Abstract
HIV-associated neurocognitive disorders (HAND), characterized by cognitive, motor, and behavioral abnormalities, are common among people living with HIV and AIDS. In combined antiretroviral therapy era in Western countries, nearly 40% of HIV-infected patients continue to suffer from HAND, mainly with mild or asymptomatic cognitive impairment. However, the prevalence and the clinical features of HAND in China are still not well known. In this study, a multi-center cross-sectional study was performed to determine the prevalence and clinical features of HAND in 134 HIV-1 infected patients in China. The International HIV Dementia Scale and a neuropsychological test battery were administered for screening and diagnosis HAND. Subjective complaints, CD4 count and viral loads in both blood plasma and cerebrospinal fluid were correlated with diagnosis of HAND. The results showed that the prevalence of HAND was approximately 37% in these patients. CD4 counts at time of sampling were significant lower in the HAND group than in the non-HAND group. But the distribution of the HAND severity did not differ by CD4 count or viral load. The presence of HAND was associated with cognitive and behavior disorder complaints (4.9- and 4.1-fold higher than those without HAND, respectively). The present data suggest that CD4 count and viral load cannot predict the severity of HAND, although the prevalence of HAND is similar to previous report in these patients. Cognitive and behavioral disorder is major complaint rather than cognitive and motor impairment. A larger prospective study is needed to obtain better estimates of HAND in China.
Keywords: AIDS, Clinical manifestation, HIV-associated neurocognitive disorders, Prevalence
Introduction
Since the beginning of the HIV/AIDS epidemic, HIV-associated neurocognitive disorders (HAND) have been commonly observed in infected populations (Heaton et al. 1995, 2011). HAND is characterized by cognitive, motor, and behavioral abnormalities and severity is classified according to patient performance in areas of neurological and behavioral functioning and neuropsychological testing (Antinori et al. 2007b; Kaul et al. 2005). Prior to the widespread use of highly active antiretroviral therapy (HAART), 20–30% of individuals in Western countries with advanced HIV infection displayed symptoms of the most severe form of HAND, HIV-associated dementia (HAD) (Kaul et al. 2005). Even in the HAART era, nearly 40% of HIV-positive patients continue to suffer from HAND and a significant number of HIV-1 infected patients show progressive loss of cognitive abilities (Antinori et al. 2007b; Boisse et al. 2008; Minagar et al. 2008). Despite the decline of severe HAND incidence, neurological complications remain an important cause of disability and death, and the overall prevalence of HAND may increase as HIV-infected individuals live longer (Kandanearatchi et al. 2003).
HAND is clinically classified as asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and dementia (Antinori et al. 2007a). The International HIV Dementia Scale (IHDS) is widely used as screening test to identify individuals at risk for HIV-associated dementia (HAD) in both the industrialized world and the developing world, and full neuropsychological (NP) testing is often required to confirm a diagnosis of HAND (Sacktor et al. 2005). The NP assessment surveys the following abilities: verbal/language, attention/working memory, abstraction/executive, memory (learning; recall), speed of information processing, sensory/perception, and motor skills (Antinori et al. 2007a). Acquired impairment in cognitive functioning is defined as involving at least two ability domains, documented by performance of at least one standard deviation below the mean for age–education-appropriate norms on standardized NP tests.
By October 2010, over 370,000 people have been found to be infected with HIV in China. Sexual contact is becoming a major mode of HIV transmission, in particular among men who have sex with men (MSM), and ethnic minorities comprise the fastest growing segment of the HIV-infected population (National Center for AIDS/STD Control and Prevention, China CDC). However, even though large numbers of people are infected in China, up to now, very few reports have documented the prevalence and the clinical features of HAND. In this study, we determined how frequently HAND could be detected in 134 HIV-1 infected patients in China, and how HAND was related to subjective complaints, CD4 counts and viral loads in blood plasma and cerebrospinal fluid (CSF).
Materials and methods
Patients
HIV-1 infected patients were selected from three sites participating in an AIDS Risk Cohort Program supported by Beijing Science and Technology Committee. These sites included the Infectious Diseases Hospital of Henan Province located in central China, Infectious Diseases Hospital of Yunnan Province located in southwest China, and the Beijing You An Hospital located in northern China. Exclusion criteria included age less than 18 years, an active or known past central nervous system (CNS) opportunistic infection, fever >37.5°C, a history of a chronic neurologic disorder, active psychiatric disorder, alcoholism, physical deficit (e.g., amputation), severe functional impairment, or severe medical illness that would interfere with the ability to perform the study evaluations. This study was approved by the Human Subjects Protection Committees of each participating hospital. Written informed consent was obtained from all study participants.
