Abstract
Migraine is a debilitating neurological disorder that affects about 12% of the population. In the past decade, the role of the neuropeptide calcitonin gene-related peptide (CGRP) in migraine has been firmly established by clinical studies. CGRP administration can trigger migraines, and CGRP receptor antagonists ameliorate migraine. In this review, we will describe multifunctional activities of CGRP that could potentially contribute to migraine. These include roles in light aversion, neurogenic inflammation, peripheral and central sensitization of nociceptive pathways, cortical spreading depression, and regulation of nitric oxide production. Yet clearly there will be many other contributing genes that could act in concert with CGRP. One candidate is pituitary adenylate cyclase-activating peptide (PACAP), which shares some of the same actions as CGRP, including the ability to induce migraine in migraineurs and light aversive behavior in rodents. Interestingly, both CGRP and PACAP act on receptors that share an accessory subunit called receptor activity modifying protein-1 (RAMP1). Thus, comparisons between the actions of these two migraine-inducing neuropeptides, CGRP and PACAP, may provide new insights into migraine pathophysiology.
Keywords: CGRP, PACAP, migraine, neuropeptides
Migraine
Clinical context of migraine
Migraine is far more than just another a headache. It is a complex and disabling neurological disorder (Goadsby et al., 2002). As defined by the International Headache Society, migraine is a headache that lasts for 4 to 72 hours and characterized by at least two of the following: unilateral localization; pulsating quality; moderate to severe pain intensity; and aggravation by movement such as walking (Headache, 2004). Furthermore, the headache must be accompanied with at least one of the following: nausea and/or vomiting; and photophobia and phonophobia (Headache, 2004). In addition, some migraineurs experience an aura, which typically precedes the headache during the premonition or prodrome phase (Headache, 2004; Kelman, 2004a). This often results in visual changes such as a scintillating scotoma that moves across the visual field (Kelman, 2004a; Purdy, 2011). The prodrome may also be accompanied by other symptoms such as fatigue, gastrointestinal issues, and mood changes (Kelman, 2004b). As a result, migraine sufferers are often incapacitated for extended periods of time.
Migraine is estimated to affect up to one in four households (Lipton et al., 2001). A highly prevalent disorder: 6 – 8% of men and 15 – 25% of women suffer from migraine (Pietrobon and Striessnig, 2003). The lifetime incidence is 43% in women and 18% in men (Stewart et al., 2008). Migraine is typically episodic (Goadsby et al., 2002); however, 3 – 5% of the general population experience chronic daily headaches occurring at least 15 days per month, often with migrainous characteristics (Couch, 2011). A 1999 study of migraine economic burden in the United States found a total of 112 million bedridden days per year by migraineurs (Hu et al., 1999). As a result, the indirect cost of migraine to employers was estimated at $11 billion annually in the US, primarily due to absenteeism (Hawkins et al., 2007). Additionally, the direct healthcare cost associated with migraine was estimated at $13 billion in the US (Hawkins et al., 2008). From a global perspective, the World Health Organization ranks migraine in the top twenty of disabling conditions. Consequently, migraine has not only a harmful impact on the individual, but also a significant impact on society.
Despite having a substantial effect on society, little advancement has been made in managing the disorder. For some migraineurs, non-steroidal anti-inflammatory drugs may be sufficient for pain relief (Diener et al., 2006; Silberstein and Goadsby, 2002). However, many migraineurs depend on oral triptans, which are 5-HT1B/D receptor agonists and the current gold standard in migraine abortive therapy, but the response rate is only 60% (Loder, 2010). Moreover, triptans have adverse effects including paresthesias, flushing, neck tightness, and chest pressure as well as possible cardiovascular risks (Loder, 2010). Preventatives such as propranolol, topiramate, and tricyclic antidepressants, such as amitriptyline, have also been used to reduce to the number of migraines attacks (Goadsby et al., 2002). Recently, botulinum toxin has been found to be an effective preventative (Rapoport, 2010). Despite these options, many migraineurs do not respond to medication (Tfelt-Hansen and Olesen, 2012) or develop chronic daily headaches due to medication overuse (Bussone, 2010).
Current understanding of migraine pathophysiology
The lack of therapeutic options in managing migraine reflects the limited understanding of the mechanisms behind migraine. Ironically, both the early development of triptan drugs and rationale for looking at CGRP in migraine were based on the old vascular migraine model. For decades, migraine had been considered a vascular disorder in which pain resulted from vasodilation of cranial arteries (Levy and Burstein, 2011). This theory was suggested after Wolff observed dilation of the superficial temporal artery during migraine (Wolff et al., 1953), an idea that gained further support due to various vasodilators found to induce migraine. These vasodilators are: CGRP, nitroglycerin, histamine, and PACAP-38 (Asghar et al., 2011; Christiansen et al., 1999; Iversen et al., 1989; Lassen et al., 1995; Schytz et al., 2009). Typically, these vasodilators have a biphasic effect with an initial mild headache experienced by both healthy controls and migraineurs, but a delayed migraine-like headache that meets the criteria described above is only experienced by migraineurs. However, the vascular theory has been challenged by evidence supporting a more neural basis of migraine (Brennan and Charles, 2010; Goadsby, 2009; Levy and Burstein, 2011). In addition, a simple role for vasodilation has been ruled out since some potent vasodilators, such as vasoactive intestinal peptide (VIP), do not induce migraine despite eliciting intracranial vascular dilation (Brennan and Charles, 2010; Rahmann et al., 2008). Importantly, while migraine-triggering vasodilators initially cause dilation in both controls and migraineurs during the initial mild headache phase, there is no increase in intracranial arterial diameter during the migraine-like headache phase (Brennan and Charles, 2010; Schoonman et al., 2008). There is though one recent study that reported CGRP-induced dilation of both the middle meningeal artery and the middle cerebral artery in migraineurs during the migraine-like attack (Asghar et al., 2011). Hence, while the story is unfinished, it seems that vascular events alone are not necessary or sufficient to induce a migraine (Brennan and Charles, 2010). In its place, the neurovascular model, which combines both neural and vascular contributions, has gained general acceptance.
The neurovascular theory of migraine centers on activation of the trigeminovascular system (Goadsby et al., 2002; Messlinger, 2009) (Figure 1). This system consists of pseudo-unipolar neurons in the trigeminal ganglion with primary afferents innervating the pial and dural meningeal vessels surrounding the brain and efferent projections synapsing with second order neurons in the trigeminal nucleus caudalis (TNC) (Liu et al., 2008; Messlinger et al., 1993). The ganglion provides sensory fibers for all anterior cranial tissues and is subdivided into three topographical divisions with the ophthalmic division playing the predominate role in migraine (Messlinger, 2009). In particular, trigeminal ganglion provides afferent innervation to the meninges including the dura mater and intracerebral arteries (Messlinger, 2009). Almost exclusively nociceptive, meningeal afferents are polymodal, thus sensitive to mechanical, thermal, and chemical stimuli including capsaicin and H+ that activate TRPV1 and ASIC3 receptors (Bove and Moskowitz, 1997; Messlinger, 2009; Yan et al., 2011). Sensory trigeminal fibers project primarily to the TNC, which extends from the medullary dorsal horn to the first two cervical segments of the spinal dorsal horn (Messlinger, 2009). Stimulation of meningeal afferents leads to c-Fos positive neurons in the TNC (Hoskin et al., 1999; Kaube et al., 1993; Strassman et al., 1986). Neurons in the TNC then synapse with neurons in the thalamus, especially nuclei in the posterior thalamus, where ascending input is integrated and relayed to higher cortical areas (Burstein et al., 1998).
Figure 1.
