Abstract
Dopamine D4 receptor (D4R) mechanisms have been implicated in several psychiatric diseases, including schizophrenia, attention-deficit hyperactivity disorder (ADHD), and autism, which are characterized by cognitive deficits. The cellular mechanisms are poorly understood but impaired neuronal synchronization within cortical networks in the gamma frequency band has been proposed to contribute to these deficits. A D4R polymorphism was recently linked to variations in gamma power in both normal and ADHD subjects, and D4R activation was shown to enhance kainate-induced gamma oscillations in brain slices in vitro. The goal of this study was to investigate the effect of D4R activation on gamma oscillations in freely moving rats during natural behavior. Field potentials were recorded in the frontal, prefrontal, parietal, and occipital cortex and hippocampus. Gamma power was assessed before and after subcutaneous injection of a D4R agonist, A-412997, in several doses between 0.3 and 10.0 mg/kg. The experiments were also repeated in a neurodevelopmental model of schizophrenia, in which rats are prenatally treated with methylazoxymethanol (MAM). We found that the D4R agonist increased gamma power in all regions at short latency and lasting for ~2 hours, both in normal and MAM-treated rats. The effect was dose-dependent indicated by the significant difference between the effects after 3 and 10 mg/kg in pair-wise comparison, whereas 0.3 and 1.0 mg/kg injections were ineffective. This study demonstrates involvement of D4R in cortical gamma oscillations in vivo and identifies this receptor as potential target for pharmacological treatment of cognitive deficits.
Introduction
Starting from the initial characterization of the DRD4 gene by Van Tol and colleagues in 1991 (Van Tol et al., 1991), the dopamine D4 receptor (D4R) has generated an exceptional level of interest for its potential relevance to schizophrenia. Later studies (Aguirre et al., 2007; Shi et al., 2008; Lung et al., 2009), including a meta-analysis (Allen et al., 2008) identified the DRD4 as a risk factor gene for schizophrenia. More recently, D4R related mechanisms and/or genetic variations have also been associated with other psychiatric diseases (Tarazi et al., 2004; Ptacek et al., 2011), notably including autism (Deth et al., 2008; Gadow et al., 2010) and attention-deficit hyperactivity disorder (ADHD)(Lynn et al., 2005; McGough, 2012).
D4Rs are particularly enriched in fast firing parvalbumin-positive (PV+) interneurons (Mrzljak et al., 1996), which play a critical role in neuronal network oscillations (Csicsvari et al., 2003; Sohal et al., 2009). Impairment of gamma oscillations, which are essential for a number of cognitive functions, such as perception, attention, working memory, etc., was demonstrated in diseases in which D4Rs are implicated, including in patients with autism (Hall et al., 2011) and schizophrenia (Uhlhaas and Singer, 2012) and their first degree relatives (Hall et al., 2011; McFadden et al., 2012). A DRD4 polymorphism was also linked to variations in gamma power (Demiralp et al., 2007) and D4R activation was shown to enhance kainate-induced gamma oscillations in hippocampal slices in vitro (Andersson et al., 2012a; Andersson et al., 2012b). However, since gamma power might show opposite changes in vitro and in vivo under the same schizophrenia-relevant experimental interventions (Gandal et al., 2012; Kocsis et al., 2013), the goal of this study was to determine whether D4R activation has an effect on gamma oscillations in vivo, in freely moving rats and whether the changes extend to neocortical networks, as well. We also studied the effect of D4R activation in a neurodevelopmental model of schizophrenia (Moore et al., 2006; Lodge et al., 2009), which recapitulates some of the characteristic alterations in gamma activity (Lodge et al., 2009; Kocsis et al., 2012) of human schizophrenia (Uhlhaas and Singer, 2012), but shows diminished capacity to generate drug-induced gamma enhancement after NMDA receptor (NMDAR) blockade (Kocsis et al., 2012; Phillips et al., 2012).
Materials and Methods
Experiments were performed on male Sprague–Dawley rats, Charles River Laboratories, MA) treated in accordance with NIH guidelines. All procedures were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center. Electrophysiological recordings and drug injections followed the protocol described in our previous studies (Kittelberger et al., 2012; Kocsis, 2012). Rats were implanted with chronic EEG and EMG electrodes under ketamine-xylazine (70–80 and 10 mg/kg, respectively) anesthesia. Field potentials were recorded using surface screw electrodes placed at equidistant locations along a rostro-caudal axis, over the left frontal, parietal and occipital cortex (AP: 1.0, -2.5, -6.5 mm, Lat: 2.0, 2.5, 3.0 mm, respectively) and with fine wires in the prefrontal cortex (PFC)(AP: 3.2, Lat: 0.2, DV: 5.1 mm, on both sides) and hippocampus CA1 and DG regions (AP: 3.7, Lat: 2.2, DV: 2.5 and 3.5mm, on the right side). Daily recordings started 7–10 days after surgery. The rats were placed in a recording box in the morning and cortical and hippocampal EEG and neck muscle EMG were continuously recorded for 24 hours. Drug injection started after several days of baseline recording during which the return of normal sleep-wake cycle was verified, the rats accommodated to the recording environment, i.e. the box and the connecting cable, and the quality of recordings on different channels were evaluated and adjusted. In the following recording days, between 4 and 5 hours after the start of control recording, during the light phase of the day, saline or the D4R agonist A-412997 (Tocris) was injected subcutaneously in one of the following doses: 10.0 (n=9), 3.0 (n=9), 1.0 (n=3), 0.3 (n=2) mg/kg. Saline was used as vehicle control in all rats (n=10). Each rat received multiple injections, the order of drug doses varied randomly and at least 2 days were allowed between consecutive injections for washout. A-412997 is a full D4R agonist with high affinity for both rat and human D4R (Ki=12.1 and 7.9 nM, respectively)(Moreland et al., 2005).
