Abstract
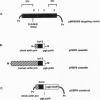
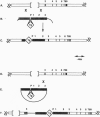
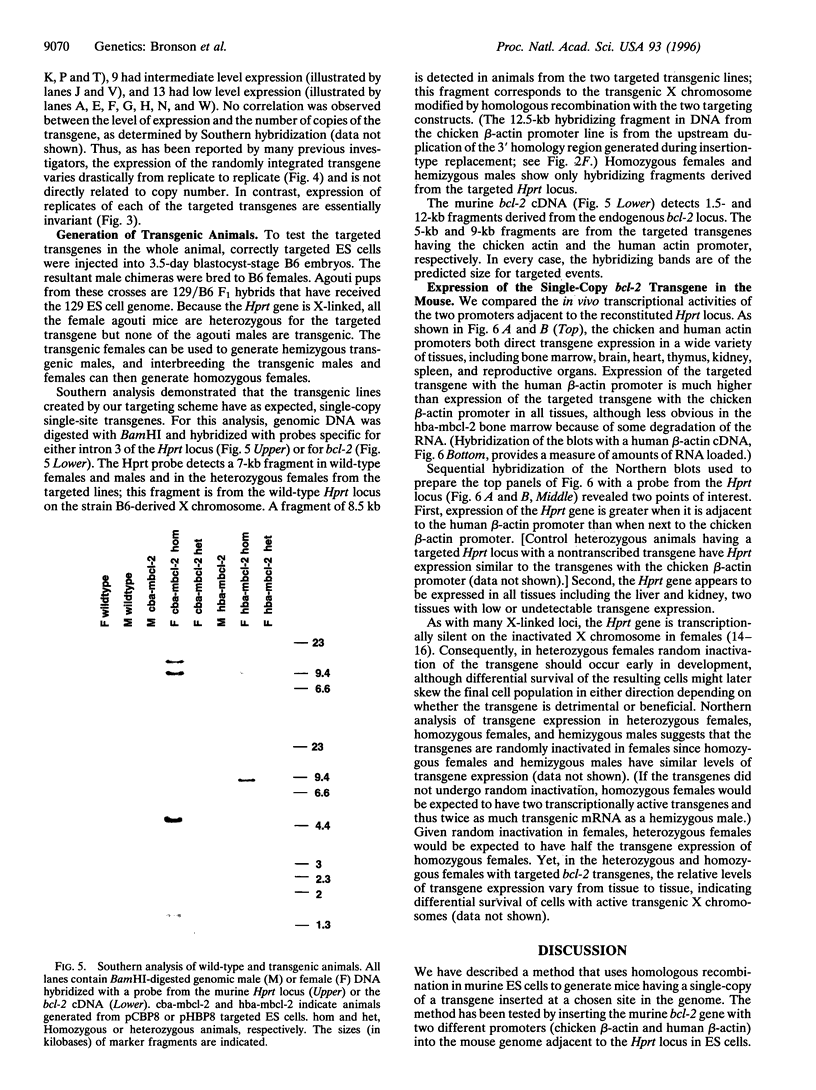
We describe a general way of introducing transgenes into the mouse germ line for comparing different sequences without the complications of variation in copy number and insertion site. The method uses homologous recombination in embryonic stem (ES) cells to generate mice having a single copy of a transgene integrated into a chosen location in the genome. To test the method, a single copy murine bcl-2 cDNA driven by either a chicken beta-actin promoter or a human beta-actin promoter has been inserted immediately 5' to the X-linked hypoxanthine phosphoribosyltransferase locus by a directly selectable homologous recombination event. The level of expression of the targeted bcl-2 transgene in ES cells is identical in independently isolated homologous recombinants having the same promoter yet varies between the different promoters. In contrast, the expression of bcl-2 transgenes having the same (chicken beta-actin) promoter varies drastically when they are independently integrated at random insertion sites. Both promoters direct broad expression of the single-copy transgene in mice derived from the respective targeted ES cells. In vitro and in vivo, the human beta-actin promoter consistently directed a higher level of transgene expression than the chicken beta-actin promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew G. R., Doetschman T., Lingrel J. B. Site-directed point mutations in embryonic stem cells: a gene-targeting tag-and-exchange strategy. Mol Cell Biol. 1993 Jul;13(7):4115–4124. doi: 10.1128/mcb.13.7.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Costantini F., Radice G., Lee J. L., Chada K. K., Perry W., Son H. J. Insertional mutations in transgenic mice. Prog Nucleic Acid Res Mol Biol. 1989;36:159–169. doi: 10.1016/s0079-6603(08)60169-5. [DOI] [PubMed] [Google Scholar]
- Crossley M., Orkin S. H. Regulation of the beta-globin locus. Curr Opin Genet Dev. 1993 Apr;3(2):232–237. doi: 10.1016/0959-437x(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Detloff P. J., Lewis J., John S. W., Shehee W. R., Langenbach R., Maeda N., Smithies O. Deletion and replacement of the mouse adult beta-globin genes by a "plug and socket" repeated targeting strategy. Mol Cell Biol. 1994 Oct;14(10):6936–6943. doi: 10.1128/mcb.14.10.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Dyer K. A., Goldman M. A. Mammalian X chromosome inactivation. Mol Genet Med. 1992;2:121–160. doi: 10.1016/b978-0-12-462002-5.50010-8. