Abstract
Heart failure in children and adults is often the consequence of myocarditis associated with Coxsackievirus (CV) infection. Upon CV infection, enteroviral protease 2A cleaves a small number of host proteins including dystrophin, which links actin filaments to the plasma membrane of muscle fiber cells (sarcolemma). It is unknown whether protease 2A–mediated cleavage of dystrophin and subsequent disruption of the sarcolemma play a role in CV-mediated myocarditis. We generated knockin mice harboring a mutation at the protease 2A cleavage site of the dystrophin gene, which prevents dystrophin cleavage following CV infection. Compared with wild-type mice, we found that mice expressing cleavage-resistant dystrophin had a decrease in sarcolemmal disruption and cardiac virus titer following CV infection. In addition, cleavage-resistant dystrophin inhibited the cardiomyopathy induced by cardiomyocyte-restricted expression of the CV protease 2A transgene. These findings indicate that protease 2A–mediated cleavage of dystrophin is critical for viral propagation, enteroviral-mediated cytopathic effects, and the development of cardiomyopathy.
Introduction
Coxsackievirus (CV) is a member of the enteroviral genus of the picornavirus family and is known to be an important cause of myocarditis and heart failure in children and adults. Enteroviral protease 2A cleaves the viral polyprotein and a small number of host cell proteins such as the cytoskeletal protein dystrophin (1) and the eukaryotic translation initiation factors eIF4G1 and eIF4G2 (2–4). Genetic deficiency of dystrophin causes cardiomyopathy in Duchenne muscular dystrophy and increases susceptibility to myocarditis (5, 6). However, the importance of protease 2A–mediated cleavage of dystrophin is not known. We hypothesized that cleavage of dystrophin by protease 2A is important in sarcolemmal membrane disruption, viral propagation, enteroviral-mediated cytopathic effects, and the development of viral myocarditis. In order to address this hypothesis, we knocked in a mutation at the protease 2A cleavage site of the dystrophin gene, thus inhibiting only the cleavage of dystrophin following CVB3 infection. When mice expressing cleavage-resistant dystrophin were infected with CVB3, there was a decrease in the sarcolemmal disruption, cardiac virus titer, and severity of myocarditis compared with control mice expressing cleavable wild-type dystrophin. In addition, the prevention of dystrophin cleavage in protease 2A–expressing transgenic mice (7) markedly inhibited the protease 2A–induced myocytopathic effect and cardiomyopathy. These findings indicate that disruption of the sarcolemma by protease 2A–mediated cleavage of dystrophin can have a critical role in the pathogenesis of viral myocarditis via alterations in viral propagation and enteroviral-mediated cytopathic effects in the intact wild-type heart.
Results and Discussion
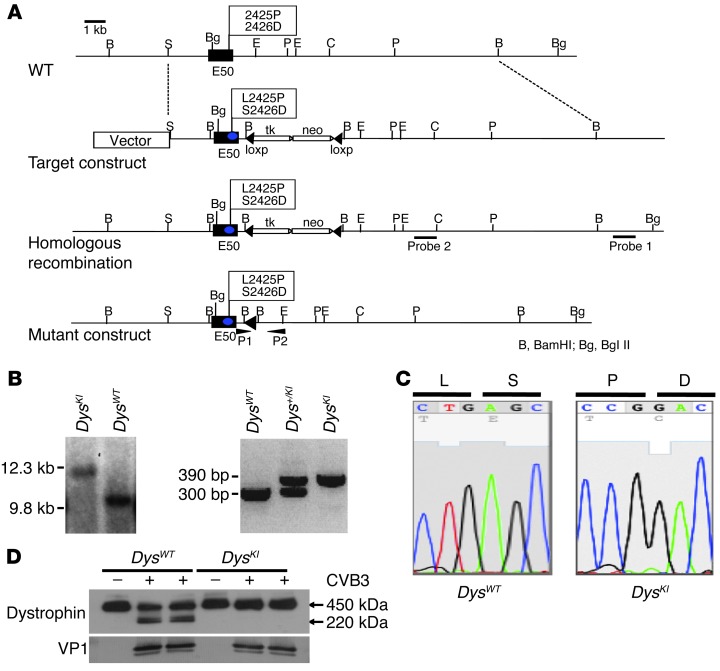
We have previously demonstrated in vitro that enteroviral protease 2A directly cleaves murine dystrophin in the hinge 3 region (8). To determine whether this cleavage by enteroviral protease 2A has an important role in the development of enteroviral-mediated heart disease in vivo, we created a genetically modified mouse line in which amino acids located in the hinge 3 region were changed from leucine to proline (L2425P) and from serine to aspartic acid (S2426D), altering the protease 2A cleavage site from LSTT-G to PDTT-G and rendering dystrophin noncleavable by enteroviral protease 2A (ref. 8 and Figure 1, A–C). To confirm that these substitutions prevented dystrophin cleavage, wild-type and mutant E16.5 embryonic cardiac myocytes were isolated and infected with CVB3. Western blot analysis from cells isolated 24 hours postinfection demonstrated cleavage of dystrophin in wild-type (DysWT) myocytes, but not in myocytes from dystrophin knockin (DysKI) mice. Similar levels of viral protein were expressed in both groups, as demonstrated by expression of the CV capsid protein VP1 (Figure 1D). The first phase of viral replication was the same in isolated DysWT and DysKI myocytes, demonstrating that viral entry and replication within the myocyte was independent of dystrophin cleavage (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI66271DS1). Thus, inhibition of protease 2A–mediated cleavage of dystrophin prevented cleavage of dystrophin, but did not substantially affect viral entry, the first cycle of viral replication, or cleavage of eIF4G1.
