Abstract
The vascular endothelium, a thin layer of endothelial cells (ECs) that line the inner surface of blood vessels, is a critical interface between blood and all tissues. EC activation, dysfunction, and vascular inflammation occur when the endothelium is exposed to various insults such as proinflammatory cytokines, oxidative stress, hypertension, hyperglycemia, aging, and shear stress. These insults lead to the pathogenesis of a range of disease states, including atherosclerosis. Several signaling pathways, especially nuclear factor κB mediated signaling, play crucial roles in these pathophysiological processes. Recently, microRNAs (miRNAs) have emerged as important regulators of EC function by fine-tuning gene expression. In this review, we discuss how miRNAs regulate EC function and vascular inflammation in response to a variety of pathophysiologic stimuli. An understanding of the role of miRNAs in EC activation and dysfunction may provide novel targets and therapeutic opportunities for controlling atherosclerosis and other chronic inflammatory disease states.
Keywords: MicroRNA, MiR-181b, Vascular inflammation, Atherosclerosis, Endothelial activation, Nuclear factor κB
Introduction
Atherosclerosis is a chronic disease of the arterial wall characterized by lipid accumulation and low-grade inflammation [1, 2]. The pathogenesis of atherosclerosis can be divided into three phases: initiation, lesion progression, and thrombotic complication [1]. Both animal evidence and human evidence suggest that the initial step in the development of atherosclerotic plaques is mediated, in part, by the monolayer of endothelial cells (ECs) lining the inner wall of the arterial vessel. When subjected to various noxious stimuli, focal areas of the endothelial monolayer are activated to express adhesion molecules and capture leukocytes. After adhering to activated ECs, leukocytes—mainly monocytes and T lymphocytes—are recruited to enter the intima by chemoattractant signals. In parallel, cholesterol-containing low-density lipoprotein (LDL) particles accumulate in the artery wall because of increased endothelial permeability and changes in the composition of extracellular matrix beneath the endothelium [3]. Monocyte-derived foam cells accumulate in lesions, and other leukocyte subsets, such as proinflammatory M1 macrophages, elaborate cytokines and growth factors. Lesion progression involves the migration and proliferation of resident smooth muscle cells (SMCs), extracellular matrix deposition, and lipid or necrotic core formation. The subsequently formed advanced plaques may cause clinical manifestations, such as angina, resulting from flow-limiting stenosis. Thrombotic complications such as myocardial infarction and stroke may be due to plaque rupture or erosion from either immature or advanced lesions associated with thin, collagen-poor fibrous caps [1].
MicroRNAs (miRNAs) are a class of single-stranded, small noncoding RNAs. They function by directly binding to the 3′ untranslated region (3′ UTR) of specific target messenger TNA (mRNA) sequences, leading to the reduction of protein expression by inhibiting mRNA translation and/or promoting target mRNA degradation. Recently, miRNAs have emerged as new regulators of atherosclerosis [4–8]. MiRNA expression is modulated by different stimuli in various cell types involved in every stage of atherosclerosis [5]. The role of miRNAs in cholesterol metabolism and atherosclerosis has been reviewed elsewhere [9, 10]. Flow-regulated miRNAs and their roles in vascular remodeling were also reviewed recently [11, 12]. Increasing evidence suggests that miRNAs are involved in the regulation of EC function, cytokine responsiveness, and vascular inflammation [13]. This review summarizes our current understanding of endothelial miRNAs as critical regulators of EC activation, vascular inflammation, and the initiation of atherosclerosis.
EC Activation and Dysfunction, and Nuclear Factor κB in Atherosclerosis
Nuclear factor κB (NF-κB) activation contributes to EC activation and dysfunction, which play critical roles during the development of atherosclerosis [1, 14, 15]. In the arterial endothelium, NF-κB signaling is activated by many risk factors for atherosclerosis, including inflammatory cytokines, diabetes, oxidized LDL, angiotensin II, and hemodynamic forces. The resulting NF-κB signaling leads to the expression of proinflammatory genes, including cytokines, adhesion molecules, and chemokines [16–21]. The mammalian NF-κB family consists of five members—p65 (RelA), RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). These transcription factors form different heterodimers and homodimers, which are retained in the cytosol by a family of inhibitory proteins known as inhibitors of NF-κB (IκB) [22, 23]. In the canonical NF-κB signaling pathway, the IκB kinase (IKK) complex is activated in response to a variety of stimuli. The activated IKK complex rapidly phosphorylates IκBα at two N-terminal serines, resulting in its ubiquitin-induced degradation by the 26S proteasome [24]. This leads to the release of NF-κB heterodimers that translocate to the nucleus, where they bind to κB elements that drive a wide range of gene expression. NF-κB signaling is tightly and precisely regulated by different mechanisms at multiple levels [25–29], and failure to control this pathway contributes to the development of many chronic diseases, such as atherosclerosis [15, 30]. Activation of NF-κB has been detected in human SMCs, macrophages, and ECs in the fibrotic-thickened intima/media and atheromatous areas of the atherosclerotic lesion, but not in vessels lacking atherosclerosis [31]. Activated NF-κB was also detected in arterial intimal cells or ECs in animals with an cholesterol-enriched diet [19, 32]. Numerous studies convincingly demonstrate that inhibition of the endothelial NF-κB signaling pathway or its target gene expression ameliorates atherosclerosis [33–36], whereas the role for myeloid NF-κB in the pathogenesis of atherosclerosis remains controversial [37–39].
Cytokine-Responsive Endothelial miRNAs and EC Activation and Dysfunction
A role for miRNAs in EC activation and dysfunction was first provided by Harris et al. [40], who detected that miR-126 reduced vascular cell adhesion molecule 1 (VCAM-1) expression in ECs by binding to its 3′ UTR, an effect that reduced leukocyte–EC interactions in response to TNF-α. Subsequent studies have shown that miR-126 inhibits VCAM-1 induced by additional stimuli such as triglyceride-rich lipoproteins isolated from subjects after consumption of a high-fat meal [41]. Control of miR-126 expression may be mediated, in part, by the E26 transformation-specific sequence (Ets) factors Ets-1 and Ets-2, which are known to regulate EC differentiation and vascular inflammation [42–44]. Ets-1 expression was induced in response to proinflammatory stimuli and, in turn, activated expression of inflammatory genes such as monocyte chemoattractant protein 1 (MCP-1) and VCAM-1 [45]. These data suggest that the induction of miR-126 could be a critical part of a negative-feedback loop to dampen Ets-1-induced inflammatory responses. MiR-126 was the most abundant miRNA in apoptotic bodies derived from ECs [46]. MiR-126 expression has also been identified in EC-derived apoptotic bodies, where it induced CXCL12 expression by targeting RGS16, a negative regulator of CXCR4. Intravenous injection of endothelial apoptotic bodies mobilized progenitor cells to peripheral blood, increased the incorporation of progenitor cells into aortic root plaques, and protected mice from atherosclerosis in an miR-126-dependent manner [46]. Clinically, the level of circulating miR-126 was reduced in young patients with stroke and patients with coronary artery disease [47, 48], suggesting that miR-126 may be an important regulator of vascular function in human disease.
