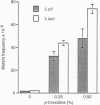
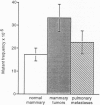
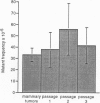
Abstract
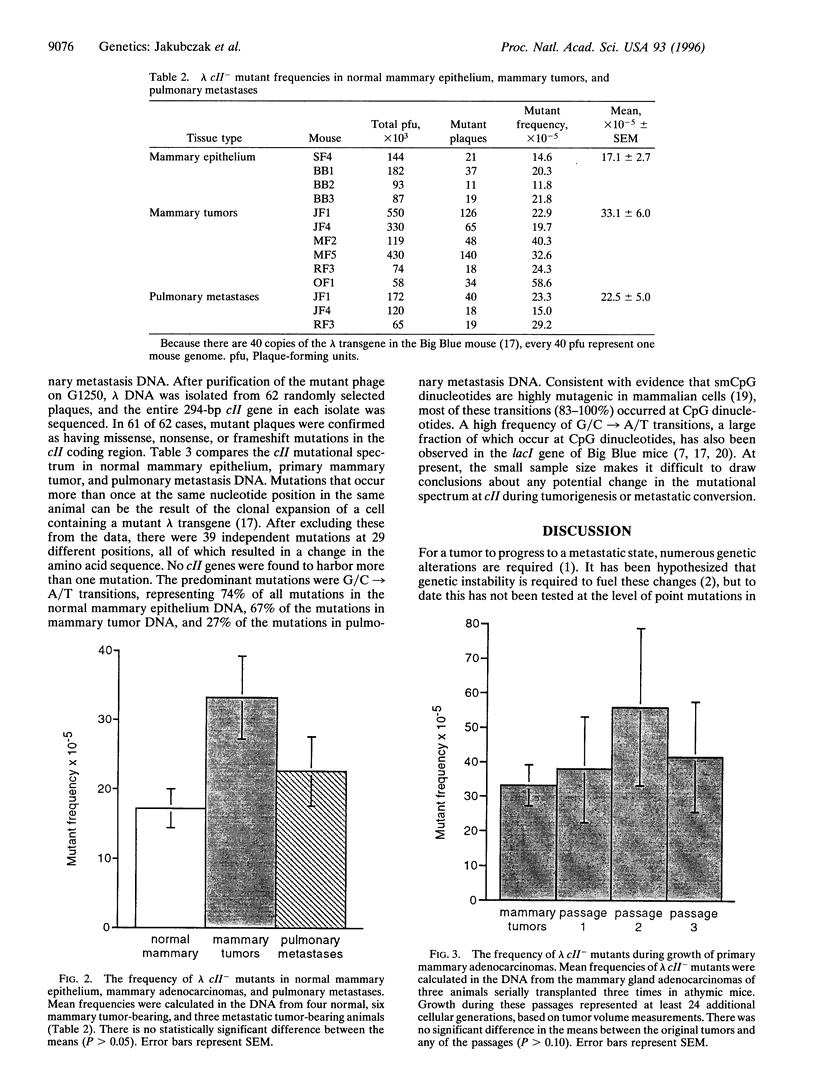
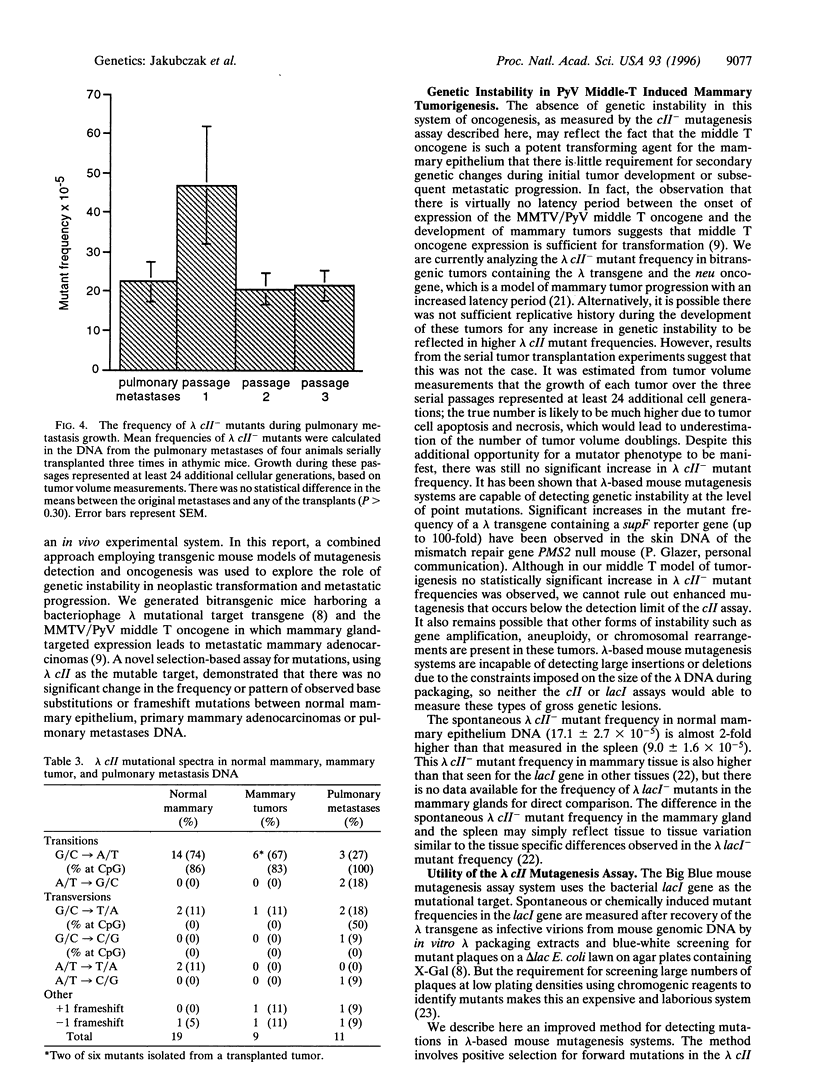
Genetic instability is thought to be responsible for the numerous genotypic changes that occur during neoplastic transformation and metastatic progression. To explore the role of genetic instability at the level of point mutations during mammary tumor development and malignant progression, we combined transgenic mouse models of mutagenesis detection and oncogenesis. Bitransgenic mice were generated that carried both a bacteriophage lambda transgene to assay mutagenesis and a polyomavirus middle T oncogene, mammary gland-targeted expression of which led to metastatic mammary adenocarcinomas. We developed a novel assay for the detection of mutations in the lambda transgene that selects for phage containing forward mutations only in the lambda cII gene, using an hfl- bacterial host. In addition to the relative ease of direct selection, the sensitivity of this assay for both spontaneous and chemically induced mutations was comparable to the widely used mutational target gene, lambda lacI, making the cII assay an attractive alternative for mutant phage recovery for any lambda-based mouse mutagenesis assay system. The frequencies of lambda cII- mutants were not significantly different in normal mammary epithelium, primary mammary adenocarcinomas, and pulmonary metastases. The cII mutational spectra in these tissues consisted mostly of G/C-->A/T transitions, a large fraction of which occurred at CpG dinucleotides. These data suggest that, in this middle T oncogene model of mammary tumor progression, a significant increase in mutagenesis is not required for tumor development or for metastatic progression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. C., Loeb L. A. Genomic instability and tumor progression: mechanistic considerations. Adv Cancer Res. 1993;60:121–156. doi: 10.1016/s0065-230x(08)60824-6. [DOI] [PubMed] [Google Scholar]
