasted day as well. The diet of individual CR mice was calculated before testing and represents 70% of the consumption of group-housed AL controls, expressed per animal. Food consumption data presented here represent amounts of food consumed while in the testing chambers on the acclimation day and fed day together (48 hours). Body weight and food consumption data were collected manually before and after, respectively, each uninterrupted 24-hour indirect calorimetry measurement and statistically analyzed using the unpaired t-test and one-way analysis of variance (Graph Pad Prism, IBM SPSS Statistics 19).
Indirect Calorimetry
Indirect calorimetry was performed using the AccuScan Instruments, Inc. PhysioScan Metabolic System. This system utilizes zirconia and infrared sensors to monitor oxygen (O2) and carbon dioxide (CO2), respectively, inside respiratory chambers in which individual mice were tested. All comparisons are based on animals studied simultaneously in eight different chambers connected to the same O2 and CO2 sensors in an effort to minimize the effect of environmental variations and calibration on data. After a 24-hour acclimation period (fasted day for EOD mice), mice were monitored in the metabolic chambers for 24 hours with access to their respective dietary allowance (CR/EOD [fed day AL]/AL) and water, and then for a second 24-hour period without food. Gas samples were collected and analyzed every 5 minutes per animal, and the data were averaged for each hour. Output parameters include VO2 (mL/kg/min) and RQ (VCO2/ VO2). Two-way analysis of variance and two-way repeated measures analysis of variance statistical analyses were performed using IBM SPSS Statistics 19.
Results
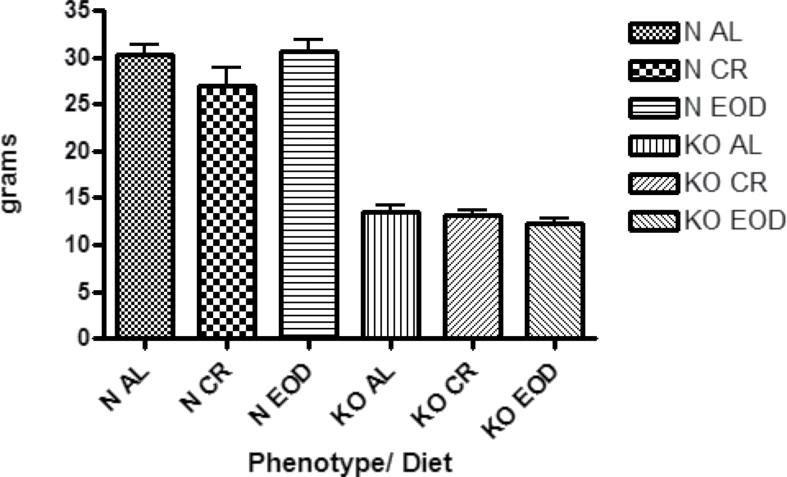
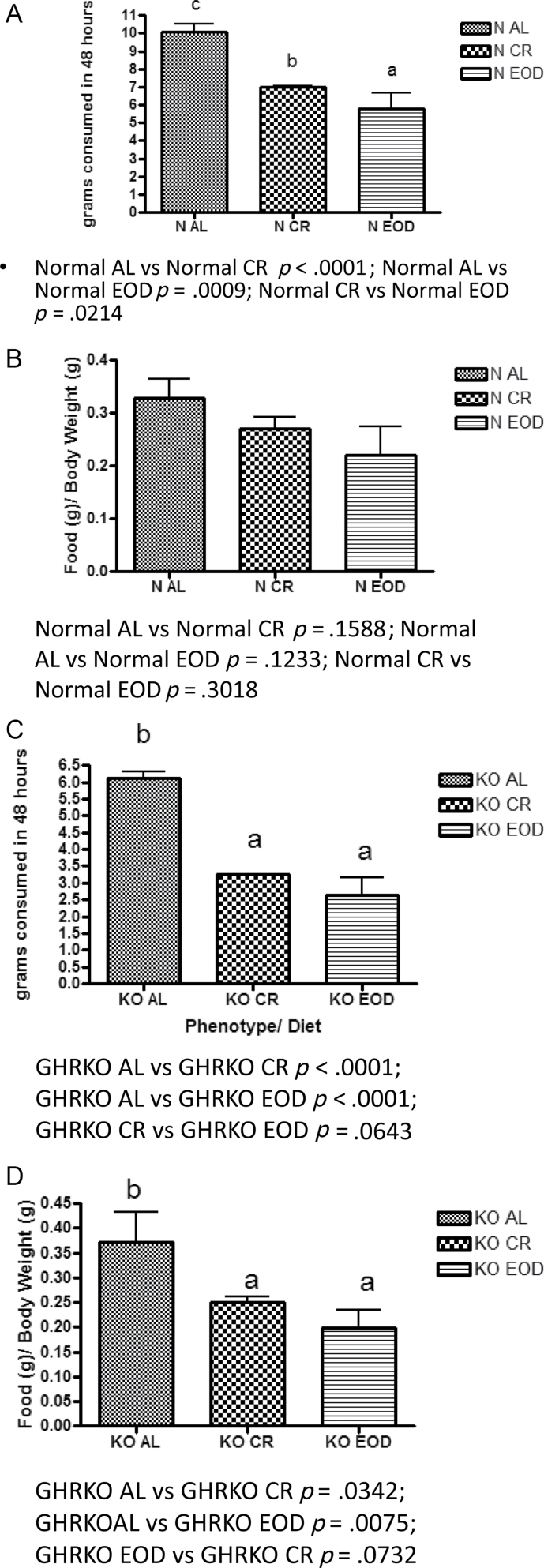