We recently identified miR-181b as a critical regulator of NF-κB-mediated vascular inflammation by virtue of miR-181b’s ability to directly target importin-α3, a protein critical for NF-κB translocation from the cytoplasm to the nucleus [49]. With use of a microarray profiling approach, miR-181b expression was rapidly reduced in response to the proinflammatory stimulus TNF-α. Both TNF-α and lipopolysaccharide reduced miR-181b expression in ECs in vitro and in the aortic intima in vivo [49]. Gain-of-function and loss-of-function studies revealed that miR-181b regulated the NF-κB signaling pathway and NF-κB-responsive gene expression in the activated vascular endothelium. Consistent with its inhibitory effect on NF-κB activation, systemic delivery of miR-181b mimics reduced EC activation, leukocyte accumulation, and lung inflammation and improved survival by approximately 50%. In contrast, miR-181b inhibition exacerbated inflammation and increased NF-κB-responsive gene expression. Microarray gene chip analysis and bioinformatic approaches validated six biological processes related to the NF-κB signaling pathway and identified an enriched set of NF-κB target genes whose expression was reduced by miR-181b in response to TNF-α. Surprisingly, there was no effect of miR-181b on upstream NF-κB mediators, including the IKK complex, IκBα, or IκBα phosphorylation, raising the possibility that miR-181b inhibited downstream NF-κB signaling by targeting the NF-κB translocation step. Emerging studies demonstrate that NF-κB translocation from the cytoplasm to the nucleus is an active process that requires a family of proteins termed importins [50–53]. Several importin-α members (e.g., importin-α3, importin-α4, and importin-α5) heterodimerize to NF-κB family members such as p65 and p50 [52, 53]. Indeed, using in silico prediction algorithms, we identified several potential importin-α members as targets; however, only importin-α3 expression and its 3′ UTR were uniquely inhibited by miR-181b in ECs [49].
Mechanistically, several pieces of experimental data confirmed that importin-α3 is a bona fide target of miR-181b in ECs in vitro and in vivo. First, miR-181b suppressed p65 nuclear translocation and activation of the NF-κB pathway by binding to two consensus sites of the importin-α3 3′ UTR and reducing its expression. Second, argonaute 2 immunoprecipitation studies verified that miR-181b associated directly with importin-α3 mRNA, which was enriched fivefold compared with a nonspecific control miRNA mimic. Third, overexpression of importin-α3 lacking its 3′ UTR could effectively rescue the miR-181b-mediated inhibition of NF-κB activity and target genes (e.g., VCAM-1, E-selectin) in vitro and in lung ECs in vivo. Fourth, small interfering RNA (siRNA)-mediated “knockdown” of importin-α3 expression “phenocopied” the inhibitory effects of miR-181b on NF-κB in ECs in vitro and in vivo. Finally, miR-181b’s inhibitory effects on NF-κB was significantly blocked in the presence of siRNA-mediated knockdown of importin-α3 in vivo [49]. Importantly, in TNF-α-treated ECs, the inhibitory effect of miR-181b was specific for the NF-κB signaling pathway as miR-181b had no effect on major mitogen-activated protein kinase (MAPK) downstream signaling pathways, including extracellular-signal regulated kinase (ERK) 1/2, p38, or c-Jun N-terminal kinase. The inhibitory effect of miR-181b was specific to ECs as no inhibition of NF-κB activity could be detected in peripheral blood mononuclear cells of endotoxemic mice. Collectively, these findings implicate miR-181b as a novel regulator of NF-κB-mediated EC activation and vascular inflammation in response to acute proinflammatory stimuli, and provide new opportunities for anti-inflammatory therapy (Fig. 1).
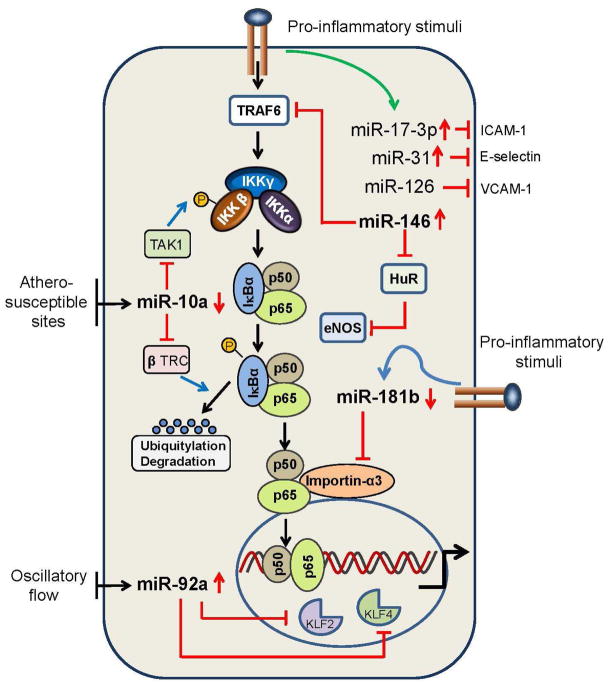
Fig. 1.
Endothelial microRNAs and vascular inflammation. MiR-17-3p, miR-31, and miR-126 target the 3′ untranslated regions (UTRs) of intercellular adhesion molecule 1 (ICAM-1), E-selectin, and vascular cell adhesion molecule 1 (VCAM-1), respectively. MiR-181b targets the 3′ UTR of importin-α3 to suppress downstream nuclear factor κB (NF-κB) signaling by inhibiting NF-κB nuclear translocation. MiR-146 targets the 3′ UTR of TNF-receptor-associated factor 6 (TRAF6) and HuR, respectively, to regulate NF-κB activation and endothelial nitric oxide synthase (eNOS) expression. The expression of miR-10a and miR-92a is regulated by shear stress. MiR-10a reduces NF-κB activation by targeting the 3′ UTR of mitogen-activated protein kinase kinase kinase 7 and the β-transducin-repeat-containing gene (β TRC). MiR-92a is involved in the regulation of endothelial activation by targeting the transcription factors KLF2 and KLF4
We have recently uncovered a protective role for miR-181b in chronic inflammation in the context of atherosclerosis [54]. MiR-181b expression was reduced in the vascular endothelium and plasma of ApoE−/− mice fed a high-fat diet. Consistent with these observations, circulating miR-181b expression in the plasma was markedly reduced in patients with coronary artery disease. Systemic delivery of liposomally encapsulated miR-181b mimics resulted in a 2.3-fold overexpression of miR-181b in the aortic intima of ApoE−/− mice and inhibited NF-κB signaling revealed by bioluminescence imaging and reduced NF-κB-responsive gene expression in the aortic arch in ApoE−/−/NF-κB–luciferase transgenic mice. Importantly, weekly intravenous injections of miR-181b significantly inhibited atherosclerotic lesion formation, proinflammatory gene expression, and the accumulation of lesional macrophages and CD4+ T cells in the vessel wall. Remarkably, miR-181b inhibited the expression of the target gene importin-α3, an effect that reduced NF-κB nuclear translocation specifically in the vascular endothelium of lesions; in contrast, leukocyte NF-κB signaling was surprisingly unaffected despite a sevenfold overexpression of miR-181b. The basis for the cell-specific inhibition by miR-181b raised the possibility that importin-α3 may not be the dominant importin-α family member in leukocytes to mediate NF-κB activation. Indeed, our studies revealed that NF-κB nuclear translocation in leukocytes is mediated by importin-α5, which miR-181b does not target, and that the ratio of importin-α5 to importin-α3 expression is significantly higher in leukocytes, whereas it is the opposite in ECs. Finally, these studies highlighted that inhibition of NF-κB signaling specifically in the vascular endothelium is sufficient to mediate miR-181b’s protective effects in atherosclerotic lesion formation. These findings are consistent with prior studies showing that inhibition of NF-κB signaling specifically in ECs (by ablating IKKγ/NF-κB essential modulator or expression of a dominant-negative IκBα) confers an atheroprotective effect in ApoE−/− mice [33]. These findings support the rationale that delivery of miR-181b may provide a novel therapeutic approach to treat chronic inflammatory diseases such as atherosclerosis.
