Sir, We read the recent review on microbleeds in Alzheimer’s disease by Cordonnier and van der Flier (2011) with interest. We agree that determining the relevant contributions of cerebrovascular disease (related to conventional risk factors including hypertension) and cerebral amyloid angiopathy to microbleeds identified on T2*-weighted imaging is an important issue, which may lead to new insights into the pathology and pathogenesis of Alzheimer’s disease. One of the difficulties in attempting to delineate the relative contributions of conventional cerebrovascular and cerebral amyloid angiopathy pathology in sporadic Alzheimer’s disease is that multiple pathologies are common at more advanced ages. An alternative strategy is to study patients with autosomal dominantly inherited familial Alzheimer’s disease: such individuals can be diagnosed definitively during life and, having younger age at symptom onset, are much more likely to have ‘pure’ Alzheimer’s disease without co-existing cerebrovascular disease. To our knowledge, no studies have attempted to assess the frequency of microbleeds in familial Alzheimer’s disease.
To investigate this question, we identified all individuals with genetically confirmed familial Alzheimer’s disease who had undergone T2*-weighted MRI as part of a longitudinal study of familial Alzheimer’s disease at the Dementia Research Centre, UCL. A total of 12 patients with familial Alzheimer’s disease (mean age: 49.4 years; range: 35.8–63.4 years; mean disease duration: 4.0 years, range: 1.1–6.7 years; 75% male) were eligible for the study. Eleven patients had a mutation in the Presenilin 1 (PSEN1) gene (three intron 4 mutations and one each of L166R, Y115H, E120K, L250S, P264L, R269H, R278I, E280G), and one patient carried the V717I amyloid precursor protein mutation. Twelve controls (mean age: 59.8 years; range: 46.9–66.5 years; 50% male) were also studied. All mutation carriers were symptomatic and fulfilled criteria for Alzheimer’s disease dementia at the time of scanning. T2* gradient echo sequences (repetition time/echo time 625/20 ms, flip angle 20°, field of view 240 × 240, matrix 256 × 320, slice thickness 5 mm) were acquired on the same 3T Siemens MRI scanner. All subjects gave written informed consent according to the Declaration of Helsinki, and the study was approved by the local ethics committee. All scans were assessed by an experienced neuroradiologist, blinded to mutation status, to evaluate the presence, number and location of microbleeds. Microbleeds were defined as small (2–10 mm diameter), round and homogenous foci of low signal intensity on T2*-weighted MRI. Microbleed mimics including basal ganglia mineralization, vessel flow voids in the subarachnoid space and artefacts at air-bone interfaces were excluded in line with standardized criteria (Gregoire et al., 2009). The number and location of microbleeds was assessed in the following regions: cortical/cortico-subcortical (frontal, temporal or parieto-occipital), infratentorial (brainstem and cerebellum) and the basal ganglia (including thalamus).
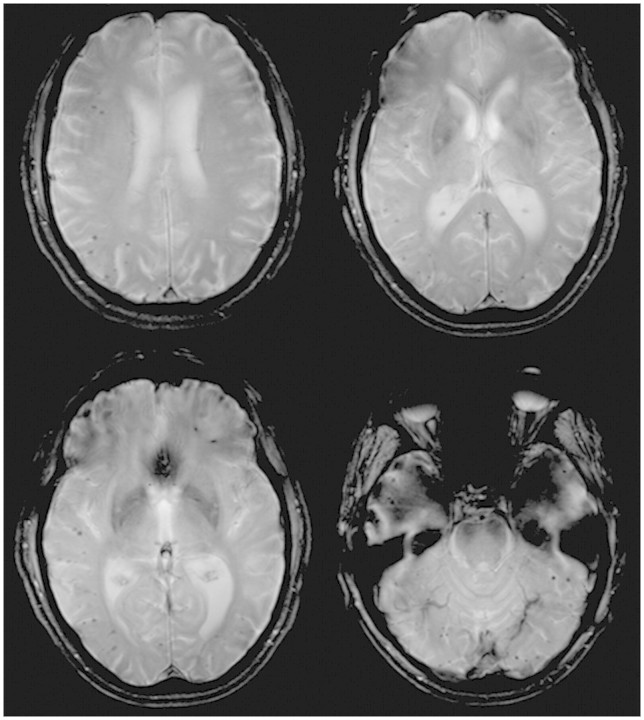