Body weights of the mice at the time of indirect calorimetry are shown in (Figure 1). Total food consumption and food consumption per gram body weight for mice on the acclimation and fed days of indirect calorimetry are shown in (Figure 2). Total food consumption was decreased by both diets in normal mice (Figure 2a); however, when food consumption is expressed per gram body weight, the differences are eliminated (Figure 2b). The GHRKO mouse’s total food consumption was also reduced by CR and EOD diet (Figure 2c); however, GHRKO mice consumed less food per gram body weight when on CR or EOD diets as well (Figure 2d). Interestingly, when expressed per percent lean mass (fat-free mass - bone weight), there were no significant differences between GHRKO and normal control mice (Supplementary Figure 1b).
Figure 1.
Body weights of mice recorded just prior to start of acclimation day of indirect calorimetry (n = 6–9 per genotype per diet group).
Figure 2.
Total food consumption on the acclimation and fed day of testing (48 hours) of normal (A) and growth hormone receptor knockout (GHRKO) (C) mice and food consumption expressed per gram body weight of normal (B) and GHRKO (D) mice. A–C—values that do not share the same letter in the superscript are statistically significant (p < .05).
Oxygen Consumption
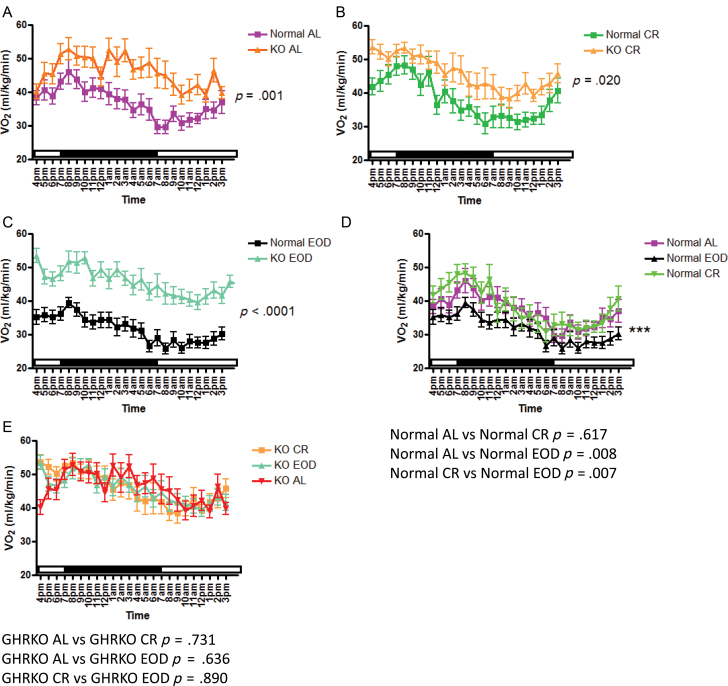
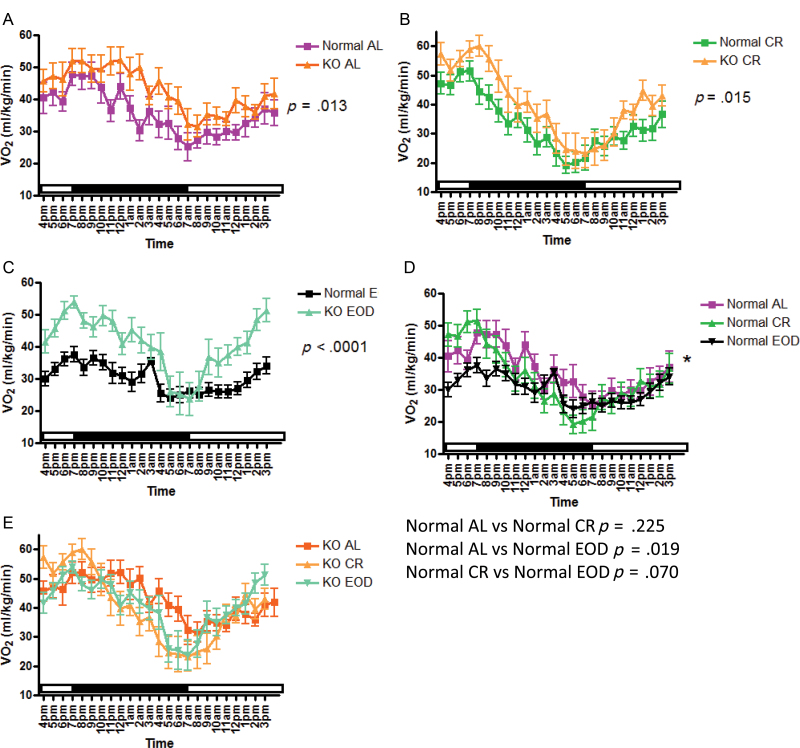
VO2 is the volume of oxygen consumed by an organism over a certain period of time and is expressed as mL/kg/min. On the fed day of testing (Figure 3), VO2 was increased in GHRKO mice compared with normal mice in animals that were fed AL, CR, or EOD diet (Figure 3a–c). Normal CR mice showed no statistically significant difference in VO2 when compared with normal AL mice (Figure 3d); however, normal EOD mice showed decreased VO2 compared with normal AL mice (Figure 3d) and normal CR mice (Figure 3d). GHRKO mice that had been on CR or EOD diets showed no statistically significant differences in VO2 