Abstract
The ability of stressful life events to trigger drug use is particularly problematic for the management of cocaine addiction due to the unpredictable and often uncontrollable nature of stress. For this reason, understanding the neurobiological processes that contribute to stress-related drug use is important for the development of new and more effective treatment strategies aimed at minimizing the role of stress in the addiction cycle. In this review we discuss the neurocircuitry that has been implicated in stress-induced drug use with an emphasis on corticotropin releasing factor actions in the ventral tegmental area (VTA) and an important pathway from the bed nucleus of the stria terminalis to the VTA that is regulated by norepinephrine via actions at beta adrenergic receptors. In addition to the neurobiological mechanisms that underlie stress-induced cocaine seeking, we review findings suggesting that the ability of stressful stimuli to trigger cocaine use emerges and intensifies in an intake-dependent manner with repeated cocaine self-administration. Further, we discuss evidence that the drug-induced neuroadaptations that are necessary for heightened susceptibility to stress-induced drug use are reliant on elevated levels of glucocorticoid hormones at the time of cocaine use. Finally, the potential ability of stress to function as a “stage setter” for drug use – increasing sensitivity to cocaine and drug-associated cues – under conditions where it does not directly trigger cocaine seeking is discussed. As our understanding of the mechanisms through which stress promotes drug use advances, the hope is that so too will the available tools for effectively managing addiction, particularly in cocaine addicts whose drug use is stress-driven.
Keywords: Cocaine, Stress, Relapse, Norepinephrine, CRF, Glucocorticoid, Review
1. Stress-related cocaine use: evidence from human addicts
An accumulating body of evidence suggests that there is a strong relationship between stress and drug use by cocaine addicts. Anecdotal reports suggesting that stressful life events can precipitate relapse are paralleled by laboratory findings that the presentation of pre-recorded individualized stress-related scripts can induce craving in cocaine-dependent individuals (Sinha et al., 1999, 2000) and by epidemiological studies demonstrating high rates of co-morbidity between cocaine dependence and a number of stress-related disorders, including depression, anxiety, and post-traumatic stress disorder (PTSD; Rounsaville et al., 1991; Chen et al., 2011). In fact, it is likely that most incidents of relapse in cocaine addicts are in some way stress-related. In the case of PTSD, morbidity is not only higher in cocaine-dependent individuals (Cottler et al., 1992), but in many cases traumatic events predate cocaine use (Brady et al., 1998) and imagery based on the traumatic episode can elicit drug craving (Coffey et al., 2002). Indeed, one study noted that 95% of individuals with co-morbid PTSD and cocaine dependence reported a functional relationship between PTSD-related symptoms and drug use, with 86% reporting that a worsening of symptoms resulted in increased cocaine use (Back et al., 2006). While it is clear that a relationship exists between stress and cocaine use, the precise contribution of stress to the addiction process remains unclear and in many cases likely involves a complex interaction between stressful stimuli, cocaine-related cues, and the effects of the drug itself and may vary across different subpopulations of addicts (Preston and Epstein, 2011). These effects of stress on cocaine use are particularly problematic due to their unavoidable nature, making stress a key target for interventions aimed at relapse prevention. Thus, understanding the neurobiological processes that mediate the influence of stress on cocaine use is important for devising new and more effective therapeutic approaches for the management of addiction.
2. Rodent models for studying stress-induced relapse
The contribution of stress to drug relapse can be studied pre-clinically using reinstatement-based designs in which the ability of various stressful stimuli to re-establish extinguished cocaine seeking is assessed in rats or mice. Despite some concerns about their construct validity (e.g., extinction of behavior in rodents is not the same as abstinence in human addicts) and their predictive validity (it is not clear that these approaches can be used to reliably identify effective relapse interventions), these approaches have been critical in shaping our understanding of the neurobiological pathways that contribute to stress-related drug use. Brief over-views of the two primary variations of these approaches, as applied in our laboratory, are provided below. Representative graphs depicting stress-induced cocaine seeking as measures using these approaches are shown in Fig. 1.
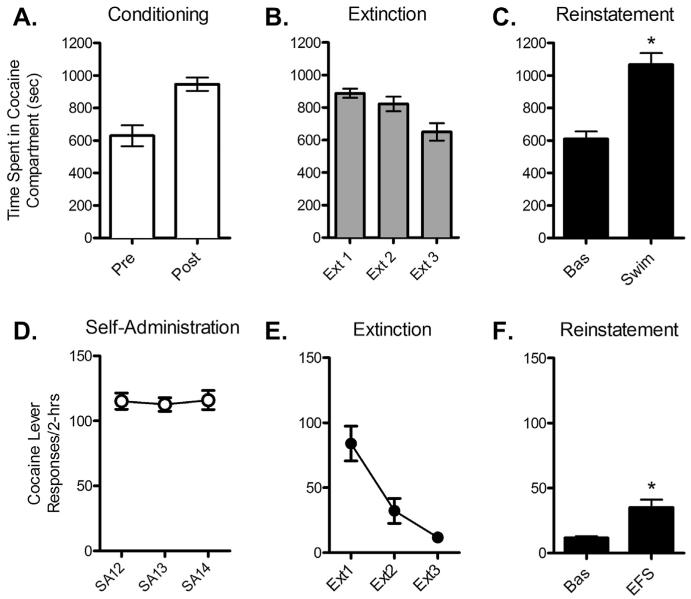
Fig. 1.
Stress-induced cocaine seeking in mouse (A–C) and rat (D–F) models. Stress-induced reinstatement of extinguished cocaine-induced CPP is shown in Figs. A-C. Preference for a cocaine-paired compartment (4 × 15 mg/kg, ip) was established (A; increase in time spent post-conditioning vs. pre-conditioning) and extinguished (B) in C57BL/6 mice (n = 4) prior to reinstatement of preference by pre-exposure to a forced swim session (C; 6-min swim in 22 °C water; *P < 0.05 vs. Bas). Stress-induced reinstatement in rats following cocaine self-administration and extinction is shown in Figs. D–F. Following training, rats (n = 5) self-administered cocaine (1.0 mg/kg/inf) by pressing a lever during daily 6-h sessions for 14 days (D) prior to undergoing extinction over a 10-day period during daily 2-h sessions (E) and reinstatement testing (F). Stress-induced reinstatement was observed as the ability of footshock stress (0.5 mA; 0.5” duration delivered an ave. of 40-s apart over a 15-min session) to increase responding on the cocaine lever (*P < 0.05 vs. Bas).
2.1. Self-administration/reinstatement approaches in rats
In rats, the study of relapse typically involves testing for the reinstatement of extinguished responding following intravenous cocaine self-administration (SA). Although a variety of stressors have been demonstrated to reinstate cocaine seeking following SA in rats, including food restriction (Shalev et al., 2003) and forced swim (Conrad et al., 2010), the most common method involves the use of uncontrollable intermittent electric footshock delivered through the grid floors of the SA chambers (Ahmed and Koob, 1997; Erb et al., 1996). Notably, while a number of laboratories have demonstrated footshock-induced reinstatement of cocaine seeking following SA in rats, the conditions under which reliable rein-statement has been observed are very specific (see e.g., Shelton and Beardsley, 2005) and, in our hands, depend on a history of SA under conditions of prolonged daily access (Mantsch et al., 2008a,b).