- Digweed M. Human genetic instability syndromes: single gene defects with increased risk of cancer. Toxicol Lett. 1993 Apr;67(1-3):259–281. doi: 10.1016/0378-4274(93)90061-2. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Gossen J. A., de Leeuw W. J., Tan C. H., Zwarthoff E. C., Berends F., Lohman P. H., Knook D. L., Vijg J. Efficient rescue of integrated shuttle vectors from transgenic mice: a model for studying mutations in vivo. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7971–7975. doi: 10.1073/pnas.86.20.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. T., Cardiff R. D., Muller W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992 Mar;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. T., Webster M. A., Schaller M., Parsons T. J., Cardiff R. D., Muller W. J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. A cII-dependent promoter is located within the Q gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1985 May;82(10):3134–3138. doi: 10.1073/pnas.82.10.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Rideout W. M., 3rd, Shen J. C., Spruck C. H., Tsai Y. C. Methylation, mutation and cancer. Bioessays. 1992 Jan;14(1):33–36. doi: 10.1002/bies.950140107. [DOI] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Putman D. L., Sorge J. A., Short J. M. Analysis of spontaneous and induced mutations in transgenic mice using a lambda ZAP/lacI shuttle vector. Environ Mol Mutagen. 1991;18(4):316–321. doi: 10.1002/em.2850180421. [DOI] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Sorge J. A., Putman D. L., Short J. M. Spectra of spontaneous and mutagen-induced mutations in the lacI gene in transgenic mice. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Kretz P. L., Fieck A., Sorge J. A., Short J. M. The use of transgenic mice for short-term, in vivo mutagenicity testing. Genet Anal Tech Appl. 1990 Dec;7(8):212–218. doi: 10.1016/0735-0651(90)90003-x. [DOI] [PubMed] [Google Scholar]
- Lee A. T., DeSimone C., Cerami A., Bucala R. Comparative analysis of DNA mutations in lacI transgenic mice with age. FASEB J. 1994 May;8(8):545–550. doi: 10.1096/fasebj.8.8.8181674. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Loeb L. A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [PubMed] [Google Scholar]