Clinical assessments
HIV-infected patients and/or primary care givers were randomly selected and subsequently questioned by an investigator about symptoms of: (1) cognitive impairments including memory dysfunction, difficulty with concentration, weak calculation, difficult to recognize his/her families, apathy, mutism, visual hallucinations, disorientation, inertia, social withdrawal and waning interest in work and hobbies; (2) motor impairments including poor hand writing, tendency to drop things easily, difficulty with hand coordination, holding instablity, insecure balance, difficulty with gait, postural tremor, dystonia, choreoathetoid movements, impairments of rapid eye movement and urinary or fecal incontinence; (3) change in behavior including change in personality, blunting of emotional responsiveness, agitation, mania and difficulties with activities of daily living or employment. Cognitive impairments and motor impairments contained 11 symptoms, respectively (each cumulative score 11), change in behavior containing five symptoms (cumulative score 5). Then these patients were evaluated using a screening test for HIV dementia (IHDS score ≤10) (Sacktor et al. 2005). The evaluations were translated into the local language, Chinese. Clinical assessments used standardized questionnaires assessing demographic information, including primary language and reading abilities, medical, psychiatric, and neurologic history, including an assessment of peripheral neuropathy symptoms, and a systematic neurological examination.
Neuropsychological and functional impairment in everyday life assessments
After questioning about neurocognitive symptoms and screening for HIV dementia, patients underwent standardized NP testing including Grooved Pegboard (dominant and non-dominant Hands), Trail Making Test: Parts A and B, and Wechsler Adult Intelligence Scale (WAIS-III) Digit Symbol. The Grooved Pegboard is a manipulative dexterity test. This unit consists of 25 holes with randomly positioned slots. Pegs, which have a key along one side, must be rotated to match the hole before the can be inserted. This test requires more complex visual–motor coordination than most pegboards. The Trail-making test is a neuropsychological test of visual attention and task switching. The task requires a subject to ‘connect-the-dots’ of 25 consecutive targets on a sheet of paper or computer screen. Two versions are available: A, in which the targets are all numbers, and B, in which the subject alternates between numbers and letters. The goal of the subject is to finish the test as quickly as possible, and the time taken to complete the test is used as the primary performance metric. WAIS-III Digit Symbol is sensitive to brain damage, dementia, age and depression and consists of digit–symbol pairs followed by a list of digits. Under each digit, the subject should write down the corresponding symbol as fast as possible. The number of correct symbols within the allowed time is measured. The combination of Grooved Pegboard, Trail Making Test: Parts A and B, and Wechsler Adult Intelligence Scale (WAIS-III) Digit Symbol was confirmed to be more sensitive and accurate in detection HAND (Antinori et al. 2007a; Carey et al. 2004; Kim et al. 2001). Results of these NP tests were assessed in relation to previously reported normative data according to age and education (Kim et al. 2001). Based on these results, HAND was categorized as ANI, MND, or HAD, according to the Revised Research Criteria for HIV-associated Neurocognitive Disorders (Antinori et al. 2007a). Reports of cognitive difficulties in everyday life were assessed using Activity of Daily Living (ADL) scale (Gandhi et al. 2011). The ADL scale includes 20 items on which participants rate themselves as having neurobehavioral difficulties in their everyday lives. A total summed score is derived, with higher scores indicating more difficulty.
Laboratory assessments
HIV infection was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. Complete blood counts and CD4+ T cells (flow cytometry) were performed. HIV RNA levels were measured in blood plasma and CSF by reverse transcriptase polymerase chain reaction (Roche Amplicor, v. 1.5; lower limit of quantization 50 copies per milliliter).
Data analysis
The results were evaluated as a mean with a standard deviation (SD) or other forms in accordance with the variable distribution. Statistical significance was determined by one-way ANOVA, with post hoc correction using the Tukey multiple comparison test. Chi-square test was used to compare the ratio change of different HAND types. Spearman correlation analysis was used to compare the relationship between two variables. Statistical software PASW statistics 18 was used in this study, and a value of p<0.05 was considered statistically significant.
