Abstract
The production of reactive aldehydes such as 4-hydroxynonenal (4-HNE) is a key event in the pathogenesis of alcoholic liver disease which ranges from simple steatosis to fibrosis. The lipid phosphatase PTEN plays a central role in the regulation of lipid metabolism in the liver. In the present study, the effects of chronic ethanol feeding and carbonylation on the PTEN signaling pathway were examined in a 9-week mouse feeding model for ALD. Chronic ethanol consumption resulted in altered REDOX homeostasis as evidenced by decreased GSH, decreased Trx1 and increased GST activity. Both PTEN expression and phosphorylation was significantly increased in the livers of ethanol-fed mice. Carbonylation of PTEN increased significantly in the ethanol-fed mice compared to pair-fed control animals corresponding to decreased PTEN 3-phosphatase activity. Concomitantly, increased expression of Akt2 along with increased Akt phosphorylation at residues Thr308, Thr450 and Ser473 was observed resulting in increased Akt2 activity in the ethanol-fed animals. Akt2 activation corresponded to a decrease in cytosolic SREBP and ChREBP. Subsequent LC-MS/MS analysis of 4-HNE modified recombinant human PTEN identified Michael addition adducts of 4-HNE on Cys71, Cys136, Lys147, Lys223, Cys250, Lys254, Lys313, Lys327 and Lys344. Computational based molecular modeling analysis of 4-HNE adducted to Cys71 near the active site and Lys327 in the C2 domain of PTEN suggest inhibition of enzyme catalysis via either stearic hindrance of the active site pocket or by prevention of C2 domain-dependent PTEN function. We hypothesize that 4-HNE-mediated PTEN inhibition contributes to the observed activation of Akt2 suggesting a possible novel mechanism of lipid accumulation in response to increased reactive aldehyde production during chronic ethanol administration in mice.
Introduction
Steatosis is an early pathologic consequence of both non-alcoholic steatohepatitis (NASH) and chronic alcoholic liver disease (ALD). In general, steatosis is considered relatively benign. However, continued hepatic insults from toxins such as ethanol leads to a transition from mild steatosis to more dynamic and advanced hepatic phenotypes including steatohepatitis, fibrosis and ultimately cirrhosis. The production of reactive aldehydes has been implicated in both NASH and ALD [1–5]. A well documented marker for increased oxidative stress in cells is the presence of elevated levels of the reactive aldehydes such as 4-hydroxynonenal (4-HNE) that originate from peroxidation of lipids including free or membrane-associated polyunsaturated fatty acids [6]. 4-HNE is a potent electrophile that will react with nucleophilic functional groups in DNA as well as Cys, Lys and His residues within proteins. Many proteins have been documented to be targets for modification by 4-HNE including protein disulfide isomerase, HSP90, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), and Akt2 [7–10]. Consistent with the potent electrophilic properties of 4-HNE, proteins modified by this biogenic aldehyde exhibit compromised function.
PTEN is a dual specificity phosphatase possessing both lipid and protein phosphatase activity and is a member of the protein tyrosine phosphatase (PTP) family of phosphatases [11, 12]. PTEN is a tumor suppressor via its ability to regulate Akt and loss of function mutations in PTEN lead to abnormal growth and proliferation as seen in patients with Cowden’s syndrome [13]. PTEN negatively regulates Akt activation through its ability to dephosphorylate the 3-position phosphate from phosphatidylinositol (3,4,5) trisphosphate (PIP3) to produce phosphatidylinositol (4,5) bisphosphate (PIP2). Production of PIP2 prevents the membrane lipid binding of the PH domain of Akt thereby preventing subsequent phosphorylation and kinase activation [14].
