Abstract
Background
Stunting is prevalent by the age of 6 mo in the indigenous population of the Western Highlands of Guatemala.
Aim
The objective of this study was to determine the time course and predictors of linear growth failure and weight-for-age in early infancy.
Study Design and Subjects
One hundred and forty eight term newborns had measurements of length and weight in their homes, repeated at 3 and 6 mo. Maternal measurements were also obtained.
Results
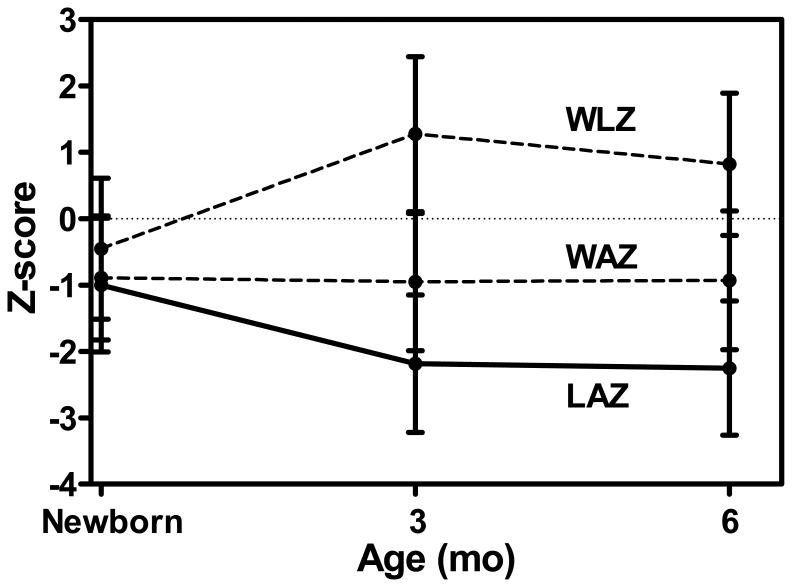
Mean ± SD length-for-age Z-score (LAZ) declined from newborn -1.0 (1.01) to -2.20 (1.05) and -2.26 (1.01) at 3 and 6 mo respectively. Stunting rates for newborn, 3 and 6 mo were 47%, 53% and 56% respectively. A multiple regression model (R2 = 0.64) demonstrated that the major predictor of LAZ at 3 mo was newborn LAZ with the other predictors being newborn weight-for-age Z-score (WAZ), gender and maternal education*maternal age interaction. Because WAZ remained essentially constant and LAZ declined during the same period, weight-for-length Z-score (WLZ) increased from -0.44 to +1.28 from birth to 3 mo. The more severe the linear growth failure, the greater WAZ was in proportion to the LAZ.
Conclusion
The primary conclusion is that impaired fetal linear growth is the major predictor of early infant linear growth failure indicating that prevention needs to start with maternal interventions.
Introduction
The association between impaired linear growth and enhanced risks of morbidity and mortality in infants and children under the age of five years from resource-poor communities has been well established (1-4). Survivors are at risk for impaired brain function, loss of economic productivity, and greater reproductive risks to adult women and their offspring (5). The community as a whole suffers from loss of human capital (5). Except in circumstances of acute under-nutrition, inadequate growth is typically manifested by linear growth failure with low length-for-age Z-scores (LAZ). According to World Health Organization (WHO) data (6), linear growth failure in low resource communities typically starts in early to mid-infancy with progressive deterioration in linear growth rates reaching a plateau at a low level at approximately two years of age. Though considered to result primarily from under-nutrition, further aggravated by infection, infant-toddler nutrition interventions designed to reverse this progressive linear growth failure have had only limited success (3) in what is regarded as the “window of opportunity” to prevent or/and treat growth failure.
Our own recent experience with the indigenous post-Mayan population in Chimaltenango in the Western Highlands of Guatemala has documented a very high incidence of stunting, approximately 60%, by as early as 6 mo of age (7, 8). The precise timing of the early growth failure and its predictors has not been explored.
Guatemala, as is typical of resource poor countries in transition, is faced with the dual challenges of growth failure and a rapidly increasing prevalence of overweight (9). There is increasing evidence that excessive weight gain in infancy is a predictor of overweight in later childhood (10).
The objectives of this project were: [1] to characterize the linear growth of the indigenous population in Chimaltenango from the newborn to 6 mo of postnatal age; [2] to identify the predictors of linear growth failure in early infancy; [3] to determine the relationship between weight-for-age Z-score (WAZ) and low LAZ in early infancy.
Experimental Design and Methods
Study Design
This was an observational longitudinal anthropometric study of newborns and infants from birth until 6 mo of age in a population known to have linear growth retardation by the age of 6 mo (7). Maternal, paternal, family, home and infant feeding data were also collected. Basic cross-sectional statistics were calculated and LAZ at 3 and 6 mo were modeled as functions of the measured variables using multiple linear regression analysis.