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Januzzi J. L., Azrolan N., O'Connell A., Aalto-Setälä K., Breslow J. L. Characterization of the mouse apolipoprotein Apoa-1/Apoc-3 gene locus: genomic, mRNA, and protein sequences with comparisons to other species. Genomics. 1992 Dec;14(4):1081–1088. doi: 10.1016/s0888-7543(05)80133-8. [DOI] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Meisler M. H. Insertional mutation of 'classical' and novel genes in transgenic mice. Trends Genet. 1992 Oct;8(10):341–344. doi: 10.1016/0168-9525(92)90278-c. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitschke W. W., Lin Z. Y., DePonti-Zilli L., Paterson B. M. The beta actin promoter. High levels of transcription depend upon a CCAAT binding factor. J Biol Chem. 1989 Jun 5;264(16):9539–9546. [PubMed] [Google Scholar]
- Reid L. H., Shesely E. G., Kim H. S., Smithies O. Cotransformation and gene targeting in mouse embryonic stem cells. Mol Cell Biol. 1991 May;11(5):2769–2777. doi: 10.1128/mcb.11.5.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Vijh K. M., Grossi C. E., Cooper M. D. Clonal origin of haematopoietic colonies in the postnatal mouse liver. Nature. 1986 Feb 6;319(6053):507–511. doi: 10.1038/319507a0. [DOI] [PubMed] [Google Scholar]
- Shaw-White J. R., Denko N., Albers L., Doetschman T. C., Stringer J. R. Expression of the lacZ gene targeted to the HPRT locus in embryonic stem cells and their derivatives. Transgenic Res. 1993 Jan;2(1):1–13. doi: 10.1007/BF01977675. [DOI] [PubMed] [Google Scholar]
- Stacey A., Schnieke A., McWhir J., Cooper J., Colman A., Melton D. W. Use of double-replacement gene targeting to replace the murine alpha-lactalbumin gene with its human counterpart in embryonic stem cells and mice. Mol Cell Biol. 1994 Feb;14(2):1009–1016. doi: 10.1128/mcb.14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Brown C. J., Carrel L., Hendrich B., Miller A. P. Epigenetic and chromosomal control of gene expression: molecular and genetic analysis of X chromosome inactivation. Cold Spring Harb Symp Quant Biol. 1993;58:315–322. doi: 10.1101/sqb.1993.058.01.037. [DOI] [PubMed] [Google Scholar]
- Wu H., Liu X., Jaenisch R. Double replacement: strategy for efficient introduction of subtle mutations into the murine Col1a-1 gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2819–2823. doi: 10.1073/pnas.91.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]