Abstract
Studies onspermatogonial stem cells (SSCs) are of unusual significance because they are the unique stem cells that transmit genetic information to subsequent generations and they can acquire pluripotency to become embryonic stem-like cells that have therapeutic applications in human diseases. MicroRNAs (miRNAs) have recently emerged as critical endogenous regulators in mammalian cells. However, the function and mechanisms of individual miRNAs in regulating SSC fate remain unknown. Here we report for the first time that miRNA-20 and miRNA-106a are preferentially expressed in mouse SSCs. Functional assays in vitro and in vivo using miRNA mimics and inhibitors reveal that miRNA-20 and miRNA-106a are essential for renewal of SSCs. We further demonstrate that these two miRNAs promote renewal at the post-transcriptional level via targeting STAT3 and Ccnd1 and that knockdown of STAT3, Fos, and Ccnd1 results in renewal of SSCs. This study thus provides novel insights into molecular mechanisms regulating renewal and differentiation of SSCs and may have important implications for regulating male reproduction.
Keywords: miRNA-20 and miRNA-106a, spermatogonial stem cells, self-renewal, STAT3 and Ccnd1
Introduction
MiRNAs have recently been identified as a novel class of short single-stranded endogenous small RNA molecules (∼22 nucleotides). Although miRNAs were first discovered in Caenorhabditis elegans in 1993 [1], it was only 12 years ago that miRNAs were identified in mammals [2]. MiRNAs are highly conserved between animals and humans, and it has been estimated that miRNAs may regulate 30% of all genes in the human genome [3]. MiRNAs act as crucial regulators for post-transcriptional gene silencing by base-pairing with the 3′-untranslated regions (UTRs) of target mRNAs to form the RNA duplexes which lead to either endonucleolytic cleavage of the target mRNA or translation suppression. Recent studies indicate that miRNAs may have critical functions in diverse biological processes, including cell proliferation [4], differentiation [5, 6], and apoptosis [7].
Spermatogenesis is a complex process by which SSCs (also called male germline stem cells) divide and differentiate into spermatozoa. Studies on SSCs are of paramount significance because they are the only stem cells that undergo renewal throughout life and transmit genetic information to subsequent generations. Furthermore, accumulating evidence indicates that SSCs can be cultured to become pluripotent embryonic stem (ES)-like cells that are able to differentiate into all cells of the three germ layers [8-13], highlighting potentially important applications of these cells for regenerative medicine. Gangaraju and Lin published an informative review on the role of miRNAs in stem cells [14] and underscored the functional importance of miRNAs in ES cells, germline stem cells, and somatic tissue stem cells. A recent study showed differential expression patterns of X-linked miRNAs in male germ cells [15]. Another report suggested that several miRNAs in the miRNA 17-92 cluster are highly expressed in gonocytes of mice at 3 days of age [16] and miRNA expression profiles have been shown in mouse SSCs, pre-meiotic germ cells, and meiotic male germ cells [17]. The role of miRNA-21 was recently shown to be important for regulating Thy1(+) enriched germ cells in the testis [18]. Thy1+ cells in mice contain the SSC population, but Thy1 is not a specific marker for SSCs. It has been reported that miRNA-221 and miRNA-222 are required for maintaining mouse spermatogonia in an undifferentiated state and the impaired function of these miRNAs leads to an in initial differentiation of SSCs into type A1-A4 spermatogonia [19]. MiRNA-146 has been shown to regulate the differentiation of mouse SSCs through the regulation of retinoic acid [20]. There are about 1,000 miRNAs present in the mouse and human genomes, and it is very likely that other miRNAs also regulate the fate of SSCs. Therefore, the function and mechanisms of individual miRNAs in regulating mammalian germline stem cell (SSC) fate determinations remain almost unknown and research on this topic is still in its infancy. Here we have for the first time explored the expression, function, and targets of miRNA-20 and miRNA-106a in mouse SSCs.
Materials and Methods
Animals
BALB/c male mice at 8-day and 60-day-old, and mothers with 6-day-old male pups were obtained from the Charles River Laboratories, Inc. All animal care procedures were performed pursuant to the National Research Council's Guide for the Care and Use of Laboratory Animals, USA. Experimental protocols were approved by the Georgetown University Animal Care and Use Committee.
Cell Isolation and Culture
Seminiferous tubules were isolated from the testes of 6-day-, 8-day- and 60-day-old mice using enzymatic digestion with collagenase IV (Sigma) and DNase I as described previously [21]. Germ cells and Sertoli cells were obtained using a second-step enzymatic digestion with collagenase IV, hyaluronidase (Sigma), trypsin (Sigma), and DNase [21]. Sertoli cells and germ cells were separated by differential plating [22]. GFRα1 positive spermatogonia and GFRα1 negative spermatogonia (the non-stem cells) were further separated from germ cells of 6-day-old mice by magnetic-activated cell sorting (MACS) using an antibody to GFRα1 pursuant to the procedure as previously described [23]. The c-kit positive spermatogonia were separated from germ cells of 8-day-old mice by MACS using an antibody to c-kit as described [23]. We chose 6-old- and 8-day-old mice to isolate GFRα1 positive spermatogonia and c-kit positive spermatogonia because As, Apr, and Aal spermatogonia are enriched in mice at 6 days of age [21], while differentiating spermatogonia (c-kit positive) predominatein mice at 8 days of age [24]. Adult germ cells were isolated from the testes of 60-day-old mice using a 2-step enzymatic digestion and differential plating [21]. Germ cells were isolated from mouse testes after transplantation of SSCs transfected with miRNA mimic control or miRNA-20 and miRNA-106a mimics according to the procedure mentioned below.
The isolated mouse SSCs (GFRα1 positive spermatogonia) were cultured on plates coated with 0.1% gelatin in StemPro-34 SFM medium supplemented with 100ng/ml GDNF, 10ng/ml human bFGF, 10ng/ml LIF, 20ng/ml EGF, 30ng/ml TGF-β, and 100ng/ml Nodal to maintain the characteristics of SSCs [25-27]. The StemPro-34 SFM medium consisted of a basal liquid medium and frozen supplement (StemPro®-Nutrient Supplement from Invitrogen) that was added at the time of use. All cultures were maintained at 34°C in a humidified 5% CO2 incubator.
RNA Extraction and RT-PCR
RNA was extracted from freshly isolated cells as mentioned above using Trizol (Invitrogen). MiRNA was isolated from C18-4 cells and SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors, or NIH 3T3 cells (a gift from Dr. Aykut Uren, Georgetown University Medical Center), using the mirVana miRNA Isolation Kit (Ambion, Austin, TX) and treated with DNase I to remove any genomic DNA contamination.
Total RNA was extracted from mouse SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors using Trizol. Reverse transcription of purified RNA was performed using oligo (dT) priming and superscript II reverse transcription according to the manufacturer's instructions (Invitrogen). PCR reaction was performed according to the procedure as described previously [27]. The primer pairs of selected genes including Pcna, Kit, Plzf, GFRα1, Pou51, Ret, Stat3, Fos, and Gapdh were designed and listed in Table S2. The PCR reaction started with triplicates at 94°C for 2 min and was performed as follows: denaturation at 94°C for 30 sec, annealing at a temperature (Tm) as indicated in Table S2 for 45 sec, and elongation at 72°C for 45 sec. After 35 cycles, the samples were incubated for an additional 7 min at 72°C. PCR products were separated by electrophoresis on 1.2% agarose gels. The gels were exposed to a Transilluminator (Fisher Scientific, Pittsburg), and pictures were taken with a Photo-Documentation Camera (Fisher Scientific). The data were scanned with an Epson Perfection 3,200 PHOTO, and densitometric analyses were processed with Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Real-time PCR
RNA was extracted from the isolated GFRα1 positive spermatogonia and c-kit positive spermatogonia, C18-4 cells, adult male germ cells, Sertoli cells, or NIH 3T3 cells, or male germ cells from recipient mice transplanted with SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors using the mirVana miRNA Isolation Kit (Ambion, Austin, TX) and treated with DNase I to digest any contaminating genomic DNA. The primers and probes of miRNA-20, miRNA-106a, miRNA-106b, miRNA-92, miRNA-93, and miRNA16 used for real-time PCR were described previously [28]. All RT and real time PCR reagents were purchased from Ambion. Real time PCR was performed as described previously [28] using miRNA-16 primers as internal control. MiRNA-16 was stably expressed in mouse testis and therefore it was used as housekeeping miRNA in this study. The relative expression level for each miRNA was calculated using the comparative Ct method (2-ΔΔCt) after normalization to the level of miRNA-16. Individual samples were run in triplicate. All samples were run on the Applied Biosystems ABI Prism 7700 system.
