Abstract
Objective
In the fasting state, plasma free fatty acids (FFA) are thought to derive almost exclusively from adipose tissue lipolysis. However, there are mixed reports as to whether the spillover of fatty acids (FA) from very low density lipoprotein triglyceride (VLDL-TG) hydrolysis contributes significantly to the plasma FFA pool. Because substantial VLDL-TG fatty acid spillover into the plasma FFA pool would profoundly impact the interpretation of isotope dilution measures of FFA flux, we investigated the contribution of VLDL-TG spillover to plasma FFA appearance.
Materials/Methods
Eighteen obese adults (15 women) participated in these studies. Each volunteer received a primed, continuous infusion of their own ex-vivo labeled ([1-14C]triolein) VLDL-TG and a continuous infusion of [U-13C]oleate (8 nmol · kg fat free mass−1 · min−1) to measure VLDL-TG and FFA rate of appearance (Ra), respectively. The presence of 14C-oleate in the plasma FFA-oleate pool was used to calculate the contribution of spillover from VLDL-TG-oleate to the plasma FFA-oleate Ra.
Results
The spillover rate of VLDL-TG-oleate into plasma FFA-oleate was 6 ± 2 μmol/min (7 ± 2% percent of [14C]oleate from VLDL-TG) and FFA-oleate flux was 240 ± 61 μmol/min. Thus, only 3 ± 1% of total plasma FFA-oleate appearance could be accounted for by VLDL-TG spillover.
Conclusion
The contribution of VLDL-TG spillover to the total plasma FFA pool is negligible and will not materially affect the interpretation of FFA flux measures as an index of adipose tissue lipolysis.
Keywords: Isotope dilution, [U-13C]oleate, [14C]triolein, hypertriglyceridemia
Introduction
Increased free fatty acids (FFA) derived from excess adipose tissue lipolysis is considered a contributor, if not a primary mediator, of insulin resistance and hypertriglyceridemia [1]. Traditionally, isotopically labeled FFA tracer infusions have been used to measure effective adipose tissue lipolysis under the assumption that the FFA appearing in systemic circulation was a direct result of adipocyte release [2–4]. However, exceptions to this have been observed in that chylomicron-triglyceride fatty acid (TGFA) spillover may contribute 20 – 30% of plasma FFA under postprandial conditions [5–7]. This may vary depending on measurement time following the meal and appears to be greater in adipose tissue than skeletal muscle [8, 9]. Although it is generally thought that postabsorptive very low density lipoprotein triglyceride (VLDL-TG) spillover of FFA is < 5% based upon studies of dogs [10], one group reported that fasting VLDL-TG spillover was ~70% in the venous plasma of subcutaneous adipose tissue in humans [11], albeit using different methodology. Given these discrepant findings and the huge impact that a large VLDL-TG spillover would have on interpretation of FFA flux results, we designed a study using independent VLDL-TG and FFA tracers to measure spillover in humans with elevated VLDL-TG.
In order to circumvent the problem of chylomicron-TGFA spillover influencing interpretation of postprandial FFA flux measurements, both an FFA tracer infusion and a meal tracer can be used [5]. Similarly, VLDL-TG spillover needs to be assessed by infusing both a VLDL-TG tracer and an FFA tracer. We employed the ex-vivo labeled VLDL-TG tracer methodology developed for humans by Gormsen et al. [12] to measure both VLDL-TG turnover and the contribution of VLDL-TG spillover to FFA flux, measured using a stable isotope FFA tracer infusion. Using this combination of approaches we were able to directly measure the contribution of post-absorptive VLDL-TGFA spillover to the plasma FFA pool.