PTP phosphatases contain a signature HCX5R motif within their active site [15]. The presence of a nucleophilic cysteine residue within the active site allows for regulation of PTEN by reactive oxidative species and oxidative stress. Besides the aforementioned 4-HNE, both hydrogen peroxide and reactive nitrogen species have been shown to modify and inactivate PTEN [16, 17]. In addition, PTEN has also been demonstrated to be a target of glutathionylation leading to a decrease in activity [18]. Inactivation of PTEN leads to sustained Akt activation in both cellular and animal models. Hepatocyte specific deletion of PTEN leads to insulin hypersensitivity, steatohepatitis and increased occurrence of hepatocellular carcinoma in mice [19]. Initiation of steatosis and hepatocyte proliferation was linked with increased Akt1/2 activation and Akt-dependent downstream activation of SREBP1c and PPARγ [20]. In demonstrating the direct link between PTEN and Akt2, concurrent hepatospecific deletion of Akt2 with PTEN deletion led to a decrease in steatosis [21, 22].
In this report, we detail the effects of chronic ethanol on carbonylation of PTEN. Our results demonstrate that chronic ethanol administration leads to an increase in carbonylation of PTEN with an accompanying decrease in PTEN activity and subsequent increase in Akt2 activity. Thus, a correlation between inhibition of PTEN, increase in Akt2 activity and increased steatosis is observed in ethanol-fed mice. Furthermore, using LC/MS/MS and in silico-based computational modeling, we identify a potential mechanism for 4-HNE mediated inhibition of PTEN activity. Consequently, this report provides new insight into the mechanisms by which lipid peroxidation products such as 4-HNE affect intracellular signaling during chronic ethanol administration.
Materials and Methods
Animal Model and dietary information
All animal care and use procedures were in accordance with the University of Colorado Institutional Animal Care and Use Committee. Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), aged 6–8 weeks, were divided into groups of 12 and pair-fed a modified Leiber-DeCarli diet (Bio-Serv, Frenchtown, NJ) for a total of 9 weeks [23]. The diet consisted of with 45% fat-derived calories, 16% protein-derived calories and the remaining balance comprised of either ethanol or maltose dextrin-derived calories. Ethanol content was increased on a weekly basis from weeks 1–7 starting with 2% v/v at week1 with ethanol concentrations peaking at 6.5% by week 7 (35.0% ethanol-derived calories) and remaining constant at 6.5% until sacrifice at week 9. The pair-fed control animals were isocalorically matched with carbohydrate calories. Food consumption was monitored daily and body weights were measured once per week. Upon completion of the study, animals were anesthetized via intraperitoneal injection with sodium pentobarbital and euthanized by exsanguination. Blood was collected from the inferior vena cava and plasma was separated via centrifugation at 4°C and assayed for alanine aminotransferase (ALT) activity (Sekisui Diagnostics, P.E.I., Canada). Excised livers were weighed and subcellular fractions obtained via differential centrifugation as previously described (6). All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus and were performed in accordance with published National Institutes of Health guidelines.
Western Blotting
Proteins from either whole liver extracts or subcellular fractions were subjected to standard SDS-PAGE and transferred to PVDF (GE Healthcare, Picataway, NJ) as previously described [7]. Membranes were blocked for 60 minutes with a tris-buffered saline solution containing 1% Tween−20 (TBST) and 5% non-fat dry milk and probed overnight with primary antibodies directed against pSer380 PTEN, pSer473 Akt, pThr308 Akt, pThr450 Akt, total Akt1, total AKT2, total PTEN (Cell Signaling, Danvers, MA), SREBP, ChREBP, Trx2, TrxR1 (Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (Sigma Aldrich, St. Louis, MO), VDAC, Trx1, TrxR2 (ABCAM, Cambridge, MA). A horseradish peroxidase conjugated secondary (Jackson ImmunoResearch Inc. West Grove, PA) was then applied and membranes developed using ECL-Plus Reagent (GE Healthcare). Chemiluminescence was visualized using film (Thermofisher).
Histological analysis
Sections of freshly excised liver tissue were placed in 10% neutral buffered formalin (Sigma Aldrich) for 16 hours, washed in 70% ethanol for 1 hr followed by incubation in 70% ethanol overnight. Samples were then processed, embedded in paraffin and mounted to slides by the UC Denver Histology Core. Sections were either stained with hematoxylin and eosin (H&E) or Immunohistochemical characterization was performed using a rabbit polyclonal antibody directed against pSer473Akt (Cell Signaling), ChREBP (Santa Cruz), 4-HNE or MDA as previously described (7). Images of H&E stained and immunohistochemically stained liver sections were captured on an Olympus BX51 microscope equipped with a four megapixel Macrofire© digital camera (Optronics, Goleta, CA) using the PictureFrame© Application 2.3 (Optronics, Goleta, CA).