Delivery of miR-181b mimics to inhibit downstream NF-κB signaling in the vascular endothelium may offer several advantages: (1) previous targeting of upstream NF-κB signaling effectors including IKKs or IκBα may lead to off-target effects owing to the vast number of interdependent signaling pathways that they regulate (e.g., MAPK signaling, insulin signaling, and p53) [55–58]; (2) miR-181b-mediated inhibition of importin-α3 expression offers targeting of a focused downstream event by suppressing NF-κB nuclear translocation; (3) miR-181-mediated targeting of importin-α3 only inhibits NF-κB activation in the vascular endothelium, and not leukocytes, of lesions because of the differential expression of importin-α3 and importin-α5 (which miR-181b does not target) in ECs and leukocytes, respectively; and (4) the cell-specific miR-181b effects on NF-κB in response to inflammatory stimuli may be advantageous, for example, to avoid inhibition of myeloid NF-κB in order to maintain optimal protection in response to various infectious pathogens.
Other regulators of cytokine-induced EC activation include miR-31 and miR-17-3p, which target the 3′ UTRs of E-selectin and intercellular adhesion molecule 1 (ICAM-1) respectively, to reduce their expression in TNF-α-stimulated ECs, an effect that inhibited adhesion of leukocytes to activated EC monolayers [59]. The expression of miR-31 and miR-17-3p is induced by TNF-α, suggesting that these miRNAs constitute a negative-feedback loop to regulate the inflammatory response by directly targeting the 3′ UTRs of inflammatory genes. Whether miR-31 and miR-17-3p regulate E-selectin and ICAM-1 expression, respectively, or impact EC function in vivo remains unknown. More recently, elegant studies by Cheng et al. [60] revealed that proinflammatory cytokines induced expression of miR-146a and miR-146b in ECs in a delayed kinetic fashion that coincided with the reduction of inflammatory gene expression. Overexpression of miR-146a ameliorated endothelial activation, and genetic or locked nucleic acid–anti-microRNA-mediated inhibition of miR-146a had the opposite effect. MiR-146 inhibited both the NF-κB pathway and the MAPK pathway by directly targeting HuR, an RNA-binding protein which exerts inhibitory effects on endothelial nitric oxide synthase (eNOS), thereby promoting endothelial activation. Thus, miR-146 is another critical component of a negative-feedback loop that controls endothelial activation and dysfunction [60].
Endothelial miRNAs in Hyperglycemia and Diabetes-Induced EC Activation and Dysfunction and Atherosclerosis
Patients with type 2 diabetes are associated with increased cardiovascular disease [21, 61, 62]. EC dysfunction and vascular inflammation are closely linked with the pathogenesis of type 2 diabetes [63]. For example, blockade of the intracellular NF-κB pathway specifically in the vascular endothelium prevents obesity and age-related insulin resistance, and extended longevity; furthermore, these effects were associated with reduced macrophage accumulation in tissues and enhanced blood flow and mitochondrial function [64]. Several miRNAs have emerged as potential regulators of EC function under diabetic conditions. Wang et al. [65] identified that high glucose concentration induced miR-320 expression in myocardial microvascular ECs. Inhibition of miR-320 improved proliferation and migration myocardial microvascular ECs, which has implications for diabetic-impaired angiogenesis. More recently, expression of miR-503 was found to be increased in cultured conditions mimicking high glucose concentration [66]. Consistently, miR-503 expression also increased in ECs enriched from ischemic limb muscles of streptozotocin-induced diabetic mice [66]. Moreover, miR-503 expression was remarkably higher in muscles and plasma from diabetic patients and inversely correlated with expression of cell division cycle 25A, a known target gene that controls cell cycle function [66]. Local inhibition of miR-503 promoted vascular wound healing and blood-flow recovery in a diabetic mouse model of limb ischemia [66]. Expression of another miRNA, miR-29c, was increased in microvascular ECs exposed to hyperglycemic conditions and in the kidney from diabetic mice [67]. Overexpression of miR-29c decreased the levels of Spry1 protein and promoted activation of Rho kinase, which suggests that miR-29c may be a key miRNA governing kidney remodeling during diabetic nephropathy [67]. Finally, miR-221 expression increased in ECs in response to advanced glycation end products or glucose, and its overexpression reduces c-kit expression [68]. In contrast, a more recent study showed that high concentrations of glucose or advanced glycation end products decreased miR-221/miR-222 expression in ECs [69]. Moreover, reduced miR-221/miR-222 expression induced cell cycle arrest owing to the upregulation of p27KIP1 and p57KIP2 (cyclin-dependent kinase inhibitors), which are known miR-221/miR-222 direct targets [69]. Whether these miRNAs discussed above are involved in the pathogenesis of diabetic-associated atherosclerosis remains unknown.
MiRNAs in Aging-Induced EC Senescence and Atherosclerosis
Aging is a major risk factor for cardiovascular diseases, including atherosclerosis. Several miRNAs, including miR-146a, miR-181a, miR-26a, and miR-221, have been found to be modulated by EC senescence as measured at early and late human umbilical vascular EC (HUVEC) cell passage [70]. MiR-146a expression was reduced in response to late EC passage, and overexpression of miR-146a improved EC senescence by reducing the predominant NADPH oxidase protein isoform NADPH oxidase 4 and β-galactosidase expression [70]. Endothelial senescence is also controlled by miR-217 and miR-34a [71–73]. SIRT1, a major regulator of longevity and metabolic disorders, is a class III histone deacetylase involved in deacetylation of many proteins, including NF-κB and peroxisome-proliferator-activated receptor γ [74], and its expression is progressively reduced in multiple tissues during aging. MiR-217 expression increased in later-passaged ECs and targeted SIRT1 3′ UTR to reduce its expression. Furthermore, in human atherosclerotic lesions, miR-217 expression inversely correlated with SIRT1 expression [73]. MiR-34a also targeted SIRT1 expression in ECs to trigger EC senescence [71]. MiR-34a expression is increased in senescent ECs and in the heart and spleen of older mice [71]. MiR-34a expression was also upregulated in atherosclerotic arteries [75]. An age-related increase in miR-34a expression also promoted cardiac senescence by reducing the expression of phosphatase 1 nuclear targeting subunit, a novel miR-34a target [76]. Whether the miR-34a/phosphatase 1 nuclear targeting subunit axis is functionally active in cardiac ECs remains unknown. Taken together, emerging studies implicate functional roles for several miRNAs, such as miR-146a, miR-217, and miR-34a, in EC senescence. Further studies will be required to determine if these miRNAs dynamically regulate atherosclerosis and its attendant consequences in the elderly.