Methods
Participants
Eighteen obese (BMI >35kg/m2) adults (15 women) aged 18–55 y scheduled for bariatric surgery participated in this study, which is part of a larger study of the relationship between hepatic fat content and VLDL-TG turnover. Participants taking lipid lowering medications (e.g. fibrates, statins, niacin) could only be included if it was deemed safe for them to discontinue their use 4 weeks prior to the study. Potential volunteers receiving beta-blockers must have been able to safely discontinue their use 3 days prior to the study in order to participate. Exclusion criteria included type 2 diabetes treated with oral medications or insulin, type 1 diabetes, presence or history of liver disease other than non-alcoholic fatty liver disease, use of nicotine, alcohol consumption exceeding 20g/day, and the use of medications known to affect lipid metabolism that could not be discontinued prior to the study. The study was approved by the Mayo Clinic Institutional Review Board and written informed consent was obtained from all volunteers.
Protocol
Approximately one week prior to the inpatient study visit, each participant was seen at the Mayo Clinic outpatient Clinical Research Unit where a 100 mL fasting blood sample was drawn under sterile conditions to be used for ex-vivo VLDL-TG labeling with [1-14C]triolein (PerkinElmer, Boston, MA) and body composition was measured using dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, Madison, WI). The following week participants were admitted to the Mayo Clinic inpatient clinical research unit the evening before the VLDL-TG turnover study. They were provided with a meal at ~ 1800 h and then remained fasting except for water overnight. The following morning an intravenous catheter was placed in a forearm vein for tracer infusions, and a retrograde intravenous catheter was placed in the contralateral hand vein to allow for collection of arterialized blood using the heated (55°C) hand vein technique [13]. At ~0700 h, after collecting a baseline blood sample, a primed, continuous infusion of ex-vivo labeled [1-14C]VLDL-TG was started to measure VLDL-TG turnover [14]. Simultaneously, a continuous infusion of [U-13C]oleate (8 nmol · kg fat free mass−1 · min−1, Isotec, Sigma-Aldrich, Miamisburg, OH) was started to measure FFA kinetics. Blood samples were obtained at 30, 60, 90, 150, 180, 210 and 240 min for measuring VLDL-TG concentration and specific activity (SA) and plasma oleate concentration and enrichment.
Ex-vivo VLDL-TG tracer preparation
Details regarding ex-vivo labeling of VLDL-TG with [1-14C]triolein have previously been published [14]. In brief, the plasma was separated by centrifugation and aliquoted into three sterile vials to which an average of 38 ± 4 μCi of [1-14C]triolein.(~12–13 μCi per vial) was added. The plasma was then shaken at room temperature for 2 h at 225 rpm (Barnstead Lab-Line MaxQ 4000 E-class shaker, Melrose Park, IL). To isolate the VLDL-TG fraction, 2.5 mL of plasma was layered beneath 3.5 mL of 0.9% normal saline (d = 1.006 g/ml) in a sterile 6 mL centrifuge tube (Quick-Seal, Beckman Coulter Inc., Fullerton, CA), which was centrifuged at 45,000 rpm for 18 h at 4°C (50.3 Ti rotor, Optima™, LE-80K, Beckman Instruments, Spinco Division, Palo Alto, CA). After centrifugation, the top 2 – 2.5 mL containing the VLDL-TG fraction were removed, filtered (Millex GV 0.22μm filter, Millipore, Billerica, MA) and mixed with normal saline to achieve the volume needed for infusion. The SA of the final infusate was determined by liquid scintillation counting and was used to calculate the total activity infused (average ± SD: 13 ± 4 μCi) and the infusion rate. All preparation involving transferring and handling of samples was performed under sterile conditions, and all samples were tested for pyrogens and sterility before infusion.
Plasma and VLDL-TG oleate SA, concentration and enrichment
The VLDL-TG fraction was isolated from plasma samples by density gradient ultracentrifugation as described above. The tubes are sliced with the Beckman tube slicer 2 cm from the top and the VLDL fraction was aspirated into a pre-weighed tube, which was then reweighed in order to calculate the total volume. For each time point, a 1 mL sample of the VLDL fraction was analyzed for specific activity by liquid scintillation counting and 0.5 mL sample was analyzed for TG concentration (mmol/L; Cobas Integra® 400 plus, Roche Diagnostics, Ltd., Indianapolis, IN) from which total plasma VLDL-TG (μmol/L) was calculated. Total VLDL-TG SA and concentration were calculated using the average steady-state values.