Results
Participants
Among the three recruitment sites, 56 participants were selected from the Infectious Diseases Hospital of Henan Province and most reported being former plasma donors (FPD), 43 participants were selected from Infectious Diseases Hospital of Yunnan Province and most reported HIV risks of sexual contact and injection drug use (IDU), and 35 participants were selected from the Beijing You An Hospital and most reported HIV risks of MSM. Participant demographics are summarized in Table 1.
Table 1.
Participant demographics
| Non-HAND (n=84) | HAND (n=50) | p value | |
|---|---|---|---|
| Age, years, mean (SD) | 38.65(9.46) | 36.77(7.91) | NS |
| Male, n (%) | 65(77.38) | 11(22) | <0.001 |
| Education, years, mean (SD) | 5.23(1.46) | 4.85(1.24) | NS |
| Race/ethnicity — minority, n (%) | 6(7.14) | 3(6) | NS |
| Estimated infective time, years, mean (SD) | 4.63(2.68) | 5.16(3.24) | NS |
| ART treatment time, months, mean (SD) | 13.26(3.25) | 12.03(4.78) | NS |
| HIV infection risks | |||
| MSM, n (%) | 14(16.67) | 8(16) | NS |
| IDU, n (%) | 8(9.52) | 3(6) | NS |
| Heterosexual, n (%) | 27(32.14) | 17(34) | NS |
| FPD, n (%) | 32(38.1) | 20(40) | NS |
| Others, n (%) | 3(3.57) | 2(4) | NS |
HAND HIV-associated neurocognitive disorders, ART antiretroviral therapy, MSM men who have sex with men, IDU injecting drug user, FPD former plasma donors, NS non-significant at p<0.05
Among 134 subjects, 50 (37.31%) were found to have HAND based on IHDS and NP testing, significantly higher than normal population which was 17% (data not published). The HAND group had a mean (SD) age of 38.65 (9.46) years, which was not different from the non-HAND group who had a mean (SD) age of 36.77 (7.91) years. Women were over-represented in the HAND group (78% in HAND group vs. 22.62% in non-HAND group; chi-square test, p<0.001). There were no differences in reported HIV infection risks (MSM, IDU, FPD and Heterosexual contact), level of education, race ethnicity and estimated duration of infection. Among 134 HIV-1 infected patients, 98 administered HAART including 59 (70.24%) in non-HAND group whose average therapy time was 13.26 months, and 39 (78%) in HAND group whose average therapy time was 12.03 months. No difference in HAART was found between two groups.
The prevalence of HIV-associated neurocognitive disorders
Participants were clinically classified into three grades according to the Centers for Disease Control (CDC) of the United States (CDC-A, CDC-B and CDC-C). Among the 134 participants, 26 were classified into CDC-A, 52 CDC-B and 56 CDC-C. There was a total of six HAND in CDC-A group (23.08%) including four with ANI (15.38%) and two with MND (7.69%). None in CDC-A group met criteria for HAD. For the CDC-B group, 20 participants demonstrated HAND (38.46%) including 13 with ANI (25%) and five with MND (9.62%) and two with HAD (3.85%). For the most advanced group, CDC-C, 24 participants had HAND (42.86%) and included 13 with ANI (23.21%), seven with MND (12.5%) and four with HAD (7.14%). As expected, the prevalence of HAND increased with the advanced stages of HIV disease, but statistical analysis showed no difference in the distribution of three HAND classifications among three CDC stages (chi-square test, p>0.05), likely secondary to sample size (Table 2).
Table 2.
