Abstract
IMPORTANCE
The Apolipoprotein E (APOE) ε4 allele is a common and well-established genetic risk factor for Alzheimer Disease (AD). Sleep consolidation is also associated with AD risk and previous work suggests that APOE genotype and sleep may interact to influence cognitive function.
OBJECTIVE
To determine whether better sleep consolidation attenuates the relation of the APOE genotype to the risk of incident AD and the burden of AD pathology.
DESIGN
Prospective longitudinal cohort study with up to 6 years of follow-up.
SETTING
Community-based.
PARTICIPANTS
We studied a volunteer sample of 698 community dwelling older adults without dementia (average age 81.7 years; 77% female) in the Rush Memory and Aging Project followed for up to 6 years.
EXPOSURES
We used up to 10 days of actigraphic recording to quantify the degree of sleep consolidation, and ascertained APOE genotype.
MAIN OUTCOME MEASURES
Subjects underwent annual evaluation for AD over a follow-up period of up to 6 years. Autopsies were performed on 201 deceased participants, and Aβ and neurofibrillary tangle (NFT) pathology were identified by immunohistochemistry and quantified.
RESULTS
Over a follow-up period, 98 individuals developed AD. In a series of Cox proportional hazards models, better sleep consolidation attenuated the effect of the ε4 allele on the risk of incident AD (HR 0.67 95%CI 0.46–0.97 p=0.036 per allele per 1SD increase in sleep consolidation). In a series of linear mixed effect models, better sleep consolidation also attenuated the effect of the ε4 allele on the annual rate of cognitive decline (interaction estimate +0.048 SE=0.012 p<0.001). In deceased individuals, better sleep consolidation attenuated the effect of the ε4 allele on NFT density (interaction estimate −0.42 SE=0.17 p=0.016), which accounted for the effect of sleep consolidation on the association between APOE genotype and cognition proximate to death.
CONCLUSIONS AND RELEVANCE
Better sleep consolidation attenuates the effect of APOE genotype on incident AD and NFT pathology. Assessment of sleep consolidation may identify APOE positive individuals at high risk for incident AD, and interventions to enhance sleep consolidation should be studied as potentially useful means to reduce the risk of AD and NFT pathology in APOE ε4+ individuals.
INTRODUCTION
A confluence of genetic, behavioral and environmental factors contributes to the risk of Alzheimer disease (AD) in old age. The Apolipoprotein E (APOE) ε4 allele is the most well established genetic risk factor for AD1–6. Meanwhile, in older adults, sleep disturbance is common7, poorer sleep consolidation is associated with worse cognition8,9, and sleep apnea, which can impair sleep consolidation, is associated with a higher risk of incident mild cognitive impairment (MCI) and dementia10. While APOE genotype is immutable, many social, environmental, and medical contributors to poor sleep are modifiable.
Previous work has suggested a potentially complex relationship between APOE genotype, sleep disruption, and cognitive impairment. Some studies suggest that the ε4 allele may predispose to sleep disruption11–13. Others suggest that sleep disruption and APOE genotype may amplify each other’s negative cognitive effects14–17.
In prior work with data from participants in the Rush Memory and Aging Project (MAP), we reported that APOE genotype is associated with cognitive decline in old age and that AD pathology mediates this association18–20. We also reported an association between sleep consolidation – the extent to which sleep is uninterrupted by repeated awakenings – and incident AD risk21. The present study extends this work and examines whether better sleep consolidation reduces the effect of APOE on the risk of incident AD and the burden of AD pathology.
METHODS
A full description of the methods is contained in the Supplementary Material.
Participants
We studied 698 participants with baseline actigraphy, APOE genotype, and serial cognitive assessments from the MAP cohort22 – a community-based cohort study of aging and dementia, whose participants agree to organ donation upon death. A full description of inclusion/exclusion criteria is contained in the Supplementary Material.
The institutional review board of Rush University Medical Center approved this study. All participants signed written informed consent and an anatomical gift act for organ donation.
Quantifying Sleep Consolidation
Sleep consolidation is the extent to which sleep is uninterrupted by repeated awakenings. We obtained up to 10 days of actigraphy in participants’ usual environments and quantified the sleep consolidation using the metric kRA, as described and validated in prior publications21,23,24. Briefly, kRA represents the probability per 15-second interval of having an arousal, indicated by movement, after a sustained (≥ ~5 minutes) period of inactivity (i.e., sleep). A lower kRA indicates better sleep consolidation (and a higher kRA indicates greater sleep fragmentation, as in our previous work21,23,24). We previously showed that kRA correlates well with polysomnographic measures of sleep consolidation including sleep efficiency and wake time after sleep onset21.