Participants
Participants were identified through the Eunice Kennedy Shriver National Institute of Child Health and Human Development Global Network Maternal and Newborn Health Registry. They were consecutive deliveries from four control clusters for the series of research projects being undertaken by this Global Network site in the Department of Chimaltenango in the Western Highlands of Guatemala. The population was indigenous, primarily post-Mayan, with a long history of deprivation and poverty. The participants were primarily resident in small rural towns, with men typically working on subsistence farms but progressively changing to laborers on larger farms or to other low to low-medium income jobs. Maize remains the dominant crop and food staple. Homes generally have a primitive electrical supply, television but no refrigerator. The water supply is variable; the majority having a running water supply of variable consistency and purity. Typically, wood fires are still used in part or whole for cooking, either in a kitchen or sometimes in the same room as the sleeping quarters. The majority of deliveries were in the home, most frequently attended by a local traditional birth attendant. All final participants were apparently healthy term or near-term neonates, based on history of last menstrual period (LMP) obtained through the Registry records. They had no acute illnesses during the six month study and 82% of participants were exclusively breast fed at 3 mo according to maternal history. Approval for this study was provided by the Comite de Etica Independente and by the University of Colorado Multiple Institutional Review Board.
Anthropometry
Crown-heel and crown-rump lengths were obtained in the recumbent position per World Health Organization (WHO) guidelines (11) by a specially trained pair of the research team. Two measurements were taken using a calibrated Harpenden infantometer (Crymych, Pembs, UK) and recorded to the nearest 0.1 cm. A third measurement was made if the difference was >0.4 cm and the mean of the two closest measurements calculated. Duplicate newborn and infant naked weights were obtained with calibrated Salter scales and recorded to the nearest 4 g. Newborn measurements were obtained as soon after birth as possible. Infant measurements were made at the age of 3 and 6 mo. All measurements were made in the home.
Maternal height and weight were measured at 6 mo postpartum in the local health clinic. Barefoot height was obtained using a calibrated mechanical column scale with a measuring rod at the project headquarters and recorded to the nearest 0.5 cm. Maternal weight was obtained from a calibrated digital scale and recorded to the nearest 0.2 kg.
Data management and analyses
Data were recorded by two of the authors (JBB & SCD) in the participants' homes with pencil/paper and entered into an electronic excel database after verification of data accuracy. These data included socio-economic status assessed using World Bank guidelines (12)(Table 1); maternal and parental education and work; parity; obstetric history; exclusive breast feeding at 3 mo, infant and child feeding index at 6 mo; use of iron/folate supplements (IFS) or mineral/vitamin supplements, and other demographic information. Initial processing included final cleaning and conversion of infant anthropometric data to Z-scores (WHO Anthro, version 3.2.2, World Health Organization). Cross-sectional statistical measures including correlation were calculated for all the variables of interest, notably LAZ, WAZ and WLZ. These statistics and plots of selected data were used examine longitudinal characteristics and inter-variable relationships.
Table 1. Data used in computing SES score.
| Electricity, Y/N |
| Radio, Y/N) |
| Television, Y/N |
| Refrigerator, Y/N |
| Telephone, Y/N |
| Work own or family's land, Y/N |
| Principal source of drinking water |
| Principal type of toilet facility |
| Natural or man-made material used for floors in home |
| Natural or man-made material used for walls in home |
| Natural or man-made material used for roof of home |
| Type of home tenancy |
| Number of people for each sleeping room in home |
Multiple linear regression analysis was used to model LAZ at 3 and 6 mo as functions of 23 quantitative and categorical variables (Table 2). Stepwise and best subsets model selection methods were used in multiple phases of the analyses to determine the best candidates for the final models. After elimination of the explanatory variables for which there was no evidence of a relationship to the response variables, all two-way interactions between the remaining explanatory variables were added to the models being investigated. Final model selections were performed manually taking into consideration the statistical significance of the parameter estimates, Akaike Information Criterion (AIC) values, and model parsimony. Throughout the analyses regression residuals were examined to check for deviations from regression assumptions. Multicollinearity of variables was assessed using variance inflation factors (VIF) and apparent outliers were evaluated.
Table 2. Measured variables that were examined as potential explanatory variables in multiple regression analysis.