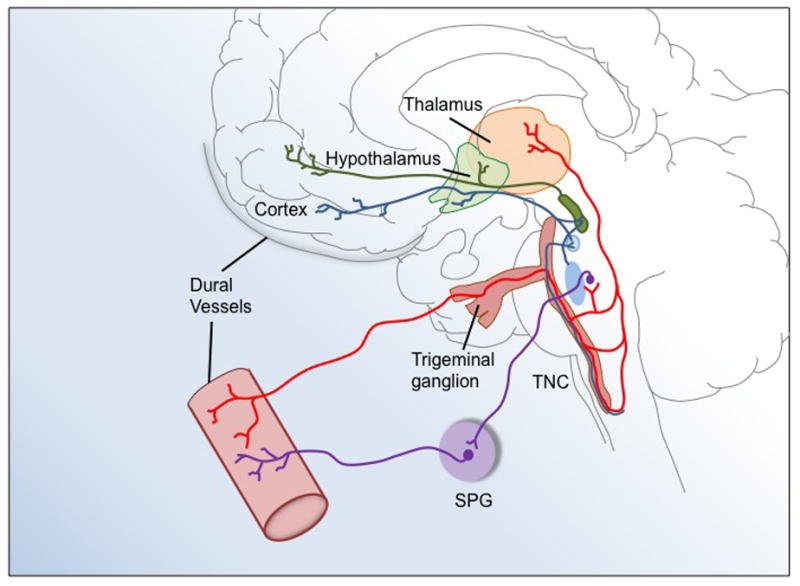
Representation of the trigeminovascular system in migraine. Trigeminal afferents innervate the dural vasculature. The first-order trigeminal neuron is located in the trigeminal ganglion. Its central terminal projects to the trigeminal nucleus caudalis (TNC) that extends from the dorsal medulla to the dorsal spinal horn of the first two cervical segments. Second-order neurons of the TNC project to the posterior thalamus. The sphenopalatine ganglion (SPG) also provides reflex parasympathetic innervation to dural vessels. This illustration has been adapted from (Goadsby et al., 2002).
In addition to the trigeminal system, innervation from the sphenopalatine ganglia has also been implicated in the neurovascular model of migraine (Figure 1) (Bolay et al., 2002). This ganglion contains parasympathetic nerves involved in autonomic control of cranial vessel tone. Interestingly, the sphenopalatine ganglia and nerves contain PACAP and its receptors (Csati et al., 2012). As discussed below, both CGRP and PACAP may play roles in the trigeminal-autonomic reflex during migraine attacks.
Some studies have suggested trigger sites in the brainstem could activate the trigeminovascular system (see (Messlinger, 2009)), or that defective central processing allows non-noxious trigeminovascular input to be perceived as painful (Lambert, 2010; Lambert and Zagami, 2009). While these studies suggest the brainstem plays a role in migraine, the mechanisms are not yet clear.
In summary, the exact mechanisms of migraine remain elusive. This is not surprising given migraine is a very complex disorder with a variety of triggers and phenomena occurring throughout the CNS and intracranial tissues. While the old vascular theory has fallen from favor, it seems likely that the vasculature will still play a role and that model led to the finding that vasodilators, such as CGRP, can trigger migraine-like headaches. Importantly, other vasodilators do not, which raises the question as to what else CGRP does. Using that same logic, we have compared those activities with those of another migraine-inducing vasodilator, PACAP, to potentially reveal overlapping roles of CGRP and PACAP in migraine.
Calcitonin gene-related peptide (CGRP)
CGRP background
CGRP is a neuropeptide involved in a number of physiological and pathological processes in the body. CGRP is found in neurons throughout the body (Recober and Russo, 2009; van Rossum et al., 1997; Wimalawansa, 1996). Approximately 50% of trigeminal neurons express CGRP (Eftekhari et al., 2010; Tajti et al., 1999). There are two genes encoding nearly identical forms of CGRP. The CALCA gene encodes α-CGRP, a 37 amino acid peptide (Amara et al., 1982), which we will refer to simply as CGRP. This gene also encodes the originally identified splice variant, calcitonin, a hormone peptide produced in thyroid C-cells (Amara et al., 1982; Moya et al., 1975). The CALCB gene encodes β-CGRP, which differs from α-CGRP by only one amino acid. While the two peptides have nearly indistinguishable activities, they are differentially regulated and expressed in a distinct, but overlapping pattern (Russo et al., 1988). Note that trigeminal ganglia predominantly express α-CGRP (Amara et al., 1985).
The CGRP receptor consists of three separate subunits: calcitonin-like receptor (CLR); RAMP1; and receptor component protein (RCP) (Archbold et al., 2011; Barwell et al., 2012) (Figure 2). CLR, a G protein-coupled receptor, and RAMP1 are required for trafficking to the cell surface and for CGRP binding (McLatchie et al., 1998). RCP is an intracellular subunit that is required for Gαs coupling to the receptor (Barwell et al., 2012; Evans et al., 2000; Prado et al., 2002). RAMP1 appears to be the functional rate-limiting subunit of the receptor (Zhang et al., 2006; Zhang et al., 2007). Additionally, there is evidence that 2 RAMP1 subunits may cooperatively bind to a CLR dimer (Heroux et al., 2007; Zhang et al., 2007). Binding of RAMP2 or 3 subunits to CLR forms adrenomedullin receptors, AM1 or AM2, respectively, which have low affinity for CGRP (Walker et al., 2010). RAMP1 with the calcitonin receptor forms amylin subtype 1a receptor (Hay et al., 2006). After binding of CGRP to the cleft between CLR and RAMP1, a signal is transduced through the receptor, which can lead to activation of multiple pathways that modulate gene expression and ion channel activity (Walker et al., 2010). Via autoregulation, CGRP can increase CGRP promoter activity and mRNA levels (Zhang et al., 2007). Consequently, CGRP may initiate a positive feedback loop, which may in part explain why peripheral injection of CGRP leads to a delayed migraine-like headache several hours after injection.
Figure 2.
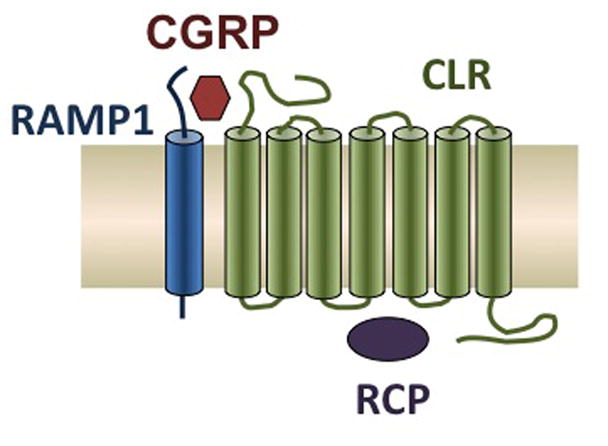
Schematic of the CGRP receptor complex, which contains three subunits: CLR, RAMP1, and RCP. This figure has been modified slightly from (Russo et al., 2009).
Localization of CGRP and its receptor provides insight into the various actions that CGRP has throughout the body. Many of these areas have been implicated in migraine (Raddant and Russo, 2011). CGRP-immunopositive cell bodies and fibers are found in hypothalamic nuclei, ventral tegmental area, cerebellum, and various brainstem nuclei, including the periaqueductal grey (PAG) (Dobolyi et al., 2005; Edvinsson et al., 2011; Kresse et al., 1995; Ma et al., 2003). Furthermore, CGRP-binding sites have been localized throughout the CNS including the cortex, limbic areas (e.g. amygdala), hypothalamus, and brainstem, including PAG (Skofitsch and Jacobowitz, 1985a; van Rossum et al., 1997; Yashpal et al., 1992). In addition to neurons, satellite glia and possibly non-myelinating Schwann cells contain CGRP receptors although they do not normally produce CGRP (Eftekhari et al., 2010).
CGRP and migraine
Over the past years, a strong role has emerged for CGRP in the pathogenesis of migraine (Goadsby, 2005, 2006; Villalon and Olesen, 2009; Waeber and Moskowitz, 2005). This is primarily based on three lines of evidence from clinical studies.
Serum and salivary CGRP levels have been reported to be elevated in migraineurs during a spontaneous attack as well during a nitric oxide-induced attack (Bellamy et al., 2006b; Cady et al., 2009; Gallai et al., 1995; Goadsby et al., 1990; Ho et al., 2010; Juhasz et al., 2003). CGRP levels have been reduced by triptans, coincident with pain relief (Goadsby and Edvinsson, 1993; Juhasz et al., 2005). Most of these studies used blood from the external jugular vein, so the most likely source of CGRP would have been perivascular release from peripheral trigeminal nerve fibers. However, another study did not find elevated CGRP during migraine (Tvedskov et al., 2005). The reason is not clear, but the lack of an increase in some patients may reflect heterogeneity of the pathology or different severity of the symptoms. So, while elevated CGRP in migraine seems likely, it remains controversial (Tfelt-Hansen and Le, 2009).
Intravenous injection of CGRP led to migraineurs experiencing a delayed headache roughly 2 – 4 h after injection with some of those headaches meeting criteria as a migraine (Asghar et al., 2011; Hansen et al., 2010; Lassen et al., 2002). Whereas, non-migraineurs only described an initial mild headache or fullness of head, and none met criteria for experimentally induced migraine (Hansen et al., 2010; Petersen et al., 2005). Interestingly, administration of sumatriptan was effective in reversing the CGRP-induced migraine (Asghar et al., 2011). The triptans are 5-HT1B/D receptor agonists that act by inducing vasoconstriction and inhibiting release of CGRP and other neuropeptides (Loder, 2010). They have been shown to down regulate nociceptive signaling in the spinal trigeminal nucleus (Levy et al., 2004), although the sites of triptan actions are not yet fully known. Sumatriptan also reversed a nitric oxide-induced migraine and reduced CGRP levels coincident with pain relief (Juhasz et al., 2005).