Figure 1. Generation of enteroviral protease 2A cleavage–resistant DysKI mice.
(A) Genomic structure and design of DysKI mouse. We changed the wild-type exon 50 (E50) amino acid residues 2425 and 2426 from leucine to proline (L2425P) and serine to aspartic acid (S2426D), respectively. (B) Confirmation of DysKI recombination by Southern blotting of mouse embryonic stem cells. Genomic DNA isolated from embryonic stem cells was used to confirm that homologous recombination had occurred as opposed to random insertion. Southern blotting with probe 1 was performed with a BglII digest, demonstrating loss of the wild-type genome at 9.8 kb (left panel). Female mice were genotyped using PCR primers P1 and P2 (right panel). Data are shown for mice with wild-type dystrophin (DysWT), heterozygous dystrophin knockin (Dys+/KI), and homozygous dystrophin knockin (DysKI). (C) Sequence analysis of exon 50 showing replacement of nucleotides in DysKI mouse genomic DNA as expected. (D) Western blot showing dystrophin in embryonic cardiac myocytes 24 hours after CVB3 infection, in which the cells were isolated from DysWT and DysKI mice. Dystrophin cleavage was inhibited in myocytes from DysKI mice. Comparable levels of viral infection of the cells are demonstrated by the presence of the CVB3 capsid protein VP1.
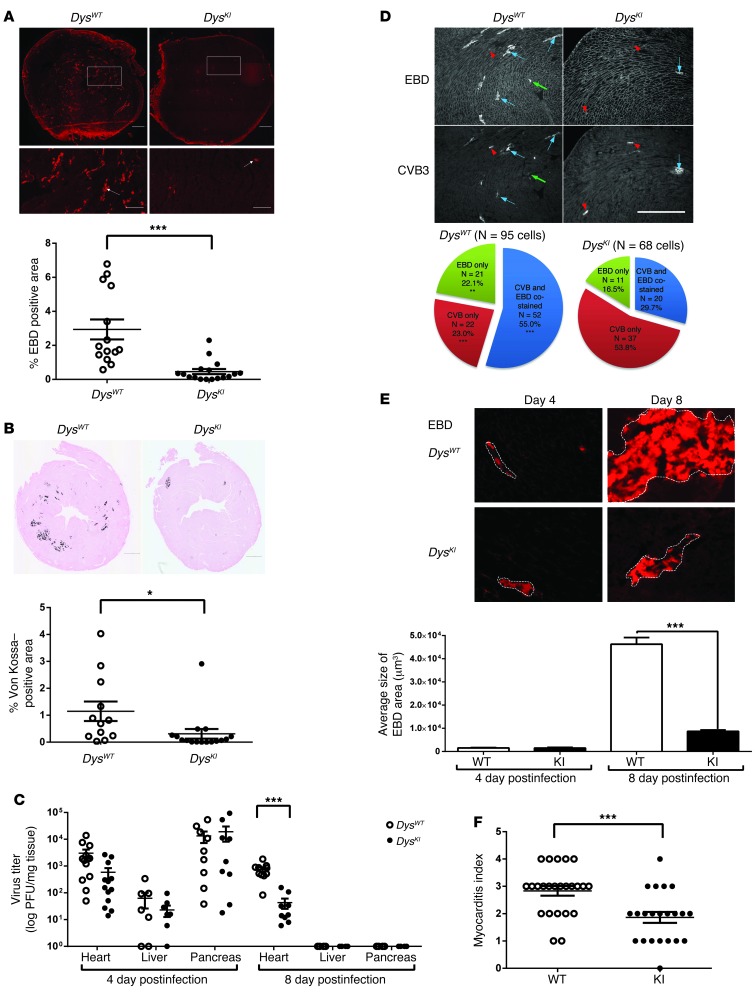
We backcrossed the DysWT and DysKI mice into the C3H mouse strain. Five- to 6-week-old littermates were then infected with 0.2–1 × 104 PFU of CVB3. Eight days postinfection, we injected the mice with Evans blue dye (EBD) to identify cells with disrupted sarcolemmal integrity. There was significantly less disruption of the sarcolemma in the infected DysKI mice than in the DysWT mice following CVB3 infection (Figure 2A). We found significantly less Von Kossa staining, a marker of dystrophic calcification in myocyte necrosis or increased cell membrane permeability (9–11), in infected DysKI mice than in DysWT mice (Figure 2B). To determine whether the ability to cleave dystrophin would affect viral titers, we measured titers in the heart, liver, and pancreas of infected mice. Titers in the hearts of DysKI mice tended to be decreased on day 4 postinfection, but did not reach statistical significance. However, viral titers were significantly lower in the DysKI mice 8 days postinoculation. As expected, in tissues not expressing dystrophin, such as the liver and pancreas, we found no significant difference in viral titers (Figure 2C). In order to determine the efficiency of membrane disruption in CVB3-infected myocytes, we assessed the pattern of EBD and CVB3 staining. In the DysWT mice on day 4 postinfection, the majority of CVB3-infected cells were also EBD positive, while in the DysKI mice, most of the CVB3-infected cells were EBD negative (Figure 2D). We anticipated that the size of the focal areas of EBD-positive staining would be different