MiRNA Microarray and cDNA Microarray
GFRα1 positive stem spermatogonia and GFRα1 negative spermatogonia (non-stem cells) were separated from testes of 6-day-old mice by MACS as described above. Total RNA was extracted from GFRα1 positive spermatogonia and GFRα1 negative spermatogonia using Trizol (Invitrogen), and a RNA clean-up was performed for all samples using the RNeasy MinElute Cleanup Kit (Qiagen) following a specific protocol preserving the miRNA. The quality of total RNA was checked by gel and electropherogram (data not shown), and miRNA was further extracted from these cells for miRNA microarray. MiRNA microarrays were conducted on miRXplore™ Microarrays (MiltenyiBiotec) with duplicates. Fluorescence signals of the hybridized miRXplore™ Microarrays were detected using a laser scanner from Agilent (Agilent Technologies). The cDNA microarrays of GFRα1 positive spermatogonia and GFRα1 negative spermatogonia were performed with duplicates according to the procedure as described previously [23].
In Situ Hybridization
Fluorescence in situ hybridization (FISH) in testes of 6-day-old and 60- day- old mice was performed by adapting miRNA localization protocols [29]. The fluorescence probes to miRNA-20 and miRNA-106a were obtained from Exiqon (Woburn, MA) and hybridized to mouse testis sections. All reagents and apparatus used were DEPC treated. Hybridizations were conducted overnight at 58°C. Sections were stained with DAPI, and the in situ signal was visualized by confocal microscopy.
Transfection of Pre-miR™ MiRNA Precursors (mimics) and Anti-miR™ MiRNA Inhibitors into Mouse SSCs
Pre-miR™ miRNA precursors (mimics) and anti-miR™ miRNA inhibitors for miRNA-20 and miRNA-106a were purchased from Ambion. MiRNA mimics are chemically synthesized RNAs designed to mimic individual endogenous mature miRNAs. Mouse SSCs were cultured as mentioned above and they were transfected with miRNA mimic control, miRNA-20 mimic, miRNA inhibitor control, miRNA-20 inhibitor, miRNA-106a mimic, or miRNA-106 inhibitor, using siPORT™ NeoFX™ transfection agent (Ambion). Optimization experiments were performed using various miRNA mimic or inhibitor concentrations. In this study, we used 100 nM of miRNA-20, miRNA-106a mimics and inhibitors and found that this concentration was sufficient for their long-term biological effects. After 48 hours of culture, cells were harvested for the analysis of miRNA-20 and miRNA-106a expression and for determining phenotypes of various genes and proteins.
Proliferation Assays
Mouse SSCs were seeded at a density of 2,000 cells/well in 96-well microtiter plates coated with 0.1% gelatin in StemPro-34 SFM medium supplemented with 100 ng/ml GDNF and transfected without miRNA or with miRNA mimic control, miRNA-20 mimic or miRNA-106a mimic, miRNA inhibitor control, miRNA-20 inhibitor, or miRNA-106 inhibitor. After 5 days of culture, proliferation assays were performed using the Non-Radioactive Cell Proliferation Assay (Promega) as measured by the amount of absorbance at 490 nm according to the procedure as described previously [27].
Bromodeoxyuridine (BrdU) Incorporation Assay
The isolated mouse SSCs were grown on cover slips in 6-well plates coated with 0.1% gelatin in StemPro-34 SFM medium supplemented with 100ng/ml GDNF. The cells were transfected without miRNA or with miRNA mimic control, miRNA-20 mimic or miRNA-106a mimic, miRNA inhibitor control, miRNA-20 inhibitor, or miRNA-106 inhibitor, and then 30 μg/ml BrdU (Sigma) were added to the medium. After 24 hours of culture, immunocytochemistry was performed using an antibody to BrdU (Sigma) according to the protocol as described previously [30].
Transplantation of Mouse SSCs Transfected with MiRNA-20 or MiRNA-106a Mimics or Inhibitors into Infertile Mice
Eighty male Ncr Swiss nude mice were treated with busulfan (44 mg/kg body weight) 2 months prior to transplantation to deplete endogenous germ cells. Viral vector AAV2-CMV-eGFP was shown to efficiently and stably label donor SSCs [31]. To distinguish transplanted cells from endogenous germ cells, SSCs were exposed to AAV2-CMV-eGFP (Vector core of the University of Pennsylvania) for 3 hours before transplantation. Mouse SSCs were transfected with miRNA mimic control, miRNA-20 mimic, or miRNA-106a mimic, miRNA inhibitor control, miRNA-20 inhibitor, or miRNA-106 inhibitor, and cultured for 1 day or 5 days with the media as mentioned above in proliferation assay, and 106 cells were transplanted to each testis of busulfan-treated nude mice (n=6 each group) pursuant to the procedure for germ cell transplantation [32]. Recipient mice with DMEM injection but without mouse SSC transplantation served as controls. Two months after transplantation, recipient mice were sacrificed for determining phenotypic features of SSC markers in testicular cells.
Immunohistochemistry
Testes sections were prepared from mice transplanted with or without SSCs that were transfected with miRNA mimic control, miRNA-20 mimic, or miRNA-106a mimic, as mentioned above. For immunohistochemistry, 5 μm sections were dewaxed in xylene and rehydrated through a series of graded alcohols. Antigen retrieval was performed using the antigen retrieval citra plus solution (BioGenex Laboratories Inc., San Ramon, CA), and the endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After permeabilization with Triton X-100 and blocking with 10% normal serum, testis sections were incubated with primary antibodies, including rabbit polyclonal antibody to GFRα1 (Abcam Inc., Cambridge, MA) or VASA (Abcam Inc., Cambridge, MA) at a 1:100 dilution or mouse monoclonal antibody to PCNA (proliferating cell nuclear antigen) (EMD Biosciences Inc., San Diego, CA) at a 1:200 dilution, pursuant to the procedures described previously [27]. Testis sections were incubated with secondary antibody to rhodamine-conjugated anti-rabbit or anti-mouse IgG at a 1:200 dilution for 30 min at 34 °C and washed 3 times with PBS. All the secondary antibodies were purchased from Jackson ImmunoResearch laboratories. The 4′, 6′-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei of the cells in the testes, and the sections were observed for epifluorescence using an Olympus Fluoview 500 Laser Scanning Microscope (Olympus, Melville, NY).
Immunofluorescence and Confocal Microscopy
Germ cells were isolated from mice after transplantation of mouse SSCs transfected with miRNA mimic control, miRNA-20 mimic, or miRNA-106a mimic, using a 2-step enzymatic digestion and differential plating as described above [22]. Immunofluorescence by double staining was performed on these germ cells using antibody to GFRα1 (Abcam Inc., Cambridge, MA) and GCNA1 (a gift from Dr. George C. Enders, University of Kansas) according to the procedure as described previously [30]. After 3 washes in PBS, the cells were incubated with the secondary antibody, including fluorescein (FITC)-conjugated donkey anti-rabbit IgG and Rhodamine-conjugated goat anti-rat IgG at a 1:200 dilution for 30 min at 34°C, and washed 3 times with PBS. All the secondary antibodies were purchased from the Jackson ImmunoResearch laboratories. The cells were observed for epifluorescence using an Olympus Fluoview 500 Laser Scanning Microscope (Olympus). Images were acquired and analyzed using the accompanying Fluoview software.