when compared with GHRKO AL mice, or each other (Figure 3e). On the fasted day of testing (Figure 4), VO2 was increased in GHRKO mice compared with normal mice, whether fed AL, CR, or EOD diet (Figure 4a–c) similar to findings on the fed day. Under fasted conditions, normal CR mice showed no significant differences in VO2 from normal AL mice (Figure 4d); however, normal EOD mice had significantly lower VO2 than normal AL mice (Figure 4d). The differences in VO2 between normal CR mice and normal EOD mice seen on the fed day were eliminated on the fasted day of testing (Figure 4d). Similar to the fed day, GHRKO mice showed no significant alterations in VO2 on any diet on the fasted day (Figure 4e). Importantly, differences between normal AL and GHRKO AL mice remained significant when VO2 was expressed per percent lean mass (fat-free mass − bone weight) derived from our previously published dual-energy x-ray absorptiometry measurements of body composition of mice of the same age, sex, and background as used in this experiment (Supplementary Figure 1a). These data show that although body fat percentage is increased, lean tissue percentage reported as fat-free mass minus bone tissue is 32% in GHRKO mice and 36% in controls (11).
Figure 3.
Oxygen consumption (VO2; mL/kg/min) on fed day of indirect calorimetry plotted at 1-hour intervals of growth hormone receptor knockout (GHRKO) and normal AL (A) caloric restriction (CR)- (B) and every other day (EOD) (C) -fed mice and inter-genotypic comparison of dietary effects in normal (D) and GHRKO (E) mice.
Figure 4.
Oxygen consumption (VO2; mL/kg/min) on fasted day of indirect calorimetry plotted at 1-hour intervals of growth hormone receptor knockout (GHRKO) and normal AL (A) caloric restriction (CR)- (B) and every other day (EOD) (C) -fed mice and inter-genotypic comparison of dietary effects in normal (D) and GHRKO (E) mice.