MiRNAs in Hypertension, EC Activation, and Atherosclerosis
Hypertension also affects endothelial function and is a significant contributor to atherosclerosis and cardiovascular disease. In spontaneously hypertensive rats, expression of miR-125a-5p and miR-125b-5p decreased and, as a consequence, resulted in increased expression of endothelin 1, a potent vasoconstrictive peptide that promotes endothelial inflammation and atherosclerosis [77]. In miR-21 endothelial-specific knockout mice, diastolic blood pressure was reduced and acetylcholine-induced endothelium-dependent relaxation of the aorta was impaired [78]. In addition, miR-21 deficiency decreased the aortic elastin content and increased the wall thickness of the thoracic aorta media layer and aortic stiffness. At the molecular level, deletion of miR-21 increased the expression of Smad7, connective tissue growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 10, while decreasing the expression of Smad2, Smad5, and tissue inhibitor of metalloproteinase 4. These data suggest that endothelial miR-21 may play a critical role in vascular remodeling through regulating transforming growth factor (TGF) β1 signaling [78].
Angiotensin II regulates multiple aspects of EC function. In response to angiotensin II, the expression of Ets-1, a transcription factor that plays a pivotal role in the vascular inflammatory response, and its target genes VCAM-1, MCP-1, and Fms-like tyrosine kinase 1 (FLT-1), is increased, which can contribute to atherosclerotic lesion formation. Several studies have implicated an important role for miR-155 in regulating angiotensin II and Ets-1 signaling in ECs. For example, in response to angiotensin II, miR-155 (and miR-221) directly targeted Ets-1, an effect that reduced VCAM-1, MCP-1, and FLT-1 expression with a concordant reduction in leukocyte–EC interactions and EC migration [79]. In addition, miR-155 also reduced ERK1/2 activation in aortic adventitial fibroblasts [80] and ECs [81], and attenuated apoptotic factors by targeting the angiotensin II type 1 receptor [82]. MiR-155 was also found to increase expression of heme oxygenase 1 (HO-1), a stress-inducible enzyme that exerts anti-inflammatory effects in the endothelium, and plays a protective role in cardiovascular diseases, including atherosclerosis. The induction of HO-1 expression was due to the ability of miR-155 to inhibit the expression of BACH1, a transcriptional repressor of the HO-1 gene [83]. Although these studies suggest a protective role for miR-155 in EC function, other reports suggest that it may promote EC dysfunction. In HUVECs, miR-155 targeted the 3′ UTR of eNOS. MiR-155 overexpression decreased whereas miR-155 inhibition increased eNOS expression and NO production in ECs and acetylcholine-induced endothelium-dependent vasorelaxation in human internal mammary arteries [84]. Expression of miR-155 was also induced by TNF-α and decreased by simvastatin [84]. Finally, miR-155 expression was elevated in the atherosclerotic thoracic aorta from ApoE−/− mice, and in plasma samples from patients with coronary artery disease [85].
The role of miR-155 in atherosclerosis remains controversial. Although endothelial-specific overexpression or deletion of miR-155 has not been reported, hematopoietic deficiency of miR-155 led to more advanced and inflammatory atherosclerotic lesions in LDLR−/− mice harboring miR-155-deficient bone marrow cells [85]. In contrast, leukocyte-specific miR-155 deficiency reduced plaque size and the number of lesional macrophages after partial carotid artery ligation in ApoE−/− mice receiving miR-155-deficient bone marrow cells [86]. BCL6, a negative regulator of NF-κB signaling, was identified as a direct target of miR-155 that may underlie these effects [86]. In contrast, systemic intravenous delivery of miR-155 “agomiRs” to overexpress miR-155 reduced atherosclerotic lesion formation in ApoE−/− mice and inhibited inflammatory cytokine production as part of a negative-feedback loop by targeting MAPK kinase kinase 10 [87]. Taken together, the disparate effects of miR-155 on atherosclerosis may be due to the role of miR-155 in specific cell types or stage-dependent effects. Indeed, the opposing effects of miR-155 on proinflammatory gene expression (protective) and eNOS (inhibitory) may suggest distinct roles for miR-155 in EC adhesion and vasoreactivity, respectively. Further studies will be required to interrogate the functional role of miR-155 in different cell types, tissues, and stages of atherosclerotic lesion severity to assess whether miR-155 could serve as a target for therapy.
MicroRNAs in Shear-Stress-Induced EC Activation and Atherogenesis
Both human and animal atherosclerotic lesions preferentially develop at arterial branch points, bifurcations, and the lesser curvature of the aorta [20, 88, 89]. The phenomenon is explained, in part, by the presence of disturbed wall shear stress that results from flow separation and reattachment, reciprocating flow, low shear stress, and flow reversal during the cardiac cycle [89]. The ECs at atherosclerosis-prone regions exhibit increased permeability and a highly proliferative state. Disturbed flow enhances endothelial permeability by modulating the expression and distribution of intracellular junctional proteins, including connexins and vascular endothelial cadherin [90–94]. In particular, these ECs are proinflammatory, with increased signaling (e.g., NF-κB activation), gene expression (e.g., increased VCAM-1 and ICAM-1 expression), and function (e.g., leukocyte adhesion) [95, 96].
MiRNAs are differentially expressed in response to different flow patterns both in vitro and in vivo. In HUVECs following 12 h of high laminar shear stress at 12 dyn/cm2 (approximating the hemodynamic force in the straight part of arteries), 35 miRs including miR-19a, miR-181b, miR-10a, and miR-29c were upregulated and 26 miRs were downregulated compared with the static control cells. Among these regulated miRNAs, miR-19a expression increased after laminar shear stress and miR-19a reduced cyclin D1 expression by binding to its 3′ UTR [97], thereby suggesting a new mechanism by which high laminar shear stress keeps ECs in a low proliferative state. Given the role of miR-181b in suppressing NF-κB in ECs [49], it will be interesting to assess if it also contributes to EC quiescence under laminar flow. In a similar study, miRNA profiling identified eight upregulated and 13 downregulated miRNAs in response to 24 h of pulsatile shear flow (12 ± 4 dyn/cm2), with a significant forwarding direction in comparison with the static condition [98]. MiR-23b was one of the upregulated miRNAs, and it suppressed endothelial proliferation by reducing E2F1 expression and Rb phosphorylation [98]. In fibroblast-like synoviocytes, miR-23b inhibited NF-κB activation by targeting TGF-β-activated kinase 1/MAPK kinase kinase 7 binding protein 2, TGF-β-activated kinase 1/MAPK kinase kinase 7 binding protein 3, and IKK-α [99]. However, it is unknown if miR-23b regulates NF-κB signaling in ECs. Laminar shear stress also increased miR-27a/b expression in ECs; overexpression of miR-27a/b promoted EC sprouting, whereas inhibition of miR-27a/b impaired sprouting and induced EC repulsion, an effect mediated by targeting semaphorin 6A [100]. Laminar shear stress (12 dyn/cm2; 12 h) also induced the expression of miR-101, which reduced the expression of the mammalian target of rapamycin gene by targeting its 3′ UTR, and inhibited EC proliferation [101].