Western Blots
GFRα1 positive spermatogonia and c-kit positive spermatogonia were isolated from testes of 6-day-old mice and 8-day-old mice respectively by MACS as described above [23]. Western blots of STAT3 expression in GFRα1 positive spermatogonia and c-kit positive spermatogonia as well as STAT3 and Ccnd1 expression in mouse SSCs transfected with miRNA mimic control, or miRNA-20 mimic, miRNA-106a mimic, miRNA inhibitor control, miRNA-20 inhibitor, or miRNA-106 inhibitor, or with Stat3 siRNAs or Ccnd1 siRNAs, were carried out using antibody against STAT3 or Ccnd1 (Santa Cruz, Inc.) according to the protocol described previously [30]. The membranes were stripped with Restore Western Blot Stripping Buffer (Pierce Biotechnology, Inc.) and re-probed with antibody to ACTB (β-actin) (IMGENEX Corp., San Diego, CA).
RNA Interference
SiRNAs against Stat3, Fos, and Ccnd1 and negative control siRNA were purchased from Shanghai GenePharma Co., Ltd and their sequences were listed in Table S3. Mouse SSCs were isolated and cultured in the medium as mentioned above, and Stat3, Fos, or Ccnd1siRNA, or negative control siRNA was transfected into the cells using Lipofectamine™ 2019400 (Invitrogen) according to the procedure described previously [27]. RT-PCR analysis of Stat3, Fos, and Plzf and Western blots of STAT3 and Ccnd1 were performed at 48 h after transfection according to the procedure mentioned above. Proliferation assay of SSCs with or without siRNA treatment was carried out after 5 days of culture.
Statistical Analysis
One-way analysis of variance (ANOVA) and Tukey post-tests using the SPSS statistical software were performed and the P value was calculated for each experiment. For all experiments with error bars, standard deviation was calculated to indicate the variation.
Results
Identity of spermatogonial subpopulations used in this study
The GDNF/GFRα1 signaling system is the only known functional regulator of SSCs as shown initially by Meng and colleagues [25]. GFRα1, the receptor for GDNF, is expressed on the cell surface of mouse SSCs, and selection of mouse germ cells expressing GFRα1 is an excellent method to obtain a population of highly enriched SSCs in vitro (cell purity of greater than 95%)[33]. The starting cells we used in this study were GFRα1 positive SSCs. In a series of publications, Yoshida and colleagues confirmed that the Asingle (As) spermatogonia, the GFRα1 positive spermatogonia, are the actual SSCs. They also reported that Apaired (Apr) and Aaligned (Aal) spermatogonia could revert back to SSCs, and thus these cells would also have the GFRα1 receptor [34-36]. This spermatogonial dedifferentiation is almost identical to that reported earlier for the Drosophila male germline [37]. Thus, we are using GFRα1 positive SSCs in this study. This population of cells containsAs (mostly), Apr, and Aal spermatogonia, but they are almost all SSCs. In the early nineties, Yoshinaga and colleagues demonstrated that c-kit is a surface receptor marker for differentiated spermatogonia (A1 to A4)[38]. C-kit is not present in SSCs. We are using the c-kit positive cells for the experiments with differentiated spermatogonia.
Expression of MiRNA 17-92 Cluster in Mouse Germ Cells and Somatic Cells
We first probed the expression of the miRNA 17-92 cluster in various types of cells in adult mouse testes, including the SSCs. Among a number of miRNAs examined in the miRNA 17-92 cluster, we found distinct expression using real time PCR. Notably, we found that miRNA-20 and miRNA-106a were expressed at much higher levels in mouse SSCs (GFRα1 positive spermatogonia) and a mouse SSC line (C18-4 cells) [39], compared to differentiating spermatogonia (c-kit positive spermatogonia) or adult male germ cells (Figures 1A and 1B). There was no statistical difference in expression of miRNA-106b and miRNA-92 among mouse SSCs, the SSC line, the differentiating spermatogonia, and adult male germ cells (Figure 1C; Figure S1), while miRNA-93 was expressed at a lower level in SSCs compared to the differentiating spermatogonia (Figure S1).
Figure 1.
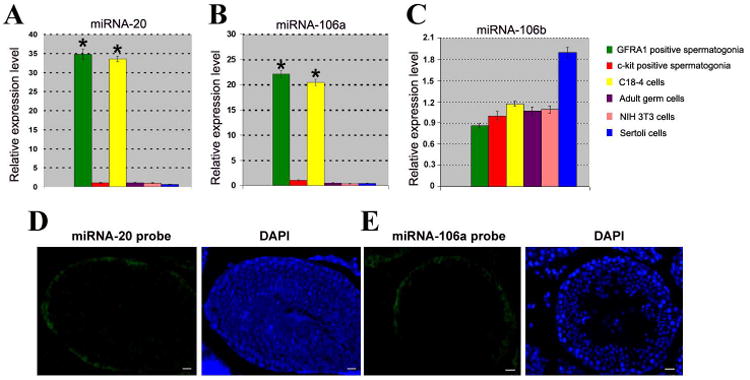
Distinct expression of miRNAs in various types of cells and subcellular localization in adult mouse testes. (A-C) Real time PCR showed the expression of miRNA-20, miRNA-106a, and miRNA-106b in GFRα1 positive spermatogonia, c-kit positive spermatogonia, C18-4 cells, adult male germ cells, NIH 3T3 cells, and Sertoli cells. House keeping miRNA-16 served as a loading control of total miRNAs. To compare the expression of miRNA-20, miRNA-106a and miRNA-106b in different types of cells, the expression of these miRNAs in c-kit positive spermatogonia was set as 1. Compared to c-kit positive spermatogonia, “*” indicated significant difference (P<0.05). (D-E) Fluorescein in situ hybridization displayed restricted expression of miRNA-20 (D) and miRNA-106a (E) only to spermatogonia at the basement membrane of the seminiferous tubules in adult mouse testes. DAPI was used to show cell nuclei. Scale bars = 20 μm (D, E).
MiRNA Microarray and Fluorescence In Situ Hybridization Reveal That MiRNA-20 and MiRNA-106a Are Expressed Preferentially in SSCs
MiRNA microarray further reveled that miRNA-20 (also known as miRNA-20A) and miRNA-106a were expressed at higher levels in mouse SSCs (GFRα1positive spermatogonia) compared to non-stem cells (GFRα1 negative spermatogonia) (Table S1). High quality of the isolated RNA used for miRNA microarray was shown by gel imaging and electropherograms and reproducibility of the microarrays was verified (data not shown). Using fluorescence in situ hybridization (FISH), we detected that miRNA-20 and miRNA-106a were largely confined to the spermatogonia along the basement membrane of adult mouse seminiferous tubules (Figures 1D and 1E). FISH analyses also showed that miRNA-20 and miRNA-106a were expressed in spermatogonia of immature mice (Figures S2A and S2B). Testis sections without miRNA probes in immature and adult mice (Figures S2C and S2D) were used to verify specific expression of miRNA-20 and miRNA-106a in immature and adult mouse testes.
MiRNA-20 and MiRNA-106a Promote Proliferation and DNA Synthesis of SSCs
The “Holy Grail” of adult stem cell research is to modulate endogenous stem cells in vivo, possibly with small molecules specifically targeted to respective stem cell populations, as reviewed by Matzuk and colleagues [40]. Obviously, endogenous miRNAs regulating stem cell fate must be thoroughly understood before such in vivo studies can be initiated. We next explored the function of miRNA-20 and miRNA-106a in the regulation of SSC fate determinations using miRNA mimics and inhibitors (antagomirs). Pre-miR™ miRNA precursors are small chemically modified double-stranded RNA molecules designed to mimic endogenous mature miRNA. Anti-miR™ miRNA inhibitors are chemically modified single stranded nucleic acids designed to specifically bind to and inhibit endogenous miRNA. Pre-miR™ miRNA mimic controls and inhibitor controls were random sequence molecules that didn't produce any identifiable effects on known miRNA function. The transfection efficiency of miRNA-20 or miRNA-106a mimics or inhibitors in mouse SSCs was about 75% as assessed by co-transfection of fluorescently labeled miRNA. RT-PCR analysis showed that miRNA-20 expression was markedly increased (3.5 fold) in SSCs after miRNA-20 mimic transfection compared to cells treated with miRNA mimic control (1.0 fold, Figure 2A). Conversely, miRNA-20 expression was decreased by 80% in SSCs treated by miRNA-20 inhibitor transfection compared to SSCs with miRNA inhibitor control (100%, Figure 2A). Likewise, miRNA-106a expression was enhanced (3.1 fold) in SSCs with miRNA-106a mimic transfection compared to the cells with miRNA mimic control (1.0 fold, Figure 2B). MiRNA-106a expression was reduced by 75% in SSCs with miRNA-106a inhibitor transfection compared to the cells with miRNA inhibitor control (100%, Figure 2B). These data illustrated the effective specificity of miRNA mimics and inhibitors to miRNA-20 and miRNA-106a in mouse SSCs.