Prevalence of HIV associated neurocognitive disorders
| Clinical stage
|
p value | Recruitment site
|
p value | Viral load (copies/ml)
|
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CDC-A (n=26) | CDC-B (n=52) | CDC-C (n=56) | Beijing (n=35) | Henan (n=56) | Yunnan 3 (n=43) | <50 (n=26) | > or =50 (n=108) | ||||
| ANI, n (%) | 4 (15.38) | 13 (25) | 13 (23.21) | NS | 10 (28.57) | 8 (14.29) | 12 (27.91) | NS | 5 (19.23) | 25 (23.15) | NS |
| MND, n (%) | 2 (7.69) | 5 (9.62) | 7 (12.5) | NS | 4 (11.43) | 6 (10.71) | 4 (9.3) | NS | 2 (7.69) | 14 (12.96) | NS |
| HAD, n (%) | 0 (0) | 2 (3.85) | 4 (7.14) | NS | 2 (5.71) | 1 (1.79) | 3 (6.98) | NS | 1 (3.85) | 3 (2.78) | NS |
| HAND, n (%) | 6 (23.08) | 20 (38.46) | 24 (42.86) | NS | 16 (45.71) | 15 (26.79) | 19 (44.19) | NS | 8 (30.77) | 42 (38.89) | NS |
CDC the Centers for Disease Control of the United States, ANI asymptomatic neurocognitive impairment, MND mild neurocognitive disorder, HAD HIV-associated dementia, HAND HIV associated neurocognitive disorders, NS non-significant at p<0.05
The prevalence of HAND in three recruitment sites was also compared. In 50 HAND patients, 16 (45.71%) came from Beijing including ten with ANI (28.57%) and four with MND (11.43%) and two with HAD (5.71%); 15 (26.79%) came from Henan including eight with ANI (14.29%) and six with MND (10.71%) and only one with HAD (1.79%); and 19 (44.19%) from Yunnan including 12 with ANI (27.91%) and four with MND (9.3%) and three with HAD (6.98%). The rates of HAND in Beijing (45.71%) and Yunnan (44.19%) were close and higher than that in Henan (26.79%), but this difference was not significant (chi-square test, p>0.05) (Table 2).
Since HIV levels are a marker of disease status and can be used to monitor the effectiveness of HAART, we also evaluated blood viral loads in relation to the presence or absence of HAND. First, 134 participants were divided into 26 with blood plasma viral loads <50 copies/ml (undetectable) and 108 with viral loads ≥50 copies/ml). Among 50 patients with HAND, eight (30.77%) had undetectable blood viral loads including five with ANI (19.23%) and two with MND (7.69%) and one with HAD (3.85%), while 42 (38.89%) had detectable blood plasma viral loads including 25 with ANI (23.15%) and 14 with MND (12.96%) and three with HAD (2.78%). Prevalence of overall HAND and the constituent HAND types between two groups were not different (chi-square test, p>0.05) (Table 2).
The symptomatic features of HIV-associated neurocognitive disorders
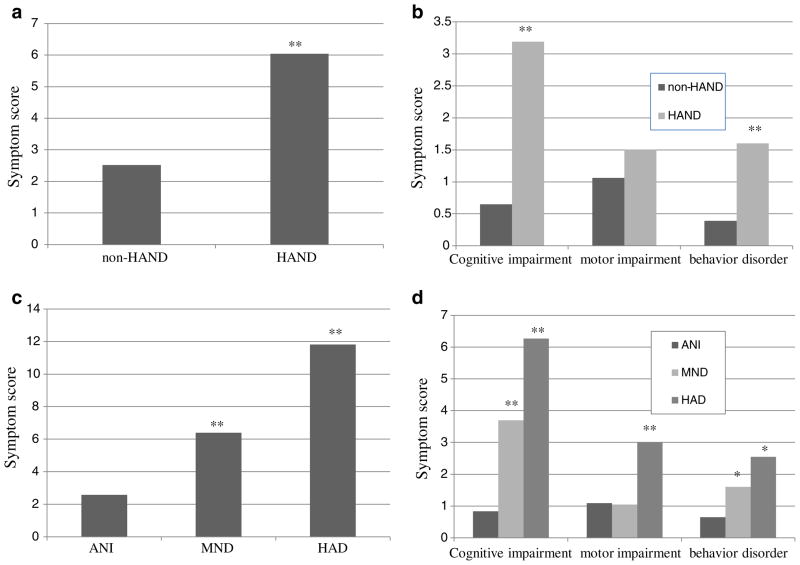
In this study, we investigated the relationship of patient’s complaints and NP results. The total symptom scores of cognitive and motor and behavior were 2.52 in non-HAND group, and 6.04 in HAND group (ANOVA, p< 0.01) (Fig. 1a); thereby, demonstrating that HAND was associated with greater participant symptomatology. Specifically, as expected the symptom of cognitive disorder was more significant in HAND group (3.19 vs. 0.65, ANOVA, p<0.01), followed by that of behavior disorder (1.60 vs. 0.39, ANOVA, p<0.01). But the symptom of motor impairment was not different between two groups (1.50 vs. 1.06, ANOVA, p>0.05) (Fig. 1b). The cumulative symptom scores were also different between ANI, MND and HAD (2.58 vs. 6.40 vs. 11.82, ANOVA, p<0.01) (Fig. 1c). Further analysis found that cognitive impairment scores (0.83 vs. 3.70 vs. 6.27, ANOVA, p<0.01) and behavior disorder scores (0.65 vs. 1.65 vs. 2.55, ANOVA, p<0.05) increased in the advanced stages of HIV disease, i.e., CDC classes. Although the symptom of motor impairment was more serious in HAD compared with that in ANI and MND (3.0 vs. 1.09 and 1.05, ANOVA, p<0.01), no difference was found between ANI and MND (Fig. 1d).