Respiratory Quotient
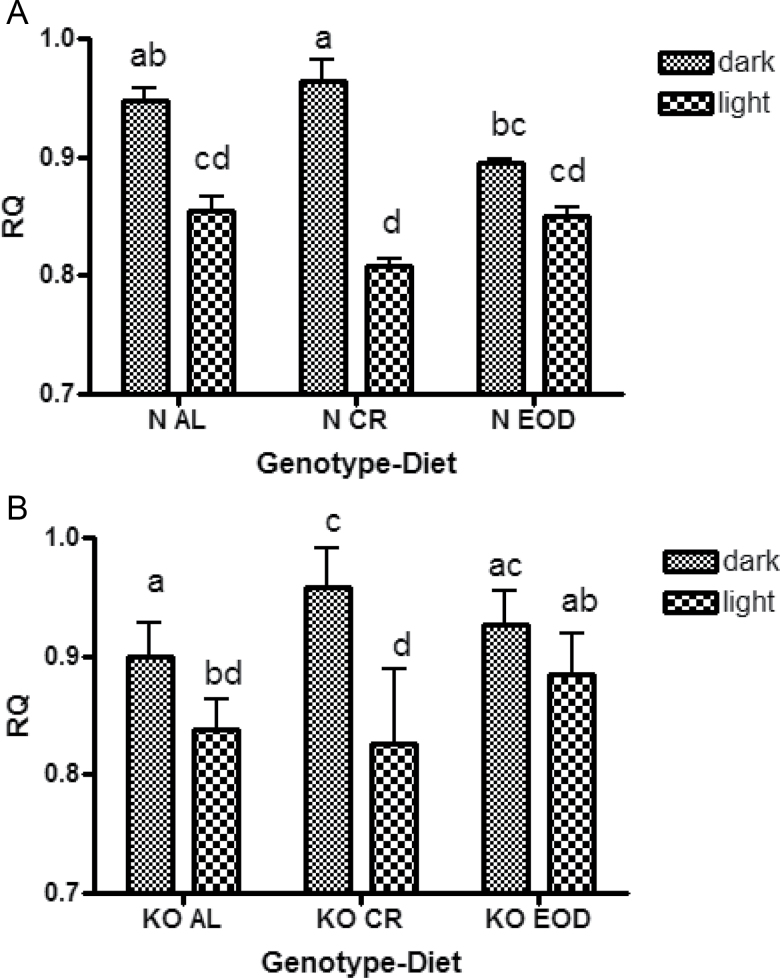
RQ is the ratio comparing the volume of carbon dioxide an animal produces over time (VCO2) to VO2 (RQ = VCO2/VO2). This dimensionless ratio gives an estimate of the type of fuel substrate being oxidized for an animal’s energy needs at a given time. This ratio is useful for monitoring an animal’s transition between lipid (RQ = 0.7) and carbohydrate (RQ = 1.0) oxidation. Under fed conditions, all mice showed the expected diurnal fluctuation in RQ. This pattern was exemplified by an increased tendency to oxidize carbohydrate during the dark period, when these nocturnal animals are active and feeding, and increased lipid oxidation during the light period when they are sleeping and consume little food (Figure 5). At old age, GHRKO mice had significantly lower RQ on AL diet (p = .0002) indicating that increased fat metabolism is a trait that persists through life span in these long-lived mice. In normal mice, daily RQ was altered in different ways by CR and EOD diet. In response to CR, these animals had increased RQ during the dark period on the fed day compared with normal EOD mice (Figure 5a). Normal and GHRKO EOD mice uniquely did not show a significant difference in RQ when comparing the dark period to the light period of the fed day (Figure 5a and b). The RQ of GHRKO mice was also differentially altered by CR or EOD diet when measured. Under fed conditions, GHRKO CR mice had increased RQ compared with GHRKO AL mice during the dark period (Figure 5b), whereas during the light period, GHRKO CR mice had significantly decreased RQ compared with GHRKO EOD mice (Figure 5b). On the fasted day, RQ values were reduced in normal and GHRKO mice on all diets (Supplementary Figure 3a and b). CR caused a severe decrease in the RQ of normal and GHRKO mice on the fasted day abrogating the difference between the dark and light period of testing (Supplementary Figure 3a and b).
Figure 5.
Respiratory quotient (RQ) values plotted as 12-hour averages representing either dark or light periods of indirect calorimetry of normal AL caloric restriction (CR) and every other day (EOD) mice (A) and growth hormone receptor knockout AL CR and EOD mice (B) during the fed day of indirect calorimetry. a–d—values that do not share the same letter in the superscript are statistically significant (p < .05).