Determination of APOE Genotype
DNA was extracted from peripheral blood lymphocytes and APOE genotype determined as described previously25 and in the Supplementary Material. Participants with ≥1 copies of the ε4 allele (i.e., ε3/ε4, ε2/ε4, and ε4/ε4) were considered ε4+. All others were considered ε4−.
Assessment of Cognition and Dementia
As described previously26 and in the Supplementary Material, a composite measure of global cognitive function was computed based on 19 cognitive tests administered annually. Individuals were classified as having AD by NINDS-ADRDA criteria27.
Neuropathological Assessment
At the time of these analyses, 201 participants with actigraphy, APOE genotype, and longitudinal cognitive data had died and undergone autopsies. As described previously28–31 and in the Supplementary Material, we quantified and computed summary measures of the percent area occupied by Aβ, the density of neurofibrillary tangles (NFT), and the density of neuritic plaques (NP) in a series of defined cortical regions. We also noted the presence/absence of Lewy bodies (LB) and gross infarcts.
Assessment of Covariates
Age, sex, education, congestive heart failure (CHF), peripheral vascular disease (PVD), diabetes, smoking, hypertension, stroke, Parkinson disease (PD), medications, depression, and total daily activity were ascertained as described in the Supplementary Material.
Statistical Analyses
As described in the Supplementary Material, we used Cox proportional hazards models to assess the relationship between APOE genotype, baseline sleep consolidation, and AD risk. Next, we used linear mixed effect models, which account for differences in baseline cognition, to examine the relationship between baseline sleep consolidation, APOE genotype, and the annual rate of cognitive decline. Finally, we used linear and logistic regression models to assess the relationship between APOE genotype, sleep consolidation, postmortem pathology, and cognition proximate to death.
All analyses were carried out using R32. All models were validated graphically and analytically.
RESULTS
Characteristics of the Study Population
Baseline characteristics of the 698 study participants are shown in Table 1.
Table 1.
Study Baseline Characteristics of Persons who Did or Did Not Develop Alzheimer Diseasea
| Characteristic | Developed AD (n=98) | Did not Develop AD (n=600) | ||
|---|---|---|---|---|
| APOE ε4− (n=67) | APOE ε4+ (n=31) | APOE ε4− (n=482) | APOE ε4+ (n=118) | |
| Age (years) | 87.5 (6.0) | 84.2 (6.0) | 81.3 (7.0) | 80.0 (7.6) |
| Female, % | 51/67 (76%) | 22/31 (71%) | 369/482 (77%) | 94/118 (80%) |
| Education (years) | 14.0 (2.9) | 14.6 (2.7) | 14.8 (2.9) | 15.3 (2.9) |
| MMSE Score | 25.6 (3.4) | 26.0 (2.5) | 28.3 (1.5) | 28.3 (1.7) |
| Composite Global Cognition | −0.52 (0.56) | −0.34 (0.52) | 0.27 (0.45) | 0.23 (0.55) |
| Depressive Symptoms | 1.9 (2.2) | 1.39 (1.63) | 0.98 (1.52) | 1.01 (1.57) |
| Hypertension, % | 44/67 (66%) | 16/31 (51%) | 286/482 (59%) | 70/118 (59%) |
| Smoking, % | 22/67 (33%) | 10/31 (32%) | 194/482 (40%) | 50/118 (42%) |
| Diabetes, % | 10/67 (15%) | 4/31 (13%) | 65/482 (13%) | 7/118 (6%) |
| Stroke % | 2/67 (3%) | 3/31 (10%) | 26/482 (5%) | 10/118 (8%) |
| Peripheral Vascular Disease, % | 17/67 (25%) | 4/31 (13%) | 64/482 (13%) | 19/118 (16%) |
| Coronary Artery Disease, % | 11/67 (16%) | 2/31 (6%) | 59/482 (12%) | 14/118 (12%) |
| Congestive Heart Failure, % | 3/67 (4%) | 0/31 (0%) | 28/482 (6%) | 6/118 (5%) |
| Parkinson Disease, % | 0/67 (0%) | 0/31 (0%) | 7/482 (1%) | 1/118 (1%) |
| Days of Actigraphy | 9.4 (0.8) | 9.2 (0.7) | 9.3 (0.9) | 9.2 (0.8) |
| Total Daily Activity (x105 counts) | 2.3 (1.2) | 2.7 (1.5) | 3.1 (1.6) | 3.2 (1.6) |
| Sleep Consolidation kRA | 0.028 (0.007) | 0.033 (0.011) | 0.029 (0.007) | 0.028 (0.008) |
All data presented as mean (standard deviation) unless otherwise indicated
Abbreviations: MMSE = mini mental state examination
APOE Genotype, Sleep Consolidation, and Incident AD
In a linear model adjusted for age, sex, and education, baseline sleep consolidation did not differ by APOE genotype (p=0.29 for ε4+ vs. ε4−).