2.2. Conditioned place preference/reinstatement approaches in mice
Methodological considerations limit the utility of SA-based approaches in mice. Instead, relapse is commonly studied in mice by examining the ability of stressors to re-establish preference for a cocaine-paired environment following the extinction of cocaine-induced conditioned place preference (CPP). As is the case with the SA approach in rats, a number of stressors, including social stress (Ribeiro Do Couto et al., 2006) and uncontrollable electric footshock (Redila and Chavkin, 2008), have been demonstrated to reinstate extinguished drug-induced CPP in mice. However, the most frequently applied approach, and the one used in our laboratory, involves a brief (6-min) forced swim at 22 °C prior to transfer to the conditioning apparatus (Kreibich and Blendy, 2004; Mantsch et al., 2008a,b).
3. Anatomy of stress-induced relapse
3.1. Overview of the neurocircuitry implicated in stress-induced reinstatement
Using the reinstatement approach in rodents, we and others have begun to define the neurocircuitry involved in stress-induced cocaine seeking. A putative circuit for stress-induced cocaine use is included in Fig. 2. Like other reinstating stimuli (e.g., priming injections of cocaine and cocaine-associated cues; see Kalivas and McFarland, 2003 for review) stress-induced reinstatement appears to involve a corticostriatal glutamatergic projection from the prelimbic cortex to the nucleus accumbens (NAc) core which likely regulates striato-pallidal outputs to produce drug seeking. Accordingly, McFarland et al. (2004) demonstrated that inhibition of the NAc core using a GABA receptor agonist cocktail blocks footshock-induced reinstatement in rats while several groups have found that dopamine (DA) D1 receptor antagonism in the medial prefrontal cortex (mPFC) prevents stress-induced cocaine seeking (Capriles et al., 2003; Sanchez et al., 2003; McFarland et al., 2004). In the PFC, D1 DA receptors are expressed on pyramidal cells, including those that project to the NAc core, where they promote excitability primarily by increasing NMDA receptor-mediated excitability (Lewis and O’Donnell, 2000; Seamans et al., 2001). However, D1 receptors are also found on fast-spiking GABAergic interneurons which they inhibit to further increase pyramidal cell excitability (Gonzalez-Islas and Hablitz, 2001). The latter mechanism could account for fMRI studies in human addicts showing that BOLD signal is reduced during stress-induced craving (Sinha et al., 2005). Dopaminergic projections to the mPFC arise from the ventral tegmental area (VTA), which appears to be a key integration site at which inputs that relay stress- and drug context/cue-related information converge. Brain structures upstream from this mesocortical pathway that are necessary for stress-induced relapse appear to include the bed nucleus of the stria terminalis (BNST), the central nucleus of the amygdala (CeA), and the NAc shell (McFarland et al., 2004; Briand et al., 2010). Collectively, these regions are often considered to comprise a larger complex, termed the extended amygdala, based on their anatomical similarities. Recently, a number of laboratories, including ours, have begun to explore the mechanisms within the VTA through which stressors regulate cocaine-seeking behavior as well as the potential role of inputs from components of the extended amygdala – particularly the BNST (Geisler and Zahm, 2005; Dong and Swanson, 2006) – in promoting drug use during periods of stress.
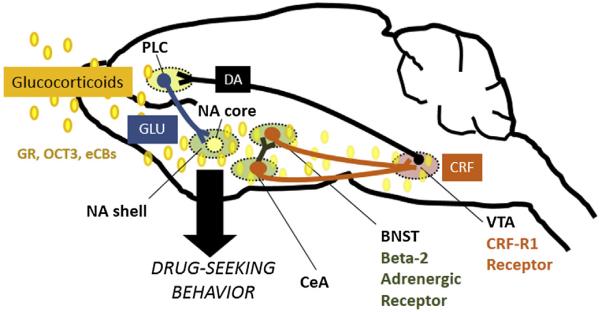
Fig. 2.
Ongoing areas of research on the mechanisms through which stress promotes cocaine use. One putative pathway that underlies stress-induced reinstatement is a key corticotropin releasing factor- (CRF-) releasing pathway from the bed nucleus of the stria terminalis (BNST) to the ventral tegmental area (VTA) that is regulated by norepinephrine released from noradrenergic projections primarily from medullary noradrenergic cells groups and appears to be partly under the control of an input from the central nucleus of the amygdala (CeA). In the BNST, beta adrenergic receptors and CeA projections appear to induce cocaine seeking via local release of CRF and BNST CRF-R1 receptor activation. In the VTA, BNST inputs converge with afferents from other regions, including the nucleus accumbens (NA) shell and the CeA to regulate dopamine (DA) release in the prelimbic cortex (PLC). DA in the PLC cortex promotes excitation of a glutamatergic pathway to the NA core that has been proposed to function as a common final pathway through which a range of stimuli induces cocaine seeking. Current areas of research focus in our lab include investigation of: 1) the CRF-releasing pathway from the BNST to the VTA and its regulation by beta-2 adrenergic receptors; 2) the mechanisms through which CRF-R1 receptor activation in the VTA regulate DA projections; 3) glucocorticoid-dependent processes in these regions through which stress can “set the stage” for cocaine use, including glucocorticoid inhibition of the monoamine transporter, organic cation transporter 3 (OCT3) and glucocorticoid regulation of endocannabinoid (eCB) production; and 4) glucocorticoid-dependent neuroplasticity in these pathways that increases susceptibility to cocaine seeking and contributes to the onset of addiction. Other abbreviations: glutamate (GLU).
3.2. A BNST to VTA projection
Although the neuroanatomy of stress-induced cocaine use is complex and almost certainly involves the coordinated activity of numerous inputs that converge upon the VTA to integrate emotion-, context-, and memory-related signals, one pathway that stands out as a key regulator of drug use is a projection from the BNST to the VTA. The BNST has long been known to serve as an important site at which stress-related responses are integrated. Towards this end, the BNST receives dense innervation from a number of structures involved in stressor-responsiveness, most notably heavy brainstem noradrenergic inputs from the locus coeruleus and medullary cell groups (see Smith and Aston-Jones, 2008 for review) and a prominent projection from the CeA (Zahm et al., 1999). Indeed, the BNST, through its projections to the hypothalamus, is a key regulator of the hormonal stress response (Forray and Gysling, 2004). Additionally, the BNST has reciprocating connectivity with the VTA from which it receives DA inputs, thus positioning it as a potential interface between stress and motivated behavior and therefore as a site at which stressors could promote drug use. Accordingly, inhibition of the BNST attenuates stress-induced reinstatement in both rat (McFarland et al., 2004) and mouse (Briand et al., 2010) models. Recent work from our lab and others suggests that brainstem adrenergic inputs into the BNST, likely in coordination with projections from the CeA, regulate a pathway that ultimately releases the neuropeptide corticotropin-releasing factor (CRF) into the VTA. A major focus of the current research in our lab involves understanding the mechanisms through which CRF in the VTA and norepinephrine (NE) in the BNST promote cocaine seeking.
4. A role for CRF in stress-induced relapse
CRF is a 41 amino acid neuropeptide that plays a key role in regulating both the hormonal and behavioral responses to stressors. CRF produces it effects via two receptors, both of which are Gs G-protein coupled but may also activate other signaling pathways cite (see Behan et al., 1996 for review): 1) the CRF-R1 receptor, which has a higher affinity for CRF and has a relatively widespread expression pattern in the brain and 2) the CRF-R2 receptor, which has a lower affinity for CRF and a more restricted pattern of expression. Notably, the CRF-R2 receptor preferentially binds to a family of CRF-related peptides, the urocortins, and may interact with CRF more effectively via a mechanism that also involves the CRF binding protein.