Minimum and Range of VO2
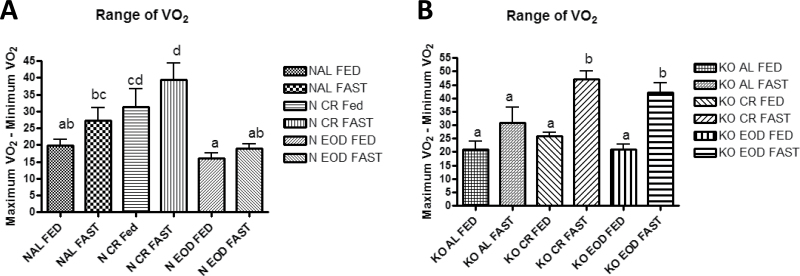
Interesting interactions between genotype and diet can be seen when comparing both the minimum VO2 and the range of VO2 (maximum VO2 − minimum VO2) throughout the day. Normal CR mice had increased range of VO2 compared with normal AL- and EOD-fed mice on both the fed and fasted days of testing (Figure 6a), whereas both GHRKO CR- and EOD-fed mice had increased range on the fasted day only (Figure 6b). Interestingly, GHRKO EOD-fed mice had increased range of VO2 compared with normal EOD-fed mice on the fasted day only (p < .0001).
Figure 6.
Range of oxygen consumption (VO2 [maximum VO2 − minimum VO2]) for normal mice (A) and growth hormone receptor knockout (B) mice. a–d—values that do not share the same letter in the superscript are statistically significant (p < .05).
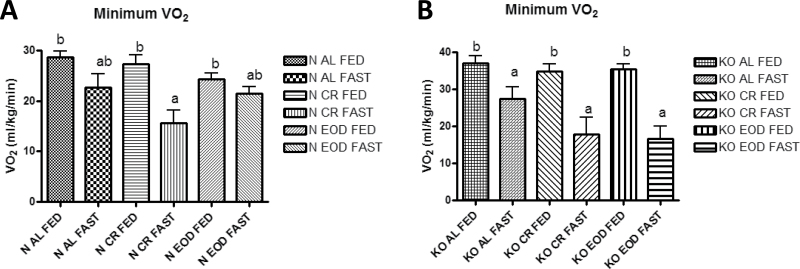
GHRKO mice on all diets had significantly increased minimum VO2 compared with their respective normal control mice on the fed day (p ≤ .043) but not on the fasted day. Of the normal mice, only normal CR mice had significantly decreased minimum VO2 on the fasted day compared with the fed day (Figure 7a), whereas GHRKO mice on all diets have decreased minimum VO2 on the fasted day (Figure 7b).
Figure 7.
Minimum oxygen consumption (VO2) for normal (A) and growth hormone receptor knockout (B) mice.
Discussion
Studies examining the interaction of longevity-conferring genes with longevity-conferring diets provide opportunities to identify candidate mechanisms by which increased life span is achieved. CR and EOD diets increase insulin sensitivity and longevity in normal mice yet fail to do so in already insulin-sensitive and long-lived GHRKO mice. Here, we have examined metabolic changes that accompany interactions between genotype and diet, as well as directly compared genotypic and dietary differences utilizing indirect calorimetry.
