Abstract
Withaferin A (WA), a bioactive constituent of Ayurvedic medicine plant Withania somnifera, is a potent apoptosis inducer in cancer cells but the mechanism of cell death induction is not fully characterized. The present study was undertaken to determine the role of mitogen-activated protein kinases (MAPK), including c-jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 MAPK, and anti-apoptotic protein myeloid cell leukemia-1 (Mcl-1) in regulation of WA-induced apoptosis using human breast cancer cells. Exposure of MCF-7 (estrogen responsive) and SUM159 (triple negative) human breast cancer cells to WA resulted in increased phosphorylation of ERK, JNK, and p38 MAPK, but these effects were relatively more pronounced in the former cell line than in SUM159. Overexpression of manganese-superoxide dismutase conferred partial protection against WA-mediated hyperphosphorylation of ERK, but not JNK or p38 MAPK. Cell death resulting from WA treatment in MCF-7 cells was significantly augmented by pharmacological inhibition of ERK and p38 MAPK. Interestingly, the WA-induced apoptosis in MCF-7 cells was partially but significantly blocked in the presence of a JNK-specific inhibitor. Pharmacological inhibition of ERK or JNK had no effect on WA-induced apoptosis in SUM159 cells. The WA-treated cells exhibited induction of long and short forms of Mcl-1. RNA interference of Mcl-1 alone triggered apoptosis. Furthermore, the WA-induced cell death in MCF-7 cells was modestly but significantly augmented by knockdown of the Mcl-1 protein. These observations indicate that: MAPK have cell line-specific role in cell death by WA, and Mcl-1 induction confers modest protection against WA-induced apoptosis.
Keywords: Withaferin A, Breast Cancer, MAPK, Mcl-1, Apoptosis
INTRODUCTION
Constituents of Ayurvedic medicine, which has been practiced in India for thousands of years, are an attractive source of potential anti-cancer agents [1]. Withania somnifera (commonly known as Ashwagandha in Ayurvedic medicine) is one such plant with a variety of pharmacological effects, including cardioprotection from ischemia reperfusion injury, inhibition of 6-hydroxydopamine-induced Parkinsonism, and anticancer effects [2–5]. For instance, leaf extract of Withania somnifera caused growth retardation of human fibrosarcoma cells implanted in athymic mice [5]. Anticancer property of Withania somnifera is attributed to withanolides, including withaferin A (WA) [6] that was the first discovered member of the withanolide family [7]. At the cellular level, anti-cancer effects of WA have now been documented against different cancer types, including prostate [8,9], breast [10], blood [11], colon [12], head and neck [13], cervical [14], and melanoma [15] cells. In vivo activity of WA has also been reported. For example, WA was shown to cause destruction of Ehrlich ascites tumor cells in vivo by causing immune activation [16]. Very impressive results have been reported for WA in a chemically-induced rodent cancer model where oral administration of WA (20 mg/kg body weight) for 14 weeks resulted in complete protection of 7,12-dimethylbenz[a]anthracene-induced oral cancer in hamsters [17]. We have also shown previously that intraperitoneal administration of 4 mg WA/kg mouse significantly retards growth of MDA-MB-231 human breast cancer cells implanted in female athymic mice [10]. In another study, the WA treatment was shown to inhibit breast cancer invasion and metastasis at sub-cytotoxic doses [18].
Because of impressive in vivo anti-cancer activity [8–10,17,18] elucidation of the mechanism by which WA causes destruction of cancer cells has been the topic of intense research over the past several years. Mechanisms underlying anti-cancer effects of WA are not fully understood but known anti-cancer responses to WA treatment in cultured cancer cells include G2 phase and mitotic arrest [19], apoptotic cell death [9–11,13,15,20], and induction of autophagy [21]. While the in vivo significance of cell cycle arrest or autophagy is still unclear, the WA-mediated inhibition of cancer cell growth in vivo is associated with apoptosis induction [10]. Moreover, WA treatment has been shown to suppress multiple oncogenic signaling pathways in cultured cancer cells including, Akt [11], nuclear factor-κB [22], signal transducer and activator of transcription 3 [23], estrogen receptor-α [24], and vimentin [18,25]. Critical role for reactive oxygen species (ROS) in apoptosis induction by WA has also been proposed [15,26].
The present study was undertaken to determine the role of mitogen-activated protein kinases (MAPK) and myeloid cell leukemia-1 (Mcl-1) in apoptosis regulation by WA using human breast cancer cells as a model. Impetus for these studies stemmed from the following observations: (a) WA was shown to induce apoptosis by activating p38 MAPK in lymphoid and myeloid leukemia cells [20], but it was unclear if these observations were unique to the leukemic cells, and (b) WA treatment was shown to cause marked induction of anti-apoptotic protein Mcl-1 in MCF-7 cells [10], but functional significance of this observation in the context of apoptosis induction was not studied.