Selective CGRP receptor antagonists are effective in the management of migraine. In a phase II study, 66% of migraineurs responded to 2.5 mg of IV olcegepant (BIBN4096) compared to 27% subjects who received placebo (Olesen et al., 2004). It was superior to placebo based on a 2 h pain free rate as well as improved photophobia, phonophobia, and nausea (Olesen et al., 2004). In several phase III clinical trials, an oral antagonist, telcagepant (MK-0974) has been show to be effective and was as efficacious as rizatriptan and zolmitriptan (Connor et al., 2009; Ho et al., 2008a; Ho et al., 2008b).
In summary, these studies have demonstrated CGRP to be elevated during a migraine and that CGRP can induce a migraine-like state in migraineurs only, suggesting that sensitivity to CGRP may play a role in migraine susceptibility. Moreover, blocking the actions of CGRP can effectively reverse the symptoms of a migraine. However, somewhat surprisingly, CGRP and its receptor have yet to be genetically associated with migraine (Anttila et al., 2010; Chasman et al., 2011; Menon et al., 2011). This may reflect not only the heterogeneity of migraine, but may also reflect the possibility that other modifiers are needed in concert with CGRP. Furthermore, both the triptans and CGRP receptor antagonists are not effective for all patients and each drug class has limitations and drawbacks. For these reasons, more targets need to be investigated.
Given that CGRP is a key player in migraine, to identify new targets, one strategy is to consider how CGRP might be contributing to the symptoms? To understand how CGRP plays a role in migraine requires an appreciation for the multifunctional roles of this peptide. As a starting point, we have outlined CGRP actions in light aversion, nociceptive sensitization, neurogenic inflammation, cortical spreading depression, and nitric oxide generation.
CGRP and light aversion
Over the past few years, studies in animal models have revealed an unexpected role for CGRP in light aversive behavior that is suggested to be analogous to photophobia experienced by migraineurs. Sensitized to CGRP as the result of RAMP1 over-expression, nestin/hRAMP1 mice demonstrated a significant decrease in time in the light zone of a dim light-dark box compared to vehicle-treated nestin/hRAMP1 mice and CGRP-treated control mice (Recober et al., 2010; Recober et al., 2009). Furthermore, CGRP decreased locomotor activity in the dark zone, which may reflect exacerbation of pain by movement that is often experienced during a migraine (Recober et al., 2010). Wildtype mice have also demonstrated these CGRP-induced behaviors, but required bright light and habituation to the chamber (Kaiser et al., 2012). While there is a difference in sensitivity in this assay between wildtype and nestin/hRAMP1 mice, it demonstrates that endogenous CGRP receptors are sufficient to convey this behavior. Triptans have attenuated the CGRP-induced behaviors in wildtype and nestin/hRAMP1 mice suggesting that light aversion in mice likely provides a preclinical model of migraine-type photophobia (Kaiser et al., 2012; Schwedt and Goadsby, 2010).
CGRP in central and peripheral nociceptive sensitization
CGRP plays a role in the nervous system as a neuromodulator of nociception. This is particularly evident in the trigeminal system. Trigeminal sensory fibers are CGRP-immunopositive at a higher ratio than sensory fibers in extracranial tissues (Messlinger, 2009). Consequently, CGRP is released from primary afferents in both the periphery and the TNC (Eberhardt et al., 2008; Ebersberger et al., 1999; Eltorp et al., 2000; Goadsby et al., 1988; Jenkins et al., 2004; Offenhauser et al., 2005). CGRP receptors are also located in the soma of trigeminal ganglion cells (Lennerz et al., 2008; Tajti et al., 1999). In the TNC, there is evidence of CGRP receptors on primary afferent terminals suggesting a potential role in modulating presynaptic activity to facilitate nociceptive transmission (Lennerz et al., 2008; Messlinger, 2009). Second-order neurons in the TNC project to the posterior thalamus (Burstein et al., 1998). In the thalamus, CGRP-immunopositive neurons are located in the peripeduncular nucleus, subparafascicular nucleus, and posterior thalamic nuclear group as well as areas ventromedial to this group (Kresse et al., 1995). Moreover, the ventral posteromedial nucleus is known to contain CGRP receptors, and CGRP receptor antagonists inhibit nociceptive activation of the ventral posteromedial nucleus (Summ et al., 2010). Interestingly, somatosensory and nociceptive activity is integrated and relayed from ascending pathways to higher cortical areas via CGRP-containing neurons of the subparafascicular thalamus and the caudal part of the posterior thalamic group (de Lacalle and Saper, 2000; Gauriau and Bernard, 2004). These CGRP containing fibers project to the secondary somatosensory cortex, amygdala, insula, and hypothalamus (Campeau and Watson, 2000; de Lacalle and Saper, 2000; Gauriau and Bernard, 2004) indicating roles in nociception, stress, autonomic responses, anxiety, and auditory perception (Raddant and Russo, 2011). Also the posterior thalamus has been implicated in migraine-type photophobia (Noseda et al., 2010). In efferent pathways, CGRP has also been found in the nucleus raphe mangus of rats leading to anti-nociceptive effects (Huang et al., 2000) and in A11 dopamine neurons located in the posterior hypothalamus that provide descending innervation to spinal gray matter including the TNC (Charbit et al., 2009).
CGRP release from central terminals of the trigeminal nerve can modulate second order-nociceptive neurons in the TNC (Fischer, 2010). This also occurs in the spinal dorsal horn, which can lead to central sensitization resulting in mechanical allodynia (Marquez de Prado et al., 2009). CGRP and its receptor co-localize with AMPA-type glutamate receptors in post-synaptic neurons in the spinal dorsal horn (Gu and Yu, 2007). Furthermore, CGRP was also found to enhance synaptic transmission of NMDA- and AMPA-type glutamate receptors (Ebersberger et al., 2000). Consequently, CGRP is thought to play a role in central sensitization (Seybold, 2009), which is a component in mitigating headache pain in migraine and likely also contributes to hypersensitivity to other senses leading to photophobia, phonophobia, and allodynia in migraine (Ho et al., 2010).
CGRP and neurogenic inflammation
Neurogenic inflammation includes vasodilation, plasma protein extravasation, mast cell degranulation, and release of proinflammatory and inflammatory molecules due to sensory nerve activation (Markowitz et al., 1987; Raddant and Russo, 2011). The process can activate meningeal nociceptors (Messlinger, 2009).
CGRP plays multiple roles in neurogenic inflammation. It is the most potent vasodilator peptide (Brain and Grant, 2004; McCulloch et al., 1986). Due to its effects on the coronary arteries, CGRP has been shown to be cardioprotective by limiting cardiac ischemia-reperfusion injury (Huang et al., 2008). Importantly, the CGRP receptor is found throughout the cerebral vasculature (Oliver et al., 2002) with CGRP acting as a particularly potent vasodilator in intracranial arteries, even more so than in coronary arteries (Edvinsson et al., 2002; Moreno et al., 2002). CGRP has been found to induce dilation of the middle cerebral artery and middle meningeal artery in migraineurs coincident with the induced migraine (Asghar et al., 2011); however, this has not been found in other studies (Goadsby, 2009; Lassen et al., 2008; Levy and Burstein, 2011). CGRP is indirectly involved with plasma extravasation, which is primarily caused by substance P and neurokinin A (Holzer, 1998). These peptides are often co-released with CGRP (Lee et al., 1985; Lundberg et al., 1985; Skofitsch and Jacobowitz, 1985b) and CGRP can increase substance P release (Zhang et al., 2007). CGRP in conjunction with substance P can trigger mast cell degranulation to release proinflammatory and inflammatory compounds (Brain and Grant, 2004; Ottosson and Edvinsson, 1997; Theoharides et al., 2005). Recently, it has been confirmed that dural mast cells express the CGRP receptor (Lennerz et al., 2008). Mast cell activation and degranulation leads to release of histamine, which has been shown to induce migraine (Lassen et al., 1995), along with prostaglandins, TNF-α, and other compounds. Mast cells can activate dural primary afferents, which subsequently leads to c-Fos immunoreactivity in the TNC (Levy et al., 2007). Similar to mast cells, satellite glia, which surround neurons in trigeminal ganglia (Hansson and Ronnback, 2003), contain CGRP receptors (Eftekhari et al., 2010; Lennerz et al., 2008). In response to CGRP, satellite glial cells can release proinflammatory cytokines such as TNF-α and IL-6 leading to sensory neuron sensitization (Capuano et al., 2009; De Corato et al., 2011; Thalakoti et al., 2007; Vause and Durham, 2010). Similar cascades occur in other inflammatory pain conditions (Cao and Zhang, 2008; Watkins et al., 2001). Moreover, satellite glia and neurons have been proposed to participate in a positive feedback loop of CGRP synthesis and release maintaining a state of heightened inflammation and sensitization (Raddant and Russo, 2011). This positive feedback loop may explain why transgenic mice overexpressing RAMP1 have higher CGRP levels in cerebrospinal fluid (Recober et al., 2009). Consequently, CGRP is apparently a key mediator of neurogenic inflammation in the trigeminovascular system.