There are several key novel findings regarding VO2 from this study. First, the increased VO2 and decreased RQ displayed by young adult male (6–12 months old) and female (17 months old) GHRKO mice (9,12,13) are conserved in old-aged male GHRKO mice. Further, GHRKO mice display increased VO2 under all dietary conditions examined in this study, and on both fed and fasted days of indirect calorimetry testing whether expressed per body mass, or lean mass (Supplementary Figure 1) derived from our previously published dual-energy x-ray absorptiometry measurements of body composition of age- and sex-matched GHRKO mice, indicating that this metabolic difference is a consistent characteristic of these mice under a variety of conditions thus measured. When traditional metabolic scaling exponents (M2/3 and M3/4) (14,15) are applied to VO2 comparisons between GHRKO and normal control mice, the differences seen are eliminated (Supplementary Figure 2). However, measured values of functional metabolic body mass (11,16) show that these allometric scaling exponents err in opposite ways for mutant and normal mice (overestimating metabolic body weight in GHRKO mice and underestimating metabolic body weight in normal animals), making these scaling exponents inappropriate for comparisons dealing with GHRKO mice. Further, the increases seen when expressing VO2 per percent lean mass are of similar magnitude as those observed when expressing VO2 per gram, indicating that in comparisons of GHRKO to normal mice the isometric analysis more accurately corresponds to metabolic rate per functional metabolic mass than the allometric scaling exponent estimates. The evidence that long-lived GHRKO mice have increased VO2 and increased life span goes against the “rate of living” theory of aging (17), which predicts that increased metabolism leads to an increased rate of aging. Because roughly 90% of VO2 is mitochondrial (18), the increase in VO2 in GHRKO is a direct reflection of mitochondrial activity. As mitochondrial activity is increased, harmful by-products of mitochondrial oxidation called reactive oxygen species are also increased. This was thought to be a mechanistic link between the rate of aging and metabolic rate called the “free radical” theory of aging (19). However, GHRKO mice have increased metabolic rate while experiencing a decreased rate of aging. One theory which may explain this phenomenon, termed “mitohormesis,” is the idea that increased mitochondrial usage results in increased reactive oxygen species production within the mitochondria causing an adaptive response, which subsequently increases stress resistance. This heightened resistance is assumed to ultimately cause a long-term reduction of oxidative stress (20,21).
Interestingly, old GHRKO mice on either dietary regimen did not display a significant decrease in body weight indicating that the body composition of these mice remained similar to controls. In fact, autopsy findings and studies of body composition previously conducted confirm that GHRKO CR mice are not depleted of adipose tissue (11). This would suggest that the GHRKO mouse would not display a relative decrease in VO2 after normalization per lean mass under conditions of CR. The lack of a decrease in VO2 in GHRKO fed either CR or EOD in this study implies that the ability of GHRKO mice to increase metabolic efficiency (13) is pushed even further by the decrease in overall calories because locomotor activity of GHRKO mice on CR is not decreased (RM Westbrook, PhD, unpublished data, 2010).
The second key finding regarding VO2 is that normal EOD-fed mice displayed significantly decreased VO2 compared with normal AL and normal CR mice on the fed day, and compared with normal AL mice on the fasted day of indirect calorimetry, whereas the EOD diet failed to significantly alter VO2 in GHRKO mice. This decrease is likely to remain significant when VO2 is expressed per lean body mass as there is only a minute difference in body weight between EOD- and AL-fed mice. Because locomotor activity is likely unchanged, or increased as observed in some studies (22) by EOD diet, the decrease in VO2 seems to exemplify an alternate metabolic strategy of EOD-fed mice to decrease metabolic rate in response to reduced caloric intake in order to maintain homeostatic control of body composition. This striking difference in metabolism is noteworthy as the search continues for the precise mechanisms mediating the beneficial effects of CR and EOD diets. These findings raise the hypothesis that EOD diet may cause increased longevity through a mechanism predicted by the “rate of living,” and “free radical” theories of aging (17,19), whereas CR may act through a different mechanism. Recent studies have shown differing responses to insulin in rat livers (23) and differing levels of life span extension in the rotifer Brachionus manjavacas (24) from CR and EOD indicating the impact of these diets on metabolism and life span is incompletely understood and warrants further study.
Modest 30% CR throughout life span did not significantly alter the VO2 expressed per gram body weight of normal or GHRKO mice when measured in old age. This result agrees with studies showing that CR causes a transient short-term decrease in metabolic rate in rats (25), but metabolic rate returns to normal indicating that decreased metabolic rate is not necessary for the life-prolonging benefits of CR (25). Because locomotor activity is not decreased by CR (26), it is probable that after the transient decrease in metabolism in response to CR, an increase in metabolic efficiency occurs to compensate for the caloric deficiency while metabolic rate is maintained (27). This increase in metabolic efficiency is supported by data showing that CR can increase mitochondrial proliferation and density while decreasing mitochondrial membrane potential and VO2 of individual mitochondria. At the same time, adenosine triphosphate production is maintained and damaging free radical production is decreased in vitro (28). CR has also been shown to increase mitochondrial biogenesis, adenosine triphosphate production, and VO2 in vivo (29).