Over a mean (SD) follow-up of 3.5 (1.8) years, 98 participants developed AD. In Cox proportional hazards models adjusted for age, sex, and education, the ε4 allele was associated with higher incident AD risk while better sleep consolidation was associated with lower risk (Table 2 Models A–B). Combining both in the same model (Model C) resulted in negligible change in the effect estimates compared to Models A and B, suggesting that sleep consolidation is not in the causal pathway linking APOE genotype to AD risk.
Table 2.
Effect of Degree of Sleep Consolidation and Presence/Absence of the APOE ε4 Allele on the Risk of Incident AD
| Effect on Risk of Incident AD | ||||
|---|---|---|---|---|
| Predictor | Model A | Model B | Model C | Model D |
| Sleep Consolidation | 0.84 [0.71–1.00] p=0.05 |
0.84 [0.71–0.99] p=0.04 |
0.83 [0.63–1.10] p=0.19 |
|
| APOE Genotype | 2.21 [1.44–3.40] p=<0.001 |
2.22 [1.44–3.42] p<0.001 |
2.70 [1.51–4.83] p<0.001 |
|
| Sleep Consolidation x APOE Genotype | 0.67 [0.46–0.97] p=0.04 |
|||
Hazard Ratio [95% Confidence Interval] p-values. Effects of sleep consolidation expressed per 1 standard deviation increase. Effects of APOE genotype expressed for presence vs. absence of the ε4 allele. All models adjusted for age at baseline, sex, and education
We next examined whether the relationship between APOE genotype and AD risk varies depending on the degree of sleep consolidation by adding a sleep x APOE interaction term (Model D). This was significant. Each 1SD increase in sleep consolidation attenuated the impact of APOE genotype on AD risk by nearly 50%.
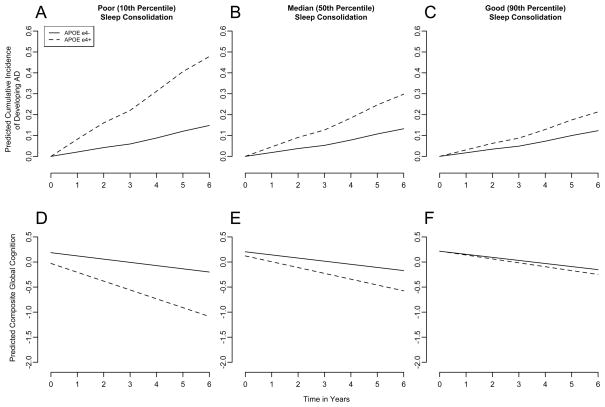
To illustrate this, we compared model predictions for hypothetical average (82 year old women with 15 years of education) APOE ε4− and ε4+ individuals with poor (10th percentile), median, and good (90th percentile) sleep consolidation (kRA = 0.037, 0.027 and 0.021; Figure 1A–C). APOE ε4 was associated with a higher risk of AD irrespective of the degree of sleep consolidation. However, the effect size varied. With poor sleep consolidation, APOE ε4+ was associated with a predicted HR of 4.1 for incident AD compared to APOE ε4−. With median sleep consolidation, this was attenuated to 2.5, and with good sleep consolidation this was further attenuated to 1.8.
Figure 1. APOE Genotype, Sleep Consolidation, Cumulative Incidence of AD and Rate of Cognitive Decline.
The model predicted cumulative incidence of AD and rate of cognitive decline based on the entire cohort are illustrated for hypothetical average APOE ε4+ and ε4− participants with poor (A,D 10th percentile), median (B,E 50th percentile), and good (C,F 90th percentile) sleep consolidation (kRA = 0.037, 0.027 and 0.021).
In separate sensitivity analyses, the sleep-APOE interaction remained significant after excluding individuals with the lowest 5% of baseline global cognition, with baseline MCI, who developed AD within the first year, or ε2/ε4 heterozygotes (eTable 1 Models A–D).
