Abstract
Human self-reports often indicate that changes in mood are a major contributor to drug relapse. Still, arguments have been made that instances of drug-seeking following abstinence in animal models (i.e. relapse/reinstatement) may be outside of hedonic control. Therefore, the present study utilized ultrasonic vocalizations in the rat in order to evaluate affect during cocaine self-administration (S-A) and contextual reinstatement of cocaine-seeking in a pre-clinical model of drug relapse (abstinence-reinstatement model). Results show that while subjects effectively reinstated drug-seeking (lever pressing) following 30 days of abstinence, and spontaneously recovered/reinstated drug seeking following 60 days of abstinence, ultrasonic vocalizations did not increase over baseline levels during either reinstatement session. These results are consistent with previous results from our laboratory and current theories of addiction suggesting that cues that are weakly-associated with drug consumption can motivate drug-seeking behavior that is outside of hedonic processing.
Keywords: Affect, Cocaine, Dopamine, Ultrasonic Vocalizations
Introduction
Addiction is a chronically relapsing disorder with three major characteristics: 1) compulsion to seek and use drug, 2) loss of control over drug intake (excess drug consumption) and 3) negative emotional states following the cessation of drug use (Koob & LeMoal 1997, 2008; Koob, 2008). Clinical and preclinical models of addiction focus on understanding these facets in order to develop therapies which prevent relapse.
One set of powerful tools in human studies of addiction are retrospective self-reports (Breiter et al., 1997). While the accuracy of these measures is sometimes questioned (McNagny et al., 1992), self-report data have inarguably uncovered a role for positive and negative affective states in drug relapse that is difficult to quantify using other measures (Bijlsma et al., 2010; Mendelson & Mello, 1996; Hodgins et al., 1995). Indeed, it seems that drug seeking can be driven by pursuit of the positive reinforcement that users recall from early drug experiences (Bijlsma et al., 2010) but can also be driven by negative reinforcement as individuals seek to alleviate a negative mood or state (Baker et al., 2004, Witkiewitz & Marlatt, 2004). It is likely that—for stimulant users—affect can shift rapidly from positive to negative both prior to drug use (Epstein et al., 2009) and following drug use (Breiter et al., 1997). Importantly, this same duality is present in preclinical data and theories derived from animal models of addiction (Koob, 2000; Robinson & Berridge, 1993; Wheeler et al., 2008, 2011).
Positive symptoms associated with cocaine use include euphoria, alertness, and increased confidence (Levinthal 2010 pp 90–115), while negative symptoms include dysphoria, irritability, paranoia, insomnia, and depression (Mendelson & Mello 1996; Williamson et al., 1997). While self-reports can be used to gather indices of affective state from human drug users, analogous measures in preclinical models have recently gained attention (Panksepp et al., 2002; Covington and Miczek., 2003; Thompson et al., 2006; Wheeler et al., 2008, 2011; Ahrens et al., 2009; Mu et al., 2009; Ma et al., 2010; Williams & Undieh, 2010; Simola et al., 2010; Barker et al., 2010; Maier et al., 2010; Wright et al., 2010, 2012; Taracha et al., 2012;; Meyer et al., 2012; Mahler et al., 2013).
One technique for measuring affect in preclinical models is the use of ultrasonic vocalizations (USVs) in the rat. Rat USVs serve as a convenient dependent measure of affect as their bi-modal frequency distribution has been reliably segmented into two frequency ranges: one from ~18–33kHz (“22-kHz range”) that has been repeatedly correlated with negative/aversive outcomes (Brudzynski et al., 1991, 1993; Brudzynski and Ociepa 1992; Knutson et al., 2002; Burgdorf et al., 2000; Vivian and Miczek 1999; Barros and Miczek 1996; Mutschler and Miczek 1998a, 1998b) and the other ranging from ~38–80-kHz (“50-kHz range”) that has been reliably correlated with positive/appetitive outcomes (Barfield and Thomas 1986; Schwarting et al., 2007; Knutson et al., 1999; Burgdorf et al., 2000; Knutson et al., 2002; Brudzynski 2005, 2008; Burgdorf et al., 2011).
Recent preclinical studies have utilized relapse and reinstatement paradigms in concert with USVs (Browning et al., 2011; Mahler et al., 2013), but a test of cocaine contextual reinstatement that includes USVs has yet to be conducted. Theories of addiction posit that all types of cues (paraphernalia, odors and sounds, availability of cocaine, drug-use partners, contexts, stress, etc.) present during cocaine self-administration (S-A) become learned and increase the probability that relapse will occur when an abstinent user is confronted with these cues (Jaffe, 1990; Ahmed & Koob, 1997). Moreover, compounds of multiple cues have been shown to produce additive effects on drug responding (Liu & Weiss, 2002). Thus, it is important to ascertain the contribution of various types of drug-related cues.
The goal of the present study was to examine affective responses during a contextual reinstatement paradigm following long-access cocaine S-A (Ahmed & Koob, 1999). Although reinstatement often includes an extinction phase, forced abstinence was chosen over an extinction procedure given the relevance of the abstinence-reinstatement model to drug dependence and relapse in the clinical setting (Fuchs et al., 2009). In the present experiment, subjects were trained in the absence of other programmed cues (i.e. CS+ or SD) in order to obtain a cue-free assessment of the contribution of affect to contextual reinstatement following 30 and 60 days of abstinence.