Treatment with methylazoxymethanol acetate (MAM) followed previously developed and commonly used protocols (Moore et al., 2006; Lodge et al., 2009; Kocsis et al., 2012; Phillips et al., 2012). Pregnant rats were obtained on gestational day 10 and housed individually. On GD17 dams were injected intraperitoneally with MAM at a dose of 22 mg/kg, or saline. Male pups were weaned 30 days after birth and housed in pairs under standard conditions. Experiments were carried out when the animals were 3–6 months old.
EEG signals were filtered between 0.1–100 Hz and sampled at 256 Hz. Power spectral density (PSD) was calculated using fast Fourier transform on consecutive 4 s long windows. Gamma oscillations were assessed using the average spectral power in two frequency bands, the first to cover all changes in the spectrum (20–65 Hz, see Results for details) and second to limited the evaluation to gamma frequencies (30–65 Hz). Two 20 min-long pre-injection segments representing non-active and active behaviors were selected and compared with the first 20min of post-injection recording. For presenting the time course of fluctuations in gamma band power (Figs. 2A and 3A), gamma activity was averaged over 5 min-long periods and normalized using a 1 hr-long quiet episode before injection. Student’s t-test and two-way ANOVA with post-hoc Bonferroni pair-wise comparisons were used for statistical analyses.
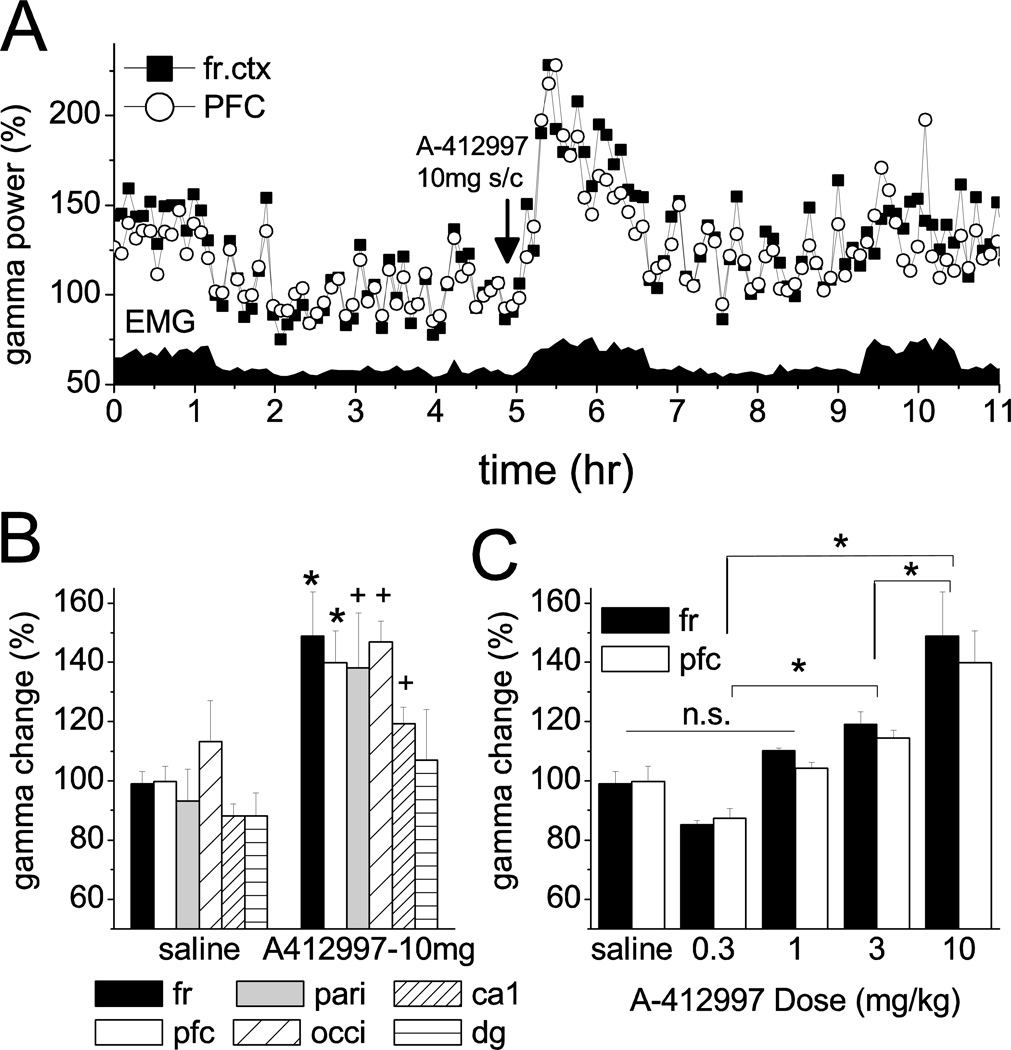
Figure 2.
