Abstract
Our laboratory is investigating the effects of protein–energy malnutrition (PEM) on cognitive outcome following global ischemia. Here, we investigated whether PEM independently impairs working memory in the T-maze and if the associated food reward reverses PEM. Gerbils were fed 12.5% (control diet) or 2% protein. A loss of body weight (20.1%) in the 2% protein group and decreased food intake and serum albumin concentration compared to controls (17.5% and 18.2%, respectively) indicated that PEM was achieved. Based on T-maze criterion frequently used in ischemia studies, no difference was observed in the mean (±SEM) number of trials required (control 5.2 ± 0.7; PEM 4.9 ± 0.4; p = 0.758) or the number of animals reaching criterion (control 10/12; PEM 12/12; p = 0.140). Using more stringent criterion, PEM animals required fewer trials (control 7.3 ± 0.7; PEM 5.4 ± 0.4; p = 0.035), and more reached criterion (control 8/12; PEM 12/12; p = 0.028). PEM may increase motivation to obtain a food reward.
Keywords: Protein–energy malnutrition, gerbil, T-maze, reward, nutrition, global ischemia
Introduction
The elderly population is at highest risk for stroke, and their prognosis may be worsened by the presence of a nutritional deficiency. Approximately 16% of elderly stroke patients have protein–energy malnutrition (PEM) upon admission to hospital, and this condition can worsen during hospital stay (Axelson et al. 1988; Davalos et al. 1996; Davis et al. 2004). Using a model of global ischemia in the gerbil, in which the CA1 region of the hippocampus is particularly susceptible to damage (Corbett and Nurse 1998), our laboratory has demonstrated that functional outcome as measured in the open field is impaired by PEM (Bobyn et al. 2005). The T-maze is another behavioural test that reliably detects impairments in working memory post-ischemia but requires a nutritive reward (as reviewed in Corbett and Nurse 1998). Before utilizing this test for studies of global ischemia, we wanted to first establish if PEM affects performance of the adult gerbil in the T-maze prior to induction of ischemia.
Prenatal and early postnatal malnutrition has well-established detrimental effects on the developing hippocampus with effects in adult life that are not reversed when malnutrition is corrected later. This is best described in one particular series of studies. Although the dietary paradigm has been described as protein deficiency (Tonkiss and Galler 1990; Tonkiss et al. 1990; Blatt et al. 1994; Diaz-Cintra et al. 1994; Almeida et al. 1996; Bronzino et al. 1997; Cintra et al. 1997a,b; Fukuda et al. 2002; Hernandes and Almeida 2003; Lister et al. 2005), the level of casein fed in these studies (6%) often results in a voluntary reduction in food intake (Kanarek et al. 1986; Zeman et al. 1986; Langley and Jackson 1994). Therefore, the deficits described more likely represent the effects of a mixed protein–energy deficiency. Unfortunately, food intake was not reported, and biochemical indices such as serum albumin and liver lipid were not measured to clarify nutritional status. Regardless, these studies reported structural and biochemical alterations in the CA1 and CA3 regions of the hippocampus of the adult rat as a result of prenatal protein or protein–energy deficiency (Blatt et al. 1994; Diaz-Cintra et al. 1994; Bronzino et al. 1997; Cintra et al. 1997a,b; Morgane et al. 2002; Lister et al. 2005). Perinatal malnutrition was also reported to impair hippocampal-dependent functions, including significant impairment in the water maze (Fukuda et al. 2002). Deficits in inhibitory avoidance associated with both pre- and postnatal PEM are also believed to result from alterations to the hippocampus (Almeida et al. 1996; Hernandes and Almeida 2003). Prenatal PEM causes behavioural inflexibility during tests that require the animal to change its response strategy (Tonkiss and Galler 1990). Possible explanations include dysfunction of the prefrontal cortex, to which the CA1 pyramidal cells project, or an imbalance in the ratio of interneurons to pyramidal cells, leading to reduced output from the hippocampus (Strupp and Levitsky 1995; Lister et al. 2005).