Materials and Methods
Subjects/Surgery
Subjects (N= 8) were catheterized and cared for as described previously (Root et al., 2009; Root et al., 2011). Male Long-Evans rats (Charles River, Wilmington, MA) were singly housed on a 12h: 12h light: dark cycle with dawn at 1030 h. All Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals and have been approved by the Institutional Animal Care and Use Committee, Rutgers University.
Apparatus
All experiments were conducted in Plexiglas chambers measuring 24 cm × 34.5 cm × 34.5 cm and housed inside a larger sound attenuating chamber (~76 cm3). Animals were attached to an intravenous fluid delivery system consisting of a syringe pump (Razel Scientific, St. Albans, VT) which connected to a fluid swivel. A spring leash was connected to the bottom of the swivel and extended to the head of the animal through the top of each chamber. Intravenous catheters were contained inside the spring leash and continued through a steel cannula on animals’ heads into the right jugular vein. For self-administration sessions, glass levers (4.7cm × 2.5 cm) were inserted through a hole 4 cm off the floor of the chamber and 6 cm from the door. Levers were set so that 0.049 Newtons of force were required to activate each lever. Experimental apparatuses were controlled by a PC running MED-Associates hardware and software (St. Albans, VT).
Self –Administration
Self-administration training began daily at 10:30 hours, immediately following the commencement of the light cycle. At the start of the session, a single non-retractable response lever was mounted on one wall of the operant conditioning chamber. For the first ten infusions, drug was available during an unsignaled 120 s availability period, followed by a 40s timeout (TO). A response during the availability period produced a 0.71mg/kg infusion of cocaine and initiated the next TO. If no responses occurred for two minutes, the availability period was terminated and the next TO was initiated. The initial 40 s timeout promoted shaping in early sessions and allowed subjects to rapidly load on cocaine during the remainder of training. Following the 10th infusion, for the remainder of the session, unsignaled availability periods (120 s) were presented after a 1–6 minute variable TO. All shaping and training sessions lasted for 6 hours or 80 response-contingent infusions, whichever occurred first. Subjects received post-session feeding in order to maintain weights of approximately 320–340 grams. Water was available ad libitum except during self-administration sessions. Animals were trained for 14 days (7 days/week) with no breaks between training sessions. Subjects were then given a series of five probe sessions (data published elsewhere) consisting of 90 minute periods of normal S-A followed by a manipulation in which animals’ drug-levels were transitioned (over 30 min) to and subsequently maintained within a finite range for the remaining 4 h of the session using a series of computer controlled micro-infusions (0.0064mg/ 5.33ml/ infusion)—similar to methods reported by Root et al. (2011)—over the 5–10 days following normal training. For all but two of the probe sessions, levers were present (but had no programmed response). Each probe session was followed by a one session return to normal self-administration contingencies. Importantly, a study by Kippin et al. (2006) demonstrated that this type of manipulation has little effect on reinstatement responding.
Abstinence/Contextual Reinstatement Tests
Following the final self-administrations session, the catheter was disconnected at the level of the headstage, and animals were returned to their home cages for 60 days of abstinence. At 30 (CR1) and 60 days (CR2) of abstinence animals were returned to the S-A chamber for contextual reinstatement testing. For both tests, the lever was inserted prior to testing and the session began by placing the animals in the chamber immediately following illumination of the houselight. All reinstatement sessions lasted for 6 hours. Lever presses during these sessions were recorded, but had no consequence.
USV Recordings
A condenser microphone (CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany) was inserted into the sound-attenuating chamber 15–30 minutes before recording sessions. The microphone was suspended ~2.5 cm from a set of small holes in the top of the S-A chamber inside of a Plexiglas tube that increased the signal: noise ratio of the recordings. Following the start of the session, recordings were triggered by a 5V TTL pulse sent from MED-PC IV to the recording hardware (Ultrasound Gate 116H, Avisoft Bioacoustics, Berlin, Germany). Sonorous activity was recorded at a 250-kHz sampling frequency (16-bits) using Avisoft Recorder software (Avisoft Bioacoustics, Berlin, Germany) and stored for offline analysis. Recorded “.wav” files were then analyzed using Avisoft SASLab Pro (Avisoft Bioacoustics, Berlin, Germany).
Recording Sessions
Baseline Recordings
Prior to surgery, subjects were allowed to live in the S-A chamber for 3 days. On the fourth day, USVs were recorded for all subjects during a 6-hour baseline period. These recordings occurred from 10:30 AM to 16:30 PM.
S-A USVs Recordings
USVs were sampled at two time points across weeks of self-administration training: days 5 and 10. Day 5 was used as an early sample after the initial 1–3 day shaping period. Day 10 was chosen as an equally spaced sample that occurred late in training. The USV recording was triggered at the start (time zero) of the session and continued until the end of the session.
Contextual Reinstatement
For both the 30 and 60 day reinstatement tests, recordings were triggered immediately after animals were placed in the chamber, and were recorded continuously until the end of the session.
Characterization of USVs
Avisoft SASLab Pro (Avisoft Bioacoustics, Berlin Germany) was used for post-hoc analysis. Each “.wav” file was opened as a spectrogram with a Fast Fourier Transform (FFT) Length of 512 samples and a flat-top window with 50% overlap. Spectrograms were visually scanned for patterns resembling USVs. Once a putative, individual USV had been identified, the mean frequency was calculated by averaging the minimum and maximum frequencies of the call. While USVs can be differentiated into several call-types based on the presence or lack of frequency modulation (Wright et al., 2010; Wöhr & Schwarting 2007), no trends related to call-type were observed in the present data. Moreover, the inclusion of call type in the present data problematically increased zero-inflation of the data for statistical analysis. Thus, only call frequency was included for statistical analysis.