Effect of Clinical and Demographic Covariates
Sleep consolidation may be marker of general health. Thus we conducted an additional analysis to examine potential confounding by depression, vascular diseases (CHF, stroke, PVD), and vascular risk factors (diabetes, smoking, hypertension), on the sleep-APOE interaction. The strength of the interaction was materially unchanged (eTable 1 Model E).
Stroke and PD can affect sleep and cognition. However, the sleep-APOE interaction remained significant after excluding individuals with these conditions (eTable 1 Model F).
Psychotropic medications can affect sleep and cognition. However, the sleep-APOE interaction was essentially unchanged after adjusting for use of antidepressants, sedative-hypnotics, and anxiolytics (eTable 1 Model G).
Total daily activity is associated with incident AD33 and may plausibly affect sleep. In a model adjusted for total daily activity, the sleep-APOE interaction remained significant (eTable 1 Model H).
APOE Genotype, Sleep Consolidation, and Cognitive Decline
To ensure that our results were not an artifact of the diagnostic process, we considered the effects of APOE genotype and sleep consolidation on the annual rate of decline of composite cognitive function, a continuous outcome, using linear mixed models incorporating participant-specific estimates of intercepts and slopes to account for baseline cognitive differences. In the base model (eTable 2 Model A), global cognition declined by 0.130 units/year. The ε4 allele was associated with poorer baseline cognition and more rapid decline while better baseline sleep consolidation was associated with better baseline cognition and slower decline. In a model with sleep-APOE interaction terms (eTable 2 Model B), better baseline sleep consolidation attenuated the ε4 effect on both the baseline cognitive level and subsequent decline.
To illustrate these effects, we compared model predictions for hypothetical average ε4−, and ε4+ individuals with poor, median and good sleep consolidation (Figure 1D–F). Irrespective of sleep consolidation, the ε4 allele was associated with poorer baseline cognition and more rapid decline. However, better sleep consolidation attenuated these effects.
APOE Genotype, Sleep Consolidation, Neuropathological Findings, and Cognition
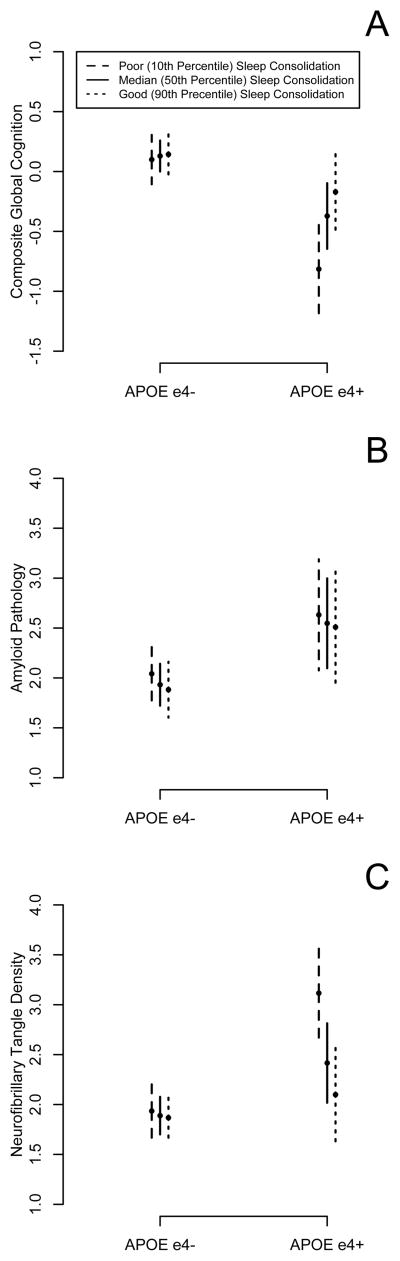
At the time of these analyses, 201 participants with actigraphy, APOE genotype, and longitudinal cognitive data had died and undergone autopsy. Characteristics of these subjects are shown in eTable 3. In this subset, we used linear and logistic regression models to examine the effect of sleep consolidation and APOE genotype on AD and non-AD pathology. Sleep consolidation was last quantified on average 17.9 months (SD 13.7) before death. Neither sleep consolidation nor APOE genotype was associated with infarcts at autopsy (eTable 5). The ε4 allele was associated with more Aβ pathology, and greater NP and NFT density (eTable 4), and a higher likelihood of having LB (eTable 5). Better sleep consolidation attenuated the effect of APOE genotype on NFT density, but not the other pathological findings (Table 3, eTable 4; eTable 5; Figure 3).