More relevant to our studies is the influence that changes in protein–energy status occurring during adulthood has on behaviour. A growing body of evidence has suggested that protein deprivation during adulthood can also cause important structural and biochemical alterations in the hippocampus, including a reduction in the number of pyramidal neurons, synapses and arborizations in the CA1 and CA3 regions (Andrade et al. 1996a,b). Decreased expression of neurotrophic factors as well as a reduction in the number of cholinergic and GABAergic neurons has also been reported in the hippocampus (Andrade and Paula-Barbosa 1996; Mesquita et al. 2002). The behavioural consequences have been less studied although in adult rats fed 8% casein diet for 8 months, performance in the open field and the water maze indicated significant impairments of hippocampal-dependent functions such as emotionality, habituation and spatial learning (Lukoyanov and Andrade 2000).
While these findings from a nutritional insult imposed during adulthood are exciting, there are some major flaws in these studies. The nutritional model used in this series of studies (8% casein) was intended to induce protein deficiency, yet weight gain was unaltered and no biochemical evidence of protein deficiency was provided. Although food intake was reported to be unchanged (Andrade and Paula-Barbosa 1996; Andrade et al. 1996a,b; Lukoyanov and Andrade 2000; Mesquita et al. 2002), it is difficult to interpret these data because the low protein diet was of entirely different composition than that of the control diet and thus likely of different energy and micronutrient density. Further, since diets were not matched for nutrients other than protein, the structural and behavioural deficits cannot be specifically attributed to protein status.
Despite the limitations, the studies cited do suggest that the model of adult gerbil PEM used in our laboratory might impair working memory measured in the T-maze. Our nutritional paradigm of PEM is characterized by decreased food intake, weight loss, decreased liver glutathione, increased liver lipid (Bobyn et al. 2005), and a decline in serum albumin (Harmon et al. 2006). The severity of protein–energy deficiency and age and species of rodent differ between our model and the previous studies reviewed, making it difficult to extrapolate previous results to our model. This is further hindered by the experimental flaws discussed. Therefore, our first objective was to investigate whether working memory in the T-maze is impaired by a model of PEM induced by feeding a diet containing 2% protein to the adult gerbil. Since the T-maze requires a nutritive reward, a second objective was to examine whether the use of sunflower seeds would interfere with the induction of PEM.
Materials and methods
Animals and diets
Twenty-four male Mongolian gerbils (Charles River Canada, Saint-Constant, QC), 11–12 weeks of age, were acclimatized for 5 days during which time they were fed standard laboratory rodent chow. Animals (60.8–72.2 g) were then randomised to receive either control diet (CON group, 12.5% protein, n = 12), or protein deficient diet (PEM group, 2% protein, n = 12) for 28 days prior to behavioural testing. Diets were obtained from Dyets, Inc. (Bethlehem, PA) and were based on the AIN-93M diet (Reeves et al. 1993) with t-butylhydroquinone omitted as previously described (Bobyn et al. 2005). Gerbils fed the 2% protein diet voluntarily reduce food intake, resulting in a mixed PEM (Bobyn et al. 2005). Gerbils remained on diet throughout the behavioural testing period, resulting in a total of 57 days spent on diets. Gerbils were caged in groups of four at 22°C with a 12-h light/dark cycle, and allowed free access to food and water. Body weight was recorded weekly and food intake per cage daily. All animal procedures were in compliance with the guidelines of the Canadian Council on Animal Care.
Behavioural testing
Gerbils underwent behavioural testing in the T-maze in order to assess working memory as previously described (Farrell et al. 2001). The structure measured 47 cm (stem) × 30 cm (arms) × 10 cm (width). Environmental cues (e.g. experimenter, shelving, lighting) were kept constant during testing. Since previous pilot study data suggested that sucrose tablets would be an acceptable reward for use in the T-maze, habituation was begun using this reward at 28 days. When it was quickly discovered that this reward was not sufficiently motivating, gerbils were placed back in their home cages for an additional 14 days during which time there was no behavioural testing. Gerbils continued on the same diets.
On day 42, testing resumed in the T-maze with a reward of sunflower seed portions, which contained on average 0.01 g protein/seed and 0.33 kcal/seed. Habituation was carried out for 5 days, during which the animal was placed in the maze and allowed to explore for three 5-min sessions per day. During this period, sunflower seed portions were initially distributed throughout the maze, and then gradually localized to the ends of the arms by day 5. After day 5, gerbils underwent 10 pairs of win-shift trials/day for 10 days, during which they received sunflower seed portions as a reward. In the first trial, the animal was allowed access to one arm of the maze where a reward was received (win trial). In the second trial, both arms of the maze were accessible, but a reward was only received if the animal entered the opposite arm (shift trial). The maze was cleaned with 70% ethanol after each trial. The criterion for learning the strategy was set at an average of 9/10 trials correct over three consecutive days to mimic criteria commonly used for global ischemia studies (Farrell et al. 2001). Results based on a more stringent criterion of a minimum of 9/10 trials correct on each of three consecutive days were also analysed.