Effect of D4R agonist on gamma band power in cortical EEG. A. Time course of gamma power (20–65 Hz) in the frontal and prefrontal cortex in a representative experiment, along with neck muscle EMG. A-412997 (10 mg/kg) was injected 5 hours after the start of the recording. Power is expressed as percent change relative to a 1 hr quiet period with no muscle activity between hrs 3 and 4. B. Increase in gamma band activity post-injection, relative to pre-injection level in active waking. C. Dose-response effect. Relationship between dose of A-412997 and increase in gamma activity in the frontal and prefrontal cortex of the group of “normal” rats (n=6; C: *: p<0.05; +: 0.05<p<0.1 t-test; D: *: p<0.05 post-hoc Bonferroni)
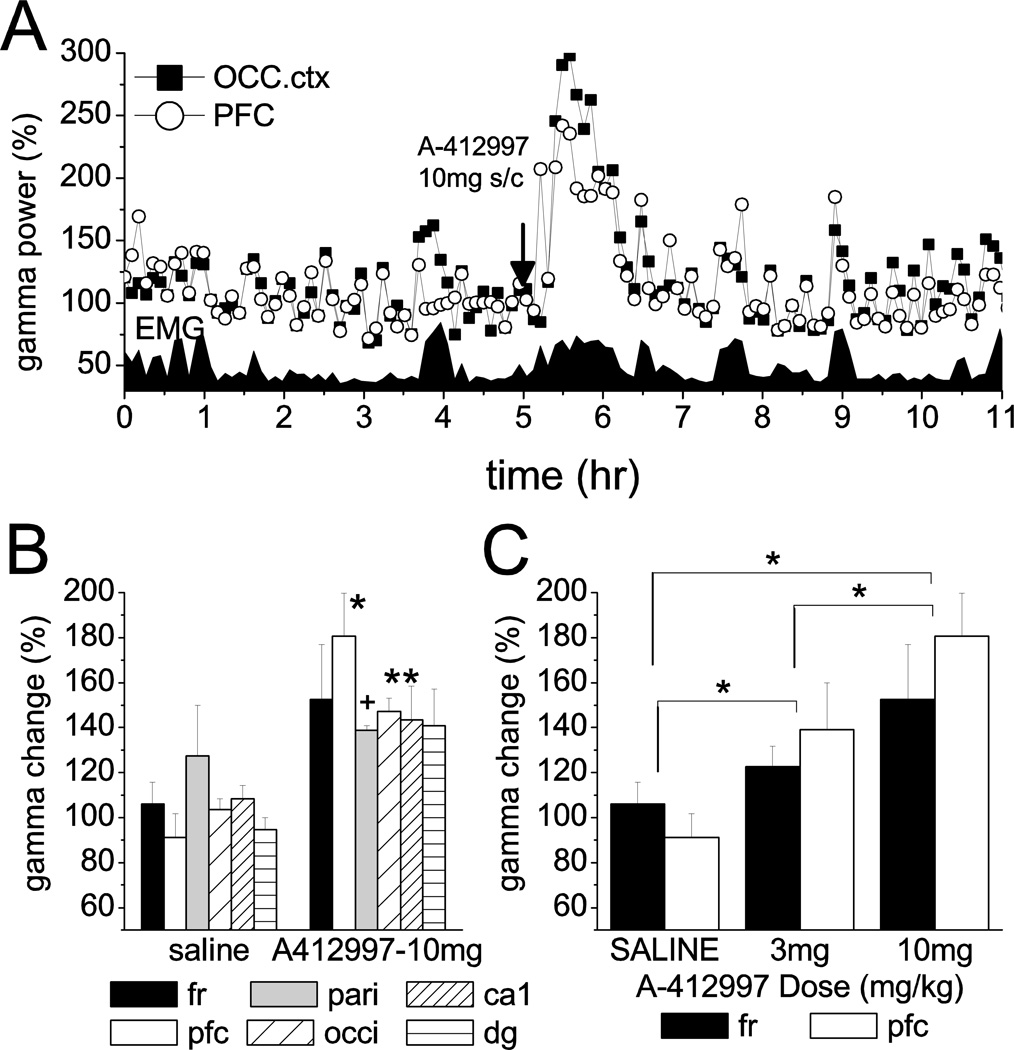
Figure 3.