Fig. 1.
The symptomatic feature of HIV-associated neurocognitive disorders in these patients. a The total symptom score of HAND group was higher than that of non- HAND group (6.04 vs. 2.52). b The symptom score of cognitive impairment was very higher in HAND group (3.19 vs. 0.65), followed by that of behavior disorder (1.60 vs. 0.39). But the symptom score of motor impairment was not different between two groups (1.50 vs. 1.06). c The total symptom scores were different among ANI and MND and HAD (2.58 vs. 6.40 vs. 11.82). d Cognitive impairment scores increased as the disease development (0.83 vs. 3.70 vs. 6.27). Similarly, behavior disorder scores also increased as the disease development (0.65 vs. 1.65 vs. 2.55). Although the symptom score of motor impairment was higher in HAD compared with that in ANI and MND (3.0 vs. 1.09 and 1.05), no difference of that was found between ANI and MND. *Statistically significant at p< 0.05; **statistically significant at p<0.01
The relationship of HIV-associated neurocognitive disorders, CD4 count and HIV load
Among the cohort, average HIV loads (HIV RNA log10 copies/ml) were 3.87 (SD 1.50) in blood plasma and 3.36 (SD 1.41) in CSF. Blood and CSF viral loads were significantly correlated (Spearman correlation analysis, r=0.59, p<0.01), but no correlations were found between IHDS score and CSF viral load (Spearman correlation analysis, r=−0.046, p>0.05), IHDS score and blood viral load (Spearman correlation analysis, r=−0.219, p>0.05), and IHDS score and CD4 cell counts (Spearman correlation analysis, r=0.175, p>0.05). CD4 counts at time of sampling were significant lower in the HAND group (368.34±77.22) than in the non-HAND group (745.38±121.74) (ANOVA, p<0.05), but HIV loads in blood plasma or in CSF were not different between the two neurocognitive (NC) groups (ANOVA, p>0.05). Further analysis did not demonstrate any differences in the CD4 counts and blood plasma and CSF viral loads between the three HAND subgroups (ANI, MND and HAD; ANOVA, p>0.05, Table 3).
Table 3.
Relationships between HIV associated neurocognitive disorders, CD4 count and HIV load
| Groups
|
p value | HAND Subtypes
|
P value | ||||
|---|---|---|---|---|---|---|---|
| Non-HAND | HAND | ANI | MND | HAD | |||
| CD4 count (106/l), mean (SD) | 745.38 (121.74) | 368.34 (77.22) | <0.05 | 415.07 (111.85) | 316.48 (96.5) | 286.36 (56.97) | NS |
| HIV load in plasma (log10 copies/ml), mean (SD) | 3.48 (1.42) | 4.13 (1.51) | NS | 4.08 (1.40) | 4.35 (1.62) | 4.24 (1.73) | NS |
| HIV load in CSF (log10 copies/ml), mean (SD)a | 3.32 (1.45) | 3.38 (1.40) | NS | 3.32 (1.46) | 3.36 (1.33) | 3.65 (1.48) | NS |
HAND HIV-associated neurocognitive disorders, SD standard deviation, ANI asymptomatic neurocognitive impairment, MND mild neurocognitive disorder, HAD HIV-associated dementia, CSF cerebrospinal fluid, NS non-significant
Correlation of HIV load between blood plasma and CSF
Discussion
Prior to the availability of HAART, HAND was relatively common among patients with advanced HIV disease. With the use of HAART, the prevalence of HAND decreased, and the overall health of HIV-infected patients improved; however, HAND is still a problem, even among patients receiving HAART and with good CD4+ counts and undetectable blood plasma viral load (Robinson-Papp et al. 2009). In fact, the prevalence of HAND has been described as close to 40% of HIV-positive patients in the HAART era (Antinori et al. 2007b; Boisse et al. 2008; Minagar et al. 2008). The results in this Chinese cohort were similar to these data, although a larger sample is needed to further confirm the results. Interestingly, compared with other two sites, the prevalence of HAND seemed to be lower at the Henan site where patients mainly consisted of patients with the FPD risk factor and had lower educational levels. This observation is significantly different from previous report (Heaton et al. 2008), although it did not reach statistical significance. Further, it is also different from previous report in our study that women were susceptible to HAND, which probably contributed to small sample size and variable treatment agents and time. Compared with male patients, lower education level in female patients probably affected NP results, although NP results have been normalized according to age and education.