To determine the oleate SA and concentration in VLDL-TG, 1 mL of the VLDL fraction from the 210 time point during infusion was extracted with chloroform:methanol (2:1) and the TG fraction was collected using solid phase extraction columns (Supelco Supelclean LC-NH2 columns, Sigma-Aldrich, St. Louis, MO). After hydrolyzing the TG fraction with methanol:heptane (80:20) and sodium hydroxide, the FA were extracted using Dole solution, derivatized and analyzed by HPLC to determine oleate SA [3].
Plasma FFA-oleate concentration and 14C SA were also analyzed using HPLC [3]. Plasma oleate enrichment at M+18 was measured using liquid chromatography/mass spectrometry [15] and used to calculate FFA-oleate rate of appearance (Ra).
Calculations and Statistics
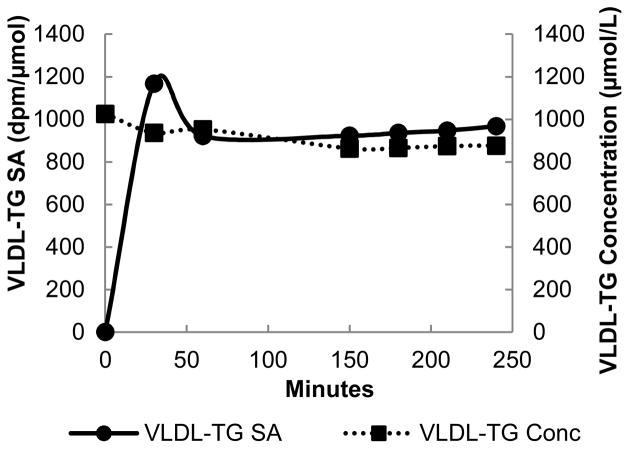
Descriptive, FFA and VLDL-TG kinetic data are presented as mean ± SD. The average [14C]VLDL-TG SA (dpm/umol) and [U-13C]oleate enrichment (mpe) during steady-state was used to calculate VLDL-TG Ra and FFA Ra, respectively, using steady-state formulas where Ra equals rate of disappearance. Each participant had at least one hour of simultaneous steady-state VLDL-TG and FFA-oleate kinetics between 150 – 240 min from starting the infusion. A typical set of individual results are provide in Figure 1.
Figure 1.
VLDL-TG SA (dpm/μmol; —●—) and VLDL-TG concentration (μmol/L; ···■···) during steady-state.
Results
The subject characteristics are provided in Table 1. Participants were ~39 y of age with an average BMI of ~47 kg/m2. Of the participants included in the study, two had hypertension treated with beta-blockers that were discontinued three days prior to the study and three had hyperlipidemia treated with statins that were discontinued at least one month prior to the study. Four participants had impaired fasting glucose but were not on oral hypoglycemic medications.
Table 1.
Participant characteristics.
| Mean ± SD or % | |
|---|---|
| Age (years) | 39 ± 10 |
| Sex (%) | 83% female; 17% male |
| BMI (kg/m2) | 47.1 ± 9.5 |
| Fat (%) | 53 ± 4 |
Table 2 provides the plasma VLDL-TG concentrations and Ra, as well as plasma FFA concentrations and kinetics. By design, the plasma VLDL-TG concentrations were above the usual normal range [12] in this group of obese volunteers. Oleate averaged 1/3 of VLDL-TGFA.
Table 2.