Effect of D4R agonist on gamma band power in cortical EEG in MAM-treated rats. A. Time course of gamma power (20–65 Hz) in the occipital and prefrontal cortex in a representative experiment, along with neck muscle EMG. A-412997 was injected in a dose of 10 mg/kg. Power is expressed as percent change relative to a 1 hr quiet period with no muscle activity between hrs 2 and 3. B. Increase in gamma band activity post-injection, relative to pre-injection level in active waking. C. Dose-response effect. Relationship between dose of A-412997 and increase in gamma activity in the frontal and prefrontal cortex of the group of MAM-treated rats (n=4; *: p<0.05; +: 0.05<p<0.1 t-test; D: *: p<0.05 post-hoc Bonferroni)
Results
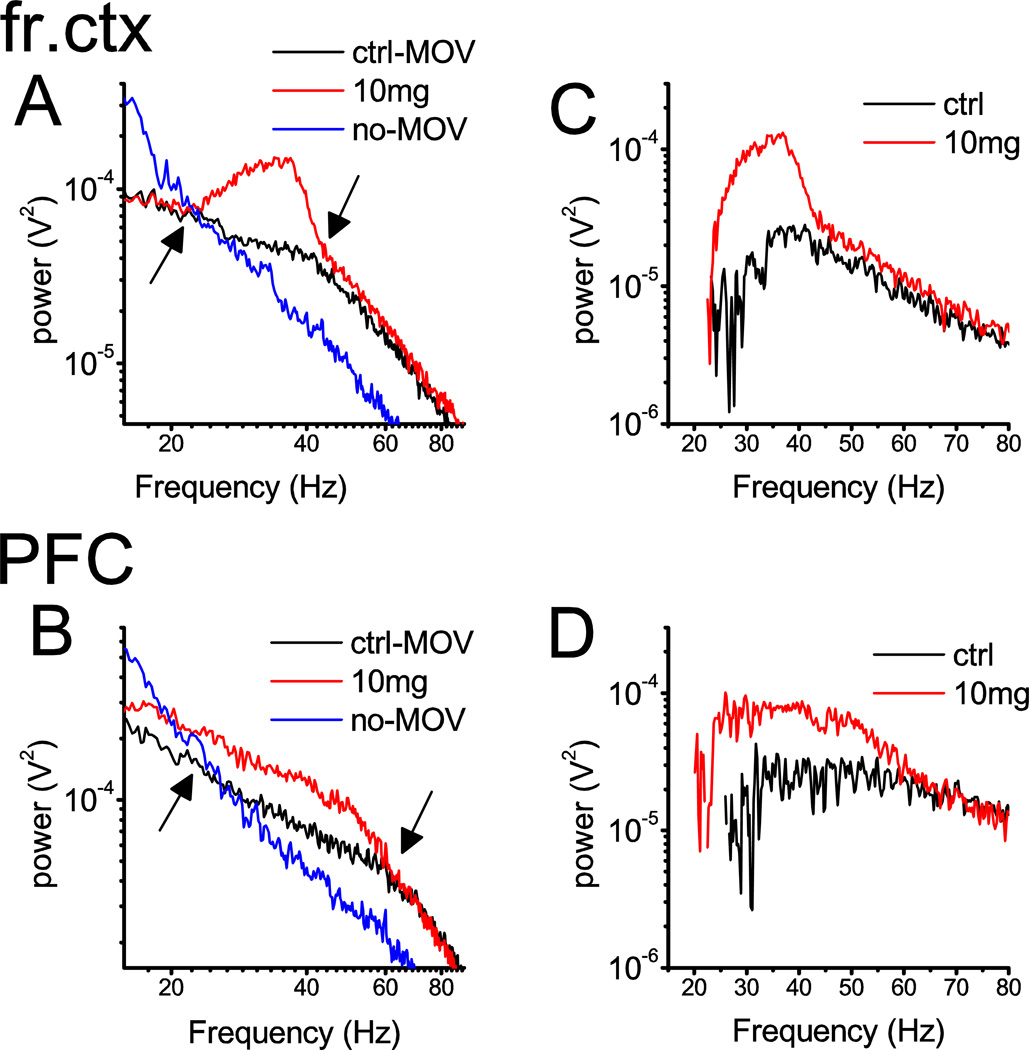
The spectral composition of EEG signals after D4R activation was compared with two types of pre-injection control EEG patterns. First, a ~20 min segment of quiet waking/slow wave sleep was selected in which there was no EMG activity, no theta activity in the hippocampal EEG, and frontal cortex EEG was dominated by irregular delta band activity. The PSD of these segments (blue traces in Fig 1A and B) showed the well-known 1/f-like characteristics in the frequency range between ~15 Hz and 90 Hz, i.e. above the characteristic slow oscillatory components and below the filter cut-off frequency of 100 Hz (Wright et al., 2001). The second segment (~20 min long) was selected from a period when the rats showed active motor behavior, indicated by high EMG activity and an EEG pattern characteristic for a high vigilance state. The PSD of these EEG segments (black traces in Fig. 2A and B) contained two linear components on the log-log plot, both showing 1/f-like decline of power with increasing frequency, but at different rates. The “knee point”, at which the slope of the spectra changed to a steeper decline, differed from one cortical area to the next, being between 44 and 47 Hz in the frontal, parietal and hippocampal EEG but a somewhat higher frequencies in occipital cortex (52±3 Hz) and PFC (59±1 Hz) (see Table S1). The two control PSDs (see blue and black lines in Fig 1A and B) crossed between 21 and 24 Hz in all recording locations (Table S1). Activation of D4Rs increased spectral power (red traces in Fig 1A and B) between these two characteristic frequencies (see arrows); outside of this range, slopes of the 1/f-like activity were similar to the active waking control EEG. Thus, D4R agonist primarily affected gamma frequencies in a wide range, and the effect somewhat extended into the upper part of beta band. There was no change at lower beta frequencies and the peak of enhanced power always fell into the gamma band, i.e. above 30 Hz. indicating a primary involvement of gamma oscillations. Spectral analysis included the 20–65 Hz range to cover the entire range of enhanced power, and the conclusions regarding gamma activity were also verified by repeating the analysis in the 30–65 Hz range.
Figure 1.
Spectral characteristics of the changes in EEG induced by D4R activation. A and B. Power density spectra of field potential recordings in two cortical areas, frontal (A) and prefrontal (B) cortex, in a representative experiment during 20 min long continuous episodes of pre-injection quiet waking/slow-wave sleep (blue; no-MOV), and active waking (black; MOV) and after drug injection (10 mg/kg A-412997)(red) between 15 and 90 Hz. Note 1/f-like PSD patterns associated with non-active states (blue) and two-segment composite PSDs in active waking (black) with a “knee” point at ~42 Hz in frontal and at ~60 Hz in prefrontal cortex and crossing of the two spectra at ~20 Hz (marked by arrows). Spectral power increased between these two characteristic frequencies. C and D. Differences between PSD in active and quiet pre-injection control episodes (black; ctrl) and between post-injection EEG and quiet pre-injection control (red).