MATERIALS AND METHODS
Cells, Antibodies, and Reagents
MCF-7 cell line was purchased from American Type Culture Collection (Manassas, VA), whereas SUM159 cell line was obtained from Asterand (Detroit, MI). The cells were cultured as recommended by the supplier. Generation of MCF-7 cells stably transfected with empty pcDNA3.1 vector or the same vector encoding for manganese-superoxide dismutase (Mn-SOD), and their culture conditions have been described previously [27]. Cell culture reagents and Oligofectamine were from Life Technologies (Grand Island, NY). Anti-actin antibody, anti-α-tubulin antibody, and N-acetylcysteine (NAC) were from Sigma-Aldrich (St. Louis, MO). Antibodies against phospho-(Thr183/Tyr185)-JNK, total JNK, and cleaved poly-(ADP-ribose)-polymerase (PARP) were from Cell Signaling Technology (Danvers, MA). Antibodies against phospho-(Tyr204)-ERK, total ERK, phospho-(Tyr182)-p38 MAPK, total p38 MAPK, phospho-(Ser63/73)-c-jun, and Mcl-1, and small-interfering RNA (siRNA) targeted against Mcl-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Non-specific siRNA was obtained from Qiagen (Valencia, CA). Pharmacological inhibitors of MAPK, including SB202190 (p38 MAPK inhibitor), SP600125 (JNK inhibitor), and PD98059 (inhibitor of an upstream kinase in ERK signaling pathway) were purchased from EMD-Millipore (Billerica, MA). WA (purity ≥ 99%) was purchased from Enzo Life Sciences (Farmingdale, NY). WA was dissolved in dimethyl sulfoxide (DMSO), and diluted with complete media immediately before use. Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was from GeneTex (Irvine, CA).
Western Blotting
DMSO-treated control or WA-treated cells were lysed and total lysates were subjected to sodium dodecyl-sulfate polyacrylamide gel electrophoresis followed by western blotting as described previously [28]. The resolved proteins were visualized by enhanced Chemiluminescence technique. Change in protein level was determined by densitometric scanning of the band and normalized against a loading control.
Detection of Apoptosis
Apoptosis induction was assessed by quantitation of histone-associated DNA fragment release into the cytosol, which is a widely used technique for determination of apoptotic death in cells, using a kit from Roche Applied Science (Indianapolis, IN). This assay was performed according to the manufacturer’s instructions.
RNA Interference of Mcl-1
Cells were seeded in six-well plates and transfected at 50% confluency with a control (non-specific) siRNA or Mcl-1-targeted siRNA. Twenty-four hours after transfection, the cells were treated with DMSO (control) or specified concentration of WA for 24 h. The cells were then collected and processed for western blotting and DNA fragmentation assay.
RESULTS
WA Treatment Caused Activation of MAPK in Human Breast Cancer Cells
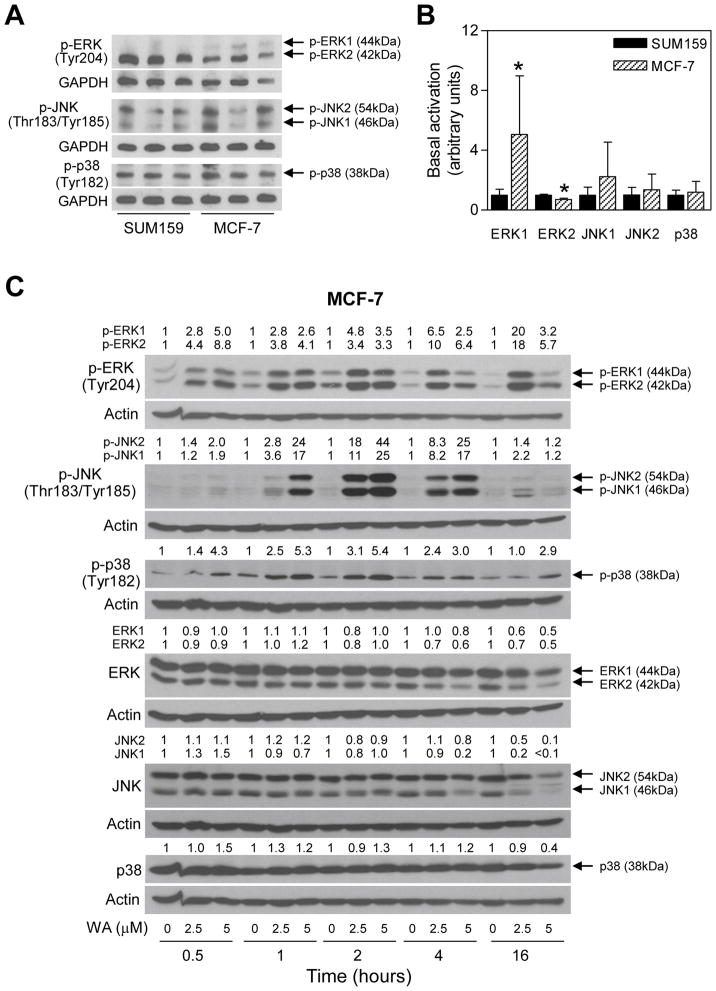
Initially, we compared basal activation of different MAPK in MCF-7 and SUM159 cells. As can be seen in Figure 1A and 1B, constitutive ERK1 activation was significantly higher in MCF-7 cells compared with SUM159, whereas basal ERK2 activation was lower in the MCF-7 cell line than in SUM159. The basal activation of JNK and p38 MAPK did not differ significantly between SUM159 and MCF-7 cells (Figure 1B). As shown in Figure 1C, exposure of MCF-7 cells to WA resulted in a rapid (as early as 30 min) and sustained activation of ERK (up to 16 h) as evidenced by an increase in the levels of Tyr204 phosphorylated protein. The WA-mediated increase in Thr183/Tyr185 phosphorylated JNK peaked between 2 and 4 h after treatment (Figure 1C). The WA-treated MCF-7 cells also exhibited dose-dependent and sustained activation of p38 MAPK with Tyr182 hyperphosphorylation persisting for the duration of the experiment (16 h) especially at the 5 μM concentration (Figure 1C). These results indicated that WA treatment resulted in hyperphosphorylation of ERK, JNK, and p38 MAPK in MCF-7 cells, which was not due to induction of these proteins except for slight but inconsistent elevation of the total p38 MAPK protein at some time points.