Plasma VLDL-TG and FFA concentrations, kinetics and spillover.
| Mean ± SD | |
|---|---|
| VLDL-TG Concentration (μmol/L) | 852 ± 421a |
| VLDL-TG Ra (μmol/min) | 93 ± 38b |
| Plasma FFA-Oleate Concentration (μmol/L) | 243 ± 52 |
| FFA-Oleate Ra (μmol/min) | 240 ± 61 |
| Spillover Rate (μmol/min) | 6 ± 2 |
| Fractional VLDL-TG Spillover in Plasma FFA Pool (%) | 7 ± 2 |
| VLDL-Oleate Fractional Appearance in Plasma FFA Pool (%) | 3 ± 1 |
VLDL-TG concentration: 76 ± 37 (mg/dl,);
VLDL-TG Ra: 87 ± 38 (mg/kg FFM/hr).
We found that only 7 ± 2% of oleate from VLDL-TG appeared in the plasma FFA-oleate pool, representing 3 ± 1% of the plasma FFA-oleate appearance. The spillover rate and oleate flux rate are also in Table 2.
Discussion
The purpose of this report is to provide the contribution of VLDL-TGFA to the plasma total FFA pool in postabsorptive, hypertriglyceridemic humans. Using independent VLDL-TGFA and FFA tracers, we found that VLDL-TGFA spillover into the FFA pool was not a significant source of plasma FFA. Therefore, VLDL-TGFA spillover will have minimal impact on the interpretation of FFA flux as an effective marker of adipose tissue lipolysis.
Previous studies documented that postprandial chylomicron-TGFA spillover is a significant source of plasma FFA, contributing 20–30% of the plasma FFA pool [5–7]. Nelson et al. [16] reported that the spillover of FA from a chylomicron-like tracer was also ~30% in obese postabsorptive humans. Although postabsorptive plasma FFA are proposed to derive primarily from adipose tissue lipolysis [4, 17], if VLDL-TG spillover was as prominent as chylomicron-TGFA, this would present a significant source of non-suppressible FFA. A recent report by Ruge et al. [11] suggested that fasting VLDL-TG spillover of FA in the venous supply of subcutaneous adipose tissue was ~70% in lean males, while an older study showed that <5% of VLDL-TGFA were found in plasma [10]. Given the latter study was conducted in dogs and the former study using indirect/modeling approaches to estimate spillover, we elected to directly measure whole body spillover using a validated ex-vivo VLDL labeling technique [12, 14] and a stable isotope FFA turnover approach. Although it seems as if the findings of Ruge et al. [11] are in stark contrast to ours and the previous study in dogs [10], in actuality, 70% spillover in the subcutaneous adipose tissue venous supply would likely contribute only a small fraction to systemic FFA.
Although it could be argued that our study of severely obese volunteers with high VLDL-TG concentrations limits the generalizability of our results, it seems that if VLDL-TG spillover were to make a major contribution to FFA appearance rates, it would be in this population. Therefore, we suggest that the relatively small contribution of VLDL-TGFA to FFA in this population indicates that the effects of spillover on the interpretation of FFA kinetic results will be likewise negligible in metabolically normal populations. Furthermore, our results indicate that LPL-mediated hydrolysis of VLDL-TG results in the efficient, almost direct transfer of fatty acids to the underlying local tissues and very little in the way of mixing with the FFA pool. This is somewhat in contrast to chylomicron-TG hydrolysis, which can contribute a relatively large fraction of plasma FFA under some conditions.
In conclusion, the contribution of FA from VLDL-TG spillover to the plasma FFA pool is negligible. This indicates that isotope dilution studies of FFA release can be used as a legitimate index of effective adipose tissue lipolysis.
Acknowledgments
We would like to express our sincere appreciation to the individuals that participated in this study. Additionally, we would like to thank our research and laboratory team, Barb Norby, Carley Vrieze, Christy Allred, Deb Harteneck, Darlene Lucas, and Lendia Zhou as well as the CTSA Clinical Research Unit staff and core laboratories.