Figure 2A shows the temporal variations of gamma band (20–65 Hz) power, along with integrated EMG activity, in the first 11 hours of a representative experiment in which the D4R agonist A-412997 was injected in the highest dose (10 mg/kg), after 5 hours of control recording. At the beginning of the experiment, after the rats were tethered and moved from their home cage in the recording box, they engaged in active motor behavior for the first 20 to 90 min (e.g. high EMG activity in the first 70 min in Fig 1), even though they had been familiarized with their own individual recording boxes earlier. Their behavior during the next 3–4 hours was dominated by slow wave sleep and quiet waking, which were regularly interrupted by rapid eye movement sleep and short episodes of active waking. After injection, the rats were again awake for a period which lasted from 31±3 min after vehicle, to 112±10 min after highest dose of A-412997 (see Table S2). Longer periods of active waking appeared later during the day (e.g. 9.5–10.5 hr in Fig. 2A) and more during the night (not shown). Motor activity was always accompanied by a 26+7% and 21+4 % (in frontal cortex and PFC respectively) increase (p<0.05) in gamma band power, but this increase was significantly exceeded by a lasting gamma enhancement induced by the D4R agonist (~75–100 % in the example in Fig 1A).
For statistical comparison, pre- and post-injection gamma power was calculated in the 20–65 Hz (Fig. 2B and C), as well as in the 30–65 Hz frequency range, which gave similar results. Since the rats were awake and showed motor activity after drug injections, the active control segment served as pre-injection control. After injection of the D4R agonist at the highest dose, gamma power increased in each region (frontal cortex: 49±15%, PFC: 40±11%, parietal: 38±18%, occipital: 47±7%, hippocampus CA1: 19±6%)(Fig. 2B)(see Fig. S1 and Table S3 for the effect after other doses). The gamma increase reached significance (t-test, p<0.05) in the frontal cortex and PFC and showed a strong tendency for increase (0.05<p<0.1) in the remaining locations. Two-way ANOVA confirmed a significant drug effect (F[4,89]=15.31, p=2.4E-9, statistical power at alpha=0.05: 1), whereas the difference between cortical areas was close to a significant level (F[5,89]=2.32, p=0.0507). Post-hoc Bonferroni testing showed significant differences in all pair-wise dose comparisons, except for pairs composed of saline, and the lower doses (0.1 and 0.3 mg/kg). The effect was dose-dependent indicated by the significant difference between the effects after 3 and 10 mg/kg in pair-wise comparison, whereas 0.3 and 1.0 mg/kg injections were ineffective (Fig. 2C). The results were similar and led to identical statistical conclusions (Table S4) when the analysis was limited to frequencies above 30 Hz.
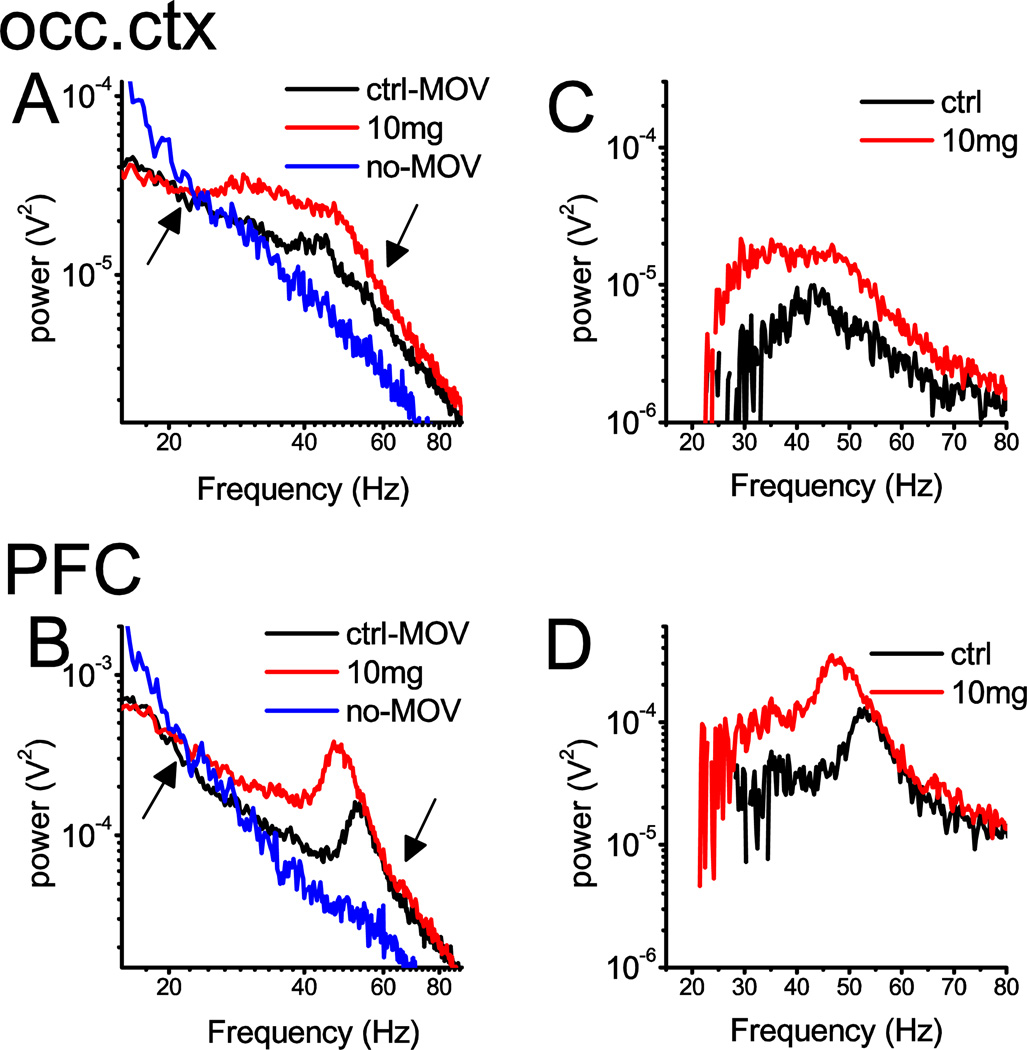
D4R activation also elicited a massive enhancement of gamma band power in MAM-treated rats (Fig. 3A). Baseline power was higher in MAM-treated rats than in normal controls in the PFC and occipital cortex (t-test, p<0.05). Drug-induced increase in gamma activity was highest in PFC where it reached 81±19% (t-test, p=0.004), after injecting 10 mg/kg A412997. In other areas, gamma increased between 38 and 52% (Fig. 3B)(see Table S3 for 20–65 Hz and S4 for 30–65 Hz). The effect was dose-dependent (Fig. 3C); two-way