Figure 2.
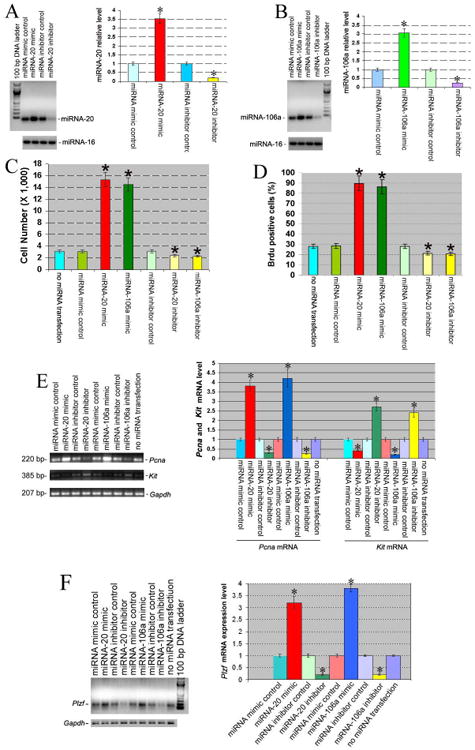
Differential expression of miRNA-20, miRNA-106a, Pcna, Kit, and Plzf in mouse SSCs transfected with or without miRNA-20 or miRNA-106a mimics and inhibitors. (A-B) RT-PCR revealed miRNA-20 (A) and miRNA-106a (B) in mouse SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors. Compared to miRNA mimic control, “*” indicated a significant difference (P<0.05).(C-D) Proliferation (C) and BrdU incorporation (D) assays of mouse SSCs transfected without miRNA or with miRNA mimic control, or miRNA-20 mimic or miRNA-106a mimics or inhibitors. Statistically significant differences (P <0.05) between miRNA-20 mimic or miRNA-106a mimic or inhibitor-treated cells and miRNA mimic control were indicated by an asterisk.(E-F) RT-PCR showed Pcna(E), Kit(E), and Plzf(F) transcriptsin mouse SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors. Compared to miRNA mimic control, “*” indicated a significant difference (P <0.05).
Next, we explored the effects of mimics of miRNA-20 and miRAN-106a in controlling the renewal and/or differentiation of the mouse SSCs. MiRNA mimic and inhibitor controls were used to assure that transfection of the mimics or inhibitors didn't affect SSC maintenance. Proliferation assays demonstrated that miRNA-20 mimic and miRNA-106a mimic induced a 5.1- and 4.8- fold increase of cell number in mouse SSCs after culture for 5 days, respectively, compared to miRNA mimic control or without miRNA transfection (1.0 fold, Figure 2C). Mouse SSCs transfected with miRNA-20 or miRNA-106a mimics could be cultured for more than 45 days with an increase of cells by 452- and 364- fold, respectively, compared to miRNA mimic controls. In contrast, miRNA-20 and miRNA-106a inhibitors resulted in a 23% and 26% reduction of cell number in mouse SSCs, respectively, compared to miRNA inhibitor control or without miRNA transfection (Figure 2C). DNA synthesis in mouse SSCs by miRNA-20 and miRNA-106a mimics was assessed immunocytochemically using BrdU incorporation assays. An increase in the BrdU-positive cells was seen in SSCs with miRNA-20 mimic (3.2 fold) and miRNA-106a mimic (3.1 fold) compared to miRNA mimic control (1.0 fold, Figure 2D). Conversely, miRNA-20 and miRNA-106a inhibitors led to a 25% and 29% reduction of BrdU-positive cells in mouse SSCs, respectively, compared to miRNA inhibitor control or without miRNA transfection (Figure 2D). These results implicate the role of miRNA-20 and miRNA-106a in promoting proliferation and DNA synthesis of SSCs.
Pcna, Kit, and Plzf Expression In SSCs Transfected with MiRNA-20 or MiRNA-106a Mimics and Inhibitors
We determined the phenotypic changes of SSCs transfected with miRNA-20 or miRNA-106a mimics and inhibitors. Pcna (Figure 2E) and Plzf (Figure 2F) mRNA was markedly elevated in SSCs with miRNA-20 or miRNA-106amimic transfection compared to the cells with miRNA mimic control. Conversely, Pcna and Plzf transcriptswere reduced in SSCs with miRNA-20 or miRNA-106ainhibitor transfection compared to the cells with miRNA inhibitor control (Figures 2E and 2F). In direct contrast to Pcna and Plzf transcripts, Kit mRNA (Figure 2E) was reduced in SSCs with miRNA-20 or miRNA-106amimic transfection compared to the cells with miRNA mimic control. Kitm RNA was enhanced in SSCs with miR-20or miRNA-106ainhibitor transfection compared to the cells with miRNA inhibitor control (Figure 2E). PCNA has been used extensively in the identification of proliferating spermatogonia in the testes of rodents and primates [41], while Plzf is required for renewal of mouse SSCs [42]. Kit has been used as a marker for differentiating spermatogonia, and type A1-A4 spermatogonia divisions are known to be Kit-dependent [24]. Consideredtogether, our results suggest that miRNA-20 and miRNA-106a are required for renewal of mouse SSCs.
MiRNA-20 or MiRNA-106a Mimics Enhance Colony Formation of SSCs and Induce Expression of GFRα1, PCNA, Pou5f1, Plzf, and Ret in Mouse Testes In Vivo
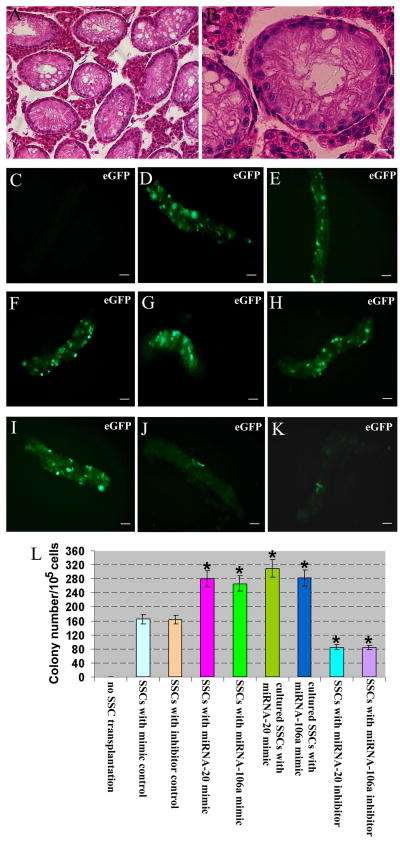
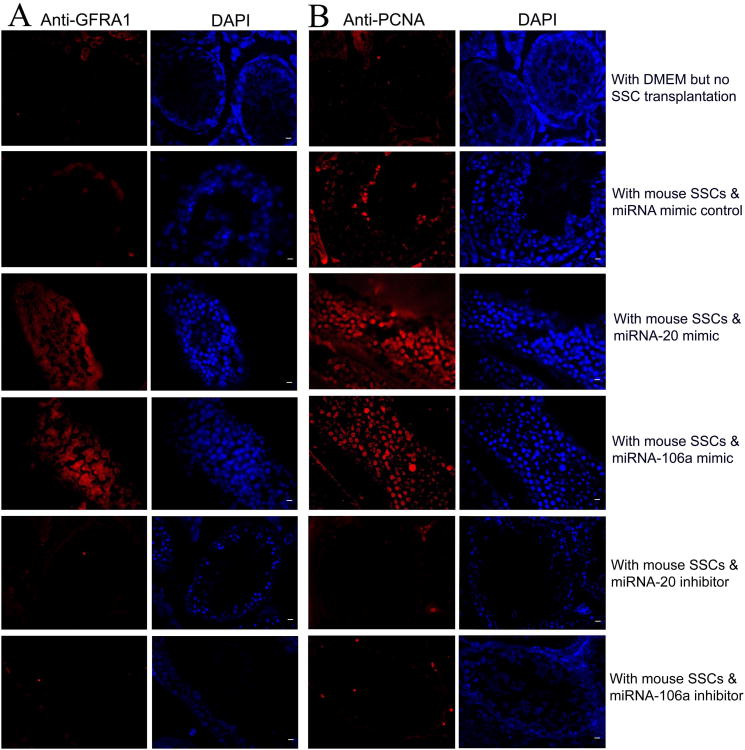
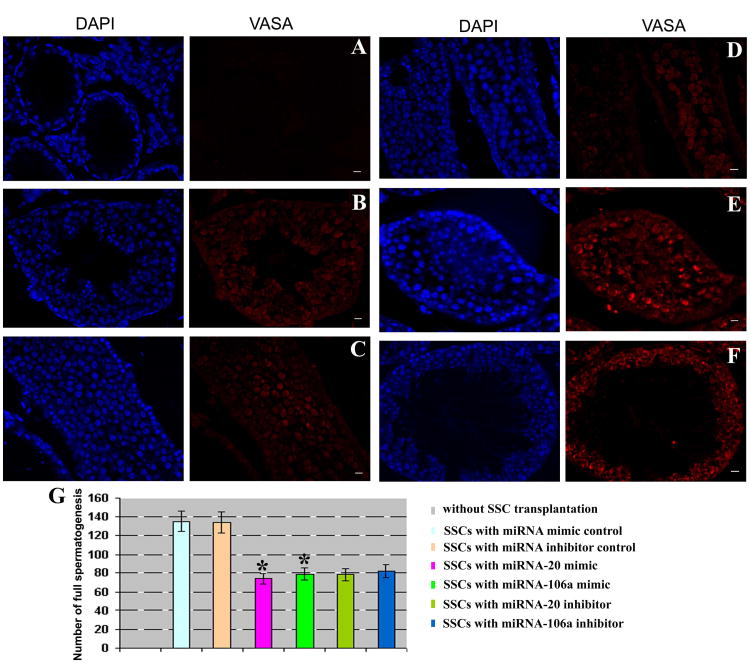
To address the function of miRNA-20 and miRNA-106a in regulating SSCs in vivo, we transplanted fresh and cultured mouse SSCs with miRNA mimic control, or transfection with miRNA-20 mimic or inhibitor, or with miRNA-106a mimic or inhibitor into seminiferous tubules of sterile busulfan-treated nude mice. Two months after busulfan treatment, no male germ cells remained in the testes of recipient null mice (Figures 3A and 3B). AAV2-CMV has been shown to be effective in transducing mouse SSCs for 12 months [31] and pig SSCs for 5 and 7 months [43].To visualize donor-derived colonization in recipient testis, we exposed SSCs (GFRα1 positive spermatogonia) to AAV2-CMV-eGFP before transplantation and transfection efficiency of AAV2-CMV-eGFP in mouse SSCs was around 80% (Figure S3A). Transfection of miRNA inhibitors or mimics into SSCs could have long-term effects on SSC self-renewal and differentiation in vivo, as evidenced by recent findings that transfection of miRNA-21 inhibitor reduces colony number of SSCs at 2 months after transplantation [18]. Two months after transplantation, colony