However, a role for neurogenic inflammation in migraine has been diminished in part due to the lack substance P found in venous outflow during a migraine attack (Goadsby et al., 1990) and the lack of substance P receptor antagonist efficacy in migraine clinical trials (Diener, 2003; Ho et al., 2010). Still several animal models of migraine utilize an inflammatory soup consisting of bradykinin, 5-HT, histamine, and prostaglandin E2 buffered at low pH, which leads to sensitization of second-order neurons in the TNC (Burstein et al., 1998; Levy et al., 2004; Strassman et al., 1996). Consequently, these animal models have been widely used to model central sensitization in migraine (Messlinger, 2009). Other than a known role for histamine inducing migraine, it remains to be seen whether neurogenic inflammation plays a role in migraine in humans.
CGRP and cortical spreading depression
A potential trigger that may engage neuropeptides in the trigeminovascular system during migraine is cortical spreading depression (CSD), which is associated with aura (Ayata, 2010). CSD is a self-propagating wave of neuronal and glial depolarization initiated in the occipital lobe that spreads rostrally over the cortex at rate of 2 – 6 mm per min; this is followed by a prolonged neuronal suppression during repolarization (Olesen et al., 2009; Raddant and Russo, 2011). CSD leads to the release of various noxious substances such as glutamate, potassium, and adenosine triphosphate, which diffuse into the meninges, activating nociceptors, releasing CGRP and substance P from peripheral terminals, and triggering neurogenic inflammation (Levy, 2010). Activation of meningeal nociceptors after CSD (Bolay et al., 2002; Zhang et al., 2011; Zhang et al., 2010) leads to c-Fos expression in the TNC (Kunkler and Kraig, 2003; Moskowitz, 1993). Interestingly, animal models have shown CSD suppression in response to common migraine prophylaxis (Ayata, 2010; Ayata et al., 2006).
CGRP may play a role in CSD. It may lead to the initial hyperemia during CGRP based on studies with animal models of CSD in which CGRP receptor antagonists blocked pial dilation (Colonna et al., 1994; Wahl et al., 1994). A recent study using rat brain slices found that endogenous CGRP was released during CSD and that CGRP receptor antagonists inhibited CSD in vitro (Tozzi et al., 2012). In addition, one clinical study reported that CGRP infusion induced an aura in some subjects although this needs further study (Hansen et al., 2010).
CGRP and nitric oxide
As discussed above, CGRP can induce a migraine-like headache in migraineurs, but nitrovasodilators, which are nitric oxide (NO) donors, such as glyceryl trinitrate or sodium nitroprusside can elicit the same effect. Remarkably, CGRP levels were elevated following glyceryl trinitrate induced migraine (Juhasz et al., 2003). Nitrovasodilators have also been studied in animals (Messlinger, 2009). Interestingly, sodium nitroprusside was found in rats to induce an initial, transient phase of neuronal activation and then a delayed, long phase of high neuronal activity in the TNC, which closely mimics clinical trials with migraineurs (Koulchitsky et al., 2004). Furthermore, glyceryl trinitrate induced c-Fos immunoreactivity in the TNC (Martin and Martin, 2001; Tassorelli and Joseph, 1995a, b). This may occur in part because NO donors were found to increase the release of CGRP in rat dura in vitro (Strecker et al., 2002). Moreover, pre-treatment of trigeminal ganglia cultures with NO donors was also found to increase CGRP release in response to both depolarization and inflammatory mediators, which may have occurred due to NO donors increasing CGRP promoter activity (Bellamy et al., 2006a; Eberhardt et al., 2008). While NO donors have some functions independent of CGRP, their function in migraine may strongly relate to promoting CGRP production and release, adding to the CGRP-influenced pathways in migraine.
Pituitary adenylate cyclase-activating peptide (PACAP)
PACAP background
Like CGRP, PACAP is a multifunctional vasodilatory peptide that has recently been implicated in migraine pathogenesis. PACAP has roles in neurodevelopment, neuroprotection, neuromodulation, neurogenic inflammation, and nociception (Hashimoto et al., 2006; Vaudry et al., 2000). PACAP belongs to the VIP-glucagon-growth hormone releasing factor-secretin superfamily (Vaudry et al., 2000). PACAP is encoded by ADCYAP1 gene, which expresses two forms containing either 27 or 38 amino acids with PACAP-38 being the more prevalent, representing 90% of PACAP forms in mammalian tissues (Bourgault et al., 2008). We will generally refer to PACAP-38 simply as PACAP. It is expressed throughout the CNS (Masuo et al., 1992; Uddman et al., 2002) as well as in peripheral organs and glands (Borzsei et al., 2009; Fahrenkrug and Hannibal, 2011; Koves et al., 1990; Reglodi et al., 2012). As a result, PACAP has various roles in cardiovascular, respiratory, gastrointestinal, and reproductive systems in addition to the nervous system (Vaudry et al., 2000). PACAP binds to three different G-protein coupled receptors, which have affinity for VIP as well. Receptors VPAC1 and VPAC2 have varying affinity for the following peptides: VIP > PACAP-38 > PACAP-27 (Laburthe and Couvineau, 2002). PAC1 is selective for PACAP-38 and PACAP-27 with 102 to 103 lower affinity for VIP (Laburthe and Couvineau, 2002). These different receptor affinities are likely to underlie the ability of PACAP-38, but not VIP, to induce migraine.
Like the CGRP core subunit CLR, the VPAC1 receptor can associate with all three RAMP subunits, including RAMP1, in a cell-based assay (Figure 3) (Christopoulos et al., 2003; Morfis et al., 2003). In contrast, VPAC2 did not show any interaction (PAC1 was not tested). Binding of PACAP and VIP to VPAC1 was not affected by any of the RAMPs (Christopoulos et al., 2003; Morfis et al., 2003). Interestingly, co-expression of VPAC1 and RAMP2 increased agonist-mediated phosphoinositide hydrolysis with no change in cAMP stimulation compared with VPAC1 receptor alone. While direct modulation by RAMP1 was not observed, we suggest that elevated RAMP1 could compete with RAMP2 association with VPAC1. This would effectively shift receptor coupling towards cAMP production. Such modulation of signaling will be dependent on the cell type, but it suggests a possible synergistic effect of increased RAMP1 levels on both CGRP and PACAP receptor cAMP signaling.
Figure 3.
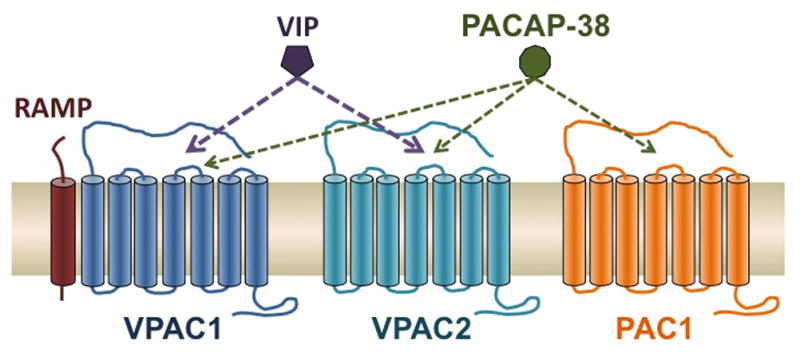
Schematic of the VPAC1, VPAC2 and PAC1 receptors, which bind VIP and PACAP-38 with varying affinities. Compared to PACAP, VIP binds to PAC1 with 100–1000-fold lower affinity. PACAP-27 can also bind all three receptors (not shown). All three RAMPs can associate with VPAC1, but apparently not VPAC2 (PAC1 not known).