ANOVA confirmed a significant drug effect (F[2,57]=23.22, p=7.3E-8, statistical power at alpha=0.05: 1) and a post-hoc Bonferroni test showed significant differences in all pair-wise comparisons, between saline, 3 mg/kg and 10 mg/kg A-412997. D4R effect on gamma activity in MAM-rats was generally stronger than in normal rats (two-way ANOVA F[1,121]=5.27, p=0.02), although in pair-wise comparisons the group differences only came close to significant level in the PFC (t-test, p=0.056). The character of gamma band PSD also differed between the two groups. In MAM-treated rats, there were prominent gamma peaks at 51±1 Hz in pre-injection active control EEG recordings which was most remarkable in the PFC in all rats (Fig. 4B). After injection of D4R agonist, the peak gamma frequency in PFC decreased (46±2 Hz; in Fig. 4) while its amplitude increased (Fig. 3B). In other areas (e.g. occipital cortex in Fig. 4A) gamma enhancement was more uniform over the entire gamma band.
Figure 4.
Spectral characteristics of the changes in EEG induced by D4R activation in a MAM-treated rat. A and B. Power density spectra of field potential recordings in the occipital (A) and prefrontal (B) cortex, in pre-injection quiet waking/slow-wave sleep (blue; no-MOV), and active waking (black; MOV) and after drug injection (10 mg/kg A-412997)(red) between 15 and 90 Hz. Note 1/f-like PSD patterns associated with non-active states (blue) and two-segment composite PSDs in active waking (black) with a “knee” point at ~60 Hz, with a gamma peak at ~55 Hz riding on the top; note also crossing of the two spectra at ~20 Hz (marked by arrows). Spectral power increased between these two characteristic frequencies. C and D. Difference between PSD in active and quiet pre-injection control episodes (black; ctrl) and between post-injection EEG and quiet pre-injection control (red).
Discussion
A few years after the discovery of high affinity binding of clozapine to the D4R (Van Tol et al., 1991) and altered D4R expression in postmortem schizophrenic brains (Seeman et al., 1993), the original enthusiasm regarding D4R weakened when highly selective antagonists were found ineffective as antipsychotic agents in neuroleptic-sensitive patients (Kramer et al., 1997) and failed to improve scores on the positive and negative symptom scales for schizophrenic patients (Corrigan et al., 2004). The present findings, along with those in a recent in vitro study (Andersson et al., 2012a; Andersson et al., 2012b), suggest, however, that D4Rs may be involved in other aspects, namely cognitive impairment, in schizophrenia and possibly other psychiatric diseases, such as autism and ADHD (Deth, 2003; Tarazi et al., 2004; Deth et al., 2008). These conditions share common features including abnormal gamma oscillations (Yordanova et al., 2001; Hall et al., 2011; McFadden et al., 2012; Uhlhaas and Singer, 2012), which are essential for a variety of cognitive functions. Thus, the current findings of a robust effect of a D4R agonist on gamma activity in different cortical areas, including the PFC, of freely moving rats, and in hippocampal slices in vitro, raises the potential of targeting the D4R for treatment of cognitive symptoms in these disorders. D4R agonists including A-412997, was effective in restoring cognitive deficits in specific tasks in preclinical studies (Browman et al., 2005; Woolley et al., 2008).