numbers of SSCs were enhanced in fresh SSCs treated with miRNA-20 mimic (Figures 3F and 3L) or miRNA-106a mimic (Figures 3G and 3L) and in cultured SSCs with miRNA-20 mimic (Figures 3H and 3L) or miRNA-106a mimic (Figure 3I and 3L) compared to miRNA mimic control (Figures 3D and 3L). In contrast, colony formation was reduced in SSCs treated with miRNA-20 inhibitor (Figures 3J to 3L) or miRNA-106a inhibitor (Figures 3K and 3L), compared to miRNA inhibitor control (Figures 3E and 3L). These results suggest that miRNA-20 and miRNA-106a are essential for renewal of SSCs. Immunohistochemistry demonstrated that miRNA-20 and miRNA-106a mimics resulted in a remarkable increase of male gem cells that were positive for GFRA1 (Figure 4A) and PCNA (Figure 4B), a hallmark for proliferating spermatogonia [41], whereas miRNA-20 and miRNA-106a inhibitors led to rare cells expressing GFRA1 (Figure 4A) and PCNA (Figure 4B). To probe whether miRNA-20 and miRNA-106a have an effect on the differentiation of SSCs, we determined the expression of VASA, a marker for germ cells ranging from spermatogonia to spermatids [44]. Immunohistochemistry with antibody to VASA revealed that there was a statistical decrease in the differentiation of SSCs into round spermatids by miRNA-20 and miRNA-106a mimics or inhibitors compared to miRNA mimic or inhibitor controls (Figures 5A to 5G).
Figure 3.
Effects of miRNA-20 and miRNA-106a mimics or inhibitors on colony formation in recipient mice. (A-B) Testis morphology of mice at 2 months after busulfan treatment. Only Sertoli cells remained in the seminiferous tubules. Scale bar in A = 20 μm; Scale bar in B = 10 μm.(C-K) Expression of eGFP from seminiferous tubules of recipient mice without SSC transplantation (C), or from seminiferous tubules after transplantation of SSCs with miRNA mimic control (D), or with miRNA inhibitor control (E), or with miRNA-20 mimic (F), or with miRNA-106a mimic (G), or withmiRNA-20 inhibitor (J); or withmiRNA-106a inhibitor (K). (H-I) Transplantation of cultured SSCs for 5days with miRNA-20 mimic (H) or miRNA-20 mimic (I). Scale bars in C-K = 100 μm. (L) Colony number of recipient mouse testes without SSC transplantation, or from transplantation of SSCs with miRNA mimic control, or miRNA inhibitor control, or miRNA-20 mimic, or miRNA-106a mimic, or miRNA-20 inhibitor, or miRNA-106a inhibitor, or from transplantation of cultured SSCs for 5days with miRNA-20 mimic, or miRNA-106a mimic. Compared to miRNA mimic control, “*” indicated a significant difference (P <0.05).
Figure 4.
Biochemical phenotypes of mouse testes transplanted with or without SSCs that were transfected with miRNA mimic control, or miRNA-20 mimic, or miRNA-106a mimic, or miRNA-20 inhibitor, or miRNA-106a inhibitor. (A-B) Immunohistochemistry showed that GFRα1 (A) and PCNA (B) expression was increased in mouse testes transplanted with miRNA-20 or miRNA-106a mimics. Scale bars = 10 μm (A, B).
Figure 5.
VASA expression in recipient mouse testes.(A-F) Immunohistochemistry revealed VASA expression in mouse testes transplanted without SSCs (A) or with SSCs that were transfected with miRNA mimic control (B), or miRNA-20 mimic (C), or miRNA-106a mimics (D), or miRNA-20 inhibitor (E), or miRNA-106a inhibitor (F). Scale bars = 20μm. (G) Number of tubule cross sections with full spermatogenesis in recipient mouse testes without SSC transplantation, or from transplantation of SSCs with miRNA mimic control, or miRNA inhibitor control, or miRNA-20 mimic, or miRNA-106a mimic, or miRNA-20 inhibitor, or miRNA-106a inhibitor. Compared to miRNA mimic control, “*” indicated a significant difference (P <0.05).
We checked the expression of miRNA-20 and miRNA-106a in male germ cells recovered from recipient mouse testes 2 months after transplantation of SSCs transfected with miRNA mimic control or miRNA-20 mimic, or miRNA-106a mimic, or with miRNA inhibitor control or miRNA-20 inhibitor, or miRNA-106a inhibitor. Real time PCR analysis revealed that miRNA-20 and miRNA-106a expression was markedly increased in male germ cells from recipient mice transplanted with SSCs after miRNA-20 or miRNA-106a mimic transfection compared to cells treated with miRNA mimic control (Figures S3B and S3C). In contrast, miRNA-20 and miRNA-106a expression was reduced in male germ cells recovered from recipient mice transplanted with SSCs after miRNA-20 or miRNA-106a inhibitor transfection compared to cells treated with miRNA inhibitor control (Figures S3B and S3C). Confocal microscopy by doubling immunostaining using anti-GFRA1 and anti-GCNA1 (germ cell nuclear antigen 1, a marker for germ cells) showed that GFRA1 was expressed in very few spermatogonia but not in other germ cells (e.g., pachytene spermatocytes and round spermatids) from mice with transplantation of mouse SSCs transfected with miRNA mimic control (Figure 6A). Importantly, many more (>90% of the germ cells) spermatogonia were positive for GFRA1 in the mice with transplantation of mouse SSCs transfected with miRNA-20 mimic (Figure 6B) ormiRNA-106a mimic (Figure 6C). Collectively, these data indicate that over expression of miRNA-20 and miRNA-106a leads to an accumulation of undifferentiated SSCs in vivo.