Analysis
S-A behavior was analyzed across training sessions for the first two weeks of training. Behavioral data were analyzed using a repeated measures ANOVA (SAS PROC GLIMMIX, SAS Institute Inc., 2005, Cary, NC) with Sidak adjustments for post-hoc comparisons. Pairwise comparisons were made between session 1 and each subsequent session of training (2–14). Lastly, a Sidak adjusted “plateau contrast” was designed to test whether behavioral measures fit an asymptotic learning curve (contrast coefficients: [−6, −2.25, −1, 0.01, 0.5, 0.75, 1, 1, 1, 1, 1, 1, 1, 1]). Lever presses during reinstatement conditions were analyzed across all 6 hours to test for extinction and subsequently, spontaneous recovery.
USVs were analyzed using 3-way repeated measures ANOVAs examining Hour X Call Frequency X Condition. Hour consisted of six, one-hour bins for each session. Call Frequency consisted of the 22- and 50-kHz ranges as well as a “33-kHz” range [33–38.99 kHz; inserted as a buffer between the 22-kHz (18–32.99 kHz) and 50-kHz (38–80 kHz) ranges]. This buffer is necessary, as graphical analyses have shown that the 50- and 22-kHz frequency distributions overlap in this region and become uninterpretable (e.g. Barker et al 2010). The inclusion of this middle range allows all USVs to be included in the model but assigns no affective state to USVs in this ambiguous range. Condition consisted of each session during which USVs were recorded. Post-hoc comparisons focused on the first hour of each experimental condition. This allowed reinstatement sessions to be directly compared to both S-A and baseline rates of calling, but reduced the influence of extinction learning on USVs during reinstatement sessions, which has been shown to cause a shift from appetitive to aversive calling (Burgdorf et al., 2000).
Results
Self-administration
Behavioral measures included the number of lever presses, number of infusions earned, percent hit [calculated as: (earned infusions/total number of cocaine availability periods)*100; colloquially defined as the percentage of opportunity periods during which animals responded, thereby administering an infusion], total drug consumption (mg/kg), peak drug level (mg/kg), and bodyweight (grams). Behavioral data for self-administration training are shown in Figure 1.
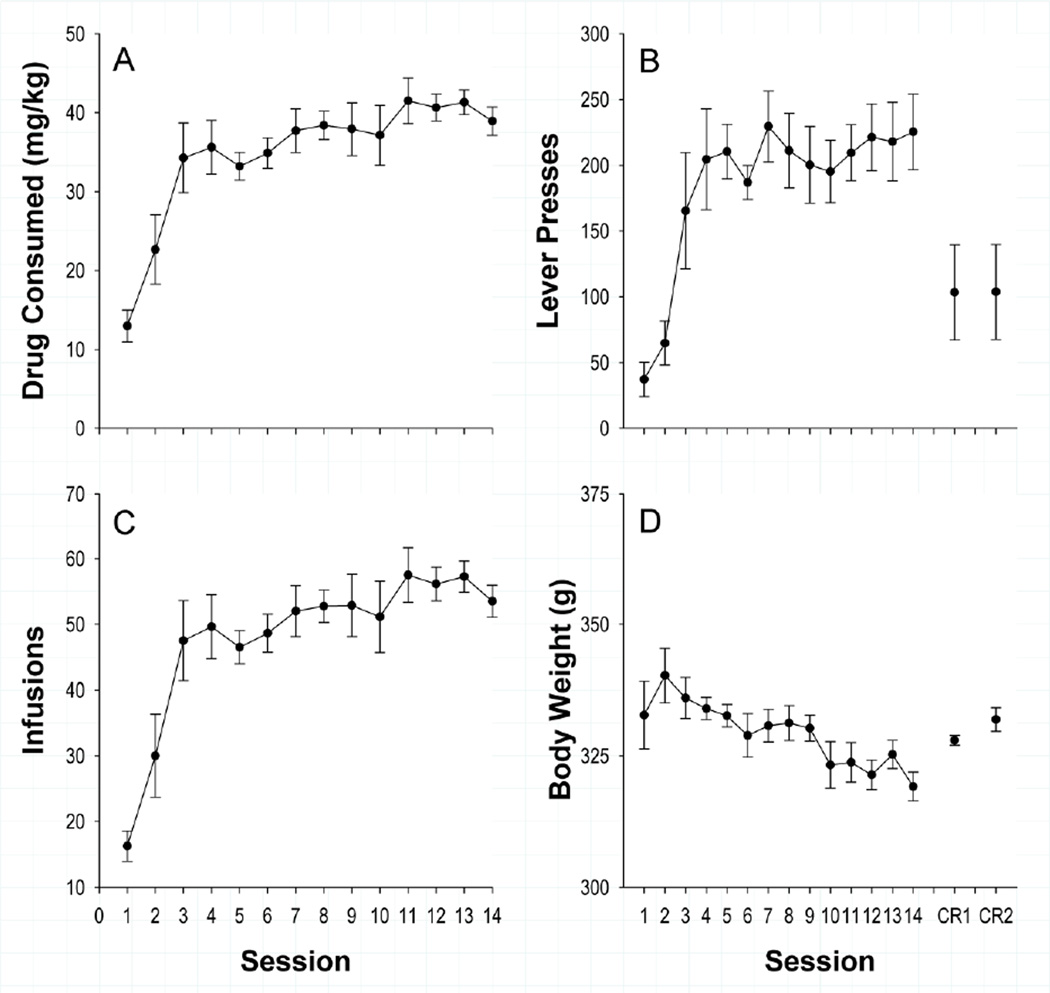
Figure 1.