Triglycerides
Liver triglycerides were determined using a 2:1 chloroform:methanol extract of whole liver sections. Triglyceride content was then quantified using a commercially available kit from Diagnostic Research Inc . Protein concentrations were determined using a Lowry Protein Assay from Bio-Rad (Hercules, CA).
Glutathione S-transferase, glutathione peroxidase and glutathione reductase activity
Glutathione S-transferase (GST), glutathione peroxidase (GPX) and glutathione reductase (GSR) activities were performed as previously described [24].
PTEN and Akt activity assays
PTEN, total Akt, Akt1 and Akt2 were immunoprecipitated from 150 µg of whole liver extracts and activity assays were performed as previously described [7, 8]. For in vivo identification of PTEN studies, total PTEN was immunoprecipitated from 4.0mg of whole cell hepatic extracts from control and ethanol fed animals. Immunoprecipitation was performed using 100mg of rabbit polyclonal anti-PTEN antibodies. Antibody was bound to Aminolink plus immunoprecipitation columns (Pierce) and immunoprecipitation performed according to the manufacturer’s instructions.
LC/MS/MS analysis of 4-HNE modified PTEN
Adduction and trypsin digest of recombinant human PTEN by 4-HNE
Ten μg of recombinant human PTEN (Cayman Chemical, Ann Arbor MI) was treated with 4-HNE at molar ratios of 1:1, 5:1 and 10:1 for 30 min at 37°C. 0.5 micrograms were removed and loaded onto an SDS-PAGE gel and Western blotted using anti-4-HNE polyclonal antibodies. To verify PTEN adduction, membranes were then stripped for 15 min using Restore™ stripping buffer (Pierce, Rockford, IL), washed twice in TBST and blocked once again for 1 h in TBST 5% NFDM. Following blocking, membranes were incubated overnight with mouse anti-PTEN (Cell Signaling) and subsequently processed. The rest of the sample was treated with 10mM sodium borohydride in 100mM NaOH for 30 min at 37°C. Samples were boiled in 5X SDS loading buffer and subjected to SDS-PAGE. Gels were stained for 15 min with Coomassie Blue R250 and destained overnight in 10% acetic acid, 20% MEOH. From the gel, each band was excised and washed briefly in a solution of 50% acetonitrile/20 mM ammonium bicarbonate, then placed in a 5 mM dithiothreitol reducing solution and incubated at 70°C for 30 minutes. A solution of 20 mM iodoacetamide was then added to alkylate cysteine residues and samples were incubated in the dark at room temperature for 45 minutes. Three 15 minute washes were then performed using distilled water, 50% acetonitrile, and 100% acetonitrile, respectively. The samples were dried in a vacuum concentrator then underwent incubation periods of 30 minutes at 4°C and overnight at room temperature in 5 ng/µl sequencing grade trypsin (Promega, Madison, WI). Two serial extractions of tryptic peptides were performed using 50% acetonitrile/1% formic acid. Samples were then concentrated to volumes of ∼20 µl to be used for LC-MS/MS analysis.
A nano-flow HPLC system (Agilent 1200, Palo Alto, CA) coupled with LTQ Orbitrap Velos Hybrid FT mass spectrometer and nanospray ion source (Thermo Fisher; San Jose, CA) was used for LC-MS/MS analysis. An 8 µl volume of samples was run on a ZORBAX 300SB-C18 trap column (5 µm i.d. × 5 mm, Agilent Technologies, Santa Clara, CA) to remove salts and contaminating analytes and reverse phase separation of peptides was performed on a C18 column (100 µm i.d. x 17 cm length) packed in- house using a 4 µm 80 Å pore size matrix (Synergi, Phenomenex, Torrance, CA). Mobile phase A was composed of 99.9% HPLC grade water/0.1% formic acid and mobile phase B of 99.9% acetonitrile/0.1% formic acid. Peptides were eluted from the column using a linear gradient with mobile phase B increasing from 2–90% over 60 minutes. The positive ion mode was used for data acquisition of MS of ions m/z 300–2000 and MS2 scans were performed for the most intense ions. All data was acquired using Thermo Xcalibur software (version 2.1.0.1140, Thermo Fisher; San Jose, CA).