Figure 6.
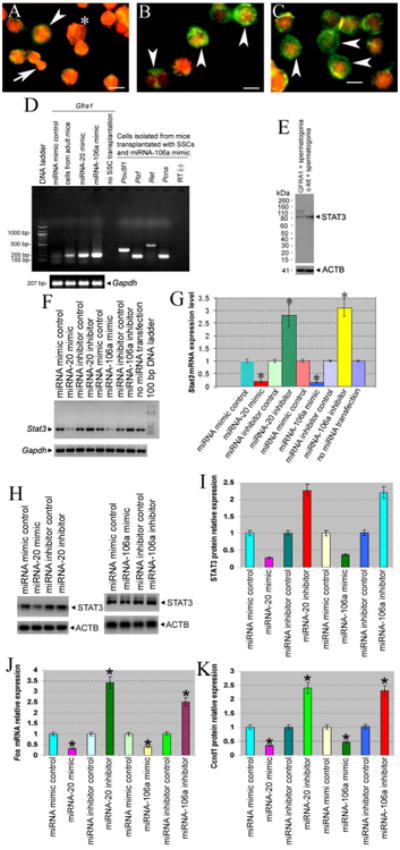
Phenotypic characteristics of testicular cells from mice transplanted with or without SSCs that were transfected with miRNA mimic control, or miRNA-20 mimic, or miRNA-106a mimic. (A-C) Confocal microscopy showed GFRα1 (green fluorescence) and GCNA1 (red fluorescence) expression in germ cells from mice with transplantation of mouse SSCs transfected with miRNA mimic control (A), or miRNA-20 mimic (B), or miRNA-106mimic (C): Note: spermatogonia (arrowheads); pachytene spermatocytes (asterisk); round spermatids (arrow). Scale bars = 10 μm (A-C). (D) RT-PCR showed expression of GFRα1, Pou5f1, Plzf, Ret, and Pcna in germ cells and Sertoli cells from mice with or without SSCs that were transfected with miRNA mimic control, or miRNA-20 mimic, or miRNA-106a mimics. Total RNA without reverse transcription (RT-) but with PCR served as negative controls. (E) Western blots showed STAT3 expression in GFRα1 positive spermatogonia and c-kit positive spermatogonia. (F-K) RT-PCR and Western blots revealed Stat3 (F, G), STAT3 (H, I), Fos (J), and Ccnd1 expression (K) in mouse SSCs transfected with or without miRNA-20 and miRNA-106a mimics or inhibitors. Compared to miRNA mimic control, “*” indicated a significant difference (P<0.05).
We further probed the biochemical phenotypes of the testicular cells isolated from mice with or without SSCs transfected with miRNA-20 or miRNA-106a mimics. Much higher levels of GFRα1 mRNA expression were observed in the cell mixture containing germ cells and Sertoli cells from the mice transplanted with SSCs and miRNA-20 mimic or miRNA-106a mimic, compared to the cells from the mice transplanted with SSCs and miRNA mimic control (Figure 6D). GFRα1 mRNA was not detected in the cells from the mice without SSC transplantation (Figure 6D). Importantly, higher levels of Pou5f1(Oct4), Plzf, Ret, and Pcna, makers for proliferation and maintenance of rodent SSCs [30, 33, 45], were seen in male germ cells and Sertoli cells from the mice transplanted with SSCs transfected with miRNA-106a mimic (Figure 6D). These data, together with GFRα1 and GCNA1 expression, further suggest that miRNA-20 and miRNA-106a are required for renewal of mouse SSCs in vivo.
STAT3 and Ccnd1 Are Direct Targets of MiRNA-20 and MiRNA-106a in SSCs
To gain an insight into the molecular mechanisms of miRNA-20 and miRNA-106a in SSCs, we identified the targets of these miRNAs. Using different miRNA prediction software programs [3] we predicted that STAT3was a potential target of the miRNA 17-92 cluster in mouse SSCs. Western blots showed that STAT3 was expressed at a lower level in mouse SSCs (GFRα1 positive spermatogonia) compared to the differentiating spermatogonia (c-kit positive spermatogonia) (Figure 6E). RT-PCR showed that Stat3 was significantly decreased in mouse SSCs transfected with miRNA-20 or miRNA-106a mimics compared to the cells with miRNA mimic controls (Figures 6F and 6G). Conversely, Stat3 mRNA was increased markedly in SSCs with miRNA-20 and miRNA-106a inhibitor transfection compared to the cells with miRNA inhibitor controls (Figures 6F and 6G). Likewise, Western blots revealed that STAT3 protein was significantly decreased in mouse SSCs transfected with miRNA-20 or miRNA-106a mimics compared to the cells with miRNA mimic controls (Figures 6H and 6I), whereas STAT3 protein was increased markedly in SSCs with miRNA-20 or miRNA-106a inhibitor transfection compared to the cells with miRNA inhibitor controls (Figures 6H and 6I). Thus, STAT3 is a binding target of miRNA-20 and miRNA-106 in mouse SSCs. To define downstream targets of miRNA-20 and miRNA-106, we examined expression of cell cycle regulators. We observed that miRNA-20 or miRNA-106a mimics reduced the transcription of Fos and translation of Ccnd1 (Figures 6J and 6K), whereas miRNA-20 or miRNA-106a inhibitors increased Fos and Ccnd1 expression (Figures 6J and 6K), indicating that Ccnd1 is a direct target of miRNA-20 or miRNA-106a.
We further explored the function of STAT3, Fos, and Ccnd1 in regulating renewal of mouse SSCs. Using small interfering RNA (siRNA), we found that siRNAs against Stat3, Fos, and Ccnd1 specifically knocked down expression of Stat3 mRNA (Figures 7A and 7B), STAT3 protein (Figures 7D and 7E), Fos (Figures 7G and 7H), and Ccnd1 (Figures 7J and 7K). Stat3 silencing led to reduction of Plzf transcription of SSCs (Figures 7A and 7C). Significantly, STAT3, Fos and Ccnd1 knockdown resulted in an obvious increase of SSCs (Figures 7F, 7I, and 7L), suggesting that STAT3 and Ccnd1promote renewal of SSCs.
Figure 7.