Mean (±SEM) A) daily drug consumption B) lever presses C) earned infusions and D) body weight across sessions (y-axis). Subjects escalated drug intake over sessions (A & C) and became more task proficient (B) across sessions. As is typical of chronic drug administration (Bozarth & Wise, 1985), subjects’ bodyweight decreased across sessions.
Subjects acquired the task rapidly, with lever presses increasing from 37.13 ± 13.12 (Mean ± SEM) in session one to 225.38 ± 28.79 in session fourteen[F (7, 91) = 5.01, p < 0.001], the number of earned infusions increasing from 16.25 ± 2.31 in the first session to 53.50 ± 2.44 in the fourteenth session [F (7, 91) = 51.73, p < 0.001] and total drug consumption increasing from 12.96 ± 1.99 mg/kg to 38.91 ±1.82 mg/kg [F (7, 91) = 13.92, p < 0.001]. Subjects also became more efficient in the task, increasing the percentage of opportunities where subjects received an infusion from 13.41 ± 1.71% in session one to 61.07 ± 2.89% in session fourteen (earned infusions/number of opportunities) [ F (7, 91) = 27.03, p < 0.001]. This demonstrates that subjects increased their drug consumption over sessions, but also missed opportunities to administer drug on nearly 40% of trials. Results from our laboratory (Root et al., 2011) would suggest that these misses correspond to periods when the animal’s drug level is at or above drug-satiety.
Peak drug levels increased from 2.97 ± 0.38 mg/kg in session one, to nearly double that (5.61 ± 0.30) by the end of training [F (7, 91) = 16.97, p < 0.001]. As has been reported previously (Bozarth & Wise, 1985), repeated S-A training also corresponded with a significant decrease in body weight over days [332.75 ± 6.42 g to 319.14 ± 2.76 g; F (7, 91) = 13.80, p < 0.001].
Sidak post-hoc comparisons indicated that the number of lever presses was significantly greater in sessions 3–14 compared to session one [all |t (91) | ≥ 3.97, p ≤ 0.01]. Similarly, drug consumption, peak drug level, earned infusions and percent hit were significantly greater than session one in all subsequent sessions [Sessions 2–14, all |t (91) | ≥ 3.24, p ≤ 0.01]. All behavioral measures except bodyweight fit an asymptotic curve, as evidenced by a significant plateau contrast [all |t (91)| > 3.03, p ≤ 0.01]. This demonstrates that animals increased task efficiency over early training sessions and reached stable behavioral performance and drug consumption by the end of training.
Contextual Reinstatement
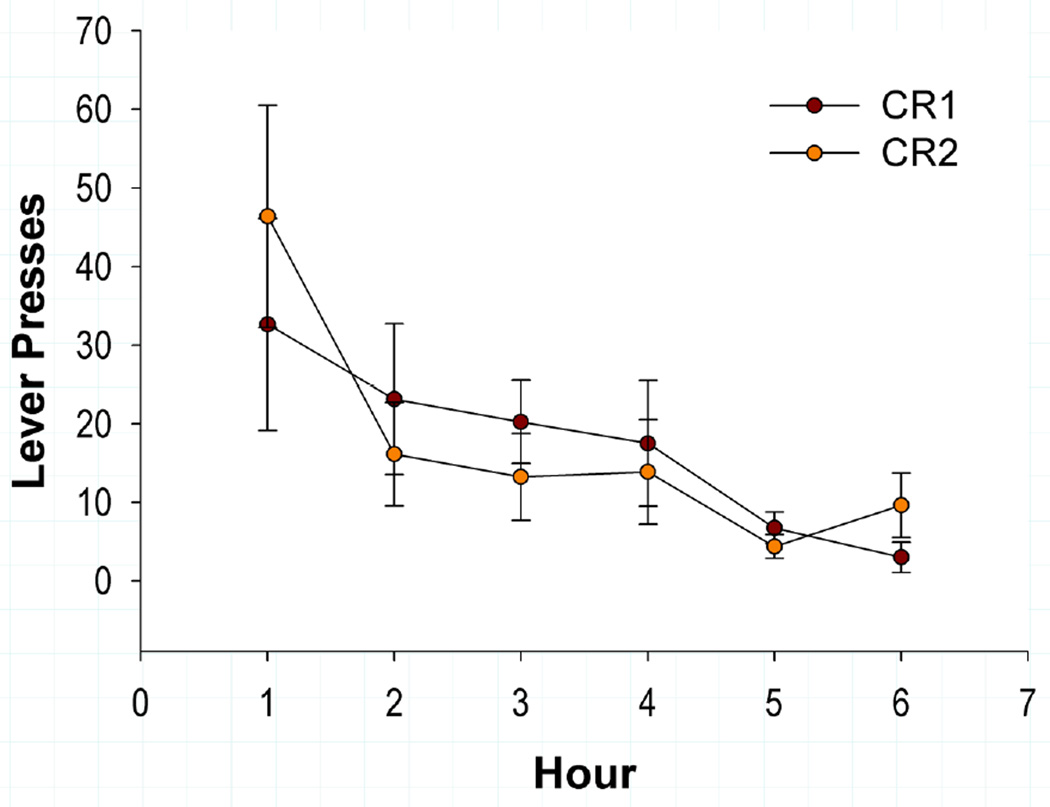
The highest number of lever presses occurred during the initial hours of re-exposure to the cocaine-associated context (Figure 2). The number of responses decreased in CR1 from 32.63 ± 13.48 lever presses in hour 1, to 3.00 ± 1.94 in hour 6. Indeed, animals extinguished responding within the reinstatement session, then exhibited spontaneous recovery of responding between hour 6 of CR1 and the first hour of CR2 [|t(7)| = 2.97, p < 0.05] (Figure 2). Similar to CR1, responding in CR2 decreased from 46.375 ± 14.12 lever presses in hour 1 to 9.63 ± 4.47 in hour 6.