Figure 1.
WA treatment activates MAPK in MCF-7 human breast cancer cells. (A–B) Determination of basal activation of MAPK in MCF-7 and SUM159 cells. The cells (5×105 cells per dish) were plated in triplicate in 6-cm dish. After overnight incubation, the cells were collected and subjected to western blotting for phosphorylated forms of ERK, JNK, and p38 MAPK. Quantitation of basal level of phosphorylated MAPK in MCF-7 and SUM159 from two-independent experiments is shown in the bar graph. Results shown are mean S.D. (n = 6). Statistical significance (*, P<0.05) of difference in expression level between MCF-7 and SUM159 cells was analyzed by unpaired Student’s t-test. (C) Effect of WA treatment on activation of MAPK in MCF-7 cells. Lysates from MCF-7 cells treated with the indicated concentrations of WA for specified time periods were subjected to western blotting for phosphorylated and total forms of ERK, JNK, and p38. The number on top of the band represents fold change in level compared to corresponding DMSO-treated control.
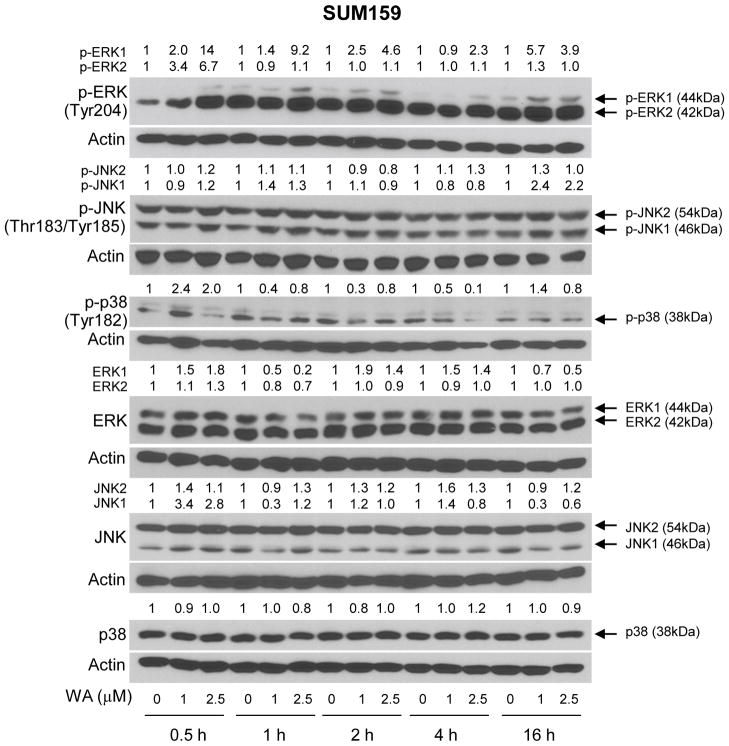
Next, we raised the question of whether activation of MAPK after WA treatment was unique to the MCF-7 cell line or influenced by the hormone receptor status. We addressed this question using a triple negative human breast cancer cell line (SUM159). Generally, the MCF-7 cell line was relatively more sensitive to WA-mediated hyperphosphorylation of MAPK (Figure 1C) compared with SUM159 cells (Figure 2). Cell line-specific differences between MCF-7 and SUM159 were also discernible with respect to kinetics of the MAPK activation. For example, in contrast to the MCF-7 cells (Figure 1C), hyperphosphorylation of only ERK1 persisted for the duration of the experiment (16 h) in SUM159 cells (Figure 2). Likewise, a clear-cut increase in phosphorylation of only JNK1 was observed in SUM159 cells only after 16 h treatment with WA (Figure 2). The WA-mediated hyperphosphorylation of p38 MAPK was transient in SUM159 cells and peaked between 30 min and 1 h after treatment (Figure 2). Interestingly, WA treatment caused a decrease in the levels of Tyr182 phosphorylated p38 MAPK at 1–4 h time points, which was not due to downregulation of the total protein (Figure 2).