By comparative miRNA microarray profiling of ECs from the atherosusceptible region of the inner aortic arch and the atheroprotected region of the descending thoracic aorta in swine, Fang et al. [102] found that the expression of miR-10a was lower in the atherosusceptible region. MiR-10a bound to the 3′ UTRs of MAPK kinase kinase 7 and the β-transducin repeat-containing gene, two key regulators of IκBα degradation, and reduced their expression [102]. Subsequently miR-10a suppressed proteasomal degradation of IκBα and inhibited the canonical NF-κB signaling pathway in ECs in vitro [102]. A functional role for miR-10a in regulating atherosclerosis will require further investigation. Another miRNA, miR-21, was induced in response to oscillatory shear stress (0.5 ± 4 dyn/cm2) in comparison with laminar shear stress (12 ± 4 dyn/cm2) [103]. Induced miR-21 enhanced the expression of proinflammatory genes, including VCAM-1 and MCP-1 and, consequently, enhanced leukocyte–EC interactions [103]. This proinflammatory effect of miR-21 resulted from targeting the 3′ UTR of peroxisome-proliferator-activated receptor α expression and activation of the AP-1 signaling pathway [103]. Oscillatory shear stress induced miR-21 expression through AP-1 activation and c-Jun-mediated transcription, thereby suggesting that sustained induction of miR-21 contributes to the proinflammatory response in ECs through a positive-feedback loop. A recent study demonstrated that miR-663 was the most significantly induced miRNA in response to oscillatory shear stress (±5 dyn/cm2 at 1 Hz) for 24 h compared with unidirectional laminar shear stress at 15 dyn/cm2 [104]. Overexpression of miR-663 increased monocyte–EC adhesion likely by altering the expression of an array of transcriptional regulators [104]. Finally, miR-92a expression was reduced by pulsatile laminar shear stress and increased by oscillatory shear stress [105]. Overexpression of miR-92a inhibited NO production in ECs by targeting the transcription factor KLF2, a positive regulator of eNOS expression. Indeed, flow-mediated vasodilation was suppressed in a mouse carotid artery transfected with miR-92a [105]. Inhibition of miR-92a also dampened TNF-stimulated EC activation and leukocyte–endothelial interactions, effects that were attributable to enhanced KLF4 expression [106]. MiR-92a can reduce both KLF2 expression and KLF4 expression by directly targeting their 3′ UTRs [106]. Consistent with this inverse relationship, miR-92a expression was elevated and both KLF2 expression and KLF4 expression were decreased at atherosusceptible sites in swine aortic arch relative to the protected thoracic aorta. In further studies it will be interesting to assess whether in vivo administration of any of these shear-stress miRNAs will alter the pathogenesis of atherosclerosis.
An emerging paradigm in cardiovascular biology is the ability of miRNAs to exhibit paracrine effects through either direct or indirect communication from one cell to another within tissues. For example, atheroprotective laminar shear stress induces the release from ECs of miR-126, which acts as a key intercellular mediator to increase SMC turnover [107]. Similarly, in response to overexpression of KLF2 or shear-stress-stimulated ECs, extracellular vesicles were released containing miR-143 and miR-145, which induced an atheroprotective SMC phenotype [108]. Systemic delivery of these extracellular vesicles also inhibited atherosclerotic lesion formation in an miR-143/145 dependent manner [108]. Taken together, these studies suggest that miRNAs are important regulators of EC function and signaling pathways in response to shear stress and may control cell phenotypes in the vessel wall through extracellular communication.
MicroRNAs in Pluripotency, Endothelial Differentiation, and Transdifferentiation
A primary event in atherosclerotic lesion progression is damage to the endothelium resulting in endothelial activation, dysfunction, and senescence [109]. MiRNAs may provide a therapeutic tool in preventing atherosclerotic lesion progression through their role in inducing differentiation, reprogramming, and transdifferentiation of cells toward a healthy endothelial phenotype.
MiRNAs are known to play a role in both maintaining the pluripotency and guiding the differentiation of embryonic stem cells (ESCs). Dicer knockout mice die early in development owing to lack of proper ESC proliferation and differentiation [110]. Pluripotency factors such as Oct4, Sox2, and Nanog bind miRNA promoters, inducing the expression of many miRNA families [111]. The miR-290 cluster is the most highly expressed miRNA in ESCs, representing more than 70% of total expression [111]. MiRNAs from that cluster (miR-291, miR-294, and miR-295) along with the miR-302 cluster are known to repress cell cycle inhibitors of the cyclin E–cyclin-dependent kinase 2 pathway, promoting a pluripotent state [112, 113]. In addition to their role in maintaining pluripotency, miRNAs reprogram cells by inducing dedifferentiation to an induced pluripotent stem (iPS) cell state. For example, members of the miR-290 cluster when expressed with the pluripotency transcription factors Oct4, Sox2, and KLF4 enhanced the reprogramming of mouse embryonic fibroblasts to an iPS cell state [114]. Inhibition of the let-7 family of miRNAs enhanced reprogramming of somatic cells [115]. Overexpression of the miR-302 cluster and miR-367 has been shown to reprogram mouse and human somatic cells even in the absence of the pluripotency factors Oct4, Sox2, and KLF4 [116].
In addition to inducing the reprogramming of cells to an iPS cell state, miRNAs also guide the differentiation of pluripotent cells. MiRNAs are implicated in regulating endothelial differentiation. Dicer-knockout mice die between days 12.5 and 14.5 of gestation, display altered vascular endothelial growth factor (VEGF), FLT-1, kinase insert domain receptor, and Tie2 expression, and fail to properly develop blood vessels in the embryo and yolk sac [117]. Endothelial-specific Dicer-knockout mice, although not displaying embryonic lethality, also possess impaired angiogenic properties after ischemic injury or in response to VEGF signaling [118]. In vitro, siRNA-induced Dicer “knockdown” in ECs reduced endothelial functional properties [119]. During directed endothelial differentiation from human ESCs, expression of a number of proangiogenic miRNAs (miR-296, miR-210, miR-133b, miR-133a, miR-139a, miR-126, let-7a, let-7b) is increased and expression of a number of antiangiogenic miRNAs (miR-222, miR-221, miR-20a, miR-20b) is decreased [120]. Although these angiogenic miRNAs are correlated with endothelial differentiation, no studies have demonstrated a causal link between the two. One study investigated whether the miR-17-92 cluster, a group of miRNAs known to play a role in neovascularization, was required for endothelial differentiation [121]. This cluster was found to be induced on endothelial differentiation. However, cholesterol-modified antagomiR “knockdown” of the miR-17-92 cluster did not impair endothelial differentiation. Further studies will need to determine which of these miRNAs who expression is increased with endothelial differentiation from ESCs are causally linked to the differentiation process. Another recent study has linked miR-99b, miR-181a, and miR-181b in inducing the differentiation of human ESCs to vascular ECs [122]. MiRNA microarray analysis of human ESCs in different stages of differentiation toward an endothelial phenotype demonstrated that miR-99b expression, miR-181a expression, and miR-181b expression were all increased in a coordinated fashion with endothelial differentiation [122]. Furthermore, overexpression of these miRNAs using a lentiviral-expression method in human ESCs enhanced endothelial differentiation by increasing mRNA and protein expression of endothelial markers (platelet endothelial cell adhesion molecule 1, vascular endothelial cadherin, and eNOS) and by improving neovascularization in vivo [122]. MiR-181a has been shown to repress Prox1, a protein characteristic of the lymphatic endothelial phenotype [123]. Thus, miR-181a may be necessary for vascular-specific endothelial differentiation. Furthermore, miR-99b, miR-20b, and let-7b have also been shown to be differentially expressed in different EC types throughout the body and may provide a clue to endothelial-specific vascular differentiation [124]. Finally, miR-126 is involved in the differentiation of mesenchymal stem cells to an endothelial phenotype through activation of the phosphatidylinositol 3-kinase/Akt and MAPK/ERK signaling pathways [125].