| Variable | Mean | Median | STD | Range |
|---|---|---|---|---|
| Newborn LAZ | -1.00 | -0.93 | 1.01 | -3.7 to 1.5 |
| Newborn WAZ | -0.88 | -0.79 | 0.94 | -3.8 to 1.4 |
| Newborn head circumference z-score | -0.53 | -0.48 | 1.16 | -3.8 to 2.7 |
| Maternal height, cm | 145 | 144 | 4.5 | 135 to 155 |
| Maternal BMI, kg/m2 | 26.7 | 26.4 | 3.7 | 18 to 39 |
| Maternal age, y | 27.5 | 27 | 6.8 | 15 to 46 |
| Maternal education, y | 4.1 | 3 | 3.3 | 0 to 12 |
| Paternal education, y | 4.7 | 5 | 3.4 | 0 to 12 |
| Socio-economic status index(12) | 0.53 | 0.59 | 0.35 | -0.53 to 1.29 |
| Food diversity index at 6 mo(50, 51) | 3.9 | 2 | 2.4 | 1 to 9 |
| Number of pregnancies | 3.9 | 3 | 2.8 | 1 to 12 |
| Number of living children | 3.5 | 3 | 2.4 | 1 to 11 |
| Number of prenatal visits | 7.3 | 7 | 3.6 | 0 to 20 |
| Distribution | ||||
| Gender, M/F | 80/68 | |||
| Exclusively breastfed at 3 mo, Y/N | 123/25 | |||
| Mother working, Y/N | 66/81 | |||
| Supplement use during pregnancy, Y/N | 124/23 | |||
| Multivitamins, Y/N | 46/101 | |||
| Iron, Y/N | 95/52 | |||
| Folic acid, Y/N | 92/55 | |||
| Pregnancy/Delivery complication, Y/N | 37/105 | |||
| Type of delivery, C-section/vaginal | 16/130 | |||
| Location of delivery, hospital/home | 36/89 | |||
Because missing data entailed the use of subsets of the complete dataset for certain analyses, a sensitivity analysis, using t-tests, ANOVA and chi-squared tests, was performed to determine whether the datasets were statistically similar.
Regression analyses were accomplished using the R statistical computing language, version 2.14 (13). Other statistical analyses were performed with GraphPad Prism (version 5, GraphPad Software, Inc. San Diego, CA) and Microsoft Excel (version 2003). Data are presented as mean ± SD unless otherwise noted. The level of significance used was 0.05.
Results
Two hundred apparently healthy newborns (108 males, 92 females) were enrolled in this project. Some did not participate through the end of the study; the total numbers of participants were 200 at birth, 176 at 3 mo and 157 at 6 mo. Six participants who completed all measurements, but were born prematurely (gestational age < 37 wk from the last menstrual period reported by mother), were excluded. In addition, three participants whose data were anomalous and could not be verified were excluded. Complete longitudinal data are reported here for the remaining 148 participants. Seventeen (11%) of these 148 term infants met criteria for intrauterine growth retardation (IUGR) with birth weights ranging from 1900g to 2500g.
Missing data for delivery location, an interaction variable in the final model of LAZ at 6 mo, caused that analysis to be restricted to 125 participants. T-tests or ANOVA were used to compare the means of all quantitative variables for the complete data and the subsets of 148 and 125 participants. Chi-squared tests were used to test whether the distributions of values of categorical variables and quantitative variables taking limited values were independent of the datasets. None of the variables were found to differ across datasets; the P-values for all tests were >0.6. Where t-tests or ANOVA were used, tests for equal variances produced P-values >0.1.
Newborn measurements were obtained at 5.6 ± 2.7 d after delivery. Mean ± SD length and weight for newborn and for infants at 3 and 6 mo of age are summarized in Table 3 for separate and combined sexes. The ratio of crown-rump to crown-heal length was 0.650, i.e. close to the normal term mean of 0.673 ± 0.030 (14). This ratio did not vary with birth weight (14). The mean LAZ at the three time points for combined sexes were -1.00 ± 1.01, -2.20 ± 1.05 and -2.26 ± 1.01. Forty-seven percent of newborns, 53% of infants at 3 mo, and 56% at 6 mo of age were stunted (defined as LAZ <-1 for newborn and <-2 for 3 and 6 mo). Corresponding values for severe stunting (LAZ < -3) at 3 and 6 mo were 24% and 23%, respectively. The mean LAZ for the males was always lower than the LAZ of the females, but approached significance only at 3 mo (P = 0.05) whereas the means for the males and females were -2.36 (0.97) and -2.02 (1.13), respectively.
Table 3. Anthropometry at birth, 3 and 6 mo of age1.