The current address for Jessica M. Triay is Department of Diabetes and Endocrinology, Bristol Royal Infirmary, Marlborough Street, Bristol, BS1 3NU, United Kingdom.
Grant support
Supported by grants DK40484, DK45343, DK50456 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01and UL1 TR000135. N.B. received fellowship funding from the NIH training grant T32-DK07352 and the ADA fellowship grant 7-112-MN-36. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of Abbreviations
- FFA
free fatty acids
- FA
fatty acid
- TG
triglyceride
- VLDL
very low density lipoprotein
- BMI
body mass index
- SA
specific activity
Footnotes
Conflict of interest statement: The authors have no conflict of interest to disclose at this time.
Disclosures
The authors have no conflict of interest to disclose at this time.
Author contributions
Author contributions are as follows: conception and study design (NCB and MJD), performed experiments (NCB, JMT, NWG and KCH), analyzed data (NCB, JMT and MJD), interpreted data (NCB, JMT, KCH and MJD), drafted manuscript (NCB and MJD), edited and revised (NCB, JMT, NWG, KCH and MJD), approved final copy (NCB, JMT, NWG, KCH and MJD).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Diabetes. 1995;44:863–869. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RR, Evans JE, Mullany CJ, et al. Measurement of plasma free fatty acid turnover and oxidation using [1-13C]palmitic acid. Biomed Mass Spectrom. 1980;7:168–171. doi: 10.1002/bms.1200070407. [DOI] [PubMed] [Google Scholar]
- 3.Miles JM, Ellman MG, McClean KL, et al. Validation of a new method for determination of free fatty acid turnover. Am J Physiol Endocrinol Metab. 1987;252:E431–E438. doi: 10.1152/ajpendo.1987.252.3.E431. [DOI] [PubMed] [Google Scholar]
- 4.Samra JS, Clark ML, Humphreys SM, et al. Regulation of Lipid Metabolism in Adipose Tissue During Early Starvation. Am J Physiol. 1996;271:E541–E546. doi: 10.1152/ajpendo.1996.271.3.E541. [DOI] [PubMed] [Google Scholar]
- 5.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42:1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 6.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 7.Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res. 2007;39:726–729. doi: 10.1055/s-2007-990273. [DOI] [PubMed] [Google Scholar]
- 8.Evans K, Burdge GC, Wootton SA, et al. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 2002;51:2684–2690. doi: 10.2337/diabetes.51.9.2684. [DOI] [PubMed] [Google Scholar]
- 9.Miles JM, Park YS, Walewicz D, et al. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 2004;53:521–527. doi: 10.2337/diabetes.53.3.521. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR, Shaw JH, Durkot MJ. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985;248:E732–740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]
- 11.Ruge T, Hodson L, Cheeseman J, et al. Fasted to fed trafficking of Fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab. 2009;94:1781–1788. doi: 10.1210/jc.2008-2090. [DOI] [PubMed] [Google Scholar]
- 12.Gormsen LC, Jensen MD, Nielsen S. Measuring VLDL-triglyceride turnover in humans using ex vivo-prepared VLDL tracer. J Lipid Res. 2006;47:99–106. doi: 10.1194/jlr.M500205-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991;40:406–409. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen LP, Gormsen LC, Nielsen S. VLDL-TG kinetics: a dual isotope study for quantifying VLDL-TG pool size, production rates, and fractional oxidation in humans. Am J Physiol Endocrinol Metab. 2009;297:E1324–E1330. doi: 10.1152/ajpendo.00366.2009. [DOI] [PubMed] [Google Scholar]
- 15.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761–2765. doi: 10.1194/jlr.M008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson RH, Basu R, Johnson CM, et al. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes. 2007;56:2878–2884. doi: 10.2337/db07-0812. [DOI] [PubMed] [Google Scholar]
- 17.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans [Review] J Lipid Res. 1994;35:177–193. [PubMed] [Google Scholar]