Another manner in which miRNAs may be used to enhance re-endothelialization and the maintenance of a healthy endothelium is through the process of transdifferentiation. Transdifferentiation is defined as the changing of phenotypes of a differentiated cell into a different phenotype without entering a dedifferentiated, iPS-cell-like transition state as characterized in reprogrammed cells [126]. Margariti et al. [127] first demonstrated that transdifferentiation to an endothelial phenotype was possible by treating human fibroblasts with Oct4, Sox2, KLF4, and c-Myc for 4 days. After 4 days, a partial iPS (PiPS) cell was created. The PiPS cell, although not identical to the iPS cell phenotype, was still able to differentiate to an endothelial phenotype (PiPS-EC) when placed in endothelial differentiation medium. The PiPS-EC cells enhanced neovascularization and blood flow in response to hindlimb ischemia. Li et al. [128] used a more directed transdifferentiation approach to an endothelial phenotype using a lentiviral technique to overexpress Oct4 and KLF4 in human neonatal fibroblasts in a combined endothelial and fibroblast growth medium containing differing concentrations of a number of soluble factors, including VEGF, bone morphogenic protein 4, and basic fibroblast growth factor. After 28 days in this differentiation condition, patches of ECs emerged at a low frequency (3.85%). When isolated and expanded, these cells demonstrated endothelial phenotypic mRNA and protein expression patterns and displayed endothelial functional properties. Finally, mature c-Kit− amniotic cells have also been transdifferentiated to an endothelial phenotype when grown with the Ets transcription factors ETV2, FLI1, and ERG1, along with TGF-β inhibition [129].
The role of miRNAs in transdifferentiation to an endothelial phenotype has not been investigated sufficiently. Nevertheless, an emerging role for miRNAs in transdifferentiation of other relevant cardiovascular cell types has been reported, for example, in transdifferentiating fibroblasts to cardiomyocytes [23, 130]. Jayawardena et al. [23] identified six candidate miRNAs known to play a role in cardiac muscle development and differentiation and transfected human cardiac fibroblasts with these miRNAs in various combinations of three and found that miR-1, miR-133, and miR-208 were most efficient in converting the fibroblasts to a cardiac myocyte phenotype which demonstrated mature cardiomyocyte marker expression, sarcomeric organization, and calcium flux signaling sensitivity. Nam et al. [130] also transdifferentiated cardiac fibroblasts to cardiomyocytes using miR-1 and miR-133 in combination with the four human cardiac transcription factors GATA4, Hand2, T-box5, and myocardin. Although the role of miRNA in transdifferentiating cells to an endothelial phenotype has not yet been investigated, there is likely a role for miRNAs in this process. Studies incorporating endothelial growth and transcription factors with miRNAs that are known to play a role in endothelial differentiation may provide potent transdifferentiation conditions and provide the foundation for therapies to repair the damaged endothelium in the course of atherosclerosis.
Concluding Remarks
Great progress has been achieved to treat atherosclerosis in the last several decades. Statins, a group of pharmacologic inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, have been widely used to treat patients in the primary or secondary prevention of coronary artery disease. Although statins effectively reduce LDL levels and cardiovascular risk, a considerable residual burden of coronary artery disease remains even in patients treated with statins. Novel complementary therapeutic approaches will likely be helpful for treating disease states such as atherosclerosis involving complex signaling networks. In this regard, because miRNAs can target multiple genes often in the same regulatory network, miRNAs can have tremendous effects on biological pathways, cell function, and homeostasis in the vessel wall. To develop miRNA-based therapeutics, the following are needed: (1) better understanding of the pharmacokinetics and pharmacodynamics of miRNA inhibitors or mimics in vivo; (2) identification of miRNA-specific targets, (3) better understanding of miRNA cell-type specific functions under pathophysiological stimuli, and (4) the development of various technologies to facilitate tissue-specific delivery. Indeed, miRNA therapeutics has inched toward the clinic, with miR-122 inhibitors demonstrating strong efficacy and reasonable safety for patients infected with chronic hepatitis C in a phase IIa trial [131]. Accumulating studies suggest that miRNA mimics may also be as efficacious using novel delivery strategies including liposomal, nanoparticle, or microvesicle formulations that can accumulate in the vessel wall, including the vascular endothelium [49, 54, 108]. Delivery of a cassette of miRNA mimics or inhibitors may facilitate “fine-tuning” of the vascular endothelium as an attractive therapeutic approach to prevent atherosclerosis and ischemic cardiovascular disease.
Table 1.
MiRNAs involved in endothelial cell activation, dysfunction, and vascular inflammation
| miRNAs | Targets | Functions | References |
|---|---|---|---|
| miR-10a | MAP3K7, βTRC | Inhibits upstream canonical NF-κB signaling pathway | [102] |
| miR-17-3p | ICAM-1 | Decreases leukocytes adhesion to activated ECs | [59] |
| miR-19a | Cyclin D1 | Mediates laminar flow induced cell growth arrest | [97] |
| miR-21 | PPARα | Promotes flow induced vascular inflammation | [103] |
| unknown | Modulate vascular remodeling by regulating TGF-β1 signaling | [78] | |
| miR-23b | E2F1 (indirect) | Leads to cell growth arrest in response to laminar flow | [98] |
| miR-27a/b | Semaphorin 6A | Inhibition of miR-27 impaired sprouting and induced EC repulsion | [100] |
| miR-29c | Spry1 | Induces cell apoptosis, increases ECM protein | [67] |
| miR-31 | E-selectin | Decreases leukocytes adhesion to activated ECs | [59] |
| miR-34a | SirT1 | Induces EC senescence, suppresses cell proliferation | [71] |
| PNUTs | Promotes cardiac senscence | [76] | |
| miR-92a | KLF2 | Regulates flow-meidated EC activation | [105] |
| KLF4, KLF2 | Conbributes to flow-mediacted EC activation | [106] | |
| miR-101 | mTOR | Inhibits EC proliferation | [101] |
| miR-125 | Endothelin-1 | Reduces endothelial inflammation and atherosclerosis | [77] |
| miR-126 | VCAM-1 | Inhibits leukocyte adherence to ECs | [40] |
| RGS16 | Induction of CXCL-12, atheroprotective | [46] | |
| miR-146 | Nox-4 | Reduction of miR-146a associated with EC senescence | [70] |
| HuR, TRAF6 | Increases eNOS expression Inhibits NF-κB and MAPK signaling and their target gene expression |
[60] | |
| miR-155 | AT1R, Ets-1 | Inhibits AngII-induced EC migration and leukocyte adhesion | [79] – [82] |
| eNOS | Regulates NO production and endothelium-dependent vasorelaxation | [84] | |
| BACH1 | Increases Heme oxygenase 1 expression, reduces inflammation | [83] | |
| BCL6 | Promotes NF-κB signaling and partial ligation induced vascular injury | [86] | |
| MAP3K10 | Inhibits cytokine production and atherosclerotic lesion formation | [87] | |
| miR-181a | Prox1 | Modulate vascular specific endothelial differentiation | [123] |
| miR-181b | Importin-α3 | Inhibits downstream canonical NF-κB signaling pathway Reduces vascular inflammation in vivo Decreases lung inflammation in endotoxemia mice |
[51] |
| Reduces atherosclerosis | [54] | ||
| miR-217 | SirT1 | Promoters EC senescence | [73] |
| miR-221 | c-Kit | Impairs EC migration | [68] |
| Ets-1 | Inhibits AngII-induced EC activation and leukocyte adhesion | [79] | |
| p27kip1, p57kip2 | Reduction of miR-221 inhibit cell cycle progression | [69] | |
| miR-222 | p27kip1, p57kip2 | Reduction of miR-222 inhibits cell cycle progression | [69] |
| miR-320 | unknown | Inhibits myocardial microvascular EC proliferation and migration | [65] |
| miR-503 | CCNE1, cdc25A | Inhibits EC proliferation, migration, tube formation Inhibits vascular wound healing and blood flow recovery |
[66] |
| miR-663 | KLF4 (direct?) | Regulates oscillatory shear stress induced EC activation | [104] |
Acknowledgments
This work was supported by the National Institutes of Health (HL091076, HL115141, and HL117994 to M.W.F.) and a Watkins Cardiovascular Medicine Discovery Award (to M.W.F.).