if the sarcolemmal membranes were more easily disrupted and the virus was able to exit the cell to infect adjacent cells. This was supported by the finding that the cross-sectional area of each EBD focus on day 8 postinfection was increased in the DysWT hearts compared with that in the DysKI hearts, even though there was no significant difference on day 4 postinfection (Figure 2E). This correlates with embryonic myocyte culture data showing inhibition of membrane disruption by trypan blue staining in CVB3-infected DysKI myocytes (Supplemental Figure 2). We also found a decrease in the myocarditis score in infected DysKI mice (Figure 2F and Supplemental Figure 3). However, there was no mortality by day 8 in either the DysWT or the DysKI mice (Supplemental Figure 4A). Similarly, there was no significant change in heart weights or echocardiographic fractional shortening (Supplemental Figure 4B). This may be related to the relatively mild severity of myocarditis in C3H mice. Together, these data indicate that disruption of the cell membrane is inhibited in CVB3-infected myocardium when the protease 2A cleavage site in dystrophin has been mutated. The secondary pathogenic effects of viral infection and viral replication are also affected by the ability of the virus to cleave dystrophin.
Figure 2. Decreased susceptibility to CVB3 viral infection of the heart in DysKI mice.
(A and B) Sarcolemma permeability (EBD uptake) and myocyte damage (von Kossa staining) are significantly reduced in the hearts of DysKI mice 8 days postinfection. Scale bars: 500 μm (200 μm, enlargements). Quantified results represent the mean ± SEM. *P < 0.05; ***P < 0.001; each data point is represented on graphs. (C) CVB3 titer is lower in the hearts of the DysKI mice on day 8. Data represent the mean ± SEM. ***P < 0.001; each data point is represented on the graph. (D) Relationship between CVB3 and EBD uptake in DysWT and DysKI mice. CVB3-infected hearts (n = 6) were stained with EBD, wheat germ agglutinin (WGA) (upper panel), and CVB3 (lower panel) 4 days postinfection. Blue arrows identify cells positive for CVB3 and EBD. Red arrowheads identify cells positive for CVB3 only. Green arrows identify EBD-positive cells with little or no CVB3 staining. Pie chart indicating cells that stained positive for CVB3 and/or EBD (n = 95 in DysWT and 68 in DysKI mice). ***P < 0.0001; **P < 0.05, comparing the percentage of DysWT with DysKI. Scale bar: 500 μm. (E) Foci of viral infection (outline in upper panel) are smaller in DysKI hearts on day 8 postinfection. Quantitation of randomly selected foci (n = 10) from 4 mice in each group. Data represent the mean ± SEM. ***P < 0.001. Scale bar: 200 μm. (F) Decreased myocarditis in DysKI mice 8 days postinfection. Quantitation was graded from 0+ to 4+, as described previously. Data represent the mean ± SEM. ***P < 0.001; each data point is represented on the graph.