4.1. CRF and stress-induced reinstatement
CRF is a key regulator of stress-induced reinstatement. We and others have shown that systemic or intra-cerebroventricular (icv) administration of CRF receptor antagonists prevents footshock-induced reinstatement in rats (Erb et al., 1998; Shaham et al., 1997; Graf et al., 2011) while central administration of CRF alone can induce cocaine seeking (Erb et al., 2006; Mantsch et al., 2008a,b). The role of CRF in stress-induced reinstatement is independent of its regulation of the hypothalamice–pituitary–adrenal (HPA) axis. Eliminating the plasma corticosterone response via surgical adrenalectomy and basal corticosterone replacement does not attenuate reinstatement by either footshock (Erb et al., 1998; Graf et al., 2011) or icv CRF administration (Graf et al., 2011). Although blocking CRF receptors prevents stressor-induced rein-statement, it has no effect on reinstatement by a priming injection of cocaine (Erb et al., 1998; Graf et al., 2011), suggesting that the pathways underlying stress-induced relapse may be distinct from those that mediate drug use in response to other triggers.
4.2. Role of CRF in the VTA in stressor-induced reinstatement
The VTA is a key site for CRF regulation of cocaine seeking. Both CRF-R1 and CRF-R2 receptors (Goeders et al., 1990; Van Pett et al., 2000; Korotkova et al., 2006) as well as the CRF binding protein (Wang and Morales, 2008) are expressed in the VTA which receives CRF positive projections from several brain regions, including the BNST (Rodaros et al., 2007). Correspondingly, extracellular CRF levels in the VTA, as measured using in vivo microdialysis and radioimmunoassay, are increased during footshock-induced rein-statement in rats (Wang et al., 2005). We and others have found that delivery of CRF directly into the VTA is sufficient to reinstate cocaine seeking following SA in rats, while intra-VTA CRF receptor antagonist administration prevents footshock-induced reinstatement (Wang et al., 2005, 2007; Blacktop et al., 2011).
4.2.1. Role for CRF-R1 receptors
While it is generally agreed that CRF release in the VTA is required for stress-induced reinstatement, the receptor subtype that mediates the effects of CRF on cocaine seeking is controversial. Using the SA/reinstatement approach in rats, we have found that the CRF-R1 but not the CRF-R2 receptor in the VTA is necessary for CRF- and stress-induced reinstatement, and that activation of the CRF-R1 receptor in the VTA alone is sufficient to reinstate cocaine seeking (Blacktop et al., 2011). Intra-VTA pretreatment with the CRF-R1 receptor-selective antagonists antalarmin or CP-376395 blocks reinstatement in response to delivery of CRF into the VTA or footshock without producing response-limiting sedative effects, while intra-VTA pretreatment with the CRF-R2 receptor-selective antagonist astressin2B has no effect. Similarly the reinstating effects of footshock and intra-VTA CRF are reproduced by administration of the CRF-R1 receptor-selective agonist, cortagine, into the VTA but not the CRF-R2 receptor-selective agonist, rat urocortin 2. These findings are consistent with reports that systemic administration of antalarmin also blocks stress-induced reinstatement of cocaine seeking in rats (Shaham et al., 1998) or mice (our own unpublished data) as well as stress-induced reinstatement following heroin (Shaham et al., 1997), alcohol (Lê et al., 2000), and nicotine (Bruijnzeel et al., 2009) SA.
4.2.2. Role for CRF-R2 receptors
A role for the CRF-R2 receptor in stress-induced relapse has also been reported (Wang et al., 2005, 2007). In these studies, which involved more prolonged delivery into the VTA via reverse micro-dialysis, it was found that reinstatement by footshock or intra-VTA CRF delivery was blocked by antagonists at CRF-R2 but not CRF-R1 receptors. The mechanism of action in these studies appeared to involve CRF interactions with the CRF binding protein. Agents that displace CRF from its binding protein prevented reinstatement and the corresponding neurochemical changes in the VTA while only CRF-R2 agonists that also interact with the binding protein induced cocaine seeking (Wang et al., 2007). Notably, this finding is consistent with a report that CRF can potentiate NMDA receptor mediated synaptic transmission in the VTA through a similar mechanism (Ungless et al., 2003). Differences in the mode and duration of CRF/antagonist delivery, as well as the experimental history of the rats likely contributed to the disparate findings regarding the role of CRF receptor subtypes in stress-induced cocaine seeking. Nonetheless, the implication of both receptors is not particularly surprising, considering the VTA expression profile of CRF receptors (both subtypes are expressed on DA and non-DA cells and likely on nerve terminals) as well as the complexity of CRF actions in the VTA.
4.3. CRF actions in the VTA
CRF has been reported to produce both excitatory (Wanat et al., 2008; Korotkova et al., 2006) and inhibitory (Beckstead et al., 2009) effects on DA neuronal activity in the VTA, to alter synaptic integration via intracellular calcium mobilization in DA cells (Riegel and Williams, 2008), and to promote excitatory synaptic transmission through post-synaptic trafficking of NMDA and AMPA receptors (Ungless et al., 2003; Hahn et al., 2009). Additionally, CRF promotes glutamate release in the VTA as measured using in vivo microdialysis (Wang et al., 2005), a finding that is consistent with reports that CRF also increases the frequency of AMPAR-mediated spontaneous miniature EPSCs in DA cells in slice preparations (Hahn et al., 2009). Based on its effects on excitatory neurotransmission, it has been proposed that the primary function of CRF in the VTA during stress is to excite DA cells, thereby activating mesocorticolimbic neurotransmission and promoting drug use (Wise and Morales, 2010). Consistent with this hypothesis, CRF delivery into the VTA increases local DA release measured during reinstatement testing (Wang et al., 2005), while glutamate receptor antagonism in the VTA prevents both increases in local DA levels and stressor- and CRF-induced reinstatement (Wang et al., 2005). However, with the exception of a small group of DA cells in the most ventral aspects of the VTA (Brischoux et al., 2009), the time-locked response of DA cells in the VTA to stressful/aversive stimuli in the absence of drug-associated context is a reduction in firing rate (Ungless et al., 2004, 2010). Likewise, the time-locked DA response in the NAc to such stimuli, as measured using voltammetry, is also a decrease (Badrinarayan et al., 2012; Roitman et al., 2008; Oleson et al., 2012). Taken together, these findings suggest that the mesolimbic DA response to stressful/aversive stimuli is somewhat incompatible with a model that assumes that increased CRF release into the VTA will evoke drug seeking simply by exciting dopaminergic efferents.
It is intriguing to speculate that, rather than producing direct excitatory effects in the VTA, CRF serves as a coordinator of VTA function – generally silencing DA cellular activity in the absence of drug-associated stimuli, while opportunistically promoting signaling via key excitatory inputs that relay drug-related/appetitive information to the VTA. Consistent with this possibility, it has been reported that CRF receptor antagonism increases DA neuron population activity in the VTA but attenuates evoked DA release in the NAc (Lodge and Grace, 2005). This coordinating function of CRF in the VTA could position it as a key determinant of stimulus salience and coping behaviors during periods of stress and may have implications not only for addiction but also for under-standing other conditions associated with aberrant stress-related behavioral responses.
Adding to the difficulty in understanding CRF function in the VTA is the anatomical complexity of the region. While often studied as a uniform structure in terms of cellular composition and connectivity, the VTA is comprised of both DA and non-DA (i.e., local and projecting GABAergic and glutamatergic) cell types (Steffensen et al., 1998; Dobi et al., 2010; Yamaguchi et al., 2011) that are differentially regulated during periods of stress depending on their anatomical localization, the neurochemical context at the time of stimulation and differences in their intrinsic properties that may render them differentially responsive to the same input. The potential significance of this differential responsiveness to motivated behavior is evident when considering that the putative pathway responsible for stress-induced cocaine seeking involves reciprocating connectivity between the VTA and the BNST and NAc shell that ultimately regulates the activity of a key DA projection from the VTA to the mPFC (McFarland et al., 2004). Therefore, the possibility that CRF differentially regulates distinct neuronal sub-populations in the VTA, possibly in a receptor subtype-dependent manner, cannot be ruled out. Indeed, CRF-R1 receptors are heterogeneously expressed on multiple cell types in the VTA (Korotkova et al., 2006; Refojo et al., 2011) and the behavioral consequences of CRFR-1 receptor deletion these different cell types (GABAergic vs. glutamatergic vs. dopaminergic) varies significantly (Refojo et al., 2011).