Serum albumin analysis
At the end of behavioural testing, animals were humanely killed by an overdose of isoflurane, and blood was collected for serum albumin analysis by the bromcresol green method (Doumas et al. 1971). One control animal was omitted from the serum albumin analysis due to small serum sample size.
Statistics
Statistical analysis was carried out using SPSS v13.0 for Windows. Body weight, food intake, serum albumin and the number of trials to criterion in the T-maze were analysed by unpaired t-test. The number of animals reaching criterion in the T-maze was analysed by χ 2 analysis. Differences were considered significant at p < 0.05.
Results
Indices of PEM
Mean (±SEM) initial body weight was not significantly different between the two experimental groups, being 64.9 ± 1.1 g in the control group and 65.2 ± 0.7 g in the PEM group (p = 0.813). The pattern of weight change is shown in Figure 1. There was a significant difference between groups in body weight change over 57 days (p < 0.001). Control animals increased body weight by 12.2%, while PEM animals lost 20.1% of their initial body weight by day 57. The two groups differed in body weight by day 7.
Figure 1.
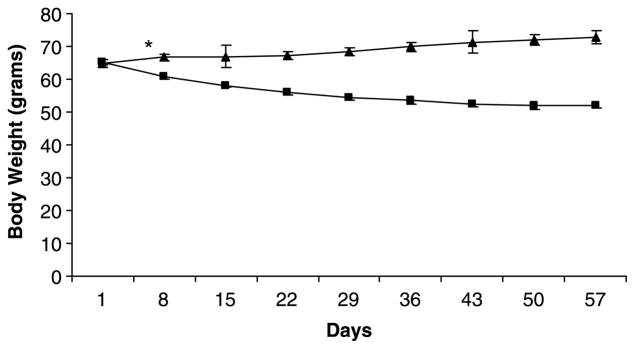
Pattern of mean (±SEM) body weight of control animals (triangles) and PEM animals (squares) over 57 days, n = 12. *Indicates the point at which a significant difference in body weight was first detected between control and PEM animals, and this was sustained throughout the feeding period.
Total food intake was decreased significantly in the PEM group to 17.5% less than that of the control group (p = 0.025; Table I). Mean (±SEM) serum albumin concentration of the PEM group declined significantly to 18.2% lower than that of the control group (p < 0.001; Table I).
Table I.
Other indices of PEM.
| Total food intake/57 days (g)* | Serum albumin concentration (g/l)† | |
|---|---|---|
| Control | 837.3 ± 13.4 | 46.7 ± 0.1 |
| PEM | 690.4 ± 39.8‡ | 38.2 ± 0.1¶ |
Values expressed as mean ± SEM;
Food intake is based on cage data [n = 3 (4 animals/cage)];
n = 11 (Control) and n = 12 (PEM);
p = 0.025 as compared to controls, based on unpaired t-test;
p < 0.001 as compared to controls, based on unpaired t-test.
Behavioural testing
There was no significant difference between diet groups on performance in the T-maze when criterion was set at an average of 9/10 correct trials over three consecutive days (Table II; p = 0.758). There was also no significant effect of diet on the number of animals reaching this criterion by 10 days—[χ 2(n = 24, df = 1) = 2.182, p = 0.140]. However, when criterion was raised to a minimum of 9/10 correct trials on each of three consecutive days, the PEM group required significantly fewer trials to reach criterion (p = 0.035), and more PEM animals reached criterion as compared to controls [χ 2(n = 24, df = l) = 4.800, p = 0.028].
Table II.