Figure 2.
WA treatment activates MAPK in SUM159 cells. Effect of WA treatment on activation of MAPK in SUM159 cells. Lysates from SUM159 cells treated with the indicated concentrations of WA for specified time periods were subjected to western blotting for phosphorylated and total forms of ERK, JNK, and p38 MAPK. The number on top of the band represents fold change in level compared to corresponding DMSO-treated control.
The Role of ROS in MAPK Activation by WA
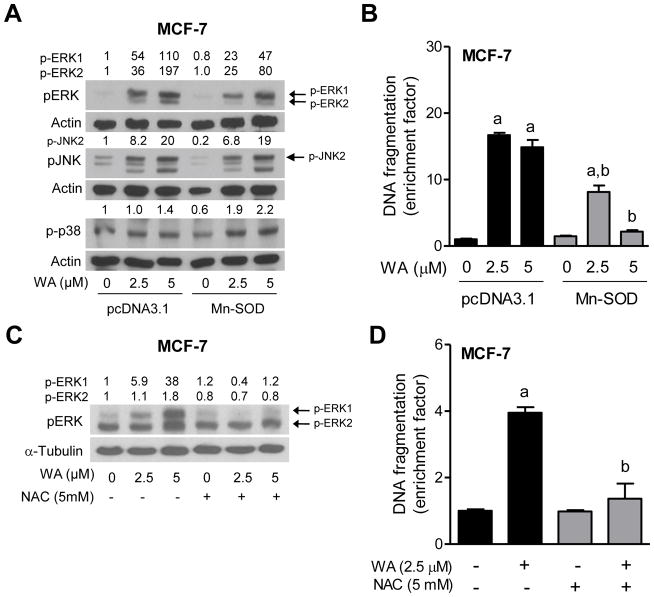
We have shown previously that the WA-induced apoptosis in human breast cancer cells, including the MCF-7 cell line is intimately linked to ROS production [26]. Moreover ROS-dependence of the WA-induced apoptosis is not unique to the breast cancer cells [15]. We therefore raised the question of whether activation of MAPK in our model was linked to ROS production. We addressed this question by determining the effect of Mn-SOD overexpression on WA-mediated activation of MAPK using MCF-7 cells. The WA-mediated activation of ERK, but not JNK or p38 MAPK was partially suppressed by overexpression of Mn-SOD (Figure 3A). In addition, overexpression of Mn-SOD conferred partial but significant protection against WA-induced apoptosis at least in the MCF-7 cell line (Figure 3B). The protection by Mn-SOD overexpression on ERK activation was consistent at the 2.5 μM WA in different experiments, but not at the higher dose. Because Mn-SOD overexpression, which will suppress superoxide anion, showed partial protective effect on ERK activation by WA treatment, we determined the effect of NAC on WA-mediated activation of ERK and apoptosis. As can be seen in Figure 3C and 3D, NAC treatment reversed the effect of WA on ERK activation as well as apoptosis. These results suggested that only ERK activation by WA treatment was sensitive to ROS production at least in the MCF-7 cell line.
Figure 3.
ROS are involved in apoptosis by WA in MCF-7 cells. (A) Effect of overexpression of Mn-SOD on WA-mediated activation of MAPK. The MCF-7 cells stably transfected with pcDNA3.1 empty vector or pcDNA3.1 vector encoding for Mn-SOD were treated with the desired concentrations of WA for 2 hours. The total lysates from these cells were subjected to western blotting for phosphorylated MAPK. Quantitation relative to DMSO-treated empty vector transfected cells is shown. (B) Detection of apoptosis (histone-associated DNA fragment release into the cytosol) in MCF-7 cells stably transfected with pcDNA3.1 empty vector or pcDNA3.1 vector encoding for Mn-SOD. The cells were treated with the indicated doses of WA for 24 h and then processed for apoptosis assay. Statistical significance (P<0.05) was analyzed one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with each control. bSignificantly different (P<0.05) between groups with the same dose. The results shown are mean ± S.D (n= 3). (C) Effect of NAC on WA-mediated activation of ERK. MCF-7 cells (5×105 cells per dish) were plated in 6-cm dish and allowed to attach. After overnight incubation, the cells were pretreated with 5 mM NAC for 2 h and then treated with indicated doses of WA in the absence or presence of NAC for 2 h. After treatment, the cells were collected and processed for immunoblotting. (D) Effect of NAC on WA-mediated apoptosis induction in MCF-7 cells. The cells were pretreated with 5 mM NAC for 2 h and then treated with 2.5 μM WA in the absence or presence of NAC for 24 h. Statistical significance (P<0.05) was analyzed by one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with each control. bSignificantly different (P<0.05) between groups with the same dose. The results shown are mean ± S.D. (n= 2–3).