4.4. CRF-releasing projections to the VTA
The sources of CRF release into the VTA appear to include projections that originate from several regions known to be critical regulators of stress-related hormonal and behavioral responses, including the CeA, the paraventricular nucleus of the hypothalamus, and BNST (Rodaros et al., 2007). CRF-containing projections to the VTA appear to be both glutamatergic and GABAergic, suggesting that co-released CRF can participate in both excitatory and inhibitory transmission (Tagliaferro and Morales, 2008). However, most of the direct innervation of tyrosine hydroxylase-positive cells appears to be glutamatergic (Tagliaferro and Morales, 2008). The nature of the connectivity with other VTA cell types has not been determined. In light of studies demonstrating that the BNST is critical for stress-induced cocaine seeking (McFarland et al., 2004; Briand et al., 2010), we have recently begun to examine the importance of this CRF-releasing BNST to VTA projection in stress-induced relapse and its regulation by noradrenergic neurotransmission. Importantly, while much research, including our own, has focused on the involvement of the BNST-to-VTA pathway, CRF-releasing projections from other regions likely also contribute to relapse. For example, it has been reported that inhibition of the CeA prevents stress-induced cocaine seeking (McFarland et al., 2004). It is tempting to speculate that the release of CRF into the VTA from multiple pathways and its actions on functionally distinct sub-populations of cells in the VTA account for the complicated and often seemingly contradicting literature describing CRF’s actions in the region.
5. Norepinephrine
Central noradrenergic signaling has been implicated in a number of stress-related processes, including stress-induced relapse. Key sources of central noradrenergic projections include the locus coeruleus (A6 region) and groups of nuclei in the pons and rostral medulla (A1, A2, A5, and A7) whose projections collectively comprise the ventral noradrenergic bundle (VNAB). Adrenergic receptors bound by centrally released NE are primarily G-protein coupled and include Gs-coupled beta-1, beta-2 and beta-3 adrenergic receptors, Gq-coupled alpha-1 adrenergic receptors, and Gi-coupled alpha-2 adrenergic receptors. Noradrenergic innervation and adrenergic receptor expression in the CNS is widespread and includes a number of regions implicated in stress-induce cocaine seeking, including the NA shell, mPFC, and the BNST (Berridge, 2008).
5.1. NE and stress-induced relapse
An important role for noradrenergic signaling in stress-induced relapse has been established. We and others have shown that functional antagonism of noradrenergic signaling via administration of alpha-2 adrenergic receptor agonists such as clonidine prevents footshock-induced reinstatement in rats (Erb et al., 2000) and swim-induced reinstatement in mice (Mantsch et al., 2010). Conversely, the activation of central noradrenergic neurotransmission through alpha-2 adrenergic autoreceptor antagonism by drugs such as yohimbine is sufficient to reinstate cocaine seeking in rats (Feltenstein and See, 2006; Brown et al., 2009), monkeys (Lee et al., 2004) or mice (Mantsch et al., 2010; Vranjkovic et al., 2012). These reinstating effects can be reproduced by central (icv) administration of NE in rats (Brown et al., 2011). Like CRF, the role of NE in reinstatement appears to be specific to stress, as alpha-2 adrenergic receptor agonists fail to attenuate cocaine-primed reinstatement in rats or mice (Erb et al., 2000; Mantsch et al., 2010). The potential utility of targeting noradrenergic neurotransmission for managing stress-induced relapse in cocaine addicts is supported by findings that alpha-2 adrenergic receptor agonists prevent stress-induced craving in human cocaine-dependent subjects (Jobes et al., 2011; Fox et al., 2012).
5.2. Role of adrenergic receptors in stress-induced reinstatement
Using the mouse CPP/reinstatement approach, we have identified an important role for beta, but not alpha-1 adrenergic receptors in stress-induced relapse. Reinstatement following forced swim or yohimbine administration is prevented by pretreatment with the beta adrenergic receptor antagonist, propranolol, but not by the alpha-1 adrenergic receptor antagonist, prazosin (Mantsch et al., 2010) and is reproduced by administration of the beta adrenergic receptor agonist, isoproterenol (Vranjkovic et al., 2012). Furthermore, forced swim fails to produce reinstatement in beta adrenergic receptor-deficient mice (Vranjkovic et al., 2012). More specifically, stress-induced reinstatement appears to require the beta-2 but not the beta-1 adrenergic receptor. Reinstatement following forced swim or alpha-2 receptor antagonism is blocked by pretreatment with the beta-2 adrenergic receptor-selective antagonist, ICI-188,551, but not the beta-1 adrenergic receptor-selective antagonist betaxolol, while administration of the beta-2 adrenergic receptor-selective agonist, clenbuterol, is sufficient to reinstate (Vranjkovic et al., 2012). In situ hybridization and auto-radiography studies have demonstrated that beta-2 adrenergic receptors are expressed in a number of brain structures that have been implicated in stress-induced relapse, including the PFC and structures that comprise the extended amygdala complex (Asanuma et al., 1991; Rainbow et al., 1984). However, the finding that beta adrenergic receptor blockade in the BNST using a cocktail of beta-1 and beta-2 receptor antagonists prevents footshock-induced reinstatement in rats (Leri et al., 2002) has led to the hypothesis that beta-2 adrenergic receptor activation of a CRF-releasing projection from the BNST to the VTA is critical for stress-induced cocaine use.
5.3. BNST connectivity with the VTA
As described above (Section 3.2), the BNST is required for stress-induced reinstatement of extinguished cocaine seeking following SA in rats (McFarland et al., 2004) and CPP in mice (Briand et al., 2010) and sends projections to the VTA that co-release CRF (Rodaros et al., 2007). Both glutamatergic and GABAergic projections from the BNST to the VTA have been characterized (Georges and Aston-Jones, 2002; Kudo et al., 2012; Jennings et al., 2013) and a recent disconnection study showing attenuated expression of CPP following contralateral inhibition of the BNST in one hemisphere and VTA of the other supports a functional role for this pathway in cocaine seeking (Sartor and Aston-Jones, 2012). Recent work by Stuber and colleagues (Jennings et al., 2013), has provided a more comprehensive picture of the anatomy and function of BNST projections to the VTA. Both glutamatergic and GABAergic projections to the VTA are activated during stress but exert functionally divergent regulation of stress-related behaviors. Notably, both circuits primarily innervate non-DA cells in the VTA and most of the direct innervation of DA cells appears to be GABAergic (Jennings et al., 2013). It has not yet been determined which of the projections from the BNST co-releases CRF, nor has the relative contribution of these distinct pathways to stress-induced cocaine seeking.