Acquisition of the T-maze win-shift trials.
| Criterion = average 9/10 correct
|
Criterion = minimum 9/10 correct
|
|||
|---|---|---|---|---|
| # Trials to criterion | # Animals to criterion | # Trials to criterion | # Animals to criterion | |
| Control | 5.2 ± 0.7 | 10/12 | 7.3 ± 0.7 | 8/12 |
| PEM | 4.9 ± 0.4 | 12/12 | 5.4 ± 0.4* | 12/12† |
Trials to criterion expressed as mean ± SEM; n = 12;
p = 0.035 as compared to controls based on unpaired t-test;
Significantly different than controls based on χ 2 analysis [χ 2(n = 24, df = 1) = 4.800, p = 0.028].
Discussion
The first issue addressed in this study was whether a sunflower seed reward used for the T-maze would elevate intake of protein and energy sufficiently to interfere with the induction of PEM. Sucrose tablets were initially tested as a reward, but, unlike sunflower seeds, they did not provide sufficient motivation for the gerbils to explore the T-maze. Based on nutrient analysis of the sunflower seeds and the highest reward consumption, it is estimated that the maximum extra protein consumed was approximately 0.03 g/day. Therefore, animals in the protein–energy malnourished group ingested a maximum of 0.10 g of protein/day, which is below the estimated requirement for maintenance of 0.20 g protein/day. This estimate was extrapolated from rat data (National Research Council 1995), since protein requirements for maintenance in the gerbil are not known.
In agreement with these calculations, the results demonstrate that the food reward associated with the T-maze did not reverse PEM achieved by feeding a 2% protein diet for 6 weeks. Body weight and food intake remained significantly decreased in the PEM animals throughout the experimental period to an extent consistent with results from a previous 4-week study in our laboratory in which no food reward was present (Bobyn et al. 2005). Serum albumin concentration was also significantly lower in the PEM animals at the end of the testing period, confirming that PEM was induced and was not interfered with by the sunflower seed reward. The decrease in serum albumin concentration of 18% is consistent with our previous data obtained in the absence of a food reward (Harmon et al. 2006).
Using criterion typically employed in studies of global ischemia, our results suggest that working memory tested in the T-maze was not impaired by 6 weeks of PEM in the gerbil. Although findings from a study by Tonkiss and Galler (1990) cannot be directly extrapolated to our own due to the difference in age at which deficiency was imposed, their results do support our findings. They investigated the effect of prenatal PEM on working memory in adulthood and reported no effect on performance on win-shift trials in the T-maze. Our results are in contrast to those of some other studies in which protein deficiency or PEM were found to have a negative effect on hippocampal function measured by the elevated T-maze and the water maze. This is not surprising given that the studies differed from ours in basal diet composition, age of rodent, and length of feeding period (Tonkiss and Galler 1990; Tonkiss et al. 1990; Strupp and Levitsky 1995; Almeida et al. 1996; Lukoyanov and Andrade 2000; Fukuda et al. 2002; Hernandes and Almeida 2003). This would have resulted in varying degrees of severity of PEM, which was often not characterized by measuring indices of protein–energy status.
When criterion in the T-maze was set more stringently, protein–energy malnourished animals required significantly fewer trials to reach criterion, and more of these animals reached criterion. We hypothesize that these results reflect increased motivation to obtain the food reward. This appears to be a novel finding as we are not aware of a previous report of PEM exerting such an effect in animals unless they were food-deprived to motivate them to perform in the T-maze. We avoided this food deprivation by using a preferred food for the gerbil as reward. Increased motivation to obtain a food reward in an operant task requiring pressing of a lever was reported in adult rats that had been protein–energy deprived during prenatal life (Tonkiss and Galler 1990). However, a major difference in study design was that the animals were food-deprived and thus presumably hungry when they performed the task. In a second study by Tonkiss et al. (1990) using a food rewarded operant task, prenatally malnourished rats also performed better when a non-nutritive reward (saccharin solution) was used, but not to the same extent as when the nutritive reward was offered.
It is possible that the increased motivation detected with the most sensitive criterion would cloud interpretation of future studies assessing how PEM influences global ischemia-induced changes in T-maze performance. However, criterion adopted for evaluating performance in the T-maze for studies of global ischemia are typically less stringent (Colbourne and Corbett 1995; Babcock and Graham-Goodwin 1996; Farrell et al. 2001). In rat models of global ischemia, this potential confounder can also be avoided by using behavioural tests such as the water maze that are sensitive to ischemic injury but do not require the use of a food reward. Unfortunately, the water maze is not ethologically well-suited for the gerbil because of the stress generated when a desert rodent is required to swim to solve a task (Corbett and Nurse 1998).