Figure 2.
The mean (±SEM) number of lever presses (x-axis) across hours (y-axis) for contextual reinstatement tests 1 (CR1) and 2 (CR2). Extinction of lever presses occurred across hours for both conditions. Subjects spontaneously recovered lever pressing as evidenced by the increase in responding during the first hour of CR2 as compared to extinguished responding in the last hour of CR1.
When lever responding was examined using a 2 × 6 ANOVA for Condition X Hour, a significant interaction was observed [F(5, 35) = 2.882, p < 0.05] as well as a significant main effect of hour [F(1.18, 8.25) = 7.087, p < 0.05] but no significant effect of condition. Although more responses were emitted during hour 1 of CR2 than hour 1 of CR1, a planned comparison between these hours showed the increase to be non-significant.
Ultrasonic Vocalizations
USVs were analyzed using an omnibus three-way repeated measures ANOVA with 5 levels of condition [Baseline, Week 1 (WK1) of S-A, Week 2 (WK2) of S-A, the 30-day Contextual reinstatement test (CR1), and the 60-day Contextual reinstatement test (CR2)], three levels of frequency, and 6 levels of hour. The average number of USVs in each hour and condition (Mean ± SEM) can be found in Table 1.
Table 1.
Condition x Call Frequency x Session Time: Mean ± SEM
| Baseline | WK1 | WK2 | CR1 | CR2 | |
|---|---|---|---|---|---|
| Hour | 22-kHz USVs |
||||
| 1 | 12.25 ± 9.31 | 27.50 ± 23.72 | 20.63 ± 11.34 | 46.63 ± 32.78 | 18.13 ± 13.81 |
| 2 | 5.38 ± 4.96 | 51.50 ± 50.22 | 16.75 ± 15.49 | 19.00 ± 13.97 | 27.00 ± 24.87 |
| 3 | 5.88 ± 4.90 | 27.50 ± 24.11 | 16.25 ± 14.02 | 21.13 ± 11.54 | 40.50 ± 22.02 |
| 4 | 5.38 ± 2.95 | 18.25 ± 17.12 | 15.63 ± 13.72 | 15.25 ± 10.75 | 16.50 ± 10.32 |
| 5 | 1.00 ± 0.63 | 18.63 ± 17.10 | 8.00 ± 5.38 | 13.00 ± 12.29 | 55.75 ± 41.68 |
| 6 | 4.88 ± 4.19 | 14.13 ± 12.85 | 5.13 ± 3.88 | 0.38 ± 0.26 | 2.25 ± 2.11 |
| 50-kHz USVs |
|||||
| 1 | 2.13 ± 1.86 | 126.13 ± 91.22 | 145.88 ± 92.73 | 19.00 ± 15.47 | 6.88 ± 4.21 |
| 2 | 0.13 ± 0.13 | 35.50 ± 35.36 | 0.75 ± 0.41 | 0.25 ± 0.16 | 1.25 ± 0.25 |
| 3 | 1.00 ± 0.38 | 6.63 ± 6.48 | 0.75 ± 0.62 | 1.25 ± 0.45 | 1.25 ± 0.73 |
| 4 | 0.88 ± 0.30 | 1.88 ± 1.88 | 0.13 ± 0.13 | 0.63 ± 0.38 | 0.88 ± 0.52 |
| 5 | 1.00 ± 0.46 | 0.38 ± 0.38 | 0.13 ± 0.13 | 0.38 ± 0.26 | 0.50 ± 0.19 |
| 6 | 0.25 ± 0.16 | 1.25 ± 1.25 | 0.00 ± 0.00 | 0.63 ± 0.50 | 0.50 ± 0.27 |
The mean (± SEM) number of USVs emitted in the 22- and 50-kHz frequency ranges during each hour of the recording conditions. WK1—week 1 of self-administration; WK2—week 2 of self-administration; CR1— first contextual reinstatement test (30 days abstinence); CR2—second contextual reinstatement test (60 days abstinence).
The repeated measures ANOVA revealed a significant three-way interaction [F(7, 623) = 8.37, p < 0.001], significant two-way interactions for frequency X hour F(7, 623) = 143.40, p < 0.001], condition X hour [F(7, 623) = 13.17, p < 0.001], and condition X frequency [F(7, 623) = 81.67, p < 0.001] and significant main effects for frequency [F (2, 623) = 16.68, p < 0.001] and hour [F(5, 623) = 90.93, p < 0.001] but not condition.