5.4. Noradrenergic neurotransmission in the BNST and stress-induced relapse
The BNST receives dense noradrenergic innervation, much of which consists of projections from medullary cell groups conducted as part of the VNAB (Shin et al., 2008). Indeed, it has been reported that disruption of the locus coeruleus fails to attenuate stress-induced drug seeking following opiate SA or opiate-induced CPP, while lesion of the VNAB or inhibition of more caudal adrenergic cell groups prevents stress-induced reinstatement (Shaham et al., 2000; Wang et al., 2001). Both alpha and beta adrenergic receptor subtypes are expressed in the BNST (Rainbow et al., 1984; Asanuma et al., 1991; Day et al., 1997; Shields et al., 2009). However, it has been found that beta adrenergic receptors in the BNST are particularly important for stress-induced cocaine seeking. Leri et al. (2002) reported that administration of a cocktail consisting of betaxolol and ICI-118,551 into the BNST dose-dependently attenuated footshock-induced reinstatement in rats. We have reproduced these findings with administration of ICI-118,551 into the BNST by itself (unpublished findings), suggesting that beta-2 adrenergic receptor activation in the BNST is necessary for stress-induced relapse. In the BNST, beta adrenergic facilitation of both GABAergic (Dumont and Williams, 2004) and glutamatergic (Egli et al., 2005; Nobis et al., 2011 regulation of neuronal function has been reported. Recently, we have provided more direct evidence for a beta-2 adrenergic receptor-regulated, CRF-releasing pathway from the BNST to the VTA; ICI-118,551 administration in the BNST of one hemisphere and contralateral CRF-R1 receptor antagonism using antalarmin in the VTA of the other hemisphere prevents footshock-induced reinstatement (unpublished data). Altogether, these findings establish the likely importance of a beta-2 adrenergic regulated CRF-releasing pathway to the VTA for stress-induced cocaine use.
5.5. NE regulation of CRF in the BNST
Although the exact mechanism through which beta adrenergic receptors regulate projections from the BNST to the VTA has not been fully characterized, recent evidence suggests that it likely involves effects on CRF release into the BNST and the resulting activation of CRF-R1 receptors. Like the VTA, CRF-R1 receptor activation in the BNST is necessary for stress-induced reinstatement (Erb and Stewart, 1999). The source of CRF in the BNST is unclear and may involve release from terminals of neurons that project into the region (Beckerman et al., 2013) or from local cell populations (Silberman et al., 2013). The BNST receives a strong projection from the CeA (Zahm et al., 1999) and this projection appears to be required for stress-induced reinstatement, since inhibition of the CeA–BNST pathway via TTX administration in the CeA in one hemisphere and contralateral CRF receptor antagonism in the BNST of the other hemisphere prevents footshock-induced reinstatement (Erb et al., 2001). In the BNST, CRF facilitates excitatory regulation of efferent projections (Kash et al., 2008; Nobis et al., 2011), including those that innervate the VTA (Silberman et al., 2013), likely via activation of CRF-R1 receptors on glutamatergic terminals (Silberman et al., 2013). We hypothesize that, ultimately, these beta-2 adrenergic receptor-regulated processes in the BNST increase the activity of CRF-releasing projections to the VTA. Although this hypothesis has not been directly tested, it has been found that icv administration of a CRF receptor antagonist blocks reinstatement by central NE delivery, while functional antagonism of noradrenergic neurotransmission via clonidine fails to affect reinstatement following icv administration of CRF (Brown et al., 2009).
6. Intake-dependent emergence of stress-induced reinstatement
The contribution of stress to drug use is not uniform across all cocaine users and appears to vary significantly based on a number of factors, including the context within which stress is encountered, genetic predisposition, and, importantly, the history of prior cocaine use. For example, it has been reported that a prior history of higher frequency cocaine use is associated with augmented stress-induced craving measured in a laboratory setting in cocaine-dependent individuals (Fox et al., 2005). A possible explanation for the relationship between heightened sensitivity to stress-induced craving and previous drug use is that repeated drug exposure establishes intake-dependent neuroplasticity that renders addicts more susceptible to stressor-induced cocaine seeking.
6.1. Long-access cocaine SA
One approach that can be used to investigate persistent drug-induced neuroplasticity related to heightened use involves the study of rats provided chronic daily prolonged access to cocaine for SA (Ahmed and Koob, 1998). Using this approach, Koob and colleagues have demonstrated that, over time, rats self-administering under these conditions display an escalating pattern of cocaine intake that is associated with and likely the consequence of the emergence of an anhedonic/dysphoric state attributable to the recruitment of a number of opponent process mediators, including dynorphin/kappa opioid (Wee et al., 2009), endocannabinoid (Orio et al., 2009), noradrenergic (Wee et al., 2008), and CRF (Specio et al., 2008) systems (see Koob, 2009 for review). We and others have found that the consequences of extended-access SA are long-lasting and affect cocaine-seeking in relapse models, as demon-strated by heightened susceptibility to reinstatement in response to cocaine priming (Mantsch et al., 2004), presentation of cocaine-associated cues (Kippin et al., 2006), and stress (Mantsch et al., 2008a).
6.2. Establishment of stress-induced reinstatement following long-access SA
Research in our laboratory has focused on how SA history alters subsequent susceptibility to stress-induced reinstatement. We have found that increasing the amount of prior cocaine SA by allowing rats to self-administer cocaine under long-access conditions (14 × 6 h/day) that result in very high levels of daily drug intake (approximately 70 mg/kg/day) augments the ability of electric footshock to reinstate cocaine seeking (Mantsch et al., 2008a). Indeed, under the SA and shock conditions that we use, robust and reliable reinstatement of extinguished cocaine seeking in response to electric footshock, only occurs when rats have a history of SA under long-access conditions and is not observed in rats with history of SA under conditions of more limited access (14 × 2 h/day; approximately 15 mg/kg daily intake; Mantsch et al., 2008a). Others have reported shock-induced reinstatement of cocaine seeking in the absence of prior testing under long-access SA conditions (e.g., Ahmed and Koob, 1997; McFarland et al., 2004). However, since susceptibility to reinstatement is likely also determined by environmental variables (e.g., food restriction, time of day, handling, and experimental context) and genetic factors (e.g., rat strain), we do not view our findings as disparate, especially considering that we have optimized our approach to study the ability of SA history to influence later cocaine seeking as measured using the reinstatement approach. Notably, other groups have similarly reported that footshock does not reliably induce rein-statement after short-access cocaine SA (e.g., Shelton and Beardsley, 2005).
6.2.1. Neuroadaptations that underlie heightened susceptibility to stress-induced relapse
Recognizing that the ability of stressors to trigger relapse is likely influenced by intake-dependent cocaine-induced neuroplasticity, we have begun to search for neuroadaptations in the pathways that underlie stress-induced cocaine seeking that are associated with long-access cocaine SA. Not surprisingly, repeated cocaine exposure has been found to produce widespread alterations in synaptic strength, structure, and regulation, many of which have the potential to influence stress-induced reinstatement and emerge in an intake-dependent manner with cocaine SA (see Koob and Volkow, 2010 for review). A complete inventory of all of these adaptations is well beyond the scope of this review. Instead, here we will briefly summarize recent findings suggesting that increased responsiveness of CRF-regulated processes may contribute to heightened relapse susceptibility.
6.2.2. Augmented CRF responsiveness in the VTA
Our laboratory has demonstrated that, like stress, the ability of intra-VTA CRF administration to reinstate cocaine seeking emerges with repeated long-access SA (Blacktop et al., 2011). Bilateral delivery of CRF directly into the VTA fails to induce reinstatement in rats with a history of short-access SA (2 h/day) but produces robust reinstatement when rats have a history of SA under long-access conditions (6 h/day), suggesting that at least some of the neuroadaptations responsible for establishing stress as a trigger for drug use involve processes downstream from the site of CRF action in the VTA. These findings are consistent with a report that CRF-R1 receptor antagonism only interferes with cocaine SA following repeated testing under-extended access conditions (Specio et al., 2008). We hypothesize that alterations in CRF receptor and/or signaling mechanisms in the VTA contribute to the heightened CRF responsiveness. However, since long-access cocaine SA also heightens cocaine-primed reinstatement – a process that is independent of CRF (Erb et al., 1998; Graf et al., 2011) - it is clear that at least some of the contributing neuroplasticity occurs in brain regions downstream from CRF receptors in the VTA, likely in the cortico-accumbens pathway required for both stress and drug-induced cocaine seeking (i.e., in the prelimbic cortex and/or NAc core). Additionally, as the NAc shell and BNST have reciprocating connectivity with the VTA that may also be regulated by CRF, adaptations in these regions cannot be ruled out.