MS/MS Data Analysis
An in-house script was used to convert raw spectral data files into mascot generic file (mgf) format. The mgf files were searched against the human Swissprot database Mascot (version 2.2, Matrix Science Inc., London, UK). The MS/MS tolerance was set at ± 0.6 Da for the searches and the peptide tolerance was set at ± 15 ppm. The search allowed for the missed cleavage of one tryptic site as well as one carbon-13. The carbamidomethylation of cysteine was searched as a fixed modification. Variable modifications included acylation of the protein N-terminus, pyroglutamic acid formation of the peptide N-terminus, oxidation of methionine, 4-HNE modification of cysteine, histidine and lysine (mass 156.22), and reduced 4-HNE modification of cysteine, histidine and lysine (mass158.24). Spectral images were obtained from the raw data files using Xcalibur and Mascot was used to assist peak assignment.
Computational-based molecular modeling
All simulations were performed using Discovery Studio software (Version 3.1; Accelrys Inc., San Diego, CA; http://www.accelerys.com). The crystallographic coordinates of the 2.1Å human activated PTEN crystal structure (PDB code: 1D5R) were obtained from the RCSB Protein Data Bank (http://wwww.rcsb.org)[25]. The 4-HNE modification of residues Cys71, Cys136, Lys147, Lys223, Cys250, Lys254, Lys313, Lys327 and Lys344 of PTEN were modified with Michael adducts of 4-HNE and the resulting structure typed with the CHARMm force field and subjected to minimization (10,000 iterations) using the conjugate gradient method to a convergence of 0.001 kcal/mol using the Generalized Born implicit solvent model [26, 27].
Statistical Analysis
Western blots were quantified using ImageJ (http://rsb.info.nih.gov/ij/) followed by statistical analysis via a paired Students t-test and Prism 4.0 for Windows (GraphPad Software, San Diego, CA). All data are expressed as mean ± standard error and p values <0.05 were considered significant.
Results
Effects of chronic ethanol administration on basic physical and biochemical parameters
As presented in Table 1, chronic ethanol led to a significant decrease in total weight gain compared to the control animals. Surprisingly, following 9 weeks of a high fat diet, the mean overall liver weight was significantly lower in the ethanol fed animals compared to control fed mice. Chronic ethanol administration resulted in a liver to body weight ratio of 4.5% compared to 4.0% in control animals.
Table 1. Physical and Biochemical analysis of serum and liver homogenates from control and ethanol pair-fed mice.
Serum ALT, liver triglycerides, GST and GSR and GPX activities were determined as described in Methods. Data were analyzed using paired Students t-test. All values are presented as the mean± SEM. Significance was determined by comparison to the control fed animals. n=5 for both groups.