Post-Hoc Tests
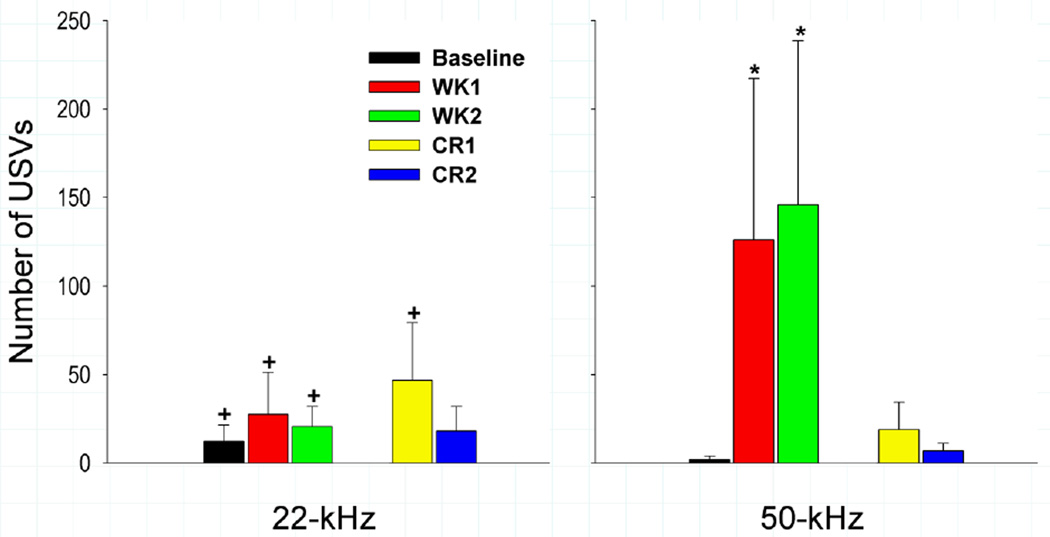
Post-hoc comparisons were planned specifically for analyzing USVs during the first hour, for which mean ± SEM are shown in Figure 3. No significant differences were found in the 33-kHz range and thus the data are not reported. Post-hoc comparisons between baseline and hour 1 of CR1 and CR2 revealed that neither 22- nor 50-kHz USVs were greater during reinstatement sessions when compared to baseline. Furthermore, neither 22- nor 50-kHz USVs correlated with rates of responding during the first hour of CR1 or CR2. There were, however, fewer 50-kHz USVs in hour 1 of CR1, CR2, as well as baseline when compared to the first hour of WK1 and WK2 of self administration [all |t (623)| ≥ 2.63, p<0.01]. No differences were observed in the 22-kHz range between baseline or S-A and reinstatement, although there were more 22-kHz USVs during CR1 than CR2 [t (623) = 3.32, p<0.001]. Thus, contextual reinstatement was not sufficient to increase 50- or 22-kHz USVs above baseline levels even though context was sufficient to produce rates of responding comparable to those seen during an average hour of self-administration.
Figure 3.
The mean ±SEM number of 22-kHz (A) and 50-kHz (B) USVs emitted during the first hour of each recording condition. WK1—week 1of self-administration; WK2—week 2 of self-administration; CR1— first contextual reinstatement test (30 days abstinence); CR2—second contextual reinstatement test (60 days abstinence). * p < 0.05 when compared to baseline. + p < 0.05 when compared within-condition to the 50-kHz range.
Comparisons of 22-kHz and 50-kHz USVs within the first hour of each condition revealed a tendency for more 22-kHz USVs than 50-kHz USVs during baseline [t (623) =5.19, p <0.001]. This difference is likely attributable to the near absence of 50-kHz USVs during the baseline condition (Table 1). Moreover, subjects emitted significantly greater numbers of 22-kHz USVs than 50-kHz USVs during CR1 [t (623) = 2.02, p<0.05], but not CR2. In contrast, there were more 50-kHz USVs than 22-kHz USVs during the first hour of both WK1 and WK2 of self-administration [all |t (623)| ≥ 2.41, p <0.05]. Importantly, the robust increase in 50-kHz USVs over 22-kHz USVs observed during S-A sessions was not observed during either baseline or reinstatement recordings.
Discussion
Similar to previous reports from our laboratory (Root et al., 2009; Barker et al., 2010), animals rapidly acquired S-A and stabilized behavior following ~3–5 days of training. Cocaine dependence in humans is characterized by weight loss, tolerance, and withdrawal symptoms (Mendelson & Mello, 1996). Thus, behavioral results from the current experiment are consistent with characteristics of cocaine dependence, as 1) animals showed weight loss over the course of drug-administration and 2) the acquisition of S-A also corresponded to an escalation of drug intake over sessions. Importantly, the escalation of drug intake—defined as an increase in drug consumption across daily sessions—is thought to serve as a pre-clinical model of drug tolerance and compulsive drug seeking (Ahmed & Koob, 1999) and occurs only following long-access training (Ahmed & Cador, 2006; Quadros & Miczek, 2009; Zernig et al., 2007). Furthermore, while animals in the present study were not tested for withdrawal, work by Miczek and colleagues recording USVs demonstrated that rats experience an aversive withdrawal state following long-access sessions (Covington & Miczek, 2003; Mutschler & Miczek, 1998a, 1998b), which has been replicated in our laboratory (unpublished observations).
An examination of lever responding during 30- and 60-day contextual reinstatement tests (CR1 & CR2 respectively) revealed that, during the first hour of each reinstatement test, subjects responded at rates comparable to those observed in S-A. Moreover, subjects extinguished responding across hours within each reinstatement session but spontaneously recovered responding in CR2, following extinction during CR1.
The literature suggests that reinstatement of drug responding could involve a positive anticipation or reaction to being returned to the drug-paired environment (Ahrens et al., 2009; Ma et al., 2010; Browning et al., 2011) and/or a means of relieving conditioned negative affect (e.g., Siegel, 1983). Moreover, 22-kHz USVs could represent a reaction to reward omission (Burgdorf et al., 2000). Thus, the most surprising result of the present study was the lack of a clear correspondence between USVs and reinstated responding. Specifically, USVs were not significantly increased from baseline rates of calling during either reinstatement test. In fact, USVs actually decreased during the first hour of CR1 compared to that of CR2 while drug-seeking (lever presses) increased.