However, there is a reasonable possibility that this conclusion would require modification if body composition data for these mice were available in order to normalize the VO2 data per gram lean mass. Despite this limitation, we can deduce that the probable cause for the observed reduction in overall body weight in normal mice on 70% CR (Figure 1) is presumably due to a loss of body fat mass, as body fat reduction is a hallmark of CR (30). Therefore, the percentage of lean body mass is likely increased in normal mice on CR compared with normal. Because a larger divisor would bring the VO2 value down relative to normal AL controls, expressing the VO2 results obtained for normal CR mice per gram lean mass may show that these mice do in fact experience a decrease in VO2 compared with normal controls as some studies have shown (31). This would suggest that in addition to increasing metabolic efficiency, normal CR mice decrease metabolic rate to deal with lower caloric intake. This supports theories linking caloric restriction to a “metabolic reprogramming” that may influence physiological effects at the cellular, tissue, and organismal level to affect the rate of aging (32).
The third novel set of findings regarding VO2 come from the comparison of both the minimum VO2 and the range of VO2 from GHRKO and normal mice on the three diets. In normal mice, CR increases both the range of VO2 and the difference in minimum VO2 between fed and fasted states, whereas EOD diet was associated with a relatively static VO2 pattern under fed and fasted states. This indicates that both dynamic and relatively steady metabolic rates are both associated with increased life span in normal mice. GHRKO mice had significantly increased range of VO2 on CR or EOD only when fasted, indicating that this increase in range may be a response to critically low energy. Interestingly, CR caused increases in the range of VO2 on the fed day in normal mice, whereas no diet significantly altered the range of VO2 of GHRKO mice under fed conditions. Together, this is further evidence that diets, which increase life span, cause major metabolic changes in normal mice under standard fed conditions (increased VO2 range in normal CR mice and decreased VO2 in normal EOD mice), but do not induce such changes in GHRKO mice and similarly do not increase life span. The observation that both dynamic and relatively steady metabolic rates are both associated with increased life span in normal mice offers a new insight into the role of metabolism in life span and raises questions regarding the importance of metabolic rate in longevity. Whether metabolic rate is a causal agent in the influence of dietary intake on life span has yet to be determined and warrants further study.
The RQ measurements produced a pair of novel findings in this study. The first is evidence that the reduced RQ found in younger GHRKO mice (9) is also present at older age. Decreased RQ is indicative of increased fatty acid oxidation and has been associated with delayed aging, protection from obesity and diabetes, and increased longevity (9,33–35). The second is that the EOD dietary regimen caused an abrogation of a significant diurnal fluctuation in RQ in both normal and GHRKO mice on the fed day of testing, which returned to normal resembling RQ of mice on AL and CR on the fasted day. The loss of a significant difference in fuel selection from dark to light possibly shows that the normal feeding pattern in which most calories are consumed at night is altered to a more frequent meal pattern. Whether this has any impact on insulin sensitivity or life span would warrant further study.
One important difference between normal mice fed either CR or EOD diet is the impact of these diets on body weight throughout life span. Similar to findings from previous experiments, in this study, the old-aged normal mice on CR had a numeric reduction in body weight (7), whereas the reduction in body weight displayed by younger EOD-fed mice disappears in old age (8). This is especially interesting because the amount of food consumed when normalized per gram body weight was not significantly altered in any dietary group from AL. It seems that the access to AL food supply for 50% of the time allows EOD-fed mice the luxury of laying fat, whereas CR mice never get enough extra calories to successfully add body fat. Further, EOD-fed mice drastically reduce VO2 in order to defend body weight, whereas for CR mice, additional homeostatic control by reducing VO2 is not necessary.
Another notable finding from this study is concordance of the VO2 data with longevity data previously collected from research examining the same genotypic and dietary interactions studied here. Just as neither CR (7) nor EOD (8) diets extended life span in GHRKO mice, neither diet significantly altered VO2 of GHRKO mice. Further, normal mice experienced an increase in life span from CR and EOD diet and a significant reduction in VO2 expressed per body mass from EOD diet, and a projected decrease in VO2 expressed per fat-free mass from EOD and CR diets. From these results, we can conclude that normal mice decrease metabolism to match calorie deficit, whereas GHRKO mice increase metabolic efficiency to deal with caloric deficits. Why the absence of GH signaling and the subsequent reduction in insulin-like growth factor-1 causes this alternate response and whether this has any impact on longevity are questions calling for further study.