Microbleeds were found in 3 out of 12 patients and none of the controls. Two PSEN1 mutation carriers had one microbleed each (L166R right parieto-occipital; intron 4 left infratentorial). Only one patient had multiple, predominantly cortical/cortico-subcortical microbleeds (>30 in total) and fulfilled Boston Criteria for probable cerebral amyloid angiopathy (Greenberg et al., 1996; van Rooden et al., 2009). This subject, a 59-year-old male harbouring the R269H PSEN1 mutation, had a 1-year history of cognitive decline at the time of referral and recruitment to the study. He was adopted and had no siblings. There was no significant past history and no hypertension or other vascular risk factors. The neurological examination revealed finger myoclonus but was otherwise unremarkable. The Mini-Mental State Examination was 24/30. Cognitive testing revealed him to have little insight into his problems, a pronounced dysexecutive syndrome, mild episodic memory impairment, and relative preservation of parietal lobe function. Three dimensional T1-weighted images showed mild generalized volume loss without predominance of medial temporal lobe atrophy. T2 Fluid Attenuated Inversion Recovery MRI depicted mild-moderate white matter disease (Fig. 1). T2*-weighted imaging revealed prominent cerebellar, parieto-occipital and temporal lobar microbleeds (Fig. 2).
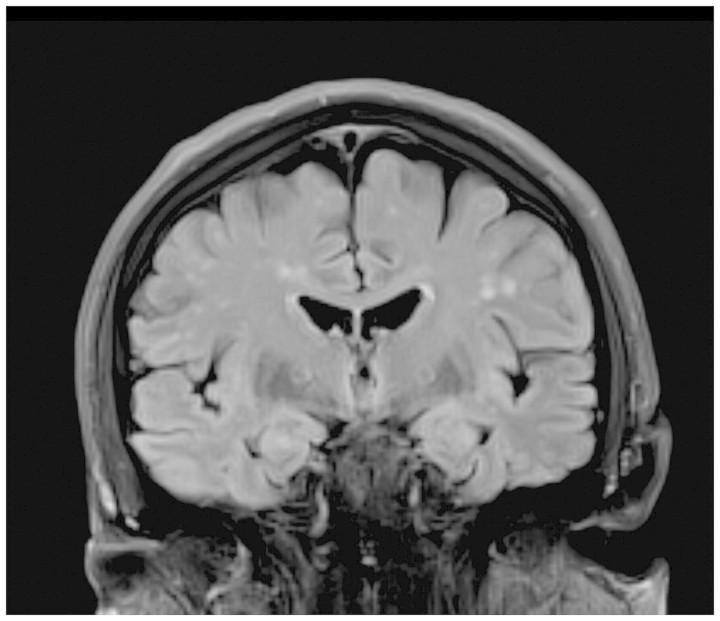
Figure 1.
3T T2-weighted FLAIR coronal MRI shows white matter lesions, but no significant medial temporal lobe atrophy.
Figure 2.
3T T2* MRI shows multiple lobar hypointensities consistent with microbleeds.
Cordonnier and van der Flier (2011) discuss that while cerebral amyloid angiopathy is found at post-mortem in up to 90% of patients with Alzheimer’s disease, only ∼23% of patients with Alzheimer’s disease have identifiable microbleeds on MRI (although as they point out the prevalence will be markedly influenced by the MRI sequence characteristics). As they comment, it is possible that microbleeds only occur when cerebral amyloid angiopathy is particularly severe; or it may be that patients with Alzheimer’s disease and microbleeds represent a subgroup in whom the pathological process is different. Pathological studies of familial Alzheimer’s disease confirm that while cerebral amyloid angiopathy is seen in the context of mutations in amyloid precursor protein, PSEN1 and PSEN2 and also in amyloid precursor protein duplications, the degree of pathological cerebral amyloid angiopathy in familial Alzheimer’s disease is also highly variable. Thus for example, in PSEN1-associated familial Alzheimer’s disease, mutations beyond codon 200 appear to be associated with particularly severe cerebral amyloid angiopathy (Mann et al., 2001). Cerebral amyloid angiopathy, when present, may also be associated with different clinical correlates: some mutations within the Aβ coding domain of amyloid precursor protein leading to significant pathological cerebral amyloid angiopathy burden are associated with recurrent cerebral haemorrhage, while others can present with a purely cognitive phenotype (Ryan and Rossor, 2010).