| Crown-Rump Length cm | Length cm | Weight g | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Newborn | Newborn | 3 mo | 6 mo | Newborn | 3 mo | 6 mo | |
| Females (n = 66) | 31.2 ± 1.52 | 48.3 ± 2.0 | 55.6 ± 2.42 | 61.0 ± 2.32 | 2929 ± 393 | 5308 ± 6902 | 6705 ± 7772 |
| Males (n = 80) | 31.9 ± 1.9 | 48.9± 1.9 | 56.7 ± 2.0 | 62.6± 2.0 | 3038 ± 448 | 5666 ± 718 | 7103 ± 862 |
| Combined | 31.6 ± 1.8 | 48.6 ± 1.9 | 56.2 ± 2.3 | 61.9 ± 2.3 | 2988 ± 426 | 5502 ± 725 | 6920 ± 845 |
Mean ± SD;
Significant difference between females and males (unpaired t test, P < 0.02)
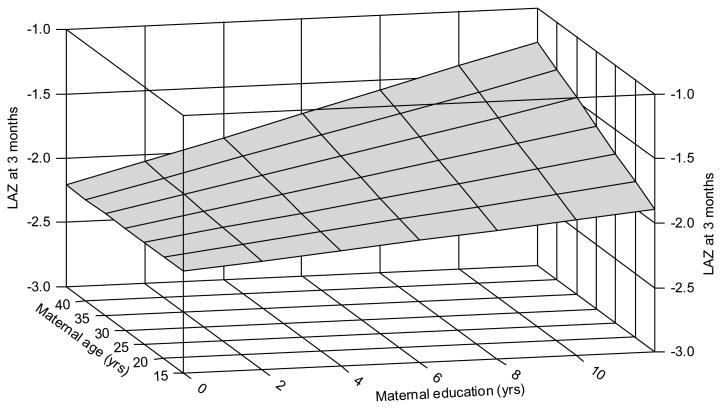
LAZ declined significantly between newborn and 3 mo of age (Figure 1). The correlation between newborn vs. 3 mo LAZ was r = +0.73 (P < 0.0001). It was concluded from the multiple regression analyses that the optimal descriptive model of LAZ at 3 mo was as a function of newborn LAZ, newborn WAZ, gender, and the interaction of years of maternal education and age (R2 = 0.64, Table 4). Regression parameters were significant (P < 0.007) and coefficients of partial determination showed that newborn LAZ was the strongest predictor of LAZ at 3 mo. All parameter estimates were positive indicating a positive relationship of all explanatory variables with LAZ at 3 mo. The interaction can be interpreted as showing that, the older the mother, the larger the increase in LAZ associated with maternal education (Figure 2). Parameter estimates were similar when the 17 infants with weight < 2500 g were excluded; the correlation between newborn and 3 mo LAZ data remaining significant (P < 0.0001) with r = 0.61.
Figure 1.
Mean (+SD) length-for-age (LAZ), weight-for-age (WAZ) and weight-for-length (WLZ) in newborn and infants at 3 and 6 mo of age.
Table 4. Model of LAZ at 3 mo.
| Explanatory variables | Parameter estimate | P-value | Coefficient of partial determination |
|---|---|---|---|
| LAZ (newborn) | 0.572 | <0.0001 | 0.27 |
| WAZ (newborn) | 0.284 | 0.0013 | 0.07 |
| Gender1 | 0.325 | 0.0024 | 0.06 |
| Maternal education *age | 0.0017 | 0.0066 | 0.05 |
| (intercept) | -1.69 | <0.0001 |
Indicator values: male = 0, female = 1
Figure 2.
A 3-dimensional plot of the relationship of the maternal education * maternal age interaction and LAZ at 3 mo predicted by the model. The plot shows that increased education was associated with higher LAZ at 3 mo and that this relationship was enhanced when the mothers were older. The model surface is calculated for females with average newborn LAZ and WAZ values. The upper corner of the surface (at high values of both education and age) extends beyond the range of the data as none of the older mothers had the highest levels of education.
Multiple regression analysis of LAZ at 6 mo showed it to be optimally modeled as a function of newborn LAZ, socio-economic status (SES), an interaction of SES and delivery location, and an interaction of SES and the use of iron/folate supplements (IFS) (R2 = 0.49,Table 5). Parameter estimates were significant (P < 0.017) and the coefficients of partial determination showed that, as with the 3 mo LAZ model, newborn LAZ was the strongest predictor of LAZ at 6 mo. SES was the second strongest predictor, with the interactions making lesser but significant contributions. The role of iron and folate in the SES*IFS interaction was modeled as iron or folate, but iron and folate produced similar results since 94% of the participants took both or neither. While newborn LAZ and SES exhibited positive associations with LAZ, both SES interactions had negative parameter estimates indicating that home delivery and/or the use of IFS lessened the influence of SES on LAZ at 6 mo. It is noted that other models also suggested a weaker association of LAZ with maternal height.
Table 5. Model of LAZ at 6 mo.