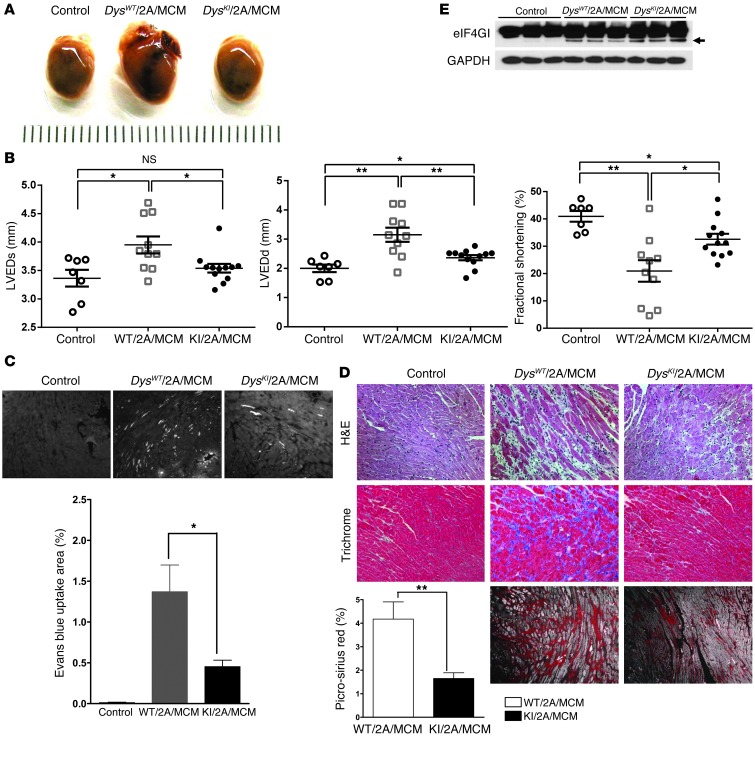
Previously (7), we demonstrated that expression of protease 2A alone can cause dilated cardiomyopathy. In order to determine whether the prevention of dystrophin cleavage can inhibit this protease 2A–mediated cardiomyopathy, we bred mice that expressed inducible protease 2A (2A/MCM) with DysKI mice (DysKI/2A/MCM) and compared them with the wild-type dystrophin controls (DysWT/2A/MCM) and with the negative control littermates that did not express protease 2A (Control). The control mice consisted of littermates with the following genotypes: DysWT or DysKI and MCM (DysWT/MCM or DysKI/MCM), or DysKI protease 2A (DysKI/2A). All mice in the current experiments were treated with tamoxifen. We found that the severity of cardiomyopathy as assessed by gross appearance and echocardiography in the DysKI/2A/MCM mice was significantly less than that seen in the DysWT/2A/MCM mice (Figure 3, A and B). This was accompanied by significantly less EBD staining and fibrosis in the DysKI/2A/MCM hearts compared with that found in the DysWT/2A/MCM hearts (Figures 3, C and D). The improvement in cardiac function in DysKI mice occurred even though there was evidence of cleavage of eIF4G1 in both the DysWT/2A/MCM and DysKI/2A/MCM hearts (Figure 3E). These findings demonstrate that the cardiomyopathic effect of protease 2A expression is markedly inhibited when dystrophin cannot be cleaved by protease 2A, even though protease 2A continues to have an effect on other cellular proteins such as eIF4G.
Figure 3. Prevention of dystrophin cleavage in DysKI/2A/MCM mice inhibits enteroviral protease 2A–mediated cardiomyopathy.