Nevertheless, this result is in line with results suggesting that compulsive drug seeking occurs following long-access self administration (Ahmed & Cador, 2006; Quadros & Miczek, 2009; Zernig et al., 2007), and that the types of compulsive drug-seeking that occur following long-term drug use can be affected by drug “wanting” (motivation to seek drug) without being affected by—or even in the absence of— hedonic responses to abused drugs (“liking”; Robinson & Berridge, 2001; Berridge & Robinson, 1995). That is, the motivation to seek drug may be able to function outside of hedonic control.
Interestingly, another study examining USVs during reinstatement has shown that USVs are prevalent at reinstatement testing (Browning et al., 2011). Browning and colleagues allowed animals two hours of daily access to cocaine under an FR-5 schedule followed by 1–2 weeks of extinction training before being tested for cue- and cocaine-primed reinstatement. It was shown that USVs increased across self-administration training (to ~700 USVs/hr) before decreasing during extinction to levels comparable to the earliest self-administration recordings (~250 USVs/hr). Ultrasonic vocalizations showed a transient increase from extinction during cue-induced reinstatement testing and a robust increase following cocaine-primed reinstatement.
A number of differences between the above mentioned study and present data might explain the differences between cue-induced and contextual reinstatement. First, the USVs observed byBrowning et al. (2011) sensitized across multiple short access sessions, while attenuation in USVs was observed across long-access sessions in the present study. Thus, the session length, quantity of daily drug consumption, and other inherent differences between long- and short-access sessions may contribute to different hedonic reactions at reinstatement. Proposed theories of addiction suggest that long-access drug use might cause certain protective allostatic changes that could be reflected in animals’ affective responses at reinstatement (Koob & LeMoal, 2001).
Second, it should not be overlooked that different types of cues become instilled with different motivational properties across training (Everitt & Robbins, 2005). Differences in the imbued properties of a CS+, which shares a proximal relationship with reward delivery, may produce a more pronounced hedonic response than contextual cues which do not share this same proximal relationship. Indeed, subjects in the present study lived in the chamber for approximately three weeks during recovery from surgery and self-administration training, making the context a poor-predictor of reward delivery. Also, the affective response to the CS+ used by Browning and colleagues was small and transient suggesting that the perhaps lesser salience of contextual cues was insufficient to produce an affective response. Nonetheless, despite failing in this regard, context was sufficient to produce reliable drug-seeking behavior (i.e., lever pressing) during the present reinstatement testing.
Finally, research has shown that different neural circuits are recruited during reinstatement that is preceded by extinction than by forced-abstinence. Indeed, Fuchs et al. (2006) demonstrated that brain regions involved in habit formation (e.g., dorsolateral striatum), but not goal-directed responding (e.g., basolateral amygdala and dorsomedial prefrontal cortex) are involved in contextual reinstatement following abstinence, while only those involved in goal-directed responding are involved in extinction-reinstatement paradigms. Thus, differences during the post-training period may contribute to the observed differences in hedonic response at test. It is certainly possible that reinstatement to contextual cues following forced abstinence from long-access training might have been habitual, whereas CS+ induced responding following short-access training might have remained goal directed.
Given the aforementioned evidence, it might be suggested that motivated responding and affect can work in concert or independently during reinstatement or relapse. Indeed, a drug-paired CS+ in a short-access extinction-reinstatement paradigm was able to produce both a hedonic and behavioral response during reinstatement (Browning et al., 2011). On the other hand, contextual cues following long-access training in an abstinence-reinstatement paradigm are imbued with similar incentive value in that they can motivate responding, but do not produce the same hedonic response (present data). Accordingly, the present findings lead to the conclusion that a hedonic reaction does not appear to be necessary for reinstatement/relapse behavior in this animal model. Moreover, the presence or absence of such a hedonic response may indicate differences in neural physiology as individuals transition from drug-use to drug-dependence, differences in cue-learning, or differences in subjects’ levels of response automaticity. These insights may be important for developing successful behavioral and pharmacological treatments, suggesting that the motivational and hedonic components of relapse might necessitate specific (and perhaps different) therapies.
Acknowledgments
This article is dedicated to the memory of Linda King, a colleague whose character and commitment to students will not be forgotten. We thank Linda King, Thomas Grace Sr., Jackie Thomas, and Kevin Coffee for technical assistance. This study was supported by the National Institute on Drug Abuse Grants DA006886 (MOW), DA029873 (MOW), DA026252 (DHR) and DA032270 (DJB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All coauthors have seen and approve of the contents of the manuscript.
Footnotes
The authors have no financial interests to be disclosed.