We used linear regression models to further examine the relationships between APOE genotype, sleep, Aβ pathology, and NFT density (Table 3). In separate models, both APOE genotype and Aβ pathology were associated with NFT density, and when combined in the same model, both remained significant, although the effect estimate for APOE genotype was attenuated (models A, B, D), statistically consistent with APOE genotype influencing NFT density through both amyloid-dependent and independent pathways. Addition of sleep to this model did not appreciably change the effect estimates, arguing against mediation or confounding (model E). However, there was a significant sleep-APOE interaction (model G) even after adjusting for Aβ pathology (model H), suggesting that sleep consolidation modifies the APOE effect on NFT density in a manner not statistically mediated by Aβ pathology.
Aβ and NFT pathology may link APOE genotype to cognition29,34, and LB and infarcts may also affect cognition. We used linear regression models to examine if Aβ pathology, NFT density, infarcts, or LBs may account for the effect of sleep on the association between APOE genotype and cognition proximate to death. As expected, better sleep attenuated the effect of APOE genotype on cognition proximate to death (eTable 5 Model B). Inclusion of terms for presence of gross infarcts, presence of LB, and burden of Aβ pathology did not substantially change this (C–E) suggesting that these pathologies do not account for the effect of sleep on the association between APOE genotype and cognition. However, adding a term for NFT density attenuated the interaction effect (F) in a manner that did not change with inclusion of Aβ pathology in the model (G). This is statistically consistent with NFT density accounting for the effect of sleep on the association between APOE genotype and cognition.
DISCUSSION
In this study of nearly 700 older persons without dementia, better sleep consolidation substantially attenuated the negative impact of the ε4 allele on incident AD risk. This environment-gene interaction was not accounted for by variation in physical activity, co-morbid medical conditions, or psychotropic medications. In additional analyses of cognitive change over time we showed that this finding was not an artifact of the diagnostic process. Finally, better sleep consolidation attenuated the negative impact of the ε4 allele on NFT density at death, which accounted for its beneficial effects on the association between APOE and cognition proximate to death. These findings highlight a thus far unappreciated biological and clinical link between sleep, APOE biology, NFTs, and AD.
There is a well-established link between APOE genotype and AD risk1–5. Furthermore, several studies support a link between sleep, cognition, and dementia risk8–10,21,24,35. However the precise relationship between APOE genotype, sleep, and AD is unclear. Several studies suggest that the ε4 allele may potentiate sleep disturbance11–13,15,36. Others suggest that sleep disruption and APOE genotype may interact to worsen cognitive function13,16,17. In this study, sleep consolidation neither mediated nor confounded the association between APOE genotype and incident AD, suggesting that it is not in the causal pathway linking APOE genotype to AD risk. However, better sleep consolidation substantially attenuated the impact of APOE genotype on incident AD risk.
APOE genotype is thought to influence the development of NFTs and Aβ pathology37, the pathological hallmarks of AD. Biomarker studies suggest that Aβ pathology accumulates early, before clinical symptoms, while tau pathology accumulates only after Aβ accumulation is established38. Considerable evidence suggests that APOE ε4 predisposes to pathological Aβ accumulation39,40, possibly by reducing clearance41. Aβ aggregation may then drive tau pathology42–44. However, APOE ε4 may also directly promote tau pathology in an Aβ-independent manner. APOE ε4 knock-in mice have higher levels of hyperphosphorylated tau compared to ε3 knock-in mice45. Moreover, in both in vitro and transgenic mouse models, APOE4 fragments can induce neuronal NFT-like inclusions46,47 even without amyloid pathology. APOE4 may preferentially activate glycogen synthase kinase (GSK) 3β compared to APOE348, leading to tau hyperphosphorylation.
Animal experiments suggest that sleep disruption may influence the accumulation of Aβ pathology35. Moreover, a recent cross-sectional study of cognitively asymptomatic individuals demonstrated an association between actigraphic sleep efficiency and CSF Aβ, a marker of cerebral Aβ pathology49. Markers of tau pathology were not assessed in this study. In the present study, better sleep consolidation attenuated the association between APOE genotype and postmortem AD pathology, specifically NFT density, which accounted in part for its beneficial effect on the association between APOE genotype and cognition. These results add to the growing body of evidence supporting a link between sleep, genetic susceptibility, and AD pathology. Moreover, they invite further investigation of the role of sleep in pathways directly linking APOE to tau pathology, not only in AD but also in primary tauopathies. In our study, although APOE genotype was strongly associated with Aβ pathology at death, sleep consolidation was not, nor did it modify the impact of APOE genotype on Aβ pathology. Several factors may account for this apparent difference compared to previous animal35 and human49 studies supporting an effect of sleep disruption on Aβ pathology. First, our participants were older than in previous human studies, and Aβ pathology was assessed only at death. Therefore, our results do not exclude an association at earlier times and in younger individuals. Second, animal studies relating sleep disruption to Aβ pathology were carried out in strongly amyloidogenic models that may not be completely representative of sporadic human AD. Third, in our study only a subset of participants had died by the time of these analyses and it is possible that data from a larger number of deceased individuals may reveal more subtle effects of sleep consolidation on Aβ pathology.