In the trigeminovascular system, PACAP is expressed in the TNC (Uddman et al., 2002) and found in the spinal cord, trigeminal ganglia, and TNC (Schytz et al., 2010). PACAP and its receptors are also expressed in sphenopalatine ganglia neurons that control dural vessel tone and cranial blood flow (Csati et al., 2012). Similar to CGRP, PACAP binds to a variety of sites throughout the CNS, including the hypothalamus, thalamus, various areas throughout the brainstem, and the dorsal horn of the spinal cord (Masuo et al., 1992; Narita et al., 1996; Tajti et al., 2001), and PAC1 is expressed throughout the brain, including the neocortex, limbic system, and brainstem (Hashimoto et al., 2006; Vaudry et al., 2000). As with CGRP, PACAP has been linked to anxiety-like behavior. PACAP and PAC1 knockout mice have decreased anxiety-like behavior (Hattori et al., 2012; Otto et al., 2001). Both transgenic mice show a variety of neurobehavioral phenotypes including increased hyperactivity, decreased depression-like behavior, and aberrant social interaction (Hattori et al., 2012; Otto et al., 2001). In addition, chronic stress increased PACAP mRNA expression within the bed nucleus of the stria terminalis, suggesting a likely role in stress and anxiety, which are both key triggers in migraine (Hammack et al., 2009; Hammack et al., 2010). Like CGRP and its receptor, PACAP is not expressed in satellite glia cells, and PAC1 and VPAC1 receptors are found in satellite glia (Csati et al., 2012).
Emerging role for PACAP in migraine
A role for PACAP in migraine is primarily based on two lines of clinical evidence. A very recent clinical study in migraineurs demonstrated that PACAP plasma levels were elevated during a migraine attack compared to their interictal levels (Tuka et al., 2013). A complication of this finding is that interictal PACAP levels were lower in comparison to healthy subjects, and ictal PACAP levels were comparable to healthy subjects (Tuka et al., 2013). The second line of evidence is that following peripheral injection of PACAP in migraineurs, 11 out 12 subjects experienced an initial headache, and 7 out of 12 subjects experienced a migraine-like headache that was delayed by 2–11 h with only one exception (Schytz et al., 2009). Injection in healthy subjects led to an initial headache in 12 out 12 subjects, and only 2 subjects experienced a migraine-like headache during the first two hours (Schytz et al., 2009). Hence, PACAP appears to have a similar ability to induce a delayed migraine-like headache as observed with CGRP. Similarly, PACAP induced an initial vasodilation of the middle cerebral artery and superficial temporal artery in both healthy and migraineur subjects, but only the migraineurs experienced a delayed migraine-like headache (Schytz et al., 2009). Like CGRP, migraineurs seems to be particularly sensitive to PACAP. This clinical evidence has garnished attention to the possible role of this peptide in migraine pathophysiology.
Comparisons of PACAP and CGRP activities relevant to migraine
A very intriguing shared activity of PACAP and CGRP is their ability to induce light aversion in mice. Peripheral injection of nitroglycerol and PACAP-38 has been shown to induce light aversive behavior in wildtype mice, but not in PACAP knockout mice (Markovics et al., 2012). These findings reflect similar studies with CGRP, which induces light aversion in CGRP-sensitized and wildtype mice (Kaiser et al., 2012; Recober et al., 2010; Recober et al., 2009).
Unlike CGRP, PACAP has been reported to be antinociceptive in the periphery (Helyes et al., 2007; Nemeth et al., 2006; Sandor et al., 2009; Sandor et al., 2010). However, a PACAP knockout mouse indicated that PACAP plays an excitatory role in pain transmission, suggesting a possible role in central sensitization (Sandor et al., 2010). Furthermore, a recent study has shown that injection of PACAP-38 into the paraventricular nucleus of the hypothalamus increased TNC activity, which could be inhibited by a PAC1 receptor antagonist (Robert et al., 2013). In addition, intrathecal injection of PACAP has been shown to induce hyperalgesia in mice (Narita et al., 1996).
Being vasodilatory peptides, both PACAP and CGRP are likely to contribute to vasodilation during neurogenic inflammation. PACAP-containing nerve fibers have been closely localized near cerebrovasculature containing VPAC1 receptors in rats (Fahrenkrug et al., 2000). PAC1, VPAC1 and VPAC2 have been localized to the smooth muscle within the cerebrovasculature of rodents (Erdling et al., 2013). This facilitates VIP-, PACAP-27-, and PACAP-38-induced vasodilation of the middle cerebral artery, but PACAP-38 is the least potent (Erdling et al., 2013). This may be due to greater abundance of VPAC1 receptors compared to VPAC2 and PAC1 receptors (Baun et al., 2011). However, in the middle meningeal artery, another study showed PACAP-38 to be as potent as VIP (Boni et al., 2009), but in the cerebellar arties, PACAP-38 was 1000-fold less potent (Syed et al., 2012). While VPAC2 and PAC1 antagonists were ineffective in blocking VIP and PACAP-38 induced vasodilation, VPAC1 antagonist PG97-269 was able to block PACAP-38 induced dilation (Boni et al., 2009), yet in another study, the same PAC1 receptor antagonist was effective in blocking vasodilation (Syed et al., 2012). Interestingly, maxadilan, a selective PAC1 receptor agonist, has not been shown to vasodilate the basilar artery or the middle meningeal artery in the rat (Baun et al., 2011). The contradicting nature of these findings leaves which of the three receptors are key in mitigating PACAP intracranial dilation unanswered. In human studies, PACAP-38 has also been shown to dilate human meningeal arteries, but not as potently as VIP (Chan et al., 2011). Considering that VIP does not induce a migraine and that PACAP38 is a likely a weak vasodilator in intracranial arties in comparison, it has been suggested that the ability of PACAP-38 to induce a migraine is not likely due its vasodilatory effects (Baun et al., 2011), but likely the result of its other central actions (Hashimoto et al., 2006).
Like its role in nociception, PACAP also has inconsistent roles in neurogenic inflammation. PACAP, like CGRP, is upregulated following inflammation in sensory neurons (Zhang et al., 1998). Also, PACAP has been reported to inhibit neurogenic inflammation in the periphery (Helyes et al., 2007; Nemeth et al., 2006; Sandor et al., 2009; Sandor et al., 2010). Yet, PACAP-38 induces dural mast cell degranulation, and was significantly more potent than VIP and PACAP-27 (Baun et al., 2012; Theoharides et al., 2005). This suggests a possible role for PACAP, like CGRP, in triggering neurogenic inflammation within the dura, which may be relevant to migraine.
With respect to CSD, to our knowledge, PACAP in CSD has not been investigated, thus it remains to be seen whether PACAP plays any role in CSD similar to CGRP.
PACAP has also been linked to nitric oxide pathways. In rats, peripheral injection of nitroglycerin increased PACAP within the TNC, and electrical stimulation of the trigeminal ganglia increased PACAP within the plasma and TNC (Tuka et al., 2012). Also, nitroglycerol induced more vasodilation and neuronal activation in trigeminal ganglia and TNC in wildtype mice compared to PACAP knockout mice (Markovics et al., 2012). This suggests that PACAP and NO, like CGRP and NO, are coupled in the trigeminovascular system.
Conclusion
CGRP clearly plays an integral role in migraine based on several clinical studies. While its exact contributions in migraine are not known, it has known roles in several events associated with migraine, light aversion, nociceptive sensitization, neurogenic inflammation, cortical spreading depression, and nitric oxide generation. Thus, CGRP is well positioned to contribute to migraine symptoms. The ability of CGRP receptor antagonists to ameliorate migraine provides conclusive evidence of the involvement of CGRP in migraine. However, CGRP alone cannot account for migraine, but instead is likely to act with other modifiers, such as PACAP.