| Explanatory variables | Parameter estimate | P-value | Coefficient of partial determination |
|---|---|---|---|
| LAZ (newborn) | 0.577 | <0.0001 | 0.38 |
| SES | 1.112 | <0.0001 | 0.15 |
| SES * Delivery location1 | -0.569 | 0.0065 | 0.06 |
| SES * (folate or Fe supplement)2 | -0.502 | 0.017 | 0.05 |
| (intercept) | -1.87 | <0.0001 |
Indicator values: hospital = 0, home = 1
Indicator values: no supplement used = 0, supplement used = 1
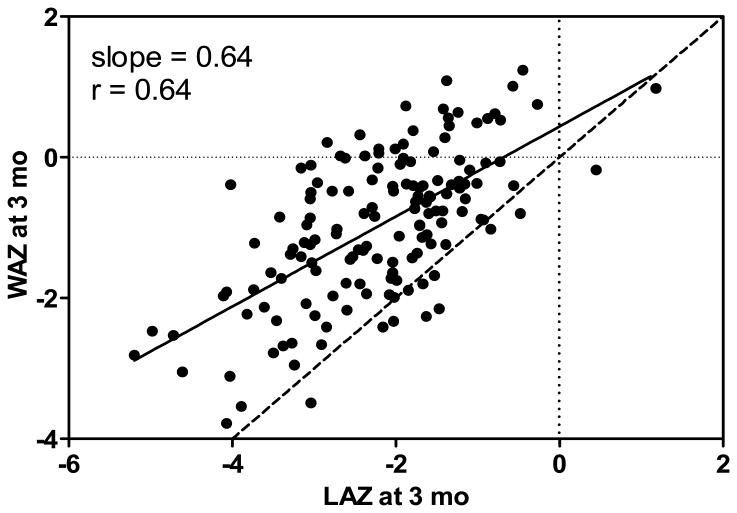
Mean WAZ at the same time points were -0.89, -0.97 and -0.94, respectively. Corresponding data for WLZ were -0.44, +1.28 and +0.82 (Figure 1). The slopes of the regression lines for WAZ vs. LAZ at 3 and 6 mo were 0.64 and 0.66 respectively (r = 0.64, P < 0.0001). A plot of the 3 mo data and regression line (Figure 3) makes evident that weight status was disproportionately high relative to length status and that this disproportionate relationship tended to be more pronounced at lower LAZ. At 3 and 6 mo, respectively, 30 and 14% of infants were overweight with WLZ ≥ 2.0.
Figure 3.
WAZ vs. LAZ at 3 mo showing the linear regression line (solid line) with slope of 0.64. Dotted lines depict zero Z-scores for WAZ and LAZ and the dashed line is the identity line. The divergence of the regression and identity lines as LAZ and WAZ decrease demonstrates that WAZ trends higher relative to LAZ (P < 0.0001).
The mean maternal height was 145 (4.5) cm and weight was 56.1 ± 9.0 kg. Maternal mean BMI was 26.7 ± 3.7; 63% were overweight (>25), including 16% who were obese (>30). Maternal height was significantly correlated with LAZ at all three measurement times (r = 0.21 (P = 0.010) at birth, r = 0.20 (P = 0.013) at 3 mo; r = 0.24 (P = 0.004) at 6 mo). Maternal height was not correlated with WLZ at any time. There was no correlation between maternal BMI and either LAZ or WLZ of offspring at any time.
Discussion
The immediate goal of the regression modeling was to determine which of the potential explanatory variables were most strongly associated with LAZ at 3 and 6 mo while controlling for the other variables. The resulting models should be viewed as descriptive models providing information on which variables appeared to have explanatory value and on the nature of their relationships with the response variables, and contributing to the larger goal of understanding some of the factors that affect infant growth
The most notable result of this study was the documentation of birth length, among multiple variables included in the analysis, being the outstanding predictor for LAZ both at 3 mo and 6 mo of age. Similar early linear growth retardation has previously been observed in Malawi, where small birth size was found to be the strongest predictor of severe stunting at 12 mo (15, 16). In both countries LAZ was approximately -1 in the newborn. This finding indicates that linear growth of the fetus or/and factors affecting fetal linear growth, at least in these populations, continue to have a prominent role in postnatal linear growth through infancy. Though linear growth of the fetus occurs throughout gestation (17), published evidence points to the special importance of the earlier months of pregnancy in determining linear growth at birth. Maternal weight gain, indicative of maternal nutritional status, during the first to the second trimester in poor indigenous communities in Eastern Guatemala is positively associated with both femur and tibial length at 17 and 30 wk gestation and with infant length at birth, while maternal weight gain from the second to third trimesters did not predict either fetal length or birth length (18). When the fetus is shorter than expected in the first trimester, there is increase in the risk of both preterm birth and intra-uterine growth retardation as documented in a recent study in the Netherlands (19). The epidemiologic evidence supporting the importance for birth size of maternal nutrition at conception is long-standing (20). The implications for early maternal environmental and especially nutrition interventions are apparent. Though human experimental data supporting the benefit to fetal growth of maternal nutrition interventions commencing well before conception are limited, (21) a strong argument can be made for studying the benefits of such interventions in order to ensure these interventions are instituted in time to improve the maternal nutrition environment to benefit the entire first trimester.
The cause of the high prevalence of early linear growth failure is likely multifactorial, but the impressive increase in height of school children from similar post-Mayan indigenous communities in the Yucatan (22) within one generation of immigration into the United States (23) provides evidence of the dominant contribution of the environment to the linear growth failure in the Guatemalan Central Highlands. Malnutrition is prominent among the environmental factors that affect linear growth adversely. Though, the current data emphasize the importance of maternal nutrition during pregnancy the quality of feeding, including the integrity and quality of exclusive breast feeding, during these early months also requires even more diligent attention. Eighty-two percent of the participants in this study were, exclusively breast fed for the first 3 mo by self-report. Even if this figure is not entirely accurate, there is nothing outstanding in either the nutrition, health or other identified environmental factors of the infant to readily account for the severity and early onset of the postnatal linear growth failure.