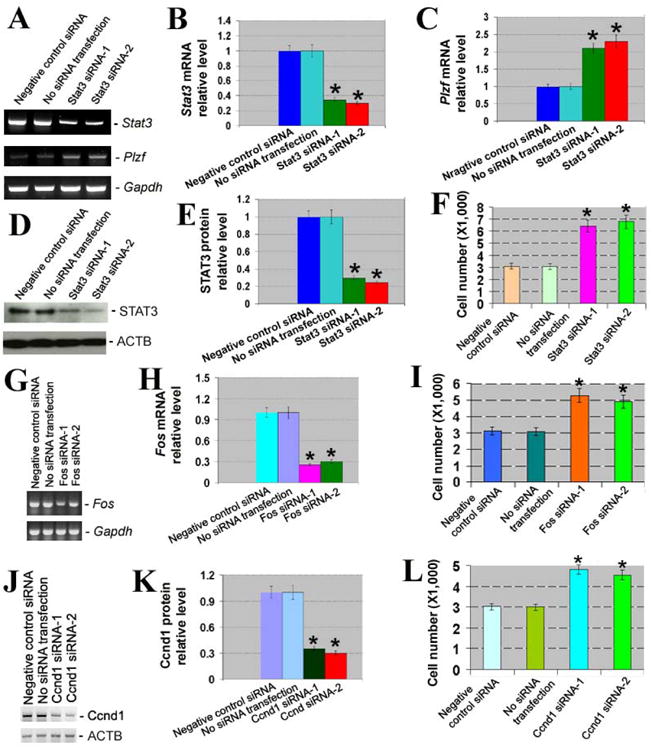
Knockdown of Stat3, Fos, and Ccnd1 and its effects on proliferation of SSCs. (A) RT-PCR showed the expression of Stat3 and Plzf after Stat3 silencing. Gapdh served as a loading control of total RNA. (B) Relative expression of Stat3 to Gapdh after silencing of Stat3. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (C) Relative expression of Plzf to Gapdh after silencing of Stat3. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (D) Western blots revealed STAT3 expression after Stat3 silencing. ACTB served as a loading control of total protein. (E) Relative expression of STAT3 to ACTB after silencing of Stat3. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (F) Proliferation assay displayed cell number of SSCs by Stat3 siRNAs. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (G) RT-PCR showed the expression of Fos after Fos silencing. Gapdh was used as a loading control of total RNA. (H) Relative expression of Fos to Gapdh after silencing of Fos. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (I) Proliferation assay displayed cell number of SSCs by Fos siRNAs. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (J) Western blots revealed Ccnd1 expression after Ccnd1 silencing. ACTB served as a loading control of total protein. (K) Relative expression of Ccnd1to ACTB after silencing of Ccnd1. Compared to negative control siRNA, “*” indicated a significant difference (P <0.05). (L) Proliferation assay displayed cell number of SSCs by Ccnd1 siRNAs.
Discussion
Several lines of evidence indicate that SSCs constitute one of the most important stem cells systems in view of a number oftheir unique characteristics. Firstly, SSCs can be considered “immortal” germ cells because they are present from birth to death and they produce sperm that transmit genetic information to subsequent generations. Thus, they represent a very valuable resource for experimental modification of the mammalian genome [46]. Secondly, SSCs self-renew throughout life and they differentiate into spermatocytes, spermatids, and eventually mature spermatozoa, and thus they can be used as an excellent model to elucidate the molecular mechanisms governing stem cell renewal versus differentiation. Thirdly, there is the clear potential to use SSC transplantation to restore fertility in cancer patients after chemotherapy or irradiation therapy [47]. Finally, recent studies have demonstrated that SSCs can acquire pluripotency to become ES-like cells that are able to differentiate into all cell lineages of the three germ cell layers [8-13]. Therefore, SSCs have great potential for cell-based and autologous organ regeneration therapy for human diseases without the associated ethical issues and immune rejection. The regulation of SSCs requires the actions of intrinsic factors and extrinsic signals, and it has been suggested that SSC renewal is regulated primarily by intrinsic factors [18]. Thus, it is imperative to uncover intrinsic molecules regulating the renewal or differentiation of SSCs before the cells can be used in the clinic.
MiRNAs play crucial roles in the regulation of cellular proliferation [4], differentiation [5, 6], and apoptosis [7]. Generating expression profiles of miRNAs in male germ cells is a prerequisite for a thorough understanding of their roles in regulating SSC fate decisions. Although several studies show the expression patterns of miRNAs in mouse germ cells [15] and mouse gonocytes [16], the expression, function, and the targets of individual miRNAs in controlling SSC fate determinations remain mostly unknown. We found using real time PCR and miRNA micro array that miRNA-20 and miRNA-106a were expressed preferentially in mouse SSCs. Our results are consistent with a recent report by Smorag and colleagues showing thatmiRNA-20 (also known as miRNA-20a) is highly expressed in mouse SSCs [17]. However, the same group also showed that miRNA-20 is present at a high level in other spermatogonia and in meiotic germ cells. This is probably due to the fact that the more differentiated spermatogonia and the isolated meiotic germ cells from Sycp3/DsRed transgenic mice may contain SSCs. Purity of the isolated cell fractions was not presented in the Smorag paper. To some extent, our results are also consistent with the report showing that miRNA-106a is present in type A spermatogonia but absent in the differentiated type B spermatogonia [15]. We further revealed cellular localization of miRNA-20 and miRNA-106a in the spermatogonia. Considered together, the expression profiles and subcellular localization of miRNA-20 and miRNA-106a in adult mouse testes suggest that miRNA-20 and miRNA-106a may play essential roles in regulating the renewal of mouse SSCs.
Interestingly, our miRNA and cDNA microarray revealed an inverse relationship in the expression of Lin28 mRNA and miRNAs Let-7G and Let-7I between mouse SSCs (GFRα1 positive spermatogonia) and the non-stem cells (GFRα1 negative spermatogonia) (Table S1), suggesting that Let-7G/Let-7I/Lin28 form a regulatory loop to regulate fate decisions of SSCs. It has been shown that Lin28 is specifically expressed in mouse As, Apr, and Aal spermatogonia and that Lin28-positive spermatogonia comprise two cell subpopulations including high stem cell potential and high differentiation commitment [48]. Taken together, Lin28 is present preferentially in spermatogonia with high differentiation commitment. Notably, our microarray data on the expression of Let-7G, Let-7I, and Lin28 are in contrast to the finding that down-regulation of Let-7 and higher levels of Lin28 are required for maintenance of ES cell pluripotency [49], highlighting that SSCs are distinct from ES cells in miRNA signatures and gene regulation networks, although SSCs can acquire pluripotency in vitro to become ES-like cells [8-13]. Moreover, our miRNA microarray showed that miRNA-21 was over-expressed (1.46 fold) in mouse SSCs (GFRα1 positive spermatogonia) compared to the non-stem cells (GFRα1negative spermatogonia), suggesting that miRNA-21 may regulate the renewal of GFRα1 positive SSCS. We also identify a list of miRNAs that are enriched in mouse SSCs compared to non-stem cells. These miRNAs may play important roles in the maintenance of SSCs.
Using miRNA mimics and inhibitors, we revealed that miRNA-20 and miRNA-106a promote renewal (proliferation and DNA synthesis) of SSCs. More importantly, functional assays by miRNA mimics and inhibitors show that these two miRNAs are essential for renewal of SSCs, as evidenced by the phenotypic changes of Pcna, Plzf, and Kit in vitro. We further demonstrated that miRNA-20 and miRNA-106a are required for renewal of mouse SSCs in vivo, as shown by the enhancement of colony formation and GFRα1, PCNA, Pou5f1, Plzf, and Ret expression. Our ability to identify miRNA-20 and miRNA-106a as endogenous regulators promoting mouse SSC renewal and proliferation may be applicable in humans in order to obtain sufficient numbers of SSCs for therapeutic applications, since human SSCs are very rare (only one or two SSCs in each 5 μm cross section) [50].
The transcription factor ETV5 is a regulator for miRNA-21 in SSCs [18]. However, nothing is known about the targets of miRNAs regulating SSCs. We predicted that Stat3 is a target of miRNA 17-92 cluster, using3 different miRNA prediction programs, namely: 1) the miRBase website employing the miRanda algorithm to predict miRNA: mRNA pairs; 2) the microInspector website using a different algorithm that allows for G:U wobbles in seed matches; and 3) custom made miRNA prediction program utilizing a variation of the TargetScans algorithm [3]. Importantly, we have for the first time identified STAT3and Ccnd1 as targets of miRNAs, specifically for miRNA-20 and miRNA-106a, which offers a novel insight into mechanism by which miRNAs act on SSC regulation. Our data are consistent with the recent finding showing that STAT3 promotes the differentiation of mouse SSCs [51]. Significantly, we demonstrate for the first time that silencing of Stat3, Fos, and Ccnd1 stimulates the renewal of SSCs.
Conclusion
In summary, we identified miRNA-20 and miRNA-106a as novel intrinsic RNA molecules that promote renewal of mouse SSCs at the post-transcriptional level via targeting STAT3 and Ccnd1. This study thus provides novel mechanisms regulating SSC fate determinations. MiRNAs are highly conserved between animals and humans [3]. Given the functional importance of miRNA-20 and miRNA-106a in spermatogenesis, these miRNAs could have great implications in offering novel therapeutic targets for treating male infertility or for male contraception. MiRNA inhibitors can be taken up by live cells and effectively exert their suppressive effects via binding the target miRNAs with high affinity and specificity [52]. Inhibitors for miRNA-20 and miRNA-106a are expected to satisfy the demand for a male contraceptive with high specificity and safety [53], since these miRNAs are expressed abundantly in SSCs in testis. Alternatively, the identification of specific miRNAs regulating SSC renewal may eventually be used to produce sufficient quantities of human SSCs and then use these adult stem cells to obtain a high yield of pluripotent ES-like cells for regenerative medicine.