| Control | Ethanol | Fold change |
Significance | |
|---|---|---|---|---|
| Change in body weight | 12.72±1.34 | 4.44±1.62 | −2.86 | *** |
| Liver Weight | 1.38±.0.04 | 1.22±0.06 | −1.13 | * |
| liver/body weight | 4.00±0.14 | 4.51±0.11 | 1.12 | p=0.1598 |
| ALT (U/L serum) | 21.67±3.96 | 37.22±5.08 | 1.72 | * |
| Liver Triglycerides (mmol/L/mg tissue) | 0.23±0.03 | 0.31±0.07 | 1.36 | p=0.0548 |
| GST activity (U/mg protein) | 6.12±0.31 | 9.69±0.26 | 1.58 | *** |
| GRX activity (U/mg protein) | 1.01±0.03 | 1.24±0.03 | 1.23 | *** |
| GPX activity (U/mg protein) | 42.75±1.23 | 34.55±1.33 | −1.24 | ** |
| GSH (µMol/g tissue) | 3.35±0.34 | 2.21±0.27 | −1.51 | * |
| GSSG (µMol/g tissue) | 0.13±0.02 | 0.13±0.01 | −1.03 | p=0.8365 |
| GSH/GSSG | 25.67±2.25 | 17.13±1.32 | −1.50 | * |
p<0.05
p<0.01
p<0.001
Histochemical analysis of mouse liver sections
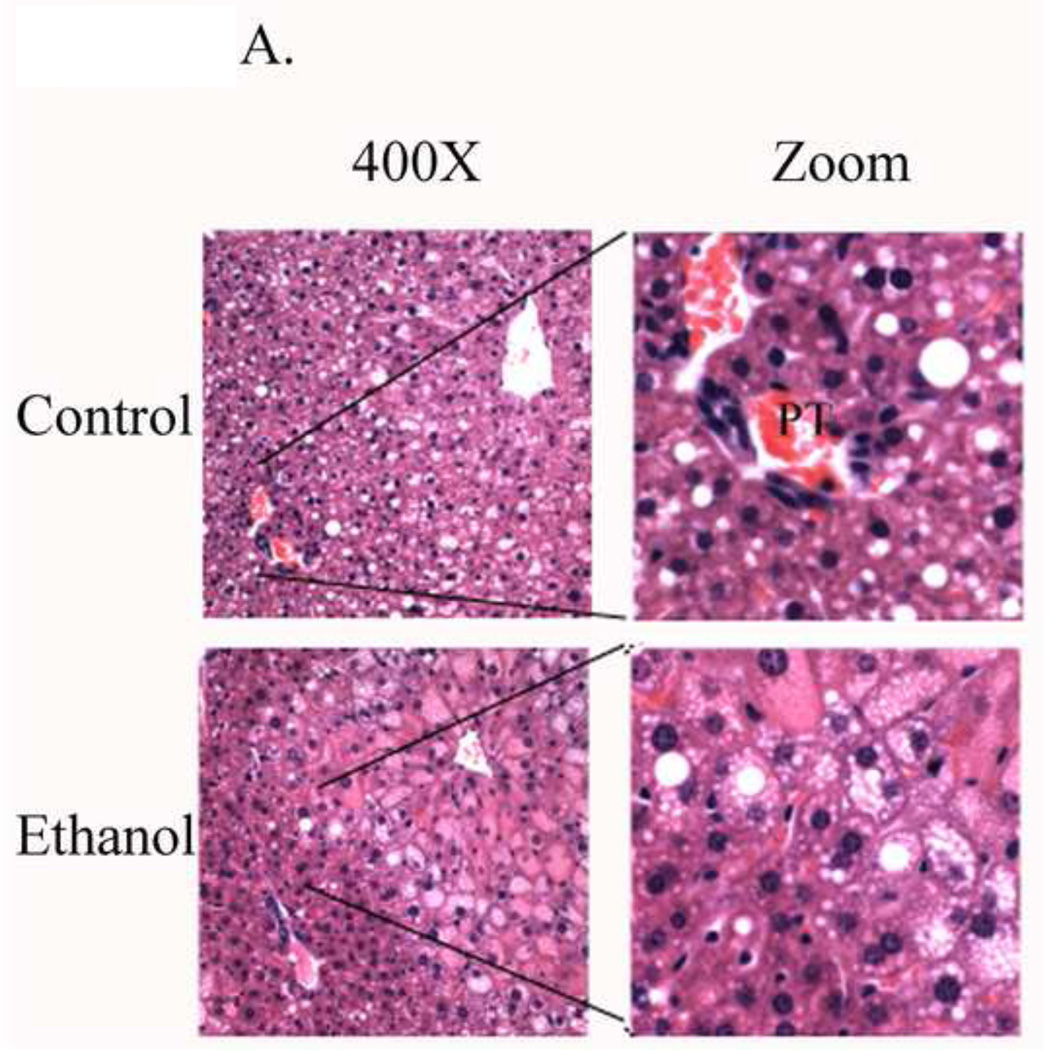
Liver sections from ethanol and control pair-fed mice were stained with hematoxylin and eosin. Both groups exhibited lipid accumulation. A closer examination (Figure 1 “zoom”) revealed an increase and redistribution of lipid primarily to zone 2 with some lipid vesicles around the periportal region following ethanol treatment. Periportal (zone 1) accumulation of lipid was evident in control-fed animals.