The results of this study should be of interest to others studying the effects of nutritional status on stroke outcome using rodent models of global ischemia. Use of the gerbil model of global ischemia is likely to decline in future given two recent reports of changes in cerebral vasculature, accompanied by loss of morphological and behavioural consistency, in a high proportion of gerbils from North American suppliers (Laidley et al. 2005; Seal et al. 2006). As investigators turn to other models of global ischemia such as two- and four-vessel occlusion in the rat, we predict that the effect of PEM on cognitive outcome will be similar to what we have previously reported in the gerbil (Bobyn et al. 2005), but this is yet to be studied. Similarly, any confounding influences of nutritional intervention on the behavioural tests used to assess functional outcome in these models of stroke would need to be assessed.
Acknowledgments
This work was supported by The Canadian Institutes of Health Research/Regional Partnership Program and the Heart and Stroke Foundation of Saskatchewan. The authors wish to thank C. Farrar for her technical assistance.
References
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behaviour of female rats in the elevated plus-maze test. Physiol Behav. 1996;60:675–680. doi: 10.1016/s0031-9384(96)80047-3. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Paula-Barbosa MM. Protein malnutrition alters cholinergic and GABAergic systems of the hippocampal formation of the adult rat: An immunocytochemical study. Neurosci Lett. 1996;211:211–215. doi: 10.1016/0304-3940(96)12734-8. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Castanheira-Vale AJ, Madeira MD. Time scale and extent of neuronal and synaptic loss in the hippocampal formation of malnourished adultrats. Brain Res. 1996a;718:1–12. doi: 10.1016/0006-8993(95)01544-2. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Castanheira-Vale AJ, Paz-Dias PG, Madeira MD, Paula-Barbosa MM. The dendritic trees of neurons from the hippocampal formation of protein-deprived adult rats. A quantitative Golgi study. Exp Brain Res. 1996b;109:419–433. doi: 10.1007/BF00229626. [DOI] [PubMed] [Google Scholar]
- Axelson K, Asplun K, Norberg A, Alafuzoff I. Nutritional status in patients with acute stroke. Acta Med Scand. 1988;224:217–224. doi: 10.1111/j.0954-6820.1988.tb19364.x. [DOI] [PubMed] [Google Scholar]
- Babcock AM, Graham-Goodwin H. Importance of pre-operative training and maze difficulty in task performance following hippocampal damage in the gerbil. Brain Res Bull. 1996;42:415–419. doi: 10.1016/s0361-9230(96)00330-9. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Chen JC, Rosene DL, Volicer L, Galler JR. Prenatal protein malnutrition effects on the serotonergic system in the hippocampal formation: an immunocytochemical, ligand binding, and neurochemical study. Brain Res Bull. 1994;34:507–518. doi: 10.1016/0361-9230(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Bobyn PJ, Corbett D, Saucier DM, Noyan-Ashraf MH, Juurlink BHJ, Paterson PG. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp Neurol. 2005;196:308–315. doi: 10.1016/j.expneurol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Austin-LaFrance RJ, Mokler D, Morgane PJ. Effects of prenatal protein malnutrition on hippocampal long-term potentiation in freely moving rats. Exp Neurol. 1997;148:317–323. doi: 10.1006/exnr.1997.6653. [DOI] [PubMed] [Google Scholar]
- Cintra L, Aguilar A, Granados L, Galvan A, Kemper T, DeBassio W, Galler J, Morgane P, Duran P, Diaz-Cintra S. Effects of prenatal protein malnutrition on hippocampal CA1 pyramidal cells in rats of four age groups. Hippocampus. 1997a;7:192–203. doi: 10.1002/(SICI)1098-1063(1997)7:2<192::AID-HIPO6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cintra L, Granados L, Aquilar A, Kemper T, DeBassio W, Galler J, Duran P, Morgane P, Diaz-Cintra S. Effects of prenatal protein malnutrition on mossy fibres of the hippocampal formation in rats of four age groups. Hippocampus. 1997b;7:184–191. doi: 10.1002/(SICI)1098-1063(1997)7:2<184::AID-HIPO5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: A six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Dávalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, Suńer R, Genís D. Effect of malnutrition after acute stroke on clinical outcome. Stroke. 1996;27:1028–1032. doi: 10.1161/01.str.27.6.1028. [DOI] [PubMed] [Google Scholar]