(A) Whole hearts from control mice (see text), mice harboring wild-type dystrophin, protease 2A, and the MerCREMer transgenes (DysWT/2A/MCM), and mice harboring the dystrophin knockin, protease 2A, and the MerCREMer transgenes (DysKI/2A/MCM). Hearts were taken from these mice 4 weeks after tamoxifen administration and demonstrate that dystrophin knockin inhibited the gross appearance of dilated cardiomyopathy. (B) Echocardiographic parameters 4 weeks after tamoxifen administration. Left ventricular end-systolic dimension (LVESd), left ventricular end-diastolic dimension (LVEDd), and fractional shortening were significantly better in DysKI/2A/MCM mice than in DysWT/2A/MCM mice 4 weeks after the induction of protease 2A expression. Data represent the mean ± SEM. *P < 0.05, **P < 0.01; each data point is represented on the graphs. (C) The percentage of overall area showing EBD uptake (white stain, see arrow) was significantly reduced in DysKI/2A/MCM mice compared with DysWT/2A/MCM mice. Data represent the mean ± SEM. *P ≤ 0.05. Scale bar: 500 μm. (D) H&E, trichrome, and picrosirius red staining demonstrate that there was less fibrosis in DysKI/2A/MCM mice compared with DysWT/2A/MCM mice. Fibrosis was quantified using picrosirius red staining in 4 DysWT/2A/MCM and 4 DysKI/2A/MCM mice. Data represent the mean ± SEM. *P ≤ 0.05; **P < 0.01. Scale bar: 200 μm. (E) eIF4GI cleavage was detected in both DysWT/2A/MCM and DysKI/2A/MCM mouse hearts (arrow), but not in control hearts without protease 2A expression. Dystrophin cleavage was not detectable by Western blotting (not shown).
These findings provide what we believe to be the first evidence that cleavage of dystrophin by protease 2A has a critical role in the development of the cardiomyopathy that occurs with enteroviral infection. Also, cleavage of dystrophin by protease 2A has an important role in disrupting the sarcolemma of infected cells, allowing mature virions to exit the infected myocyte and infect adjacent myocytes.
Our data clearly demonstrate that the ease with which the sarcolemma can be disrupted can have a substantial effect on susceptibility to viral infection and on the development of cardiomyopathy. This is consistent with our previous data demonstrating that the absence of dystrophin increased susceptibility to viral infection (5). Apart from the loss of dystrophin at the sarcolemma, which has been shown to increase susceptibility to viral infection, there are other genetic and secondary alterations in the dystrophin-glycoprotein complex (DGC) that affect sarcolemmal stability and could affect susceptibility to viral infection (12–17). Therefore, it is possible that genetic variability in the core components of the DGC or alterations that affect the level of dystrophin expression (such as occurs in Becker muscular dystrophy or X-linked dilated cardiomyopathy) (18) could have a profound permissive or inhibitory effect on susceptibility to enteroviral infection. In summary, therapies aimed at inhibiting protease 2A or stabilizing the sarcolemma could be beneficial in the treatment of cardiomyopathy associated with viral infection.
Methods
An expanded Methods section can be found in the Supplemental Methods.
Generation of a mouse expressing mutant dystrophin that cannot be cleaved by protease 2A.
Knockin of a dystrophin mutation preventing cleavage by protease 2A (DysKI) was created using a dystrophin exon 50–targeting construct, which included two-point mutations: L2425P and S2426D. Targeted ES cells were screened by Southern blotting (Figure 1B) and nucleotide sequencing of exon 50. Once germ-line transmission was confirmed, heterozygous DysKI mice were bred with protamine-Cre to remove the Neo cassette through loxP recombination. Mice were genotyped to identify the knockin construct using a Dys KI-S primer (5′-TCTCTAGGAGAGGTCTCTC) and a Dys KI-AS primer (5′-ACCCCACAATCTTGCACATG). Finally, DysKI mice were backcrossed for 3 to 11 generations with C3H mice (Charles River Laboratories) for viral infection experiments or were bred with 2A/MCM double-transgenic mice to generate DysKI/2A/MCM mice. Double-transgenic DysKI/2A/MCM and DysWT/2A/MCM mice were maintained on a mixed background and genotyped as described previously (7).
Virus.
CVB3 was derived from the infectious cDNA copy of the cardiotrophic H3 variant of CVB3. The viral titer was determined by PFU assay in HeLa cells, as described previously (5).
CVB3 infection.