The relationship between impaired linear growth of the fetus and young infant in this population suggests a common cause which continues to arrest linear growth postnatally despite a very different environment after birth. Given the evidence discussed above, this relationship is likely to extend to the earliest stages of pregnancy. We speculate that this link is attributable at least in part to an abnormal maternal, placental and fetal epigenome secondary to an imperfect maternal environment, including nutrition, during and prior to pregnancy (24-28) and which presumably does not normalize abruptly at delivery even though the placenta is no longer a factor. This hypothesis is supported by the results of recent research in the Gambia (29, 30). The notable effects of experimental dietary changes prior to conception in non-human primates on the fetal epigenome and corresponding phenotype (26) support the argument for initiating nutrition intervention studies prior to conception.
Other documentation of early linear growth retardation includes data on nearly 6000 infants in Nigeria at the time of their first immunization (1-3 mo of age), which included 30.8% stunted infants. Intrauterine growth restriction was a significant contributor both to stunting, underweight, and wasting in these young infants (31). However, the extent of this early onset linear growth failure has not been evident from global surveys in which regions of the world are combined (32, 33). Other reports, notably from the long-term longitudinal Orient, 4 village study in Eastern Guatemala (34-36) have linked low LAZ at birth or early infancy with other longer term outcomes including subsequent neurodevelopment (37-41) or later body proportions (42, 43), but have either not provided the same longitudinal data on linear growth in early infancy or have not reported lengths before 3 mo of age.
Other reports from the Guatemalan 4-village study have focused on birth weight (44) or on weight gain in early infancy (45). Birth weights and abnormalities of birth weight are much more commonly reported than birth lengths though it is impairment of linear growth that is associated with long-term enhanced vulnerability to morbidity and mortality in poor communities. In more affluent countries, most IUGR newborns have catch-up growth in infancy (19). This may occur in some poor populations; for example in a study in Nigeria low birth weight was associated with the highest weight velocities between birth and the first postnatal visit (46). Most catch up from IUGR occurs in the first 6 mo in more affluent countries (47), a time that is beneficial for long-term growth and health. In New Zealand (47), linear growth remained retarded in 20% of infants which was predicted by shortness but not low weight at birth. This high percentage of catch up growth in early infancy contrasts with the low linear postnatal growth in the cohort reported here.
Though primary attention has been directed to linear growth, the postnatal weight gain of the participants was also of note. First the maintenance of an essentially unchanging WAZ concurrently with the rapid decline in LAZ indicated that the latter could not be attributed to a lack of adequate energy intake in these young breast fed infants. This difference in pattern between WAZ and LAZ resulted, inevitably, in a notable increase in WLZ in early infancy which remained high through 6 mo (Figure 1). The WAZ, as noted in the results, was increasingly disproportionally high in relation to LAZ the more negative the LAZ, both at 3 and 6 mo. Potential concern is aggravated by the clearly excessive weight gain resulting in nearly a quarter of these infants being overweight by 6 mo. The prevalence of overweight has been noted to be higher in children who are stunted both in Guatemala (48) and other sites (49). Excessive early infancy weight gain is a potential harbinger of obesity in children and adults (10).
Conclusion
The results of this observational study indicate a strong link between linear growth of the fetus and of the young infant. Understanding of the contribution of several possible factors that could contribute to this link requires further research. However, each of these factors appears likely to relate to the maternal environment and, it is hypothesized, to the maternal epigenome. Apart from the more extensively documented adverse associations of failed linear growth, the results of this study have added to the evidence for the enhanced risk of infant overweight associated with linear growth retardation.
Acknowledgments
Role of the Funding Source: The Global Network had scientific input into the study design, collection, analyses and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Supported by NIH Eunice Kennedy Shriver NICHD, NIH, Global Network for Women's and Children's Health Research U01 HD 40657; and Rotary, Adler, and Robinson-Durst Scholarships.
No online supplementary material (OSM) is available.
Author List for Indexing: Berngard, Berngard, Krebs, Garcés, Miller, Westcott, Wright, Kindem, Hambidge
Authorship: KMH, SCB, JBB, NFK, and JW participated in study design. JBB, SCB and AG were responsible for the field studies and data collection. MK was responsible for initial statistical input and LVM for statistical analyses. LLW has overall responsibility for the Maternal and Neonatal Registry of the NICHD Global Network for Women's and Children's Health Research used in the identification of participants and contributed to manuscript review. KMH, LVM and NFK were responsible for data interpretation and, together with JEW, for writing the paper. All authors have read and approved the final manuscript.
Conflict of Interest: None of the authors have any conflict of interest to report.