Since HAND includes cognitive impairment, motor disorder and abnormal behavior, we evaluated the symptoms in each of these domains. In the pre-HAART era, HAND had more impairment in motor skills, cognitive speed, and verbal fluency, whereas in the HAART era HAND involved more memory (learning) and executive function impairment. This pattern is consistent with earliest involvement of subcortical or frontostriatal brain systems (Heaton et al. 1995). In HAART era, HIV-related HAND was generally mild and persisted at all stages of HIV infection, especially in the medically asymptomatic stage of infection, despite improved viral suppression and immune reconstitution with HAART (Heaton et al. 2011; Pascual-Sedano et al. 1999). One study reported that patients with HIV infection were at an increased risk of psychiatric illness, and that major depressive and anxiety disorder and substance abuse are more prevalent among HIV-infected individuals (Owe-Larsson et al. 2009). Our study even demonstrated that behavior disorder was more common than motor impairment, but cognitive impairment remained a persistently common complaint. Similar to a previous study in Switzerland (Simioni et al. 2010), in our study, symptoms also increased with successive HAND stages (ANI, MND, and HAD).
A previous cross-sectional cohort in the United States reported that among patients receiving HAART and with undetectable plasma HIV RNA, 38% had HAND (20%, 16%, and 2% with ANI, MND, and HAD, respectively) (Pumpradit et al. 2010). In comparison with participants who were NC normal and virally suppressed, individuals with HAND and viral suppression had shorter duration of current HAART (Cysique and Brew 2011). A history of immunosuppression (nadir CD4 cell count <200 cells/ml) was associated with an increase in prevalent of HAND and CD4 nadir is a predictor of HAND in the era of combination antiretroviral therapy (Ellis et al. 2011a; Robertson et al. 2007). Neurocognitive performance is also linked to HIV disease stages, and in both pre-HAART and HAART eras, the HAND rate is increased with later disease stages (CDC stages A, B, and C) (Antinori et al. 2007a; Heaton et al. 2011; Woods et al. 2009). Further, low CD4 counts and high HIV RNA levels in the blood usually predict poorer outcome, and HAND has been consistently associated with nadir CD4 levels (Ellis et al. 2011b; Heaton et al. 2011; Simioni et al. 2010). Our multi-center cross-sectional study revealed that the overall prevalence of HAND was over one-third of HIV-1 infected patients in China. Interestingly, in our study, although CD4 counts were lower in HAND than non-HAND, it seemed not to correlate with the severity of HAND, which probably contributed to the relative short time on HAART resulting small proportion of HIV suppressed patients. Although not assessed, the CSF penetration of HAART and patients’ adherence may have also affected the rate of HAND. Further, genotypic or phenotypic drug resistance assays were not performed, and these may have helped resolved some of the issues of possible drug resistance or infecting clade effect of HAND. Therefore, larger sample size, longitudinal observation and additional laboratory characterizations are needed to better characterize NC functioning among HIV-infected people in China.
Acknowledgments
This study has been supported by National Natural Science Foundation of China (30910103915, 30870853), Beijing Natural Science Foundation (7092045, 7101005), Twelfth Key Science and Technology Five Year Plan of China (2012ZX10001-003, 2012ZX10001-004), Major Program of AIDS of Beijing Science & Technology Committee (D09060030405912) and the U.S. National Institutes of Health MH62512, MH083552. The authors wish to thank Dongmei Hu (Dalian Medical University) for assistance in statistical analysis and Changchun Zhang (Nanjing Brain Hospital) for assistance in Neuropsychological tests.