Our data confirm that microbleeds are not an inevitable feature of even ‘pure’ genetically confirmed Alzheimer’s disease: although numbers are small, the 25% prevalence of one or more microbleed in our series is comparable with Cordonnier and van der Flier’s (2011) pooled proportion of 23% for sporadic Alzheimer’s disease. While we studied just one patient with an amyloid precursor protein mutation, it is striking that only one individual with a PSEN1 mutation had microbleeds sufficient to fulfil criteria for probable cerebral amyloid angiopathy, and that this patient had a mutation (R269H) that has been associated both with relatively late age at onset and with conspicuous amyloid overproduction (Gómez-Isla et al., 1997). We hypothesize that the atypical phenotype with a prominent early dysexecutive syndrome in this case might, in part, relate to the presence of significant cerebral amyloid angiopathy (Arvanitakis et al., 2011).
As Cordonnier and van der Flier (2011) highlight, interest in the relationship between Alzheimer’s disease and microbleeds is particularly timely in light of the findings of recent immunotherapy trials for Alzheimer’s disease. There is evidence that Aβ immunotherapy may lead to accumulation of Aβ42 in perivascular drainage pathways, with an associated increase in amyloid angiopathy and cerebral microbleeds (Boche et al., 2008). It seems that clearance of this plaque-derived Aβ42 can occur over time, but both the efficacy of the process and risk of vasogenic oedema varies and may be influenced by the degree of pre-existing cerebral amyloid angiopathy. As Alzheimer’s disease prevention trials for presymptomatic mutation carriers start to be contemplated (Bateman et al., 2011) a deeper understanding of the relationship between cerebral amyloid angiopathy and microbleeds in different familial Alzheimer’s disease mutations will become increasingly relevant. Large, multicentre studies of familial Alzheimer’s disease such as the Dominantly Inherited Alzheimer Network (DIAN), and newer scan sequences potentially more sensitive to microbleeds, will be ideally placed to address these issues.
Funding
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer’s Research UK Co-ordinating Centre and has also received equipment funded by Alzheimer’s Research UK. Genetic analysis was conducted by the Medical Research Council (MRC) Prion Unit at UCL Institute of Neurology. N.S.R. is supported by an MRC Clinical Research Training Fellowship (G0900421), N.C.F. holds an MRC Senior Clinical Fellowship (G116/143). N.C.F. and M.N.R. are NIHR senior investigators and M.N.R. is a site PI for the NIA-funded DIAN study, which receives additional support from DeNDRON, the NIHR clinical research network for dementia and neurodegenerative diseases. D.J.W. and J.M.S. are supported by Department of Health and Higher Educational and Funding Council for England Clinical Senior Lectureship Awards.
References
- Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69:320–7. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1–13. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequences of Aβ immunisation on the vasculature of human Alzheimer’s disease brain. Brain. 2008;131:3299–310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain. 2011;134(Pt 2):335–44. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Wasco W, Pettingell WP, Gurubhagavatula S, Schmidt SD, Jondro PD, et al. A novel presenilin-1 mutation: increased beta-amyloid and neurofibrillary changes. Ann Neurol. 1997;41:809–13. doi: 10.1002/ana.410410618. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46:1751–4. doi: 10.1212/wnl.46.6.1751. [DOI] [PubMed] [Google Scholar]
- Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- Mann DM, Pickering-Brown SM, Takeuchi A, Iwatsubo T. Amyloid angiopathy and variability in amyloid beta deposition is determined by mutation position in presenilin-1-linked Alzheimer’s disease. Am J Pathol. 2001;158:2165–75. doi: 10.1016/s0002-9440(10)64688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NS, Rossor MN. Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomarkers in Medicine. 2010;4:99–112. doi: 10.2217/bmm.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–72. doi: 10.1161/STROKEAHA.109.554378. [DOI] [PubMed] [Google Scholar]