Taken as a whole, our findings are compatible with two hypotheses. In the first, baseline sleep consolidation may be a marker of some other factor (e.g. a medical co-morbidity, subclinical neurodegeneration, or a genetic or environmental factor) that causally modifies the impact of APOE genotype on NFT pathology and AD. However, several observations argue against this. First, our results were unchanged after adjusting for a wide range of medical comorbidities. Second, in linear mixed effect models that allowed for differences in baseline cognitive function (a marker of baseline neurodegenerative burden), a significant sleep-APOE interaction was still seen. Third, our results were robust to the exclusion of subjects with MCI at baseline, who would have had the greatest baseline neurodegenerative burden. Even if this first hypothesis were true, it would suggest that actigraphic sleep assessment could be an inexpensive and automated means of risk stratifying ε4+ individuals. In the second hypothesis (eFigure 1), sleep causally modifies the impact of APOE genotype on NFT formation and AD. In this hypothesis, uninterrupted sleep protects against biological mechanisms liking the ε4 allele to NFT formation (an example of which might be GSK3β hyperactivation48). Under this hypothesis, interventions to improve sleep consolidation may be a potentially useful approach to attenuate the risk of NFT pathology and AD in ε4+ individuals. Future interventional studies are needed to help define the appropriate role for APOE genotyping, actigraphic sleep assessment, and sleep interventions in the clinical management of individuals at risk for AD.
This study has several limitations. First, sleep disorders, some of which may plausibly affect both sleep consolidation and cognition, were not specifically assessed. However, the expected prevalence of significant (apnea index >10) sleep apnea in community-dwelling elders aged 65–99 has been estimated at only 11%50, and the expected prevalence of significant restless legs is estimated at only 2.7%51. We think that these numbers are probably too low to have had a major impact on our results. Nevertheless, future studies should investigate whether there are specific sleep-APOE interaction effects on AD risk and pathology in patients with sleep apnea and other sleep disorders, particularly given recently reported associations between sleep apnea, cognition, and dementia10,52. Second, our cohort consisted entirely of volunteers, mostly women, which may limit generalizability. Third, although actigraphy is widely used in the ambulatory measurement of sleep, it is not identical to polysomnography. However, we and others have previously shown good concordance between actigraphic and polysomnographic sleep metrics53, including the metric kRA used in this study21. Fourth, the subset of participants who died and underwent autopsy during the study period was different than that which did not, and one must be careful in generalizing the postmortem results to the whole study population. Finally, AD pathology was examined only at death and we cannot comment on the effect of sleep consolidation and APOE genotype on the temporal trajectory of Aβ or NFT accumulation.
This study also has several strengths. Sleep consolidation was measured objectively and non-invasively in participants’ usual environments avoiding disturbance of natural sleep behavior, and confounding by poor recall or misperception. Moreover, it used a rigorous, standardized, well-characterized, and well-validated cognitive test battery administered annually for up to 6 years, allowing a high degree of certainty regarding the diagnosis of AD and the measurement of cognition. Finally, AD pathology was systematically assessed in a uniform way in the same individuals who underwent APOE genotyping and examination of sleep and cognition, allowing us to take a unique integrative approach to linking genotype, sleep, neuropathological findings, cognition, and AD in old age.
Supplementary Material
Figure 2. APOE Genotype, Sleep Consolidation, AD Pathology, and Cognitive Function Proximate to Death.
The model predicted composite global cognitive function proximate to death (A), Aβ pathology at autopsy (B) and neurofibrillary tangle density at autopsy (C) based on deceased participants are illustrated for hypothetical average APOE ε4+ and ε4− participants with poor (10th percentile), median (50th percentile), and good (90th percentile) sleep consolidation. Vertical bars indicate 95% confidence intervals.
Acknowledgments
We are indebted to the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center for this work.