Footnotes
Conflict of Interest
Xinghui Sun, Nathan Belkin, and Mark W. Feinberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25. doi: 10.1038/nature10146. This review article summarized the current understanding of atherosclerosis, and highlighted the gap between experimental animal findings and clinical applications. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 4.Rayner KJ, Moore KJ. The plaque “micro” environment: microRNAs control the risk and the development of atherosclerosis. Curr Atheroscler Rep. 2012;14(5):413–21. doi: 10.1007/s11883-012-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madrigal-Matute J, Rotllan N, Aranda JF, Fernandez-Hernando C. MicroRNAs and atherosclerosis. Curr Atheroscler Rep. 2013;15(5):322. doi: 10.1007/s11883-013-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013;33(3):449–54. doi: 10.1161/ATVBAHA.112.300279. This review discussed the role of EC miR-126, SMC miR-145, and macrophage miR-155 in the pathogenesis of atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 7.Shan Z, Yao C, Li ZL, et al. Differentially expressed microRNAs at different stages of atherosclerosis in ApoE-deficient mice. Chin Med J. 2013;126(3):515–20. [PubMed] [Google Scholar]
- 8.Wei Y, Nazari-Jahantigh M, Chan L, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127(15):1609–19. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 9.Rayner KJ, Fernandez-Hernando C, Moore KJ. MicroRNAs regulating lipid metabolism in atherogenesis. Thromb haemost. 2012;107(4):642–7. doi: 10.1160/TH11-10-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotllan N, Fernandez-Hernando C. MicroRNA regulation of cholesterol metabolism. Cholesterol. 2012;2012:847849. doi: 10.1155/2012/847849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neth P, Nazari-Jahantigh M, Schober A, Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 2013;99(2):294–30. doi: 10.1093/cvr/cvt096. [DOI] [PubMed] [Google Scholar]
- 12.Boon RA, Hergenreider E, Dimmeler S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb Haemost. 2012;108(4):616–20. doi: 10.1160/TH12-07-0491. [DOI] [PubMed] [Google Scholar]
- 13.Chamorro-Jorganes A, Araldi E, Suarez Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol Res. 2013;75:15–27. doi: 10.1016/j.phrs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133– 40. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 15•.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. This review examined the role of the NF-κB pathway in the development of inflammation-associated metabolic diseases, including obesity, diabetes, and atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Presa M, Bustos C, Ortego M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95(6):1532–41. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 18.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thrombosis. 1994;14(4):605–16. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 19.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97(16):9052–7. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies PF, Polacek DC, Handen JS, Helmke BP, DePaola N. A spatial approach to transcriptional profiling: mechanotransduction and the focal origin of atherosclerosis. Trends Biotechnol. 1999;17(9):347–51. doi: 10.1016/s0167-7799(99)01348-7. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 22.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132(3):344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nat Rev Immunol. 2008;8(11):837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 26.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen R, Smale ST. Selectivity of the NF-κB response. Cold Spring Harb Perspect Biol. 2010;2(4):a000257. doi: 10.1101/cshperspect.a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140(6):833–44. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 30•.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51. doi: 10.1161/ATVBAHA.108.179705. This review discussed the role of inflammation in aspects of experimental atherosclerosis, and the implications for the translation of these concepts to clinical practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97(7):1715–22. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor- κB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis. 2000;148(1):23–30. doi: 10.1016/s0021-9150(99)00211-7. [DOI] [PubMed] [Google Scholar]
- 33.Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-κB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8(5):372–83. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, McGregor JL. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE−/−/ICAM-1−/−) fed a fat or a chow diet. Arterioscler Thromb Vasc Biol. 2000;20(12):2630–5. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 35.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102(1):145–52. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cybulsky MI, Iiyama K, Li H, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126(14):1739–51. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 38.Park SH, Sui Y, Gizard F, et al. Myeloid-specific IκB kinase beta deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(12):2869–76. doi: 10.1161/ATVBAHA.112.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanters E, Pasparakis M, Gijbels MJ, et al. Inhibition of NF-κB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112(8):1176–85. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105(5):1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun C, Alkhoury K, Wang YI, et al. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res. 2012;111(8):1054–64. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775(2):298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Oettgen P. Regulation of vascular inflammation and remodeling by ETS factors. Circ Res. 2006;99(11):1159–66. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 44.Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(10):1990–7. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan Y, Brown C, Maynard E, et al. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115(9):2508–16. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 47.Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 48.Tan KS, Armugam A, Sepramaniam S, et al. Expression profile of microRNAs in young stroke patients. PLoS One. 2009;4(11):e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Sun X, Icli B, Wara AK, et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122(6):1973–90. doi: 10.1172/JCI61495. This study identified miR-181b as a critical negative regulator of NF-κB downstream signaling by targeting the 3′ UTR of importin-α3, which is a protein involved in NF-κB nuclear translocation. With use of a mouse model of endotoxemia, the authors provided proof-of-concept evidence that miRNA mimics can be used to target the vascular endothelium, and inhibit the deleterious effect of NF-κB signaling in acute inflammatory states in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin α3 expression. Mol Biol Cell. 2009;20(20):4412–23. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohler M, Speck C, Christiansen M, et al. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol Cell Biol. 1999;19(11):7782–91. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fagerlund R, Melen K, Cao X, Julkunen I. NF-κB p52, RelB and c-Rel are transported into the nucleus via a subset of importin alpha molecules. Cell Signal. 2008;20(8):1442–51. doi: 10.1016/j.cellsig.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-κB is transported into the nucleus by importin α3 and importin α4. J Biol Chem. 2005;280(16):15942–51. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 54••.Sun X, He S, Wara AK, et al. Systemic delivery of microRNA-181b inhibits NF-kB activation, vascular inflammation, and atherosclerosis in ApoE−/− mice. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.302089. This article demonstrated that miR-181b mimics can be delivered to the vascular endothelium in chronic inflammatory disease states such as atherosclerosis; miR-181b inhibited NF-κB activation specifically in ECs, but not leukocytes, an effect sufficient to inhibit atherogenesis in ApoE−/− mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tergaonkar V, Perkins ND. p53 and NF-κB crosstalk: IKKα tips the balance. Mol Cell. 2007;26(2):158–9. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 57.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 58.Ashida N, Senbanerjee S, Kodama S, et al. IKKβ regulates essential functions of the vascular endothelium through kinase-dependent and -independent pathways. Nat Commun. 2011;2:318. doi: 10.1038/ncomms1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184(1):21–5. doi: 10.4049/jimmunol.0902369. In this study, a negativefeedback loop was identified in which miR-31 expression and miR-17-3p expression were induced by TNF-α in ECs to inhibit EC activation by reducing the expression of adhesion molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Cheng HS, Sivachandran N, Lau A, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5(7):1017–34. doi: 10.1002/emmm.201202318. This article elegantly dissected the inhibitory role of miR-146 in EC activation and provides further support for developing miRNA-based therapies targeting vascular ECs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 62.Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93(10):1809–17. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 63.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27(4 Pt 1):436–47. [PubMed] [Google Scholar]