Although PACAP lacks some of the actions of CGRP that may contribute to migraine pathophysiology, it has some shared actions, such as ability to induce light aversion, neurogenic inflammation and neuromodulation of sensory neurons. These common features suggest CGRP and PACAP might act in concert. This commonality is even more intriguing at a molecular level since both CGRP and PACAP receptors share a RAMP1 subunit, which is functionally rate limiting for CGRP activity. Whether it is also limiting for PACAP activity remains to be tested. Much has to be learned about PACAP and its role in migraine, but it presents potential novel target in migraine therapy. It will be interesting to see if PACAP receptor antagonists are effective in acute migraine therapy as demonstrated with CGRP receptor antagonists (Charles, 2013; Schytz et al., 2010). Thus, these two migraine-inducing neuropeptides, CGRP and PACAP, may provide insight into migraine pathophysiology as future studies delve into the mechanisms shared by these peptides.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, Nyholt DR, Dimas AS, Freilinger T, Muller-Myhsok B, Artto V, Inouye M, Alakurtti K, Kaunisto MA, Hamalainen E, de Vries B, Stam AH, Weller CM, Heinze A, Heinze-Kuhn K, Goebel I, Borck G, Gobel H, Steinberg S, Wolf C, Bjornsson A, Gudmundsson G, Kirchmann M, Hauge A, Werge T, Schoenen J, Eriksson JG, Hagen K, Stovner L, Wichmann HE, Meitinger T, Alexander M, Moebus S, Schreiber S, Aulchenko YS, Breteler MM, Uitterlinden AG, Hofman A, van Duijn CM, Tikka-Kleemola P, Vepsalainen S, Lucae S, Tozzi F, Muglia P, Barrett J, Kaprio J, Farkkila M, Peltonen L, Stefansson K, Zwart JA, Ferrari MD, Olesen J, Daly M, Wessman M, van den Maagdenberg AM, Dichgans M, Kubisch C, Dermitzakis ET, Frants RR, Palotie A. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold JK, Flanagan JU, Watkins HA, Gingell JJ, Hay DL. Structural insights into RAMP modification of secretin family G protein-coupled receptors: Implications for drug development. Trends Pharmacol Sci. 2011;32:591–600. doi: 10.1016/j.tips.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Ayata C. Cortical spreading depression triggers migraine attack: Pro. Headache. 2010;50:725–730. doi: 10.1111/j.1526-4610.2010.01647.x. [DOI] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- Barwell J, Gingell JJ, Watkins HA, Archbold JK, Poyner DR, Hay DL. Calcitonin and calcitonin receptor-like receptors: Common themes with family B GPCRs? Br J Pharmacol. 2012;166:51–65. doi: 10.1111/j.1476-5381.2011.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baun M, Hay-Schmidt A, Edvinsson L, Olesen J, Jansen-Olesen I. Pharmacological characterization and expression of VIP and PACAP receptors in isolated cranial arteries of the rat. Euro J Pharmacol. 2011;670:186–194. doi: 10.1016/j.ejphar.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Baun M, Pedersen MH, Olesen J, Jansen-Olesen I. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia. 2012;32:337–345. doi: 10.1177/0333102412439354. [DOI] [PubMed] [Google Scholar]
- Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Euro J Neurosci. 2006a;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy JL, Cady RK, Durham PL. Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache. 2006b;46:24–33. doi: 10.1111/j.1526-4610.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- Boni LJ, Ploug KB, Olesen J, Jansen-Olesen I, Gupta S. The in vivo effect of VIP, PACAP-38 and PACAP-27 and mrna expression of their receptors in rat middle meningeal artery. Cephalalgia. 2009;29:837–847. doi: 10.1111/j.1468-2982.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- Borzsei R, Mark L, Tamas A, Bagoly T, Bay C, Csanaky K, Banki E, Kiss P, Vaczy A, Horvath G, Nemeth J, Szauer E, Helyes Z, Reglodi D. Presence of pituitary adenylate cyclase activating polypeptide-38 in human plasma and milk. Euro J Endocrinol. 2009;160:561–565. doi: 10.1530/EJE-08-0911. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Botia B, Couvineau A, Laburthe M, Vaudry H, Fournier A. Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides. 2008;29:919–932. doi: 10.1016/j.peptides.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Bove GM, Moskowitz MA. Primary afferent neurons innervating guinea pig dura. J Neurophysiol. 1997;77:299–308. doi: 10.1152/jn.1997.77.1.299. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiological Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Charles A. An update on the blood vessel in migraine. Current Opinion Neurol. 2010;23:266–274. doi: 10.1097/WCO.0b013e32833821c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Bussone G. Clinical considerations on chronic migraine, pharmacoresistance and refractoriness. Neurological Sci. 2010;31(Suppl 1):S83–85. doi: 10.1007/s10072-010-0294-5. [DOI] [PubMed] [Google Scholar]
- Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–1266. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ., Jr Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: An anterograde and retrograde tract tracing study combined with fos expression. J Comp Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehavioral Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: Relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Baun M, de Vries R, van den Bogaerdt AJ, Dirven CM, Danser AH, Jansen-Olesen I, Olesen J, Villalon CM, MaassenVanDenBrink A, Gupta S. Pharmacological characterization of VIP and PACAP receptors in the human meningeal and coronary artery. Cephalalgia. 2011;31:181–189. doi: 10.1177/0333102410375624. [DOI] [PubMed] [Google Scholar]
- Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: An electrophysiological and immunohistochemical study. J Neurosci. 2009;29:12532–12541. doi: 10.1523/JNEUROSCI.2887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. The evolution of a migraine attack - a review of recent evidence. Headache. 2013;53:413–419. doi: 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AM, Fendrich K, Volzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RY, Kurth T. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Colonna DM, Meng W, Deal DD, Busija DW. Calcitonin gene-related peptide promotes cerebrovascular dilation during cortical spreading depression in rabbits. Am J Physiol. 1994;266:H1095–1102. doi: 10.1152/ajpheart.1994.266.3.H1095. [DOI] [PubMed] [Google Scholar]
- Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–977. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch JR. Update on chronic daily headache. Current Treatment Options Neurol. 2011;13:41–55. doi: 10.1007/s11940-010-0104-7. [DOI] [PubMed] [Google Scholar]
- Csati A, Tajti J, Kuris A, Tuka B, Edvinsson L, Warfvinge K. Distribution of vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, nitric oxide synthase, and their receptors in human and rat sphenopalatine ganglion. Neuroscience. 2012;202:158–168. doi: 10.1016/j.neuroscience.2011.10.055. [DOI] [PubMed] [Google Scholar]
- De Corato A, Lisi L, Capuano A, Tringali G, Tramutola A, Navarra P, Dello Russo C. Trigeminal satellite cells express functional calcitonin gene-related peptide receptors, whose activation enhances interleukin-1beta pro-inflammatory effects. J Neuroimmunol. 2011;237:39–46. doi: 10.1016/j.jneuroim.2011.05.013. [DOI] [PubMed] [Google Scholar]
- de Lacalle S, Saper CB. Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience. 2000;100:115–130. doi: 10.1016/s0306-4522(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Diener HC. Rpr100893, a substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia. 2003;23:183–185. doi: 10.1046/j.1468-2982.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Diener HC, Lampl C, Reimnitz P, Voelker M. Aspirin in the treatment of acute migraine attacks. Expert Rev Neurotherapeutics. 2006;6:563–573. doi: 10.1586/14737175.6.4.563. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M. Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurology. 2005;489:92–119. doi: 10.1002/cne.20618. [DOI] [PubMed] [Google Scholar]
- Eberhardt M, Hoffmann T, Sauer SK, Messlinger K, Reeh PW, Fischer MJ. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides. 2008;42:311–317. doi: 10.1016/j.npep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- Ebersberger A, Charbel Issa P, Vanegas H, Schaible HG. Differential effects of calcitonin gene-related peptide and calcitonin gene-related peptide 8-37 upon responses to N-methyl-D-aspartate or (r, s)-alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate in spinal nociceptive neurons with knee joint input in the rat. Neuroscience. 2000;99:171–178. doi: 10.1016/s0306-4522(00)00176-7. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Alm R, Shaw D, Rutledge RZ, Koblan KS, Longmore J, Kane SA. Effect of the CGRP receptor antagonist bibn4096bs in human cerebral, coronary and omental arteries and in SK-N-MC cells. Euro J Pharmacol. 2002;434:49–53. doi: 10.1016/s0014-2999(01)01532-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Eftekhari S, Salvatore CA, Warfvinge K. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Molec Cellular Neurosci. 2011;46:333–339. doi: 10.1016/j.mcn.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–696. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Eltorp CT, Jansen-Olesen I, Hansen AJ. Release of calcitonin gene-related peptide (CGRP) from guinea pig dura mater in vitro is inhibited by sumatriptan but unaffected by nitric oxide. Cephalalgia. 2000;20:838–844. doi: 10.1046/j.1468-2982.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Erdling A, Sheykhzade M, Maddahi A, Bari F, Edvinsson L. VIP/PACAP receptors in cerebral arteries of rat: Characterization, localization and relation to intracellular calcium. Neuropeptides. 2013;47:85–92. doi: 10.1016/j.npep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. Localisation of the neuropeptide PACAP and its receptors in the rat parathyroid and thyroid glands. Gen Comp Endocrinol. 2011;171:105–113. doi: 10.1016/j.ygcen.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: Relation to PACAP and VIP containing nerves. J Cerebral Blood Flow Metabol. 2000;20:1205–1214. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Fischer MJ. Calcitonin gene-related peptide receptor antagonists for migraine. Expert Opin Investig Drugs. 2010;19:815–823. doi: 10.1517/13543784.2010.490829. [DOI] [PubMed] [Google Scholar]