Supplementary Material
Supplementary Figure 1. Expression of miRNA-92and miRNA-93 in various types of cells in adult mouse testes and NIH3T3 cells. Real time PCR showed the expression of miRNA-92 and miRNA-93 in GFRA1 positive spermatogonia, c-kit positive spermatogonia, C18-4 cells, adult male germ cells, NIH 3T3 cells, and Sertoli cells. House keeping miRNA-16 served as a loading control of total miRNAs.
Supplementary Figure 2. FISH showed expression of miRNA-20 (A) and miRNA-106a (B) in immature mouse testes. Testis sections without miRNA probes in immature mice (C) and adult mice (D) were used as negative controls. Scale bars = 10 μm (C, D).
Supplementary Figure 3. (A) Fluorescence microscopy (left figure) and phase-contrast imaging (right figure) showed transfection efficiency of AAV2-CMV-eGFP in mouse SSCs before transplantation. Scale bars = 50 μm. (B-C) Real time PCR showed expression of miRNA-20 (B) and miRNA-106a (C) in male germ cells from recipient mice transplanted with SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors. Compared to miRNA mimic or inhibitor control, “*” indicated a significant difference (P<0.05).
Acknowledgments
We thank Dr. George C. Enders, Department of Anatomy and Cell Biology, University of Kansas Medical Center, for providing an antibody to GCNA1. We also thank Dr. Aykut Uren, Georgetown University Medical Center, for providing NIH 3T3 cells. This work was supported by NIH RO1 grant HD33728 (MD). Dr. He was supported by start-up funds from the Renji Hospital, Shanghai Jiao Tong University School of Medicine and by the National Natural Science Foundation of China (31230048), Chinese Ministry of Science and Technology (2013CB947901), and the Shanghai Municipal Science and Technology Commission (12JC1405900).
Footnotes
Author Contributions: Martin Dym: Conception and design; financial support; manuscript writing; final approval of manuscript; data analysis and interpretation
Ian Gallicano: Conception and design; provision of study material (miRNA mimics and inhibitors); other (continuous guidance and discussions)
Zuping He: Collection and assembly of data; manuscript writing; data analysis and interpretation
Lin Tang: Collection and assembly of data (transplant work)
Wenxian Zeng: Collection and assembly of data (transplant work)
Ina Dobrinski: Collection and assembly of data (transplant work)
Jiji Jang: Collection and assembly of data
Maria Kokkinaki: Collection and assembly of data
References
- 1.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 3.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Brennecke J, Hipfner DR, Stark A, et al. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen CZ, Li L, Lodish HF, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 6.Yi R, Poy MN, Stoffel M, et al. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 8.Ko K, Tapia N, Wu G, et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Golestaneh N, Kokkinaki M, Pant D, et al. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad S, Renninger M, Hennenlotter J, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 11.Seandel M, James D, Shmelkov SV, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan K, Nayernia K, Maier LS, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 13.Kanatsu-Shinohara M, Inoue K, Lee J, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–125. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song R, Ro S, Michaels JD, et al. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet. 2009;41:488–493. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS ONE. 2008;3:e1738. doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smorag L, Zheng Y, Nolte J, et al. MicroRNA signature in various cell types of mouse spermatogenesis: evidence for stage-specifically expressed miRNA-221, -203 and -34b-5p mediated spermatogenesis regulation. Biol Cell. 2012;104:677–692. doi: 10.1111/boc.201200014. [DOI] [PubMed] [Google Scholar]
- 18.Niu Z, Goodyear SM, Rao S, et al. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2011;108:12740–12745. doi: 10.1073/pnas.1109987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang QE, Racicot KE, Kaucher AV, et al. MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development. 2013;140:280–290. doi: 10.1242/dev.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huszar JM, Payne CJ. MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol Reprod. 2013;88:15. doi: 10.1095/biolreprod.112.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellvé AR, Cavicchia JC, Millette CF, et al. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirami G, Ravindranath N, Pursel VG, et al. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–230. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- 23.Kokkinaki M, Lee TL, He Z, et al. The Molecular Signature of Spermatogonial Stem Cells in the 6-Day-Old Mouse Testis. Biol Reprod. 2009;80:707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohta H, Yomogida K, Dohmae K, et al. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 25.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 26.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Jiang J, Kokkinaki M, et al. Nodal Signaling via an Autocrine Pathway Promotes Proliferation of Mouse Spermatogonial Stem/Progenitor Cells Through Smad2/3 and Oct-4 Activation. Stem Cells. 2009;27:2580–2590. doi: 10.1002/stem.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang F, Hajkova P, Barton SC, et al. 220-plex microRNA expression profile of a single cell. Nature Protocols. 2006;1:1154–1159. doi: 10.1038/nprot.2006.161. [DOI] [PubMed] [Google Scholar]
- 29.Wienholds E, Kloosterman WP, Miska E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 30.He Z, Jiang J, Kokkinaki M, et al. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honaramooz A, Megee S, Zeng W, et al. Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB J. 2008;22:374–382. doi: 10.1096/fj.07-8935com. [DOI] [PubMed] [Google Scholar]
- 32.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, Sharma M, Nabeshima Y, et al. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein AM, Nakagawa T, Ichikawa R, et al. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7:214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 38.Yoshinaga K, Nishikawa S, Ogawa M, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann MC, Braydich-Stolle L, Dettin L, et al. Immortalization of mouse germ line stem cells. Stem Cells. 2005;23:200–210. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawkins SM, Buchold GM, Matzuk MM. Minireview: The roles of small RNA pathways in reproductive medicine. Mol Endocrinol. 2011;25:1257–1279. doi: 10.1210/me.2011-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steger K, Aleithe I, Behre H, et al. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol Hum Reprod. 1998;4:227–233. doi: 10.1093/molehr/4.3.227. [DOI] [PubMed] [Google Scholar]
- 42.Buaas FW, Kirsh AL, Sharma M, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 43.Zeng W, Tang L, Bondareva A, et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol Reprod. 2013;88:27. doi: 10.1095/biolreprod.112.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toyooka Y, Tsunekawa N, Akasu R, et al. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dann CT, Alvarado AL, Molyneux LA, et al. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–2937. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 46.Nagano M, Brinster CJ, Orwig KE, et al. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng K, Wu X, Kaestner KH, et al. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 50.He Z, Kokkinaki M, Jiang J, et al. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oatley JM, Kaucher AV, Avarbock MR, et al. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod. 2010;83:427–433. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 53.Nass SJ, Strauss JF., 3rd Strategies to facilitate the development of new contraceptives. Nat Rev Drug Discov. 2004;3:885–890. doi: 10.1038/nrd1526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Expression of miRNA-92and miRNA-93 in various types of cells in adult mouse testes and NIH3T3 cells. Real time PCR showed the expression of miRNA-92 and miRNA-93 in GFRA1 positive spermatogonia, c-kit positive spermatogonia, C18-4 cells, adult male germ cells, NIH 3T3 cells, and Sertoli cells. House keeping miRNA-16 served as a loading control of total miRNAs.
Supplementary Figure 2. FISH showed expression of miRNA-20 (A) and miRNA-106a (B) in immature mouse testes. Testis sections without miRNA probes in immature mice (C) and adult mice (D) were used as negative controls. Scale bars = 10 μm (C, D).
Supplementary Figure 3. (A) Fluorescence microscopy (left figure) and phase-contrast imaging (right figure) showed transfection efficiency of AAV2-CMV-eGFP in mouse SSCs before transplantation. Scale bars = 50 μm. (B-C) Real time PCR showed expression of miRNA-20 (B) and miRNA-106a (C) in male germ cells from recipient mice transplanted with SSCs transfected with or without miRNA-20 or miRNA-106a mimics or inhibitors. Compared to miRNA mimic or inhibitor control, “*” indicated a significant difference (P<0.05).