6.2.3. Potential cocaine-induced neuroadaptations in the VTA
In the VTA, repeated cocaine administration has been reported to increase CRF receptor binding (Goeders et al., 1990) and to alter CRF responsiveness. For example, it has been demonstrated that repeated cocaine establishes CRF-R1 receptor-mediate regulation of glutamatergic synaptic transmission in the VTA (Hahn et al., 2009) and attenuates CRF-R1 receptor-induced enhancement of GIRK channel-mediated inhibitory post-synaptic currents (Beckstead et al., 2009). Likewise, a prior history of cocaine SA is required for CRF regulation of extracellular DA and glutamate release in the VTA (Wang et al., 2005). Interestingly, in this study it was also found that, while CRF responsiveness in the VTA was increased by prior SA, stress-induced CRF release into the VTA was unchanged (Wang et al., 2005). Thus, cocaine-induced neuroplasticity that contributes to relapse may involve changes in VTA CRF receptors and/or downstream processes but not mechanisms (such as those in the BNST) that regulate intra-VTA CRF release. Importantly, while all of these changes may contribute to the heightened stress-induced cocaine seeking observed following long-access SA, effects of SA under these conditions on CRF receptor expression, function and downstream signaling have not yet been reported.
6.3. Induction of neuroplasticity that contributes to stress-induced relapse
In addition to identifying the neuroplastic changes that underlie heightened susceptibility to stress-induced relapse, our laboratory has focused on understanding the neurobiological processes through which these changes are put in place. Much of our research has examined the role of glucocorticoids as potential mediators of addiction-related neuroplasticity.
6.3.1. Glucocorticoids and relapse-related neuroplasticity
Glucocorticoids secreted as a consequence of activation of the stressor-responsive HPA axis play a critical role in physiological processes that enable organisms to effectively adapt to and cope with stressors (de Kloet et al., 2008; McEwen, 2008). Although elevated glucocorticoids are of short-term benefit to an organism, prolonged and/or repeated elevations of glucocorticoids as a result of chronic stress are maladaptive and lead to a number of pathological conditions, some of which may contribute to addiction. For example, we have demonstrated that daily exposure to a stressor, intermittent footshock, at the time of SA testing produces a progressive escalation of cocaine SA that is dependent on elevated glucocorticoids (Mantsch and Katz, 2007).
6.3.2. Effects of cocaine use on glucocorticoids
Like stressors, self-administered cocaine increases glucocorticoid secretion in rats (Galici et al., 2000), monkeys (Broadbear et al., 1999), and human cocaine addicts (Ward et al., 1999). When rats self-administer cocaine during prolonged sessions, increases in plasma corticosterone are greater and more persistent than they are with short-access SA (Mantsch et al., 2003) and physiological signs of increased glucocorticoid secretion (e.g., reductions in thymus weight and adrenal hypertrophy) are exaggerated. Thus, any contribution of elevated glucocorticoids to cocaine-induced neuroplasticity will likely be particularly pronounced in rats self-administering cocaine under conditions of prolonged daily drug access.
6.3.3. Role of elevated glucocorticoids during cocaine use in the induction of relapse-related plasticity
To examine the potential contribution of elevated glucocorticoids to the increased susceptibility to later stress-induced rein-statement that emerges with excessive cocaine use, we eliminated the glucocorticoid response to cocaine through surgical adrenal-ectomy along with basal corticosterone replacement before allowing rats to self-administer for 14 days under long-access conditions and subsequently testing for stress and CRF-induced reinstatement (Graf et al., 2011). While eliminating the adrenal response to cocaine SA had only very modest effects on cocaine intake and extinction, subsequent reinstatement in response to footshock or icv CRF delivery was completely abolished. By contrast, when rats underwent adrenalectomy following repeated SA but prior to reinstatement testing (i.e., after cocaine-dependent neuroplasticity was put in place), footshock- or CRF-induced cocaine seeking was unaffected, suggesting that elevated glucocorticoids at the time of cocaine use are critical for establishing neuroadaptations that render addicts susceptible to stress-induced relapse. Although elevated glucocorticoids appear to be necessary for the effects of long-access cocaine SA, increasing glucocorticoids alone during SA does not appear to be sufficient for the establishment of relapse vulnerability, as daily administration of corticosterone to adrenalectomized rats at doses that reproduce the increases plasma levels observed during long-access SA fails to restore later reinstatement (Mantsch et al., 2008b). This finding is consistent with our earlier report that elevated corticosterone is necessary but not sufficient for the effects of repeated stress on cocaine seeking (Mantsch and Katz, 2007). Notably, the inability of corticosterone alone to restore reinstatement in adrenalectomized rats suggests that, during long-access SA, corticosterone may be working in concert with another adrenal hormone to establish relapse susceptibility e a finding that is consistent with reports that cocaine-induced behavioral sensitization requires drug-induced increases in both corticosterone and epinephrine (de Jong et al., 2009). The potential interaction between glucocorticoids and other hormones/processes that are elevated/active during cocaine long-access SA requires further investigation.
6.3.4. Potential mechanisms underlying glucocorticoid-dependent cocaine-induced neuroplasticity
Glucocorticoids produce both glucocorticoid receptor (GR) mediated genomic effects via transcriptional regulation (de Kloet et al., 2008) and rapid non-genomic effects mediated via a number of mechanisms including actions at a putative membrane-associated G-protein-coupled receptors that can regulate endocannabinoid production (Tasker et al., 2006) and inhibition of the glucocorticoid sensitive monoamine transporter, organic cation transporter 3 (Gasser et al., 2006). To date, most studies have examined the potential role of GRs in the establishment of cocaine addiction-related neuroplasticity. GRs are expressed throughout the neurocircuitry implicated in stress-induced cocaine seeking, including in CRF-expressing cells in the BNST (Cintra et al., 1987), DA cells in the VTA (Harfstrand et al., 1986), stress responsive cells in the mPFC (Ostrander et al., 2003), and a variety of cell types in the NAc (Zoli et al., 1990). In these regions, GR regulation of key stress-responsive processes has been demonstrated, including CRF mRNA expression in the BNST (Schulkin et al., 1998), glutamatergic responsiveness of DA cells in the VTA (Saal et al., 2003), dendritic morphology (Wellman, 2001) and dopaminergic signaling (Butts et al., 2011) in the mPFC, and glutamatergic neurotransmission in the NAc (Campioni et al., 2009). Thus, GRs are well positioned as potential mediators of glucocorticoid-dependent neuroplasticity that establishes stress-induced cocaine seeking. Although the role of GRs in the effects of long-access cocaine SA on stress-induced reinstatement has not been examined, their role in establishing behavioral sensitization and the reinforcing effects of cocaine has. Constitutive knockout of GRs prevents cocaine-induced behavioral sensitization and attenuates the reinforcing effects of cocaine (Deroche-Gamonet et al., 2003), while forebrain GR overexpression produces the opposite effect (Wei et al., 2004). One key site of GR mediation of cocaine-induced effects is in DA receptive neurons in the NA. Selective deletion of GRs in these cells attenuates cocaine SA (Ambroggi et al., 2009) as well as cocaine-induced behavioral sensitization and CPP (Barik et al., 2010). Future studies will be required to determine if GR antagonism during long-access cocaine SA can prevent the effects on subsequent stress-induced reinstatement.