There is a rapidly growing interest in altered gamma oscillations in schizophrenia, fueled by a shift in understanding the core deficits from psychotic to negative symptoms and to cognitive deficits. The latter are unaffected by antipsychotic drugs but have stronger predictive value than the severity of positive symptoms (Tamminga et al., 1998). Abnormal cortical oscillations are commonly associated with structural changes of interneuron networks such as decreased expression of GAD67 and PV in GABAergic cells, which is one of the most reliable postmortem findings in schizophrenic brains (Woo et al., 1997). The effect of D4R activation on gamma oscillation is most likely mediated by an action on this type of neurons, which are particularly enriched with D4R. Indeed, Anderson and colleagues (Andersson et al., 2012a) provided evidence of increased synchronization of fast firing interneurons and IPSPs in pyramidal cells with gamma field potentials after D4R activation. They also proposed that the D4R effect involves NMDARs, as blockade with AP5 significantly reduced D4R agonist-induced gamma enhancement. Although the effect of NMDAR antagonists on gamma oscillations in vivo is diametrically different from in vitro (Cunningham et al., 2006; Roopun et al., 2008), both in rodents (Pinault, 2008) and human (Hong et al., 2010), our present findings are not inconsistent with a joint action of the two receptors on the same interneurons. D4R activation was previously shown to reduce NMDAR-mediated current and EPSPs (Wang et al., 2003), which may lead to enhanced gamma activity, similar to that after NMDAR blockade or genetic ablation of NMDAR in PV+ interneurons (Korotkova et al., 2010; Carlen et al., 2012).
Since research focusing on altered neuronal network dynamics in schizophrenia is primarily conducted in the framework of the NMDAR hypofunction hypothesis, it is worthwhile to compare the D4R gamma effect with that induced by NMDAR antagonists. In freely moving rats, both D4R activation and NMDAR antagonism lead to similar, steady enhancements of gamma activity, which appear at short latency, last for several hours, and are associated with active waking behavior and high EMG activity. However, the spectral characteristics of gamma activity differ between the two conditions. In the present study, gamma enhancement after D4R activation in normal rats was limited to the frequency band marked by the 1/f-like segment of the spectrum that normally appears during high vigilance states (Wright et al., 2001; Buzsaki, 2006). In contrast, enhanced gamma activity after NMDAR blockade showed two prominent peaks, one between 30–50 Hz and the other between 60–70 Hz, i.e. outside of this range (Kocsis, 2012). Interestingly, when NMDR blockade selectively targeted NR2A subunit-containing receptors, gamma enhancement was dominant in the lower gamma range (Kocsis, 2012), i.e. similar to that after D4R activation. This specific type of NMDR is predominantly expressed in PV+ interneurons and is thus most likely co-localized with D4Rs (Xi et al., 2009).
There was also a discrepancy between the gamma responses to NMDAR blockade vs. D4R activation in MAM-treated rats. This neurodevelopmental model was shown to recapitulate the essential feature of human schizophrenia (Moore et al., 2006; Lodge et al., 2009), namely, an initial damage carried from before birth leading to pathologic symptoms which only manifest after puberty (Le Pen et al., 2006). It also showed a gamma deficit during schizophrenia-relevant cognitive tasks (Lodge et al., 2009), and increased background gamma activity (Kocsis et al., 2012), matching the common pattern of gamma alterations seen in other rodent models and in human schizophrenia patients (Gandal et al., 2012; Kocsis et al., 2013). NMDAR antagonists elicit an exaggerated hyperlocomotion response in MAM-treated rats, but the concomitant gamma enhancement is weaker than in normal rats (Kocsis et al., 2012; Phillips et al., 2012). This is different from the finding of this study, in that MAM-treated rats showed no deficit in D4R-induced gamma enhancement; the reaction in the PFC was even larger than in the control group. The exact mechanism of NMDAR antagonist-induced gamma impairment remains unknown, but it is commonly associated with disfacilitation of PV+ interneurons, which show abnormalities such as low GAD67 and PV expression in schizophrenia and in different animal models, including the MAM model. D4R expression has not been studied in animal models, but was shown to be increased in schizophrenic patients (Seeman et al., 1993). Since D4Rs are predominantly expressed in interneurons (Mrzljak et al., 1996), their increase might represent a compensatory reaction to interneuron dysfunction and might explain the different reactions of oscillatory networks to the manipulation of these receptors.
Supplementary Material
Figure S1. Dose-dependent effect of D4R agonist on gamma band power in cortical EEG. A. Time course of gamma power (20–65 Hz) in the frontal and prefrontal cortex in a representative experiment, along with neck muscle EMG. Saline (A) or A-412997 was injected between 4 and 5 hours after the start of the recording (see arrow) in doses of 0.3 mg/kg (B), 1.0 mg/kg (C) and 3.0 mg/kg, i/p (cf. with 10 mg/kg injection in the same rat in Fig. 2A). Power is expressed as percent change relative to a 1 hr quiet period with no muscle activity between hrs 3 and 4. Note gamma increase only in D compared with gamma during control active behavior (~150%).