- MacPhee D. G. Mismatch repair, somatic mutations, and the origins of cancer. Cancer Res. 1995 Dec 1;55(23):5489–5492. [PubMed] [Google Scholar]
- Mattila P., Korpela J., Tenkanen T., Pitkänen K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase--an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 1991 Sep 25;19(18):4967–4973. doi: 10.1093/nar/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsalis J. C., Monforte J. A., Winegar R. A. Transgenic animal models for detection of in vivo mutations. Annu Rev Pharmacol Toxicol. 1995;35:145–164. doi: 10.1146/annurev.pa.35.040195.001045. [DOI] [PubMed] [Google Scholar]
- Morrison V., Ashby J. A preliminary evaluation of the performance of the Muta Mouse (lacZ) and Big Blue (lacI) transgenic mouse mutation assays. Mutagenesis. 1994 Jul;9(4):367–375. doi: 10.1093/mutage/9.4.367. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Piegorsch W. W., Margolin B. H., Shelby M. D., Johnson A., French J. E., Tennant R. W., Tindall K. R. Study design and sample sizes for a lacI transgenic mouse mutation assay. Environ Mol Mutagen. 1995;25(3):231–245. doi: 10.1002/em.2850250310. [DOI] [PubMed] [Google Scholar]
- Provost G. S., Kretz P. L., Hamner R. T., Matthews C. D., Rogers B. J., Lundberg K. S., Dycaico M. J., Short J. M. Transgenic systems for in vivo mutation analysis. Mutat Res. 1993 Jul;288(1):133–149. doi: 10.1016/0027-5107(93)90215-2. [DOI] [PubMed] [Google Scholar]
- Shephard S. E., Lutz W. K., Schlatter C. The lacI transgenic mouse mutagenicity assay: quantitative evaluation in comparison to tests for carcinogenicity and cytogenetic damage in vivo. Mutat Res. 1994 Apr 15;306(2):119–128. doi: 10.1016/0027-5107(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Rao G. N., Russfield A., Seilkop S., Braun A. G. Chemical effects in transgenic mice bearing oncogenes expressed in mammary tissue. Carcinogenesis. 1993 Jan;14(1):29–35. doi: 10.1093/carcin/14.1.29. [DOI] [PubMed] [Google Scholar]