7. “Stage setting” effects of stress for drug relapse
Our findings that the ability of stressors to reinstate cocaine seeking is only observed when rats have a history of long-access cocaine SA (Mantsch et al., 2008a) seem to indicate that stress-triggered relapse may only occur in cocaine addicts with a pro-longed history of excessive drug use. This should not be misinterpreted to imply that stressful life events only contribute to relapse in the most extreme addicts. In fact, in many individuals, the role of stress in their drug use is more subtle and, rather than simply functioning as a trigger for drug use, appears to involve a more complex interaction between stress and drug-associated cues and/or cocaine itself. Accordingly, there are some reports in cocaine-dependent individuals that stress imagery alone is insufficient to directly elicit drug craving (De La Garza et al., 2009) and that stressors, such as memories of traumatic events (Coffey et al., 2002) or exposure to painful stimuli (Duncan et al., 2007), function by increasing reactivity to drug-related cues rather than directly evoking craving on their own. This “stage setting” effect of stress is consistent with findings in rats demonstrating that stress-induced reinstatement of cocaine-seeking behavior requires the presence of cues previously associated with cocaine availability (Shelton and Beardsley, 2005) and that acute stressors increase the magnitude of reinstatement triggered by cocaine-associated cues (Feltenstein and See, 2006; Buffalari and See, 2009) or by administration of a dose of cocaine that by itself is insufficient to reinstate (Graf et al., 2013).
Recently, we have reported that the “stage-setting” effects of stress are prevented by surgical adrenalectomy and reproduced by administration of corticosterone at a dose that establishes plasma levels in the stress-induced range (Fig. 3; Graf et al., 2013). Thus, glucocorticoids are both sufficient and necessary for the stress-induced potentiation of cocaine-induced reinstatement. It appears that corticosterone augments reinstatement by direct actions in the nucleus accumbens that result in potentiation of the dopamine response to cocaine via a rapid, GR-independent inhibition of dopamine clearance that does not involve the dopamine transporter (DAT; Graf et al., 2013). Glucocorticoids have long been known to acutely block the clearance of monoamines via uptake2, a high-capacity transport system first characterized in peripheral tissues (Iversen and Salt, 1970). More recently uptake2 has been attributed to a collection of transporters that includes the organic cation transporters (OCTs 1, 2 and 3) and the plasma membrane monoamine transporter (PMAT). Each of these transporters is capable of transporting DA, albeit with varying efficiency (Duan and Wang, 2010; Gorboulev et al., 2005; Gründemann et al., 1998) and most are expressed in the brain (Amphoux et al., 2006; Engel et al., 2004; Vialou et al., 2008; Gasser et al., 2009). However, only one of these transporters, OCT3, is blocked by physiological, stress-induced corticosterone concentrations (Gasser et al., 2006). We have demonstrated that OCT3 is expressed on DA D1 receptor-expressing medium spiny neurons in the nucleus accumbens in close proximity to tyrosine hydroxylase positive nerve terminals (Gasser et al., 2009; Graf et al., 2013) and that administration of corticosterone or the non-glucocorticoid inhibitor of OCT3, nor-metanephrine, directly into the nucleus accumbens increases cocaine responsiveness via a mechanism that requires DA receptor activation (Graf et al., 2013).
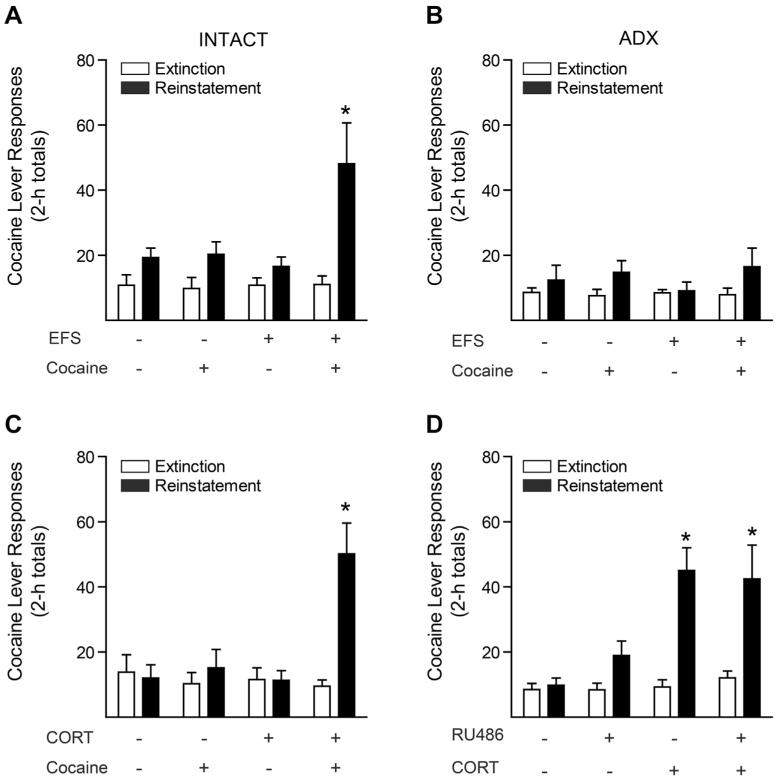
Fig. 3.
Stress-induced potentiation of cocaine-primed drug seeking behavior is corticosterone-dependent, but does not require GR activation. Reproduced from Graf et al. (2013) with permission from the Journal of Neuroscience. (A, B) Cocaine lever responses on the last day of extinction and during reinstatement testing of (A) adrenal-intact or (B) adrenalectomized rats. On the day of reinstatement testing, rats were subjected to 15 min of uncontrollable, intermittent electric footshock (EFS) or no shock, followed by injection of cocaine (2.5 mg/kg ip) or saline, and cocaine lever responses were recorded for 2 h. (C) Acute corticosterone injection mimics stress-induced potentiation of reinstatement. Rats received an injection of corticosterone (CORT; 2.0 mg/kg ip) or vehicle followed, 40 min later, by an injection of cocaine (2.5 mg/kg ip) or saline. (d) RU38486 pretreatment has no effect on corticosterone-induced potentiation of reinstatement. Rats received an injection of the GR antagonist RU38486 (12.5 mg/kg sc) or vehicle, followed 60 min later by injection of corticosterone (2.0 mg/kg ip) or vehicle, followed 40 min thereafter by an injection of cocaine (2.5 mg/kg ip). *indicates significantly different from extinction responding (P < 0.05). Error bars represent mean ± s.e.m.
While these data point to corticosterone-mediated regulation of dopamine clearance as a key mechanism through which stress can “set the stage” for cocaine use, other corticosterone-dependent mechanisms may also be involved. Current areas of investigation include corticosterone-induced increases in endocannabinoid production and resulting alterations in the excitability of the cortico-accumbens glutamatergic pathway that mediates cocaine seeking. To date, our preliminary findings have revealed important roles endocannabinoids in these “stage setting” effects in part via actions in the prelimbic cortex. Interestingly, neither glucocorti-coids (Erb et al., 1998; Graf et al., 2011) nor endocannabinoids (De Vries et al., 2001) appear to be required for reinstatement by stress alone, suggesting that the mechanisms for the relapse-triggering and promoting effects of stress may be non-overlapping.