Aknowledgements
This study was supported by MH087777, HL95491
Footnotes
Conflict of interest:
The authors declare no competing financial interests
REFERENCES
- Aguirre AJ, Apiquian R, Fresan A, Cruz-Fuentes C. Association analysis of exon III and exon I polymorphisms of the dopamine D4 receptor locus in Mexican psychotic patients. Psychiatry Res. 2007;153:209–215. doi: 10.1016/j.psychres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Andersson R, Johnston A, Fisahn A. Dopamine D4 receptor activation increases hippocampal gamma oscillations by enhancing synchronization of fast-spiking interneurons. PLoS One. 2012a;7:e40906. doi: 10.1371/journal.pone.0040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson RH, Johnston A, Herman PA, Winzer-Serhan UH, Karavanova I, Vullhorst D, Fisahn A, Buonanno A. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci U S A. 2012b;109:13118–13123. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Curzon P, Pan JB, Molesky AL, Komater VA, Decker MW, Brioni JD, Moreland RB, Fox GB. A-412997, a selective dopamine D4 agonist, improves cognitive performance in rats. Pharmacol Biochem Behav. 2005;82:148–155. doi: 10.1016/j.pbb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan MH, Gallen CC, Bonura ML, Merchant KM. Effectiveness of the selective D4 antagonist sonepiprazole in schizophrenia: a placebo-controlled trial. Biol Psychiatry. 2004;55:445–451. doi: 10.1016/j.biopsych.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Herrmann CS, Erdal ME, Ergenoglu T, Keskin YH, Ergen M, Beydagi H. DRD4 and DAT1 polymorphisms modulate human gamma band responses. Cereb Cortex. 2007;17:1007–1019. doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Deth RC. Molecular origins of attention: the dopamine-folate connection. Amsterdam: Kluwer Academic Publishers; 2003. [Google Scholar]
- Gadow KD, DeVincent CJ, Pisarevskaya V, Olvet DM, Xu W, Mendell NR, Finch SJ, Hatchwell E. Parent-child DRD4 genotype as a potential biomarker for oppositional, anxiety, and repetitive behaviors in children with autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1208–1214. doi: 10.1016/j.pnpbp.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr Bull. 2011;37:1187–1199. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Dybvik M, Harper J, Ronnestad K. Changes in background and ketamine induced gamma oscillations in the MAM-model of schizophrenia. Biological Psychiatry. 2012;71:39S. [Google Scholar]
- Kocsis B, Brown RE, McCarley RW, Hajos M. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther. 2013;19:437–447. doi: 10.1111/cns.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Last B, Getson A, Reines SA. The effects of a selective D4 dopamine receptor antagonist (L-745,870) in acutely psychotic inpatients with schizophrenia. D4 Dopamine Antagonist Group. Arch Gen Psychiatry. 1997;54:567–572. doi: 10.1001/archpsyc.1997.01830180085011. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Shu BC, Kao WT, Chen CN, Ku YC, Tzeng DS. Association of DRD4 uVNTR and TP53 codon 72 polymorphisms with schizophrenia: a case-control study. BMC Med Genet. 2009;10:147. doi: 10.1186/1471-2350-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DE, Lubke G, Yang M, McCracken JT, McGough JJ, Ishii J, Loo SK, Nelson SF, Smalley SL. Temperament and character profiles and the dopamine D4 receptor gene in ADHD. Am J Psychiatry. 2005;162:906–913. doi: 10.1176/appi.ajp.162.5.906. [DOI] [PubMed] [Google Scholar]
- McFadden KL, Hepburn S, Winterrowd E, Schmidt GL, Rojas DC. Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry. 2012;12:213. doi: 10.1186/1471-244X-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough JJ. Attention deficit hyperactivity disorder pharmacogenetics: the dopamine transporter and D4 receptor. Pharmacogenomics. 2012;13:365–368. doi: 10.2217/pgs.12.5. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland RB, Patel M, Hsieh GC, Wetter JM, Marsh K, Brioni JD. A-412997 is a selective dopamine D4 receptor agonist in rats. Pharmacol Biochem Behav. 2005;82:140–147. doi: 10.1016/j.pbb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Phillips KG, Cotel MC, McCarthy AP, Edgar DM, Tricklebank M, O'Neill MJ, Jones MW, Wafford KA. Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62:1359–1370. doi: 10.1016/j.neuropharm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wakerelated aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Ptacek R, Kuzelova H, Stefano GB. Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders. Med Sci Monit. 2011;17:RA215–RA220. doi: 10.12659/MSM.881925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Regionspecific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Buchanan RW, Gold JM. The role of negative symptoms and cognitive dysfunction in schizophrenia outcome. Int Clin Psychopharmacol. 1998;13(Suppl 3):S21–S26. doi: 10.1097/00004850-199803003-00004. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Dopamine D4 receptors: beyond schizophrenia. J Recept Signal Transduct Res. 2004;24:131–147. doi: 10.1081/rrs-200032076. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Waters KA, Reavill C, Bull S, Lacroix LP, Martyn AJ, Hutcheson DM, Valerio E, Bate S, Jones DN, Dawson LA. Selective dopamine D4 receptor agonist (A-412997) improves cognitive performance and stimulates motor activity without influencing reward-related behaviour in rat. Behav Pharmacol. 2008;19:765–776. doi: 10.1097/FBP.0b013e32831c3b06. [DOI] [PubMed] [Google Scholar]
- Wright JJ, Robinson PA, Rennie CJ, Gordon E, Bourke PD, Chapman CL, Hawthorn N, Lees GJ, Alexander D. Toward an integrated continuum model of cerebral dynamics: the cerebral rhythms, synchronous oscillation and cortical stability. Biosystems. 2001;63:71–88. doi: 10.1016/s0303-2647(01)00148-4. [DOI] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder--evidence from event-related gamma oscillations. Clin Neurophysiol. 2001;112:1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dose-dependent effect of D4R agonist on gamma band power in cortical EEG. A. Time course of gamma power (20–65 Hz) in the frontal and prefrontal cortex in a representative experiment, along with neck muscle EMG. Saline (A) or A-412997 was injected between 4 and 5 hours after the start of the recording (see arrow) in doses of 0.3 mg/kg (B), 1.0 mg/kg (C) and 3.0 mg/kg, i/p (cf. with 10 mg/kg injection in the same rat in Fig. 2A). Power is expressed as percent change relative to a 1 hr quiet period with no muscle activity between hrs 3 and 4. Note gamma increase only in D compared with gamma during control active behavior (~150%).