- Gallai V, Sarchielli P, Floridi A, Franceschini M, Codini M, Glioti G, Trequattrini A, Palumbo R. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15:384–390. doi: 10.1046/j.1468-2982.1995.1505384.x. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ. Can we develop neurally acting drugs for the treatment of migraine? Nat Rev Drug Discov. 2005;4:741–750. doi: 10.1038/nrd1822. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Migraine: Emerging treatment options for preventive and acute attack therapy. Expert Opin Emerg Drugs. 2006;11:419–427. doi: 10.1517/14728214.11.3.419. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. The vascular theory of migraine--a great story wrecked by the facts. Brain. 2009;132:6–7. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurology. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurology. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurology. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Gu XL, Yu LC. The colocalization of CGRP receptor and AMPA receptor in the spinal dorsal horn neuron of rat: A morphological and electrophysiological study. Neurosci Lett. 2007;414:237–241. doi: 10.1016/j.neulet.2006.12.056. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): Roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Molec Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann NY Acad Sci. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- Hattori S, Takao K, Tanda K, Toyama K, Shintani N, Baba A, Hashimoto H, Miyakawa T. Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Frontiers Behavioral Neurosci. 2012;6:58. doi: 10.3389/fnbeh.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins K, Wang S, Rupnow M. Direct cost burden among insured us employees with migraine. Headache. 2008;48:553–563. doi: 10.1111/j.1526-4610.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Hawkins K, Wang S, Rupnow MF. Indirect cost burden of migraine in the United States. J Occupational Environmental Med. 2007;49:368–374. doi: 10.1097/JOM.0b013e31803b9510. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, n-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-d ibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2h)-quinazoliny l) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors--the role of receptor activity modifying protein 1. Molec Pharmacol. 2006;70:1984–1991. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Headache CSotIHS. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Pozsgai G, Borzsei R, Nemeth J, Bagoly T, Mark L, Pinter E, Toth G, Elekes K, Szolcsanyi J, Reglodi D. Inhibitory effect of PACAP-38 on acute neurogenic and non-neurogenic inflammatory processes in the rat. Peptides. 2007;28:1847–1855. doi: 10.1016/j.peptides.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Heroux M, Hogue M, Lemieux S, Bouvier M. Functional calcitonin gene-related peptide receptors are formed by the asymmetric assembly of a calcitonin receptor-like receptor homo-oligomer and a monomer of receptor activity-modifying protein-1. J Biol Chem. 2007;282:31610–31620. doi: 10.1074/jbc.M701790200. [DOI] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev: Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: A randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008a;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008b;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. General Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Hoskin KL, Zagami AS, Goadsby PJ. Stimulation of the middle meningeal artery leads to fos expression in the trigeminocervical nucleus: A comparative study of monkey and cat. J Anatomy. 1999;194 (Pt 4):579–588. doi: 10.1046/j.1469-7580.1999.19440579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: Disability and economic costs. Arch Internal Med. 1999;159:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- Huang R, Karve A, Shah I, Bowers MC, DiPette DJ, Supowit SC, Abela GS. Deletion of the mouse alpha-calcitonin gene-related peptide gene increases the vulnerability of the heart to ischemia-reperfusion injury. Am J Physiol. 2008;294:H1291–1297. doi: 10.1152/ajpheart.00749.2007. [DOI] [PubMed] [Google Scholar]
- Huang Y, Brodda-Jansen G, Lundeberg T, Yu LC. Anti-nociceptive effects of calcitonin gene-related peptide in nucleus raphe magnus of rats: An effect attenuated by naloxone. Brain Res. 2000;873:54–59. doi: 10.1016/s0006-8993(00)02473-2. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Jenkins DW, Langmead CJ, Parsons AA, Strijbos PJ. Regulation of calcitonin gene-related peptide release from rat trigeminal nucleus caudalis slices in vitro. Neurosci Lett. 2004;366:241–244. doi: 10.1016/j.neulet.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25:179–183. doi: 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, Szolcsanyi J, Vitrai J, Bagdy G. NO-induced migraine attack: Strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32:15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ. Expression of c-fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993;629:95–102. doi: 10.1016/0006-8993(93)90486-7. [DOI] [PubMed] [Google Scholar]
- Kelman L. The aura: A tertiary care study of 952 migraine patients. Cephalalgia. 2004a;24:728–734. doi: 10.1111/j.1468-2982.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Kelman L. The premonitory symptoms (prodrome): A tertiary care study of 893 migraineurs. Headache. 2004b;44:865–872. doi: 10.1111/j.1526-4610.2004.04168.x. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, Fischer MJ, De Col R, Schlechtweg PM, Messlinger K. Biphasic response to nitric oxide of spinal trigeminal neurons with meningeal input in rat--possible implications for the pathophysiology of headaches. J Neurophysiol. 2004;92:1320–1328. doi: 10.1152/jn.01210.2003. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Somogyvari-Vigh A, Vigh S, Miller J. Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology. 1990;127:264–271. doi: 10.1210/endo-127-1-264. [DOI] [PubMed] [Google Scholar]
- Kresse A, Jacobowitz DM, Skofitsch G. Detailed mapping of CGRP mRNA expression in the rat central nervous system: Comparison with previous immunocytochemical findings. Brain Res Bull. 1995;36:261–274. doi: 10.1016/0361-9230(94)00201-b. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus. 2003;13:835–844. doi: 10.1002/hipo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Peptides. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Lambert GA. The lack of peripheral pathology in migraine headache. Headache. 2010;50:895–908. doi: 10.1111/j.1526-4610.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- Lambert GA, Zagami AS. The mode of action of migraine triggers: A hypothesis. Headache. 2009;49:253–275. doi: 10.1111/j.1526-4610.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Jacobsen VB, Haderslev PA, Sperling B, Iversen HK, Olesen J, Tfelt-Hansen P. Involvement of calcitonin gene-related peptide in migraine: Regional cerebral blood flow and blood flow velocity in migraine patients. J Headache Pain. 2008;9:151–157. doi: 10.1007/s10194-008-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen LH, Thomsen LL, Olesen J. Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. Neuroreport. 1995;6:1475–1479. doi: 10.1097/00001756-199507310-00003. [DOI] [PubMed] [Google Scholar]
- Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Levy D. Migraine pain and nociceptor activation--where do we stand? Headache. 2010;50:909–916. doi: 10.1111/j.1526-4610.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R. The vascular theory of migraine: Leave it or love it? Ann Neurology. 2011;69:600–601. doi: 10.1002/ana.22422. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci USA. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: Data from the American migraine study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Broman J, Edvinsson L. Central projections of the sensory innervation of the rat middle meningeal artery. Brain Res. 2008;1208:103–110. doi: 10.1016/j.brainres.2008.02.078. [DOI] [PubMed] [Google Scholar]
- Loder E. Triptan therapy in migraine. N Engl J Med. 2010;363:63–70. doi: 10.1056/NEJMct0910887. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Hua X, Hokfelt T, Fischer JA. Co-existence of substance p and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Euro J Pharmacol. 1985;108:315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Disease. 2012;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de Prado B, Hammond DL, Russo AF. Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. J Pain. 2009;10:992–1000. doi: 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RS, Martin GR. Investigations into migraine pathogenesis: Time course for effects of m-cpp, bw723c86 or glyceryl trinitrate on appearance of fos-like immunoreactivity in rat trigeminal nucleus caudalis (TNC) Cephalalgia. 2001;21:46–52. doi: 10.1046/j.1468-2982.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M, Fujino M. Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): Comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res. 1992;575:113–123. doi: 10.1016/0006-8993(92)90430-h. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: Functional role in cerebrovascular regulation. Proc Natl Acad Sci USA. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Menon S, Buteri J, Roy B, Murrell M, Quinlan S, Macmillan JC, Lea RA, Haupt LM, Griffiths LR. Association study of calcitonin gene-related polypeptide-alpha (CALCA) gene polymorphism with migraine. Brain Res. 2011;1378:119–124. doi: 10.1016/j.brainres.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Messlinger K. Migraine: Where and how does the pain originate? Exp Brain Res. 2009;196:179–193. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Baumgartel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: Ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol. 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Abounader R, Hebert E, Doods H, Hamel E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: Potential implications in acute migraine treatment. Neuropharmacology. 2002;42:568–576. doi: 10.1016/s0028-3908(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Morfis M, Christopoulos A, Sexton PM. RAMPs: 5 years on, where to now? Trends Pharmacol Sci. 2003;24:596–601. doi: 10.1016/j.tips.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43:S16–20. [PubMed] [Google Scholar]