- Davis JP, Wong AA, Schluter PJ, Henderson RD, O’Sullivan JD, Read SJ. Impact of premorbid undernutrition on outcome in stroke patients. Stroke. 2004;35:1930–1934. doi: 10.1161/01.STR.0000135227.10451.c9. [DOI] [PubMed] [Google Scholar]
- Diaz-Cintra S, Garcia-Ruiz M, Corkidi G, Cintra L. Effects of prenatal malnutrition and postnatal nutritional rehabilitation on CA3 hippocampal pyramidal cells in rats of four age groups. Brain Res. 1994;662:117–126. doi: 10.1016/0006-8993(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Farrell R, Evans S, Corbett D. Environmental enrichment enhances recovery of function but exacerbates ischemic cell death. Neuroscience. 2001;107:585–592. doi: 10.1016/s0306-4522(01)00386-4. [DOI] [PubMed] [Google Scholar]
- Fukuda MTH, Francolin-Silva AL, Almeida SS. Early postnatal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav Brain Res. 2002;133:271–277. doi: 10.1016/s0166-4328(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Harmon M, Noyan-Ashraf M, Paterson P. The effect of protein–energy malnutrition on inflammation and reactive gliosis following global ischemia in the gerbil. Appl Physiol Nutr Metab (abstract) 2006;31:344. [Google Scholar]
- Hernandes AS, Almeida SS. Postnatal protein malnutrition affects inhibitory avoidance and risk assessment behaviours in two models of anxiety in rats. Nutr Neurosci. 2003;6:213–219. doi: 10.1080/1028415031000137527. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Schoenfeld PM, Morgane PJ. Maternal malnutrition in the rat: effects on food intake and body weight. Physiol Behav. 1986;38:509–515. doi: 10.1016/0031-9384(86)90418-x. [DOI] [PubMed] [Google Scholar]
- Laidley DT, Colbourne F, Corbett D. Increased behavioural and histological variability arising from changes in cerebro vascular anatomy of the Mongolian gerbil. Curr Neurovasc Res. 2005;2:401–407. doi: 10.2174/156720205774962719. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Lister JP, Blatt GJ, DeBassio WA, Kemper TL, Tonkiss J, Galler JR, Rosene DL. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 2005;15:393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Andrade JP. Behavioral effects of protein deprivation and rehabilitation in adult rats: Relevance to morphological alterations in the hippocampal formation. Behav Brain Res. 2000;112:85–97. doi: 10.1016/s0166-4328(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Mesquita RM, Pereira PA, Andrade JP. Low levels of brain-derived neurotrophic factor and tyrosine kinase receptor B are related to loss of dentate granule cells after prolonged low-protein feeding in the rat. Neurosci Lett. 2002;330:155–158. doi: 10.1016/s0304-3940(02)00743-7. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient requirements of laboratory animals. 4. Washington, DC: National Academy of Sciences; 1995. pp. 11–79. rev. [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Seal JB, Buchh BN, Marks JD. New variability in cerebrovascular anatomy determines severity of hippocampal injury following forebrain ischemia in the Mongolian gerbil. Brain Res. 2006;1073–1074:451–459. doi: 10.1016/j.brainres.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Strupp BJ, Levitsky DA. Enduring cognitive effects of early malnutrition: A theoretical reappraisal. J Nutr. 1995;125:2221S–2232S. doi: 10.1093/jn/125.suppl_8.2221S. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Galler JR. Prenatal protein malnutrition and working memory performance in adult rats. Behav Brain Res. 1990;40:95–107. doi: 10.1016/0166-4328(90)90002-v. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Shukitt-Hale B, Formica RN, Rocco FJ, Galler JR. Prenatal protein malnutrition alters response to reward in adult rats. Physiol Behav. 1990;48:675–680. doi: 10.1016/0031-9384(90)90210-u. [DOI] [PubMed] [Google Scholar]
- Zeman FJ, Hoogenboom ER, Chase-Deesing C, Kavlock RJ, Semple JL. Effects on the fetus of maternal nitrofen exposure in the protein-deprived rat. Toxicology. 1986;38:55–68. doi: 10.1016/0300-483x(86)90172-1. [DOI] [PubMed] [Google Scholar]