The Role of MAPK Activation in Apoptosis Induction by WA
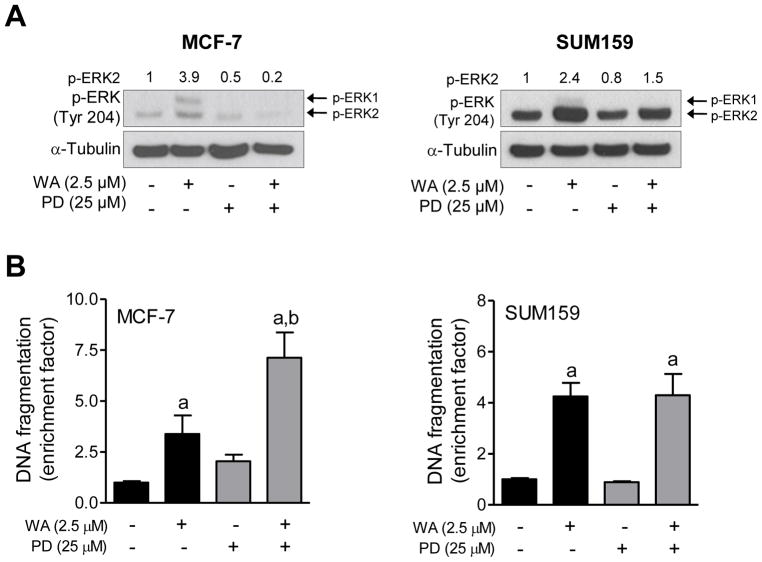
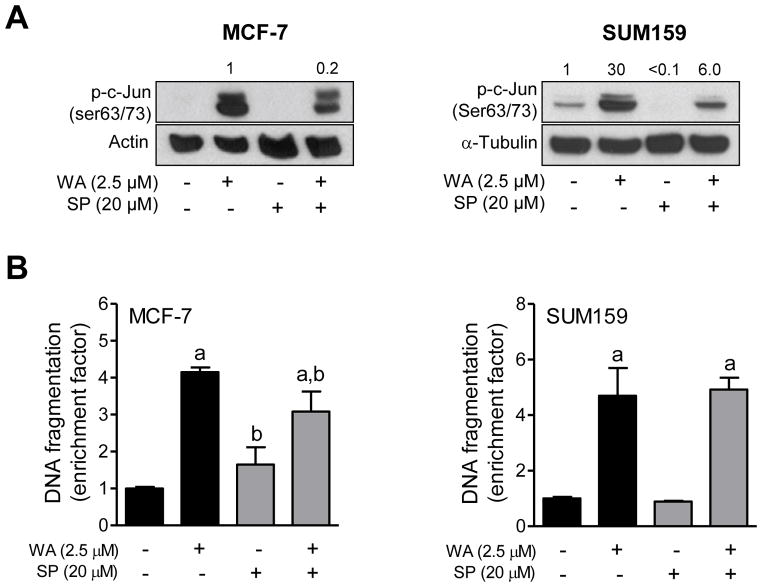
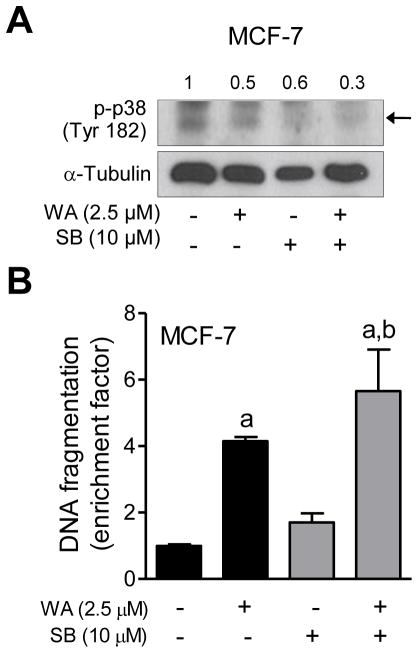
Next, we designed experiments using specific inhibitors of different MAPK to determine the functional significance of their activation in the context of WA-induced apoptosis. As shown in Figure 4A, a 1 h pretreatment with PD98059 resulted in suppression of WA-mediated hyperphosphorylation of ERK in both MCF-7 and SUM159 cells. Inhibition of ERK activation significantly augmented WA-induced apoptosis in MCF-7, but not in SUM159 cells as evidenced by analysis of histone-associated DNA fragment release into the cytosol (Figure 4B). Lack of any effect on apoptosis in SUM159 cells may be partly attributable to incomplete inhibition of the ERK. The WA-mediated activation of JNK was markedly suppressed in the presence of SP600125 (Figure 5A). Pharmacological inhibition of JNK conferred modest but significant protection against WA-induced apoptosis in MCF-7 cells (Figure 5B). Similar to PD98059 (Figure 4B), inhibition of JNK had no meaningful impact on WA-induced apoptosis in SUM159 cells (Figure 5B) possibly due to incomplete inhibition of the kinase. Because SUM159 cell line was not as sensitive as MCF-7 to WA-mediated activation of p38 MAPK, effect of its inhibition was only studied in the MCF-7 cell line. Because activation of p38 MAPK is transient and dose-dependent (e.g., activation of p38 MAPK observed only at 5 μM concentration at the 16 h time point in Figure 1) in MCF-7 cells, an increase in the levels of Tyr182 phosphorylated p38 MAPK was not evident in the western blotting experiment (Figure 6A). Nevertheless, western blotting revealed a reduction in the level phosphorylated p38 MAPK in the presence of WA and SB202190 (Figure 6A). Pharmacological inhibition of p38 MAPK resulted in a modest but significant increase in WA-induced apoptosis in the MCF-7 cell line (Figure 6B).