- Moya F, Nieto A, JLRC Calcitonin biosynthesis: Evidence for a precursor. Euro J Biochem. 1975;55:407–413. doi: 10.1111/j.1432-1033.1975.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Dun SL, Dun NJ, Tseng LF. Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord. Euro J Pharmacol. 1996;311:121–126. doi: 10.1016/0014-2999(96)00359-7. [DOI] [PubMed] [Google Scholar]
- Nemeth J, Reglodi D, Pozsgai G, Szabo A, Elekes K, Pinter E, Szolcsanyi J, Helyes Z. Effect of pituitary adenylate cyclase activating polypeptide-38 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience. 2006;143:223–230. doi: 10.1016/j.neuroscience.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhauser N, Zinck T, Hoffmann J, Schiemann K, Schuh-Hofer S, Rohde W, Arnold G, Dirnagl U, Jansen-Olesen I, Reuter U. CGRP release and c-fos expression within trigeminal nucleus caudalis of the rat following glyceryltrinitrate infusion. Cephalalgia. 2005;25:225–236. doi: 10.1111/j.1468-2982.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cerebral Blood Flow Metabol. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res: Molec Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J. Bibn 4096bs antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Therapeutics. 2005;77:202–213. doi: 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev: Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- Prado MA, Evans-Bain B, Dickerson IM. Receptor component protein (rcp): A member of a multi-protein complex required for g-protein-coupled signal transduction. Biochem Soc Transactions. 2002;30:460–464. doi: 10.1042/bst0300460. [DOI] [PubMed] [Google Scholar]
- Purdy RA. The role of the visual system in migraine: An update. Neurological Sci. 2011;32(Suppl 1):S89–93. doi: 10.1007/s10072-011-0541-4. [DOI] [PubMed] [Google Scholar]
- Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Reviews Molec Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Rapoport AM. New acute treatments for headache. Neurological Sci. 2010;31(Suppl 1):S129–132. doi: 10.1007/s10072-010-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: Implications for migraine. J Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Russo AF. Calcitonin gene-related peptide: An update on the biology. Current Opinion Neurology. 2009;22:241–246. doi: 10.1097/wco.0b013e32832b2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Horvath G, Lubics A, Laszlo E, Tamas A, Racz B, Szakaly P. Effects of pituitary adenylate cyclase activating polypeptide in the urinary system, with special emphasis on its protective effects in the kidney. Neuropeptides. 2012;46:61–70. doi: 10.1016/j.npep.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, Villanueva L. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33:8827–8840. doi: 10.1523/JNEUROSCI.0439-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF, Kuburas A, Kaiser EA, Raddant AC, Recober A. A potential preclinical migraine model: CGRP-sensitized mice. Molec Cellul Pharmacol. 2009;1:264–270. [PMC free article] [PubMed] [Google Scholar]
- Russo AF, Nelson C, Roos BA, Rosenfeld MG. Differential regulation of the coexpressed calcitonin/alpha-CGRP and beta-CGRP neuroendocrine genes. J Biol Chem. 1988;263:5–8. [PubMed] [Google Scholar]
- Sandor K, Bolcskei K, McDougall JJ, Schuelert N, Reglodi D, Elekes K, Petho G, Pinter E, Szolcsanyi J, Helyes Z. Divergent peripheral effects of pituitary adenylate cyclase-activating polypeptide-38 on nociception in rats and mice. Pain. 2009;141:143–150. doi: 10.1016/j.pain.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Sandor K, Kormos V, Botz B, Imreh A, Bolcskei K, Gaszner B, Markovics A, Szolcsanyi J, Shintani N, Hashimoto H, Baba A, Reglodi D, Helyes Z. Impaired nocifensive behaviours and mechanical hyperalgesia, but enhanced thermal allodynia in pituitary adenylate cyclase-activating polypeptide deficient mice. Neuropeptides. 2010;44:363–371. doi: 10.1016/j.npep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain. 2008;131:2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Goadsby PJ. 14th international headache congress: Basic science highlights. Headache. 2010;50:520–526. doi: 10.1111/j.1526-4610.2010.01616.x. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Olesen J, Ashina M. The PACAP receptor: A novel target for migraine treatment. Neurotherapeutics. 2010;7:191–196. doi: 10.1016/j.nurt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS. The role of peptides in central sensitization. Handbook Exp Pharmacol. 2009:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Goadsby PJ. Migraine: Preventive treatment. Cephalalgia. 2002;22:491–512. doi: 10.1046/j.1468-2982.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Autoradiographic distribution of 125I calcitonin gene-related peptide binding sites in the rat central nervous system. Peptides. 1985a;6:975–986. doi: 10.1016/0196-9781(85)90331-6. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides. 1985b;6:747–754. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- Strassman A, Mason P, Moskowitz M, Maciewicz R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986;379:242–250. doi: 10.1016/0006-8993(86)90777-8. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Strecker T, Dux M, Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J Vascular Res. 2002;39:489–496. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- Summ O, Charbit AR, Andreou AP, Goadsby PJ. Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain. 2010;133:2540–2548. doi: 10.1093/brain/awq224. [DOI] [PubMed] [Google Scholar]
- Syed AU, Koide M, Braas KM, May V, Wellman GC. Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: Implications for migraine. J Molec Neurosci. 2012;48:574–583. doi: 10.1007/s12031-012-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajti J, Uddman R, Edvinsson L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia. 2001;21:96–101. doi: 10.1046/j.1468-2982.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- Tajti J, Uddman R, Moller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Autonomic Nervous Syst. 1999;76:176–183. doi: 10.1016/s0165-1838(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. NADPH-diaphorase activity and fos expression in brain nuclei following nitroglycerin administration. Brain Res. 1995a;695:37–44. doi: 10.1016/0006-8993(95)00732-6. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. Systemic nitroglycerin induces fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995b;682:167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Le H. Calcitonin gene-related peptide in blood: Is it increased in the external jugular vein during migraine and cluster headache? A review. J Headache Pain. 2009;10:137–143. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J. Taking the negative view of current migraine treatments: The unmet needs. CNS Drugs. 2012;26:375–382. doi: 10.2165/11630590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res: Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, Bonsi P, Picconi B, Cupini LM, Materazzi S, Geppetti P, Sarchielli P, Calabresi P. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci USA. 2012;109:18985–18990. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuka B, Helyes Z, Markovics A, Bagoly T, Nemeth J, Mark L, Brubel R, Reglodi D, Pardutz A, Szolcsanyi J, Vecsei L, Tajti J. Peripheral and central alterations of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides. 2012;33:307–316. doi: 10.1016/j.peptides.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, Toth E, Kincses ZT, Vecsei L, Tajti J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia. 2013;33:1085–1095. doi: 10.1177/0333102413483931. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- Uddman R, Tajti J, Hou M, Sundler F, Edvinsson L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia. 2002;22:112–116. doi: 10.1046/j.1468-2982.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehavioral Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vause CV, Durham PL. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: Findings from array analysis. Neurosci Lett. 2010;473:163–167. doi: 10.1016/j.neulet.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Therapeutics. 2009;124:309–323. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64:S9–15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- Wahl M, Schilling L, Parsons AA, Kaumann A. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res. 1994;637:204–210. doi: 10.1016/0006-8993(94)91234-3. [DOI] [PubMed] [Google Scholar]
- Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci. 2010;31:476–483. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Spinal cord glia: New players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: Molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocrine Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- Wolff HG, Tunis MM, Goodell H. Studies on headache; evidence of damage and changes in pain sensitivity in subjects with vascular headaches of the migraine type. A.M.A. Archives Internal Med. 1953;92:478–484. doi: 10.1001/archinte.1953.00240220026006. [DOI] [PubMed] [Google Scholar]
- Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: A role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashpal K, Kar S, Dennis T, Quirion R. Quantitative autoradiographic distribution of calcitonin gene-related peptide (hCGRP alpha) binding sites in the rat and monkey spinal cord. J Comp Neurol. 1992;322:224–232. doi: 10.1002/cne.903220208. [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J Neurosci. 2010;30:8807–8814. doi: 10.1523/JNEUROSCI.0511-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dickerson IM, Russo AF. Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology. 2006;147:1932–1940. doi: 10.1210/en.2005-0918. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