8. Summary and treatment implications
In summary, recent research in our laboratory has advanced our understanding of the neurobiological processes through which stressful stimuli contribute to cocaine use. A schematic depicting some of these mechanisms is shown in Fig. 2. It is our hope that, from this better understanding, new and more effective interventions for the management of cocaine addiction will arise. Indeed, currently available medications and new drugs in development targeting adrenergic, CRF, glucocorticoid and cannabinoid receptors as well as cognitive behavioral approaches focused on stress management may represent powerful tools through which the contribution of stress to the addiction process can be minimized, at least in the case of cocaine. Notably, although the mechanisms through which stress influences drug use may vary with the class of abused drug, many of the processes likely also contribute to stress-related use of a number of drugs, including opiates, alcohol, and nicotine. For example, it has been reported that stress-induced reinstatement of drug seeking following heroin (Shaham et al., 1997), alcohol (Lê et al., 2000) or nicotine (Bruijnzeel et al., 2009) SA involves CRF, as does stress-induced reinstatement of palatable food-seeking behavior (Ghitza et al., 2006). Confirmation of the relevance of the pathways and pathways described in this review to stress-induced relapse of use of these other drugs awaits more detailed investigation. Although the contribution of stress to drug use likely varies across individual addicts, we are optimistic that advances in the diagnosis of addiction that will permit the identification of individuals whose drug use is stress-related, along with the availability of effective stress-targeting therapeutics approaches, will lead to better treatment outcomes and improve lives.
Acknowledgments
This work was supported by National Institute on Drug Abuse (NIDA) grant number DA15758. The authors would like to acknowledge Dr. Robert Wheeler for his helpful comments when preparing this manuscript.
Footnotes
This article is part of a Special Issue entitled ‘NIDA 40th Anniversary Issue’.
References
- Ahmed SH, Koob GF. Cocaine-but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:2980–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schütz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat. Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Amphoux A, Vialou V, Drescher E, Bruss M, La Cour CM, Rochat C, Millan MJ, Giros B, Bonisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Ogawa N, Mizukawa K, Haba K, Hirata H, Mori A. Distribution of the beta-2 adrenergic receptor messenger RNA in the rat brain by in situ hybridization histochemistry: effects of chronic reserpine treatment. Neurochem. Res. 1991;16:1253–1256. doi: 10.1007/BF00966654. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jaanimägi U, Jackson JL. Cocaine dependence and PTSD: a pilot study of symptom interplay and treatment preferences. Addict. Behav. 2006;31:351–354. doi: 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J. Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, Bailly A, Benecke A, Tronche F. Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biol. Psychiatry. 2010;68:231–239. doi: 10.1016/j.biopsych.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Beckerman MA, Van Kempen TA, Justice NJ, Milner TA, Glass MJ. Corticotropin-releasing factor in the mouse central nucleus of the amygdala: ultrastructural distribution in NMDA-NR1 receptor subunit expressing neurons as well as projection neurons to the bed nucleus of the stria terminalis. Exp. Neurol. 2013;239:120–132. doi: 10.1016/j.expneurol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, Grigoriadis DE, Lovenberg T, Chalmers D, Heinrichs S, Liaw C, De Souza EB. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders. Mol. Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res. Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Dansky BS, Sonne SC, Saladin ME. Posttraumatic stress disorder and cocaine dependence. Order of onset. Am. J. Addict. 1998;7:128–135. [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J. Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Cicero TJ, Woods JH. Effects of self-administered cocaine on plasma adrenocorticotropic hormone and cortisol in male rhesus monkeys. J. Pharmacol. Exp. Ther. 1999;289:1641–1647. [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Nobrega JN, Erb S. Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav. Brain Res. 2011;217:472–476. doi: 10.1016/j.bbr.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol. Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol. Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Chen KW, Banducci AN, Guller L, Macatee RJ, Lavelle A, Daughters SB, Lejuez CW. An examination of psychiatric comorbidities as a function of gender and substance type within an inpatient substance use treatment pro-gram. Drug Alcohol Depend. 2011;118:92–99. doi: 10.1016/j.drugalcdep.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Fuxe K, Härfstrand A, Agnati LF, Wikström AC, Okret S, Vale W, Gustafsson JA. Presence of glucocorticoid receptor immunoreactivity in corticotrophin releasing factor and in growth hormone releasing factor immunoreactive neurons of the rat di- and telencephalon. Neurosci. Lett. 1987;77:25–30. doi: 10.1016/0304-3940(87)90601-x. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid post-traumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol. Psychiatry. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Post-traumatic stress disorder among substance users from the general population. Am. J. Psychiatry. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr., Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J. Chem. Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, de Jong IE, Oitzl MS. Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine. Eur. J. Pharmacol. 2008;585:473–482. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Ashbrook LH, Evans SE, Jacobsen CA, Kalechstein AD, Newton TF. Influence of verbal recall of a recent stress experience on anxiety and desire for cocaine in non-treatment seeking, cocaine-addicted volunteers. Am. J. Addict. 2009;18:481–487. doi: 10.3109/10550490903205876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong IE, Steenbergen PJ, de Kloet ER. Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology. 2009;204:693–703. doi: 10.1007/s00213-009-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schütz G, Tronche F, Piazza PV. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J. Neurosci. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J. Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 2010;335:743–754. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J. Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am. J. Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J. Biol. Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J. Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology. 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav. Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res. Brain Res. Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J. Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur. J. Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J. Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J. Comp. Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J. Comp. Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J. Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Bienvenu OJ, De Souza EB. Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain. Brain Res. 1990;531:322–328. doi: 10.1016/0006-8993(90)90794-c. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J. Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Shatskaya N, Volk C, Koepsell H. Subtype-specific affinity for corticosterone of rat organic cation transporters rOCT1 and rOCT2 depends on three amino acids within the substrate binding region. Mol. Pharmacol. 2005;67:1612–1619. doi: 10.1124/mol.104.008821. [DOI] [PubMed] [Google Scholar]
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–1454. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründemann D, Schechinger B, Rappold GA, Schömig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat. Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J. Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc. Natl. Acad. Sci. U. S. A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL, Salt PJ. Inhibition of catecholamine uptake-2 by steroids in the isolated rat heart. Br. J. Pharmacol. 1970;40:528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J. Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl. 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J. Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb. Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J. Pharmacol. Exp. Ther. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a rat high-dose, extended-access self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Katz ES. Elevated glucocorticoids are necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–376. doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology. 2008a;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008b;33:814–826. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol. Psychiatry. 2011;69:1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci. 2012;32:14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J. Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res. 2003;990:209–214. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc. Natl. Acad. Sci. U. S. A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schütz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat. Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch. Gen. Psychiatry. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur. J. Neurosci. 2012;36:3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. U. S. A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int. J. Comp. Psychol. 2005;18:154–166. [Google Scholar]
- Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors hetero-synaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J. Comp. Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J. Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct. Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J. Comp. Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci. Biobehav. Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Hang S, Baker DA, Mantsch JR. β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for b1 and b2 adrenergic receptors. J. Pharmacol. Exp. Ther. 2012;342:541–551. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J. Comp. Neurol. 2008;509:302–318. doi: 10.1002/cne.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. Blockade of cocaine-induced increases in adrenocorticotrophic hormone and cortisol does not attenuate the subjective effects of smoked cocaine in humans. Behav. Pharmacol. 1999;10:523–529. doi: 10.1097/00008877-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in fore-brain: a mouse model of increased emotional lability. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., 3rd Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur. J. Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zoli M, Cintra A, Zini I, Hersh LB, Gustafsson JA, Fuxe K, Agnati LF. Nerve cell clusters in dorsal striatum and nucleus accumbens of the male rat demonstrated by glucocorticoid receptor immunoreactivity. J. Chem. Neuroanat. 1990;3:355–366. [PubMed] [Google Scholar]