This study was supported by Canadian Institutes of Health Research grant MOP125934, National Institute on Aging grants R01AG17917 and R01AG24480, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
The funding sources had no input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Lim reports no relevant conflicts of interest for this manuscript. He has served as a consultant for UCB Pharma Inc and Merck & Co. Inc. He receives research funding from the CIHR. Dr. Yu reports no relevant conflicts of interest for this manuscript. Dr. Kowgier reports no relevant conflicts of interest for this manuscript. Dr. Schneider reports no relevant conflicts of interest for this manuscript. Dr. Schneider has received consulting fees or sat on paid advisory boards for AVID radiopharmaceuticals, Eli Lilly Inc., and GE Healthcare. She is a monitoring editor of the Journal of Histochemistry and Cytochemistry and on the editorial board of International Journal of Clinical and Experimental Pathology. Dr. Schneider receives research funding from the NIH. Dr. Buchman reports no relevant conflicts of interest for this manuscript. He receives research support from the NIH. Dr. Bennett reports no relevant conflicts of interest for this manuscript. He serves on the editorial board of Neurology, Neuroepidemiology, and Current Alzheimer’s Research; has received honoraria for non-industry sponsored lectures; has served as a consultant to Nutricai, Inc., Eli Lilly, Inc., Enymotic, Ltd., Gerson Lehrman Group; and receives research support from the NIH and the Illinois Department of Public Health.
Dr. Lim has responsibility for the integrity of the work as a whole. He conceived of the study hypotheses and design, analyzed and interpreted the data, carried out statistical analysis, drafted the manuscript, and approved the final manuscript. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Yu contributed to the analysis of the data, performed statistical analysis, revised the article critically for important intellectual content, and approved the final manuscript. Dr. Kowgier contributed to the analysis of the data, performed statistical analysis, revised the article critically for important intellectual content, and approved the final manuscript. Dr. Schneider obtained funding, contributed to the study design, oversaw acquisition, analysis, and interpretation of the neuropathological data, revised the article critically for important intellectual content, provided administrative, technical, or material support, provided supervision, and approved the final manuscript. Dr. Buchman obtained funding, contributed to the study conception and design, oversaw data acquisition, contributed to data interpretation, revised the article critically for important intellectual content, provided administrative, technical, or material support, provided supervision, and approved the final manuscript. Dr. Bennett has responsibility for the integrity of the work as a whole. He obtained funding, contributed to the study conception and design, oversaw data acquisition, contributed to data interpretation, provided administrative, technical, or material support, provided supervision, revised the article critically for important intellectual content, and approved the final manuscript.
Footnotes
Authors’ Contributions:
Dr. Lim has responsibility for the integrity of the work as a whole. He conceived of the study hypotheses and design, analyzed and interpreted the data, carried out statistical analysis, drafted the manuscript, and approved the final manuscript. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Yu contributed to the analysis of the data, performed statistical analysis, revised the article critically for important intellectual content, and approved the final manuscript. Dr. Kowgier contributed to the analysis of the data, performed statistical analysis, revised the article critically for important intellectual content, and approved the final manuscript. Dr. Schneider obtained funding, contributed to the study design, oversaw acquisition, analysis, and interpretation of the neuropathological data, revised the article critically for important intellectual content, provided administrative, technical, or material support, provided supervision, and approved the final manuscript. Dr. Buchman obtained funding, contributed to the study conception and design, oversaw data acquisition, contributed to data interpretation, revised the article critically for important intellectual content, provided administrative, technical, or material support, provided supervision, and approved the final manuscript. Dr. Bennett has responsibility for the integrity of the work as a whole. He obtained funding, contributed to the study conception and design, oversaw data acquisition, contributed to data interpretation, provided administrative, technical, or material support, provided supervision, revised the article critically for important intellectual content, and approved the final manuscript.
Conflicts of Interest and Disclosures:
Dr. Lim reports no relevant conflicts of interest for this manuscript. He has served as a consultant for UCB Pharma Inc and Merck & Co. Inc. He receives research funding from the CIHR. Dr. Yu reports no relevant conflicts of interest for this manuscript. Dr. Kowgier reports no relevant conflicts of interest for this manuscript. Dr. Schneider reports no relevant conflicts of interest for this manuscript. Dr. Schneider has received consulting fees or sat on paid advisory boards for AVID radiopharmaceuticals, Eli Lilly Inc., and GE Healthcare. She is a monitoring editor of the Journal of Histochemistry and Cytochemistry and on the editorial board of International Journal of Clinical and Experimental Pathology. Dr. Schneider receives research funding from the NIH. Dr. Buchman reports no relevant conflicts of interest for this manuscript. He receives research support from the NIH. Dr. Bennett reports no relevant conflicts of interest for this manuscript. He serves on the editorial board of Neurology, Neuroepidemiology, and Current Alzheimer’s Research; has received honoraria for non-industry sponsored lectures; has served as a consultant to Nutricai, Inc., Eli Lilly, Inc., Enymotic, Ltd., Gerson Lehrman Group; and receives research support from the NIH and the Illinois Department of Public Health.