Figure 4.
The effect of pharmacological inhibition of ERK on WA-mediated apoptosis in human breast cancer cells. The MCF-7 and SUM159 human breast cancer cells were pretreated with 25 μM PD98059 for 1 h, and then exposed to 2.5 μM WA in the absence or presence of PD98059 for an additional 24 h. (A) Western blotting for phosphorylated ERK. Quantitation relative to DMSO-treated cells is shown. (B) Histone-associated DNA fragment release into the cytosol. Quantitation relative to DMSO-treated cells is shown. Combined results (n = 6) from two independent experiments are shown as mean ± S.D. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with respective control. bSignificantly different (P<0.05) between groups at the same dose.
Figure 5.
The effect of pharmacological inhibition of JNK on WA-mediated apoptosis in human breast cancer cells. The MCF-7 and SUM159 human breast cancer cells were pretreated with 20 μM SP600125 for 1 h, exposed to 2.5 μM WA in the absence or presence of SP600125 for 24 h, and then processed for western blot analysis or apoptosis detection. (A) Western blotting for phosphorylated c-jun. Quantitation relative to DMSO-treated cells is shown. (B) Histone-associated DNA fragment release into the cytosol. Quantitation relative to DMSO-treated cells is shown. Combined results (n = 6) from two independent experiments are shown as mean ± S.D. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with respective control. bSignificantly different (P<0.05) between groups at the same dose.
Figure 6.
The effect of pharmacological inhibition of p38 MAPK on WA-mediated apoptosis in MCF-7 cells. The MCF-7 cells were pretreated with 10 μM SB202190 for 1 h, exposed to 2.5 μM WA in the absence or presence of SB202190 for 24 h, and then processed for western blot analysis or apoptosis detection. (A) Western blotting for phosphorylated p38 MAPK and cleaved PARP. Quantitation for phosphorylated p38MAPK relative to DMSO-treated cells is shown. (B) Histone-associated DNA fragment release into the cytosol. Quantitation relative to DMSO-treated cells is shown. Combined results (n = 6) from two independent experiments are shown as mean ± S.D. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with respective control. bSignificantly different (P<0.05) between groups at the same dose.
The Role of Mcl-1 in WA-Induced Apoptosis
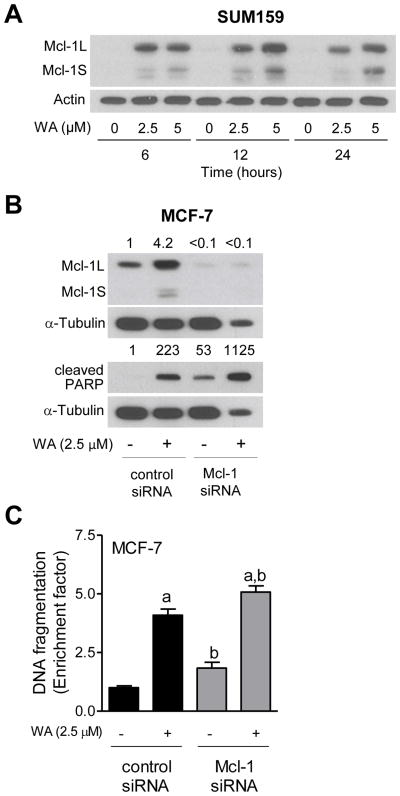
We have shown previously that WA treatment causes a marked increase in levels of long and short forms of Mcl-1, which is an anti-apoptotic protein [29], in MCF-7 cells [10]. However, the functional significance of Mcl-1 induction in the context of WA-mediated apoptosis was not clear. Moreover, it is still unclear if WA-mediated induction of Mcl-1 is unique to the MCF-7 cell line. As shown in Figure 7A, treatment of SUM159 cells with WA resulted in a dose- and time-dependent induction of both long and short forms of Mcl-1. Quantitation of treatment-related induction was not possible due to undetectable basal level of Mcl-1 in this experiment (Figure 7A). As shown in Figure 7B, the WA-mediated induction of Mcl-1 (both forms) was fully abolished by its RNA interference. Knockdown of Mcl-1 alone triggered apoptosis in MCF-7 cells (Figure 7C), which is consistent with its anti-apoptotic role [29]. Furthermore, the WA-induced apoptosis in MCF-7 cells was modestly but significantly augmented by knockdown of Mcl-1 protein as evidenced by analysis of PARP cleavage (Figure 7B) as well as histone-associated DNA fragment release into the cytosol (Figure 7C). Similar results were observed in the SUM159 cell line (results not shown).