Indirect calorimetry is a useful tool for noninvasively assessing metabolic functions, which can relate to longevity. As more information is gathered regarding the metabolic phenotypes of longevity, indirect calorimetry can be used more reliably as a predictive correlate measure for interventions purported to improve health. This can be applied to preliminary studies on suspected long-lived mutants, or studies involving longevity-engendering compounds such as CR mimetics.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by grants from the National Institute on Aging (AG019899 and U19AG023122), the Longevity Consortium, and the Ellison Medical Foundation.
Acknowledgments
Contributions of numerous colleagues, students, and lab members to the progress of our work are gratefully acknowledged. The authors would like to thank Steve Sandstrom for editorial assistance.
References
- 1. Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146 (9):3718–3723 [DOI] [PubMed] [Google Scholar]
- 2. Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37 (1):47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55 (1):69–87 [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17 (3):313–321 [DOI] [PubMed] [Google Scholar]
- 6. Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100 (10):6216–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009;8:756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64 (4):443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21 (3):188–195 [DOI] [PubMed] [Google Scholar]
- 11. Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61:562–567 [DOI] [PubMed] [Google Scholar]
- 12. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147 (6):2801–2808 [DOI] [PubMed] [Google Scholar]
- 13. Longo KA, Berryman DE, Kelder B, et al. Daily energy balance in growth hormone receptor/binding protein (GHR -/-) gene-disrupted mice is achieved through an increase in dark-phase energy efficiency. Growth Horm IGF Res. 2010;20 (1):73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubner M. Ueber den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Zeitschrift für Biologie. 1883;19:535–562 [Google Scholar]
- 15. Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–541 [DOI] [PubMed] [Google Scholar]
- 16. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14 (4):309–318 [DOI] [PubMed] [Google Scholar]
- 17. Pearl R. The Rate of Living. London, UK: University of London Press; 1928 [Google Scholar]
- 18. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77 (3):731–758 [DOI] [PubMed] [Google Scholar]
- 19. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11 (3):298–300 [DOI] [PubMed] [Google Scholar]
- 20. Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45 (6):410–418 [DOI] [PubMed] [Google Scholar]
- 21. Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45 (3):173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X, Mughal MR, Scott Hall F, et al. Dietary restriction mitigates cocaine-induced alterations of olfactory bulb cellular plasticity and gene expression, and behavior. J Neurochem. 2010;114 (1):323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donati A, Recchia G, Cavallini G, Bergamini E. Effect of aging and anti-aging caloric restriction on the endocrine regulation of rat liver autophagy. J Gerontol A Biol Sci Med Sci. 2008;63 (6):550–555 [DOI] [PubMed] [Google Scholar]
- 24. Gribble KE, Welch DB. Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera). J Gerontol A Biol Sci Med Sci. 2013;68:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCarter RJ, McGee JR. Transient reduction of metabolic rate by food restriction. Am J Physiol. 1989;257(2 Pt 1):E175–E179 [DOI] [PubMed] [Google Scholar]
- 26. Holloszy JO. Exercise and food restriction in rats. J Nutr. 1992;122(suppl 3):774–777 [DOI] [PubMed] [Google Scholar]
- 27. Summermatter S, Mainieri D, Russell AP, et al. Thrifty metabolism that favors fat storage after caloric restriction: a role for skeletal muscle phosphatidylinositol-3-kinase activity and AMP-activated protein kinase. FASEB J. 2008;22 (3):774–785 [DOI] [PubMed] [Google Scholar]
- 28. López-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103 (6):1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310 (5746):314–317 [DOI] [PubMed] [Google Scholar]
- 30. Barzilai N, Gabriely I. The role of fat depletion in the biological benefits of caloric restriction. J Nutr. 2001;131 (3):903S–906S [DOI] [PubMed] [Google Scholar]
- 31. Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128 (10):539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snitker S, Tataranni PA, Ravussin E. Respiratory quotient is inversely associated with muscle sympathetic nerve activity. J Clin Endocrinol Metab. 1998;83 (11):3977–3979 [DOI] [PubMed] [Google Scholar]
- 34. Kuhla A, Blei T, Jaster R, Vollmar B. Aging is associated with a shift of fatty metabolism toward lipogenesis. J Gerontol A Biol Sci Med Sci. 2011;66 (11):1192–1200 [DOI] [PubMed] [Google Scholar]
- 35. Haramizu S, Ota N, Hase T, Murase T. Aging-associated changes in physical performance and energy metabolism in the senescence-accelerated mouse. J Gerontol A Biol Sci Med Sci. 2011;66 (6):646–655 [DOI] [PubMed] [Google Scholar]