AIC (Akaike Information Criterion), IUGR (intra-uterine growth retardation), IFS (iron-folatesupplement), LMP (last menstrual period), LAZ (Length-for-Age Z-score), SES (socio-economicstatus), VIF (variance inflation factor), WAZ (Weight-for-Age Z-score), WLZ (Weight-for-LengthZ-score).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80(1):193–8. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361(9376):2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–40. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. The WHO Child Growth Standards. Geneva: World Health Organization; 2006. [Google Scholar]
- 7.Mazariegos M, Hambidge KM, Westcott JE, Solomons NW, Raboy V, Das A, et al. Neither a zinc supplement nor phytate-reduced maize nor their combination enhance growth of 6- to 12-month-old Guatemalan infants. J Nutr. 2010;140(5):1041–8. doi: 10.3945/jn.109.115154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambidge KM, Mazariegos M, Kindem M, Wright LL, Cristobal-Perez C, Juarez-Garcia L, et al. Infant stunting is associated with short maternal stature. J Pediatr Gastroenterol Nutr. 2012;54(1):117–9. doi: 10.1097/MPG.0b013e3182331748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder DG, Martorell R, Flores R. Infant and child growth and fatness and fat distribution in Guatemalan adults. Am J Epidemiol. 1999;149(2):177–85. doi: 10.1093/oxfordjournals.aje.a009784. [DOI] [PubMed] [Google Scholar]
- 10.Stettler N, Iotova V. Early growth patterns and long-term obesity risk. Curr Opin Clin Nutr Metab Care. 2010;13(3):294–9. doi: 10.1097/MCO.0b013e328337d7b9. [DOI] [PubMed] [Google Scholar]
- 11.Use and interpretation of anthropometric indicators of nutritional status. WHO Working Group. Bull World Health Organ. 1986;64(6):929–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Country reports on HNP and poverty. The World Bank; 2007. Socio-economic differences in health, nutrition, and population. [PubMed] [Google Scholar]
- 13.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 14.Merlob P, Sivan Y, Reisner SH. Ratio of crown-rump distance to total length in preterm and term infants. J Med Genetics. 1986;23(4):338–40. doi: 10.1136/jmg.23.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espo M, Kulmala T, Maleta K, Cullinan T, Salin ML, Ashorn P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002;91(12):1364–70. doi: 10.1111/j.1651-2227.2002.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 16.Maleta K, Virtanen SM, Espo M, Kulmala T, Ashorn P. Childhood malnutrition and its predictors in rural Malawi. Paediatr Perinatal Epidemiol. 2003;17(4):384–90. doi: 10.1046/j.1365-3016.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 17.Carrascosa A, Yeste D, Copil A, Audi L, Gusinye M, Vicens-Calvet E, et al. Fetal growth regulation and intrauterine growth retardation. JPEM. 2004;17(Suppl 3):435–43. [PubMed] [Google Scholar]
- 18.Neufeld LM, Haas JD, Grajeda R, Martorell R. Changes in maternal weight from the first to second trimester of pregnancy are associated with fetal growth and infant length at birth. Am J Clin Nutr. 2004;79(4):646–52. doi: 10.1093/ajcn/79.4.646. [DOI] [PubMed] [Google Scholar]
- 19.Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, Jaddoe VW. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA. 2010;303(6):527–34. doi: 10.1001/jama.2010.78. [DOI] [PubMed] [Google Scholar]
- 20.Smith GC, Smith MF, McNay MB, Fleming JE. First-trimester growth and the risk of low birth weight. NEJM. 1998;339(25):1817–22. doi: 10.1056/NEJM199812173392504. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen KM, Habicht JP. Maternal supplementation differentially affects the mother and newborn. J Nutr. 2010;140(2):402–6. doi: 10.3945/jn.109.114488. [DOI] [PubMed] [Google Scholar]
- 22.Varela-Silva MI, Azcorra H, Dickinson F, Bogin B, Frisancho AR. Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: a test of the intergenerational effects hypothesis. Am J Hum Biol. 2009;21(5):657–63. doi: 10.1002/ajhb.20883. [DOI] [PubMed] [Google Scholar]
- 23.Bogin B, Smith P, Orden AB, Varela Silva MI, Loucky J. Rapid change in height and body proportions of Maya American children. Am J Hum Biol. 2002;14(6):753–61. doi: 10.1002/ajhb.10092. [DOI] [PubMed] [Google Scholar]
- 24.McKay JA, Mathers JC. Diet induced epigenetic changes and their implications for health. Acta Physiologica. 2011;202(2):103–18. doi: 10.1111/j.1748-1716.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 25.Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, et al. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011;25(2):714–26. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox J, Williams S, Grove K, Lane RH, Aagaard-Tillery KM. A maternal high-fat diet is accompanied by alterations in the fetal primate metabolome. Am J Obstetric Gynecol. 2009;201(3):281. doi: 10.1016/j.ajog.2009.06.041. e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Seminars Reproduct Med. 2009;27(5):380–90. doi: 10.1055/s-0029-1237426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lillycrop KA. Effect of maternal diet on the epigenome: implications for human metabolic disease. Proc Nutr Soc. 2011;70(1):64–72. doi: 10.1017/S0029665110004027. [DOI] [PubMed] [Google Scholar]