Author Contributions
DJB, DHR and MOW were responsible for the concept and design of the study. DJB, DB, LCS, and SJS were involved in data collection and analysis, while SM and APP were involved in data analysis and statistical design. DJB and DB were responsible for writing the manuscript and all authors contributed to preparation/editing of the document.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Quarterly journal of experimental psychology. 1982;34B:77–98. [Google Scholar]
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Quarterly Journal of Experimental Psychology. 1981;33B:109–122. [Google Scholar]
- Ahmed SH, Cador M. Dissociation of Psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine but not food seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Shallert T. Repeated intravenous amphetamine exposure: rapid and persistent of 50-kHz. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barfield RJ, Thomas DA. The role of ultrasonic vocalizations in the regulation of reproduction in rats. Ann N Y Acad Sci. 1986;474:33–43. doi: 10.1111/j.1749-6632.1986.tb27996.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology. 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros HMT, Miczek KA. Withdrawal from oral cocaine in rats: ultrasonic vocalizations and tactile startle. Psychopharmacology. 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. The mind of an addicted brain: Neural sensitization of wanting versus liking. Current Directions in Psychological Science. 1995;4(3):71–76. [Google Scholar]
- Bijlsma EY, Olivier B, Groenink L. Cocaine induced changes in affective state modulate the light-enhanced startle response. Behavioral Brain Research. 2010;213:117–120. doi: 10.1016/j.bbr.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. Journal of the American Medical Association. 1985;254:81–83. [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA. Positive affective vocalizations during cocaine and sucrose self-administration: a model for spontaneous drug desire in rats. Neuropharmacology. 2011;61(1–2):268–275. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D, Bihari F. Comparison between cholinergically and naturally induced ultrasonic vocalization in the rat. J Psychiatr Neurosci. 1991;16:221–226. [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiology & Behavior. 1992;52(4):655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Bihari F, Ociepa D, Fu X. Analysis of 22 kHz ultrasonic vocalizations in laboratory rats: long and short calls. Physiol Behav. 1993;54:215–221. doi: 10.1016/0031-9384(93)90102-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Communication of adult rats by ultrasonic vocalization: Biological, sociobiological, and neuroscience approaches. ILAR J. 2008;50:43–50. doi: 10.1093/ilar.50.1.43. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50-kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neuroscience and Biobehavioral Reviews. 2011;35(9):1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioural autonomy. Philos Trans R Soc London. 1985;B308:67–78. [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66(1):88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discovery Today. 2009;30(20):1–8. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. Journal of Consulting and Clinical Psychology. 1995;63(3):400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: Pergamon; 1990. pp. 522–557. [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;182(1):60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. High frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiology & Behavior. 1999;66:639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug Abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Addiction and the brain Anti-reward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction: toward the development of new therapies. Annal NY Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic homeostatic dysregulation as a driver of drug seeking behavior. Drug Discov Today Dis Models. 2008;5(4):207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal CF. Drugs Behavior and Modern Society. Boston, MA: Allyn & Bacon; 2010. [Google Scholar]
- Liu C, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: Exacerbation by history of dependence and role of concurrent activation of corticotrophin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle C. Repeated intravenous cocaine experience: Development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behavioral Brain Research. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE. A rodent “self-report” measure of methamphetamine craving? Ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behavioral Brain Research. 2013;236(1):78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Ahrens AM, Ma ST, Schallert T, Duvachelle CL. Cocaine deprivation effect: cue abstinence over weekends boosts anticipatory 50-kHz ultrasonic vocalizations in rats. Behvaioral Brain Research. 2010;214(1):75–79. doi: 10.1016/j.bbr.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvachelle CL. The missing variable: Ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology. 2012;219(4):1141–1152. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012;219(4):999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNagny SE, Parker RM. High Prevalence of recent cocaine use and the unreliability of patient self-report in an inner-city walk-in clinic. JAMA. 1992;267(8):1106–1108. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. Drug Therapy. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalizations in rats. Neurosci Lett. 2009;453:31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from IV cocaine “binges” in rats: ultrasonic distress calls and startle. Psychopharmacology. 1998a;135:161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998b;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: A neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97(4):459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Miczek KA. Two modes of intense cocaine bingeing increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206(1):109–120. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Root DH, Fabbricatore AT, Barker DJ, Ma S, Pawlak AP, West MO. Evidence for habitual and goal-directed behavior following devaluation of cocaine: a multifaceted interpretation of relapse. PloS One. 2009;4:e7170. doi: 10.1371/journal.pone.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Barker DJ, Ma S, Coffey KR, Fabbricatore AT, West MO. Evidence for learned skill during cocaine self-administration in rats. Psychopharmacology. 2011;217:91–100. doi: 10.1007/s00213-011-2261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RKW, Jegan N, Wohr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 2007;182(2):208–222. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Siegel S. Classical conditioning, drug tolerance, and drug dependence. In: Glaser FB, et al., editors. Research advances in alcohol and drug problems. Vol. 7. New York: Plenum; 1983. [Google Scholar]
- Simola N, Ma ST, Schallert T. Influence of acute caffeine on 50-kHz ultrasonic vocalizations in male adult rats and relevance to caffeine-mediated psychopharmacological effects. Int. J. Neuropsychopharm. 2010;13(1):123–132. doi: 10.1017/S1461145709990113. [DOI] [PubMed] [Google Scholar]
- Taracha E, Hamed A, Krząścik P, Lehner M, Skórzewska A, Płaźnik A, Chrapusta SJ. Inter-individual diversity and intra-individual stability of amphetamine-induced sensitization of frequency-modulated 50-kHz vocalization in Sprague-Dawley rats. Psychopharmacology. 2012;222(4):619–632. doi: 10.1007/s00213-012-2658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Interactions between social stress and morphine in the periaqueductal gray: effects on affective vocal and reflexive pain responses in rats. Psychopharmacology. 1999;146(2):153–161. doi: 10.1007/s002130051101. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69(11):1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. JPET. 2010;332(2):463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug and Alcohol Dependence. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems. American Psychologist. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PBS. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology. 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Wright JM, Deng L, Clarke PBS. Failure of rewarding and locomotor stimulant doses of morphine to promote rat 50-kHz ultrasonic vocalizations. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2776-z. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: Models and appropriate animal laboratory tests. International journal of experimental and clinical pharmacology. 2007;80(2–3) doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]