Figure 7.
The effect of Mcl-1 knockdown on WA-mediated apoptosis in MCF-7 cells. (A) Western blot analysis for Mcl-1 using lysates from SUM159 cells treated with the indicated doses of WA for specified time periods. The number on top of the immunoreactive band represents change of protein expression level relative to corresponding DMSO-treated control. (B) Western blotting for Mcl-1 and cleaved PARP using lysates from transiently transfected MCF-7 cells with a non-specific control siRNA or a Mcl-1-targeted siRNA and treated with 2.5 μM WA for 24 h. Quantitation relative to DMSO-treated control siRNA transfected cells is shown. (C) Histone-associated DNA fragment release into the cytosol in MCF-7 cells transiently transfected with a non-specific control siRNA or a Mcl-1 targeted siRNA and treated with 2.5 μM WA for 24 h. Quantitation relative to DMSO-treated control siRNA transfected cells is shown. Combined results (n = 6) from two independent experiments are shown as mean ± S.D. Statistical significance was determined by one-way ANOVA with Bonferroni’s multiple comparison test. aSignificantly different (P<0.05) compared with respective control. bSignificantly different (P<0.05) between groups at the same dose.
DISCUSSION
Elucidation of the mechanism by which WA treatment causes apoptosis is important for two reasons: (a) this knowledge likely helps optimization of WA-based regimens for prevention and/or treatment of cancer, and (b) mechanistic studies could lead to identification of biomarker(s) predictive of WA response for application in future clinical investigations. For example, we have shown recently that activation of Notch2 and Notch4 by WA impedes its inhibitory effect on breast cancer cell migration and invasion [30], suggesting that the anti-cancer effect of WA may be augmented in the presence of Notch inhibitors. The present study indicates that MAPK activation may not be a suitable biomarker for WA because of cell line-specific contribution. The ERK or JNK activation has no meaningful impact on WA-induced apoptosis in SUM159 cells, which may be partly attributable to a lack of complete inhibition of the respective kinases in the presence of PD98059 and SP600125. On the other hand, ERK/p38 MAPK and JNK have opposing effects on cell death resulting from WA exposure in MCF-7 cells. Activation of ERK and p38 MAPK seem to confer modest protection against WA-induced apoptosis, whereas JNK activation is partially responsible for the cell death. These results are strikingly different from those in leukemia cells [20] where WA-induced apoptosis was significantly attenuated by inhibition of p38 MAPK [20]. Another interesting observation of the present study is that MCF-7 cell line is relatively more sensitive to MAPK activation compared with SUM159. Further work is necessary to answer the question of whether differential sensitivity of MCF-7 and SUM159 cells to MAPK activation by WA is related to differences in their estrogen receptor-α status. In this context, it is important to mention that a role for p38 MAPK in downregulation of estrogen receptor-α protein expression by a green tea polyphenol has been suggested previously [31]. The WA is known to cause downregulation of estrogen receptor-α protein in MCF-7 cells [24].
We have shown previously that apoptosis induction by WA treatment in human breast cancer cells is associated with ROS generation [26]. A number of examples exist to illustrate involvement of ROS in MAPK activation by naturally-occurring anti-cancer agents. For example, ROS-mediated activation of JNK is responsible for phosphorylation of Bcl-2 and apoptosis by garlic constituent diallyl trisulfide in prostate and breast cancer cells [32,33]. A similar mechanistic connection between ROS generation, JNK activation, and apoptosis induction has been noted for the garden cress constituent benzyl isothiocyanate in breast cancer cells [34]. The present study reveals that the activation of JNK or p38 MAPK in WA-treated cells is not blocked by overexpression of Mn-SOD. Thus, unlike diallyl trisulfide or isothiocyanates, the JNK or p38 MAPK activation by WA is independent of ROS production. On the other hand, the ERK activation by WA may be partly dependent on ROS generation at least in the MCF-7 cell line. However, it is important to point out that ERK inhibition leads to augmentation of WA-induced apoptosis at least in the MCF-7 cell line whereas suppression of ROS by Mn-SOD overexpression or NAC treatment is protective against cell death induction by this agent. Based on these results, we conclude that ERK-independent mechanism(s) are likely involved in ROS-dependent apoptosis by WA treatment.