- 29.Khulan B, Cooper WN, Skinner BM, Bauer J, Owens S, Prentice AM, et al. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Human Molecular Genetics. 2012;21(9):2086–101. doi: 10.1093/hmg/dds026. [DOI] [PubMed] [Google Scholar]
- 30.Cooper WN, Khulan B, Owens S, Elks CE, Seidel V, Prentice AM, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 2012 doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 31.Olusanya BO, Wirz SL, Renner JK. Prevalence, pattern and risk factors for undernutrition in early infancy using the WHO Multicentre Growth Reference: a community-based study. Paediatric Perinatal Epidemiol. 2010;24(6):572–83. doi: 10.1111/j.1365-3016.2010.01144.x. [DOI] [PubMed] [Google Scholar]
- 32.Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001;107(5):E75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- 33.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969-77) and its follow-up (1988-89) J Nutr. 1995;125(4 Suppl):1027S–41S. doi: 10.1093/jn/125.suppl_4.1027S. [DOI] [PubMed] [Google Scholar]
- 35.Habicht JP, Martorell R, Rivera JA. Nutritional impact of supplementation in the INCAP longitudinal study: analytic strategies and inferences. J Nutr. 1995;125(4 Suppl):1042S–50S. doi: 10.1093/jn/125.suppl_4.1042S. [DOI] [PubMed] [Google Scholar]
- 36.Habicht JP, Martorell R. Probability, plausibility, and adequacy evaluations of the Oriente Study demonstrate that supplementation improved child growth. J Nutr. 2010;140(2):407–10. doi: 10.3945/jn.109.114496. [DOI] [PubMed] [Google Scholar]
- 37.Kuklina EV, Ramakrishnan U, Stein AD, Barnhart HH, Martorell R. Growth and diet quality are associated with the attainment of walking in rural Guatemalan infants. J Nutr. 2004;134(12):3296–300. doi: 10.1093/jn/134.12.3296. [DOI] [PubMed] [Google Scholar]
- 38.Kuklina EV, Ramakrishnan U, Stein AD, Barnhart HH, Martorell R. Early childhood growth and development in rural Guatemala. Early Human Development. 2006;82(7):425–33. doi: 10.1016/j.earlhumdev.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Pollitt E, Gorman KS, Engle PL, Martorell R, Rivera J. Early supplementary feeding and cognition: effects over two decades. Monographs of the Society for Research in Child Development. 1993;58(7):1–99. discussion 111-8. [PubMed] [Google Scholar]
- 40.Stein AD, Wang M, DiGirolamo A, Grajeda R, Ramakrishnan U, Ramirez-Zea M, et al. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Arch Pediatrics Adolescent Med. 2008;162(7):612–8. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein RE, Arenales P, Delgado H, Engle PL, Guzman G, Irwin M, et al. Effects of maternal nutrition on fetal growth and infant development. Bull PAHO. 1976;10(4):301–6. [PubMed] [Google Scholar]
- 42.Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. International J Epidemiol. 2007;36(3):550–7. doi: 10.1093/ije/dym010. [DOI] [PubMed] [Google Scholar]
- 43.Martorell R, Stein AD, Schroeder DG. Early nutrition and later adiposity. J Nutr. 2001;131(3):874S–80S. doi: 10.1093/jn/131.3.874S. [DOI] [PubMed] [Google Scholar]
- 44.Lechtig A, Yarbrough C, Delgado H, Habicht JP, Martorell R, Klein RE. Influence of maternal nutrition on birth weight. Am J Clin Nutr. 1975;28(11):1223–33. doi: 10.1093/ajcn/28.11.1223. [DOI] [PubMed] [Google Scholar]
- 45.Rivera J, Ruel MT. Growth retardation starts in the first three months of life among rural Guatemalan children. Eur J Clin Nutr. 1997;51(2):92–6. doi: 10.1038/sj.ejcn.1600371. [DOI] [PubMed] [Google Scholar]
- 46.Olusanya BO, Renner JK. Predictors of growth velocity in early infancy in a resource-poor setting. Early Human Development. 2011;87(10):647–52. doi: 10.1016/j.earlhumdev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 47.McCowan L, Harding J, Barker S, Ford C. Perinatal predictors of growth at six months in small for gestational age babies. Early Human Development. 1999;56(2-3):205–16. doi: 10.1016/s0378-3782(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77(6):1498–505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 49.El Taguri E, Besmar F, Abdel Monem A, Betilmal I, Ricour C, Roland-Cachera MF. Stunting is a major risk factor for overweight: results from national surveys in 5 Arab countries. Eastern Mediterranean Health J. 2009;15(3):549–62. [PubMed] [Google Scholar]
- 50.Ruel MT, Menon P. Creating a child feeding index using the demographic and health surveys: An example from Latin America. Washington, D.C.: International Food Policy Research Institute; 2002. [Google Scholar]
- 51.Sawadogo PS, Martin-Prevel Y, Savy M, Kameli Y, Traissac P, Traore AS, et al. An infant and child feeding index is associated with the nutritional status of 6- to 23-month-old children in rural Burkina Faso. J Nutr. 2006;136(3):656–63. doi: 10.1093/jn/136.3.656. [DOI] [PubMed] [Google Scholar]