Male C3H DysKI mice and their male littermate control DysWT mice were inoculated by i.p. injection with 0.2–1 × 104 PFU of CVB3 at 5 to 6 weeks of age. Mice were sacrificed on day 4 or day 8 postinfection. A subset of mice (n = 10 for DysWT and n = 11 for DysKI) were assessed for cardiac function measured by M-mode echocardiography 8 days postinfection, as previously described (19). The heart, liver, and pancreas were harvested and analyzed. The mice were injected with EBD 14 hours before being sacrificed.
Tamoxifen treatment.
Tamoxifen base (Sigma-Aldrich) was dissolved in peanut oil (Sigma-Aldrich) at 100 mg/ml. Between 4 and 6 weeks of age, DysWT/2A/MCM, DysKI/2A/MCM, and control mice were treated with tamoxifen by i.p. injection for 5 consecutive days at a dose of 40 mg/kg per day (7). Four weeks after tamoxifen administration, mouse heart function was measured by M-mode echocardiography, and the hearts were harvested for histology.
Statistics.
Data are expressed as the mean ± SEM unless otherwise noted. Statistical significance was evaluated using an unpaired two-tailed Student’s t test for comparisons between two means. P < 0.05 was considered statistically significant. Bonferroni’s correction was used for multiple comparisons.
Study approval.
All procedures were performed in accordance with guidelines established by the UCSD Institutional Animal Care Program (ACP) and were approved by the IACUC of UCSD.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (5P01HL046345, to K.U. Knowlton) and by a grant from the Myocarditis Foundation Fellowship (to B.K. Lim).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(12):5146–5151. doi:10.1172/JCI66271.
References
- 1.Badorff C, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5(3):320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 2.Gradi A, et al. A novel functional human eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998;18(1):334–342. doi: 10.1128/mcb.18.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamphear BJ, et al. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J Biol Chem. 1993;268(26):19200–19203. [PubMed] [Google Scholar]
- 4.Castello A, Alvarez E, Carrasco L. Differential cleavage of eIF4GI and eIF4GII in mammalian cells. Effects on translation. J Biol Chem. 2006;281(44):33206–33216. doi: 10.1074/jbc.M604340200. [DOI] [PubMed] [Google Scholar]
- 5.Xiong D, et al. Dystrophin deficiency markedly increases enterovirus-induced cardiomyopathy: a genetic predisposition to viral heart disease. Nat Med. 2002;8(8):872–877. doi: 10.1038/nrn2154. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogeni S, et al. Myocardial inflammation in Duchenne Muscular Dystrophy as a precipitating factor for heart failure: a prospective study. BMC Neurol. 2010;10:33. doi: 10.1186/1471-2377-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong D, et al. Inducible cardiac-restricted expression of enteroviral protease 2A is sufficient to induce dilated cardiomyopathy. Circulation. 2007;115(1):94–102. doi: 10.1161/CIRCULATIONAHA.106.631093. [DOI] [PubMed] [Google Scholar]
- 8.Badorff C, Berkely N, Mehrotra S, Talhouk JW, Rhoads RE, Knowlton KU. Enteroviral protease 2A directly cleaves dystrophin and is inhibited by a dystrophin-based substrate analogue. J Biol Chem. 2000;275(15):11191–11197. doi: 10.1074/jbc.275.15.11191. [DOI] [PubMed] [Google Scholar]
- 9.Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978;28(5):439–446. doi: 10.1212/WNL.28.5.439. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. . J Clin Invest. 2007;117(9):2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konno T, et al. Heterogeneous myocyte enhancer factor-2 (Mef2) activation in myocytes predicts focal scarring in hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107(42):18097–18102. doi: 10.1073/pnas.1012826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94(8):1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- 13.Lee GH, Badorff C, Knowlton KU. Dissociation of sarcoglycans and the dystrophin carboxyl terminus from the sarcolemma in enteroviral cardiomyopathy. Circ Res. 2000;87(6):489–495. doi: 10.1161/01.RES.87.6.489. [DOI] [PubMed] [Google Scholar]
- 14.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90(4):729–738. doi: 10.1016/S0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 15.Celes MR, et al. Disruption of sarcolemmal dystrophin and beta-dystroglycan may be a potential mechanism for myocardial dysfunction in severe sepsis. Lab Invest. 2010;90(4):531–542. doi: 10.1038/labinvest.2010.3. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, et al. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281(1):213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- 17.Barresi R, et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J Med Genet. 2000;37(2):102–107. doi: 10.1136/jmg.37.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 19.Lim BK, et al. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest. 2008;118(8):2758–2770. doi: 10.1172/JCI34777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.