Previous work from our laboratory has indicated induction of Mcl-1 protein after treatment with WA in MCF-7 cells [10], which is also evident in the SUM159 cell line (Figure 7A). Another objective of the present study was to gain insight into the functional significance of Mcl-1 induction in WA-mediated apoptosis. The Mcl-1 (myeloid cell leukemia-1) belongs to the Bcl-2 family of apoptosis regulating proteins, and induced by a variety of cytokines and signaling pathways, including signal transducer and activation of transcription 3 and p38 MAPK [29,35]. Alternative splicing of Mcl-1 results in two protein isoforms, Mcl-1 long and Mcl-1 short, with opposing functions. The short form of Mcl-1 has been suggested to promote apoptosis [36]. RNA interference of Mcl-1 alone is apoptogenic in both MCF-7 and SUM159 cells (Figure 7C, and unpublished results). Because RNA interference of Mcl-1 depletes both forms of the protein and results in apoptosis induction, we can conclude that the long form predominates in function due to its abundance. The WA-induced apoptosis is only modestly increased after knockdown of both forms of Mcl-1. Based on these results, we conclude that induction of the long form of Mcl-1 confers only modest protection against WA-induced apoptosis possibly because of concomitant induction of the Mcl-1 short form.
In conclusion, the take home message from the present study is that while MAPK activation elicits cell line-specific contribution to the cell death by WA, induction of Mcl-1 long form confers modest protection at best against WA-induced apoptosis.
Acknowledgments
This study was supported by the USPHS grant RO1 CA142604-04, awarded by the National Cancer Institute. The funder had no role in the design of the experiments, data analysis, or preparation and submission of the manuscript.
Abbreviations
- WA
withaferin A
- MAPK
mitogen-activated protein kinases
- JNK
c-jun NH2-terminal kinases
- ERK
extracellular signal-regulated kinases
- ROS
reactive oxygen species
- Mn-SOD
manganese-superoxide dismutase
- siRNA
small interfering RNA
- DMSO
dimethyl sulfoxide
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- NAC
N-acetylcysteine
- PARP
poly-(ADP-ribose)-polymerase
- Mcl-1
myeloid cell leukemia-1
- ANOVA
analysis of variance
References
- 1.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: Ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Mohanty I, Talwar KK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad M, Saleem S, Ahmad AS, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 4.Devi PU, Sharada AC, Solomon FE. Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma-180. Indian J Exp Biol. 1993;31:607–611. [PubMed] [Google Scholar]
- 5.Widodo N, Kaur K, Shrestha BG, et al. Selective killing of cancer cells by leaf extract of Ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res. 2007;13:2298–2306. doi: 10.1158/1078-0432.CCR-06-0948. [DOI] [PubMed] [Google Scholar]
- 6.Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012;84:1282–1291. doi: 10.1016/j.bcp.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Lavie D, Glotter E, Shvo Y. Constituents of Withania somnifera Dun. III. The side chain of withaferin A. J Org Chem. 1965;30:1774–1778. [Google Scholar]
- 8.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 10.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JH, Lee TJ, Kim SH, et al. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis. 2008;13:1494–1504. doi: 10.1007/s10495-008-0273-y. [DOI] [PubMed] [Google Scholar]
- 12.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9:202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samadi AK, Tong X, Mukerji R, Zhang H, Timmermann BN, Cohen MS. Withaferin A, a cytotoxic steroid from Vassobia breviflora, induces apoptosis in human head and neck squamous cell carcinoma. J Nat Prod. 2010;73:1476–1481. doi: 10.1021/np100112p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–1705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 15.Mayola E, Gallerne C, Esposti DD, et al. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 16.Shohat B, Joshua H. Effect of withaferin A on Ehrlich ascites tumor cells. II. Target tumor cell destruction in vivo by immune activation. Int J Cancer. 1971;8:487–496. doi: 10.1002/ijc.2910080317. [DOI] [PubMed] [Google Scholar]
- 17.Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, Alias LM. Protective effect of Withaferin-A on tumour formation in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis in hamsters. Indian J Exp Biol. 2009;47:16–23. [PubMed] [Google Scholar]
- 18.Thaiparambil JT, Bender L, Ganesh T, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 19.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin A causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60:51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandal C, Dutta A, Mallick A, et al. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13:1450–1464. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 21.Hahm ER, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets. 2013 doi: 10.2174/15680096113139990039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaileh M, Vanden Berghe W, Heyerick A, et al. Withaferin A strongly elicits I B kinase hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–1998. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahm ER, Lee J, Huang Y, Singh SV. Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog. 2011;50:614–624. doi: 10.1002/mc.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahat G, Zhu QS, Huang KL, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS One. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakao K, Singh SV. D,L-Sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J Cell Biochem. 2012;113:599–610. doi: 10.1002/jcb.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 29.Quinn BA, Dash R, Azab B, et al. Targeting Mcl-1 for the therapy of cancer. Expert Opin Investig Drugs. 2011;20:1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136:45–56. doi: 10.1007/s10549-012-2239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Amicis F, Russo A, Avena P, et al. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol Nutr Food Res. 2013;57:840–853. doi: 10.1002/mnfr.201200560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 33.Na HK, Kim EH, Choi MA, Park JM, Kim DH, Surh YJ. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem Pharmacol. 2012;84:1241–1250. doi: 10.1016/j.bcp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agkul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, Whyte MK. Exon skipping in Mcl-1 results in a Bcl-2 homology domain 3 only gene product that promotes cell death. J Biol Chem. 2000;275:22136–22146. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]