Contributor Information
Yulin Zhang, STD/AIDS Research Center, Department of Infectious Diseases, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Luxin Qiao, Beijing Liver Disease Research Institute, Capital Medical University, Beijing 100069, China.
Wei Ding, Beijing Liver Disease Research Institute, Capital Medical University, Beijing 100069, China.
Feili Wei, STD/AIDS Research Center, Department of Infectious Diseases, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Qingxia Zhao, Department of Infectious Diseases, Infectious Diseases Hospital of Henan Province, Zhengzhou City 450015 Henan Province, China.
Xicheng Wang, AIDS Care Center, Infectious Diseases Hospital of Yunnan Province, Kunming City 650301 Yunnan Province, China.
Ying Shi, STD/AIDS Research Center, Department of Infectious Diseases, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Ning Li, STD/AIDS Research Center, Department of Infectious Diseases, Beijing You An Hospital, Capital Medical University, Beijing 100069, China.
Davey Smith, Division of Infectious Disease, University of California–San Diego, San Diego, CA, USA.
Dexi Chen, Email: dexi0963@yahoo.com, STD/AIDS Research Center, Department of Infectious Diseases, Beijing You An Hospital, Capital Medical University, Beijing 100069, China, Beijing Liver Disease Research Institute, Capital Medical University, Beijing 100069, China, HIV/AIDS Research Center, Beijing You An Hospital, Beijing Liver Disease Research Institute, Capital Medical University, Beijing 100069, China.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007a;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Trotta MP, Lorenzini P, Torti C, Gianotti N, Maggiolo F, Ceccherini-Silberstein F, Nasto P, Castagna A, De Luca A, Mussini C, Andreoni M, Perno CF. Virological response to salvage therapy in HIV-infected persons carrying the reverse transcriptase K65R mutation. Antivir Ther. 2007b;12:1175–1183. [PubMed] [Google Scholar]
- Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18:234–248. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17:176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NS, Skolasky RL, Peters KB, Moxley RTT, Creighton J, Roosa HV, Selnes OA, McArthur J, Sacktor N. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17:159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, Franklin DR, Ake C, Vigil O, Atkinson JH, Marcotte TD, Grant I, Wu Z. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008;14:536–549. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandanearatchi A, Williams B, Everall IP. Assessing the efficacy of highly active antiretroviral therapy in the brain. Brain Pathol. 2003;13:104–110. doi: 10.1111/j.1750-3639.2003.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jewison DL, Milner GR, Rourke SB, Gill MJ, Power C. Neurocognitive symptoms and impairment in an HIV community clinic. Can J Neurol Sci. 2001;28:228–231. doi: 10.1017/s0317167100001372. [DOI] [PubMed] [Google Scholar]
- Minagar A, Commins D, Alexander JS, Hoque R, Chiappelli F, Singer EJ, Nikbin B, Shapshak P. NeuroAIDS: characteristics and diagnosis of the neurological complications of AIDS. Mol Diagn Ther. 2008;12:25–43. doi: 10.1007/BF03256266. [DOI] [PubMed] [Google Scholar]
- Owe-Larsson B, Sall L, Salamon E, Allgulander C. HIV infection and psychiatric illness. Afr J Psychiatry (Johannesburg) 2009;12:115–128. doi: 10.4314/ajpsy.v12i2.43729. [DOI] [PubMed] [Google Scholar]
- Pascual-Sedano B, Iranzo A, Marti-Fabregas J, Domingo P, Escartin A, Fuster M, Barrio JL, Sambeat MA. Prospective study of new-onset seizures in patients with human immunodeficiency virus infection: etiologic and clinical aspects. Arch Neurol. 1999;56:609–612. doi: 10.1001/archneur.56.5.609. [DOI] [PubMed] [Google Scholar]
- Pumpradit W, Ananworanich J, Lolak S, Shikuma C, Paul R, Siangphoe U, Chaoniti N, Kaew-On P, Paris R, Ruxrungtham K, Valcour V. Neurocognitive impairment and psychiatric comorbidity in well-controlled human immunodeficiency virus-infected Thais from the 2NN Cohort Study. J Neurovirol. 2010;16:76–82. doi: 10.3109/13550280903493914. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and iHANDdence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Elliott KJ, Simpson DM. HIV-related neurocognitive impairment in the HAART era. Curr HIV/AIDS Rep. 2009;6:146–152. doi: 10.1007/s11904-009-0020-1. [DOI] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]