Contributor Information
Andrew S.P. Lim, Email: andrew.lim@utoronto.ca.
Lei Yu, Email: lei_yu@rush.edu.
Matthew Kowgier, Email: matthew.kowgier@oicr.on.ca.
Julie A. Schneider, Email: julie_a_schneider@rush.edu.
Aron S. Buchman, Email: aron_s_buchman@rush.edu.
David A. Bennett, Email: david_a_bennett@rush.edu.
References
- 1.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 5.Evans DA, Beckett LA, Field TS, et al. Apolipoprotein E epsilon4 and incidence of Alzheimer disease in a community population of older persons. JAMA. 1997 Mar 12;277(10):822–824. [PubMed] [Google Scholar]
- 6.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997 Oct 22–29;278(16):1349–1356. [PubMed] [Google Scholar]
- 7.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995 Jul;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006 Apr;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011 Oct;34(10):1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011 Aug 10;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CC, Lung FW. The role of PGC-1 and Apoepsilon4 in insomnia. Psychiatr Genet. 2012 Apr;22(2):82–87. doi: 10.1097/YPG.0b013e32834dc438. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004 Aug 24;63(4):664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 13.Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007 Jul 17;69(3):243–249. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 14.Cosentino FI, Bosco P, Drago V, et al. The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med. 2008 Dec;9(8):831–839. doi: 10.1016/j.sleep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal N, Ramesh V, Gozal D. Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2012 Jul 1;303(1):R19–29. doi: 10.1152/ajpregu.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005 Aug 23;65(4):642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 17.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008 Jan;56(1):45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology. 2009 Apr 28;72(17):1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–49. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Boyle PA, Schneider JA, et al. APOE e4 allele is associated with late-life cognitive change through AD pathology. Psychology and Aging. In Press. [Google Scholar]
- 21.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of Alzheimer Disease and Cognitive Decline in Older Persons. Sleep. doi: 10.5665/sleep.2802. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012 Jul;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim AS, Yu L, Costa MD, et al. Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34(11):1569–1581. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim AS, Yu L, Costa MD, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5):633–640. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009 Jan-Mar;23(1):63–69. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005 Jul;11(4):400–407. [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006 May;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003 Jan 28;60(2):246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 30.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009 Aug;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004 Apr 13;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 32.R Development Core Team. R: A language and environment for statistical computing. Vienne, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 33.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012 Apr 24;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005 Sep;76(9):1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009 Nov 13;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebes RD, Pollock BG, Perera S, Halligan EM, Saxton JA. The greater sensitivity of elderly APOE epsilon4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. The American journal of geriatric pharmacotherapy. 2012 Jun;10(3):185–192. doi: 10.1016/j.amjopharm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Science translational medicine. 2011 Apr 6;3(77):77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009 Oct 15;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fryer JD, Simmons K, Parsadanian M, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005 Mar 16;25(11):2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Science translational medicine. 2011 Jun 29;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001 Aug 24;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 43.Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001 Aug 24;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 44.Gotz J, Streffer JR, David D, et al. Transgenic animal models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004 Jul;9(7):664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M, Ishiguro K, Katoh-Fukui Y, Yokoyama M, Fujita SC. Phosphorylation state of tau in the hippocampus of apolipoprotein E4 and E3 knock-in mice. Neuroreport. 2003 Apr 15;14(5):699–702. doi: 10.1097/00001756-200304150-00008. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris FM, Brecht WJ, Xu Q, et al. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cedazo-Minguez A, Popescu BO, Blanco-Millan JM, et al. Apolipoprotein E and beta-amyloid (1–42) regulation of glycogen synthase kinase-3beta. J Neurochem. 2003 Dec;87(5):1152–1164. doi: 10.1046/j.1471-4159.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 49.Ju YE, McLeland JS, Toedebusch CD, et al. Sleep Quality and Preclinical Alzheimer Disease. JAMA neurology. 2013 Mar 11;:1–7. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991 Dec;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005 Jun 13;165(11):1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 52.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of Sleep Disordered Breathing and Cognitive Deficit in APOE epsilon4 Carriers. Sleep. 2013;36(6):873–880. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992 Oct;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.