- 64.Hasegawa Y, Saito T, Ogihara T, et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125(9):1122– 33. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 65.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36(2):181–8. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 66.Caporali A, Meloni M, Vollenkle C, et al. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123(3):282–91. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 67.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286(13):11837–48. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381(1):81–3. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Togliatto G, Trombetta A, Dentelli P, Rosso A, Brizzi MF. MIR221/MIR222-driven post-transcriptional regulation of P27KIP1 and P57KIP2 is crucial for high-glucose- and AGE-mediated vascular cell damage. Diabetologia. 2011;54(7):1930–40. doi: 10.1007/s00125-011-2125-5. [DOI] [PubMed] [Google Scholar]
- 70.Vasa-Nicotera M, Chen H, Tucci P, et al. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217(2):326–30. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 71.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398(4):735–40. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104(39):15472–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menghini R, Casagrande V, Cardellini M, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120(15):1524–32. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 74.Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 75.Raitoharju E, Lyytikainen LP, Levula M, et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219(1):211–7. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 76.Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–10. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 77.Li D, Yang P, Xiong Q, et al. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens. 2010;28(8):1646–54. doi: 10.1097/HJH.0b013e32833a4922. [DOI] [PubMed] [Google Scholar]
- 78.Zhang XY, Shen BR, Zhang YC, et al. Induction of thoracic aortic remodeling by endothelial-specific deletion of microRNA-21 in mice. PLoS One. 2013;8(3):e59002. doi: 10.1371/journal.pone.0059002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu N, Zhang D, Chen S, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–93. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 80.Zheng L, Xu CC, Chen WD, et al. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010;400(4):483–8. doi: 10.1016/j.bbrc.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 81.Cheng W, Liu T, Jiang F, et al. MicroRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int J Mol Med. 2011;27(3):393–9. doi: 10.3892/ijmm.2011.598. [DOI] [PubMed] [Google Scholar]
- 82.Liu T, Shen D, Xing S, et al. Attenuation of exogenous angiotensin II stress-induced damage and apoptosis in human vascular endothelial cells via microRNA-155 expression. Int J Mol Med. 2013;31(1):188–96. doi: 10.3892/ijmm.2012.1182. [DOI] [PubMed] [Google Scholar]
- 83.Pulkkinen KH, Yla-Herttuala S, Levonen AL. Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med. 2011;51(11):2124–31. doi: 10.1016/j.freeradbiomed.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Sun HX, Zeng DY, Li RT, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60(6):1407–14. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 85.Donners MM, Wolfs IM, Stoger LJ, et al. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7(4):e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nazari-Jahantigh M, Wei Y, Noels H, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122(11):4190–202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu J, Chen T, Yang L, et al. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7(11):e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frangos SG, Gahtan V, Sumpio B. Localization of atherosclerosis: role of hemodynamics. Arch Surg. 1999;134(10):1142–9. doi: 10.1001/archsurg.134.10.1142. [DOI] [PubMed] [Google Scholar]
- 89.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96(6):3154–9. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miao H, Hu YL, Shiu YT, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005;42(1):77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 92.Chiu JJ, Chen CN, Lee PL, et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. J Biomech. 2003;36(12):1883–95. doi: 10.1016/s0021-9290(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 93.Chiu JJ, Chen LJ, Lee PL, et al. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101(7):2667–74. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 94.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115(3):733–8. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tardy Y, Resnick N, Nagel T, Gimbrone MA, Jr, Dewey CF., Jr Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol. 1997;17(11):3102–6. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- 96.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83(2):131–51. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 97.Qin X, Wang X, Wang Y, et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2010;107(7):3240–4. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang KC, Garmire LX, Young A, et al. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci USA. 2010;107(7):3234–9. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 2012;18:1077–86. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 100.Urbich C, Kaluza D, Fromel T, et al. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119(6):1607–16. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 101.Chen K, Fan W, Wang X, Ke X, Wu G, Hu C. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun. 2012;427(1):138–42. doi: 10.1016/j.bbrc.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 102••.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107(30):13450–5. doi: 10.1073/pnas.1002120107. This study first identified miRNAs differentially expressed between the atherosusceptible and the atheroresistant regions in vivo in swine aortic artery, and found endothelial miR-10a expression is lower in the atherosusceptible regions. The study suggests the lower miR-10a expression may contribute to the proinflammatory endothelial phenotypes in atherosusceptible regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou J, Wang KC, Wu W, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA. 2011;108(25):10355–60. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300(5):H1762– 9. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105••.Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-dependent regulation of Krüppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124(5):633–41. doi: 10.1161/CIRCULATIONAHA.110.005108. This study highlights that miR- 92a expression is reduced by atheroprotective flow patterns, which resulted in the upregulation of KLF2. Overexpression of miR-92a in mouse carotid arteries exhibited impaired flow-mediated vasodilatory response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Krüppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32(4):979–87. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou J, Li JY, Nguyen P, et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113:40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 109.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135– 43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 110.Martinez NJ, Gregory RI. MicroRNA gene regulatory pathways in the establishment and maintenance of ESC identity. Cell Stem Cell. 2010;7(1):31–5. doi: 10.1016/j.stem.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marson A, Levine SS, Cole MF, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40(12):1478–83. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Card DAG, Hebbar PB, Li L, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28(20):6426–38. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27(5):459–61. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463(7281):621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280(10):9330–5. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 118.Suárez Y, Fernández-Hernando C, Yu J, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA. 2008;105(37):14082–7. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 120.Kane NM, Meloni M, Spencer HL, et al. Derivation of endothelial cells from human embryonic stem cells by directed differentiation analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30(7):1389–97. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 121.Tréguer K, Heinrich E-M, Ohtani K, Bonauer A, Dimmeler S. Role of the microRNA-17–92 cluster in the endothelial differentiation of stem cells. J Vasc Res. 2012;49(5):447–60. doi: 10.1159/000339429. [DOI] [PubMed] [Google Scholar]
- 122.Kane NM, Howard L, Descamps B, et al. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012;30(4):643–54. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood. 2010;116(13):2395–401. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 124.McCall MN, Kent OA, Yu J, Fox-Talbot K, Zaiman AL, Halushka MK. MicroRNA profiling of diverse endothelial cell types. BMC Med Genomics. 2011;4:78. doi: 10.1186/1755-8794-4-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang F, Fang Z-f, Hu X-q, Tang L, Zhou S-h, Huang J-p. Overexpression of mir-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biol Chem. 2013;394(9):1223–33. doi: 10.1515/hsz-2013-0107. [DOI] [PubMed] [Google Scholar]
- 126.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462(7273):587–94. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 127.Margariti A, Winkler B, Karamariti E, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci USA. 2012;109(34):13793–8. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J, Huang NF, Zou J, et al. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013;33(6):1366–75. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ginsberg M, James D, Ding B-S, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012;151(3):559–75. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nam Y-J, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110(14):5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]