Figure 1. Histopathology of fixed liver tissues isolated from ethanol and control fed mice.
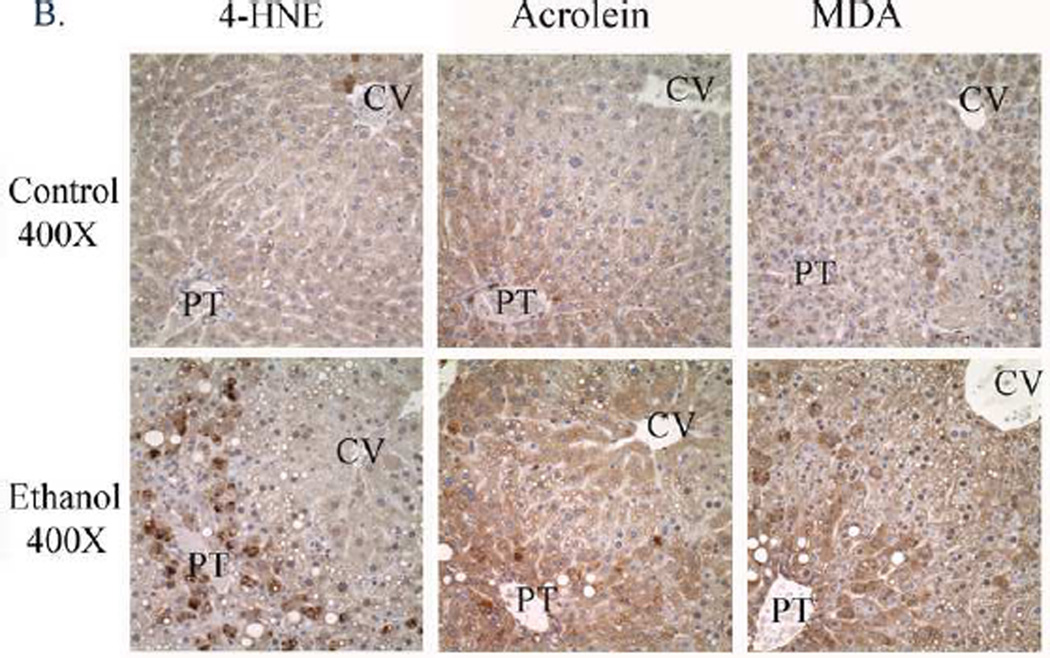
A. Hematoxylin and eosin staining demonstrates a predominantly periportal lipid accumulation in the control and predominantly centrilobular lipid accumulation in ethanol-fed mice (Arrows/Zoom). B. Increased 4-HNE, acrolein and MDA staining in the periportal region (Zones 1–2) in liver sections from ethanol fed mice. (CV, central vein, PT, portal triad). Original Magnification 400X.
Chronic ethanol consumption is known to induce accumulation of reactive aldehyde modified proteins within hepatocytes [28]. Liver sections from control and ethanol pair-fed mice were examined for accumulation of the reactive aldehydes 4-HNE, acrolein and MDA. As demonstrated in Figure 1B, immunohistochemical analysis of 4-HNE, acrolein and malondialdehyde demonstrates a low level of panlobular staining in control animals. Following chronic ethanol treatment, there is a substantial increase in periportal to midzonal staining of 4-HNE, acrolein and MDA.
Biochemical Characterization of liver parameters
The effects of chronic ethanol feeding on serum ALT, liver triglycerides and Redox capacity (GST, GSR, GPX and GSH/GSSG ratio) were examined. As presented in Table 1, chronic ethanol led to a significant increase in overall liver damage as indicated by a 1.72-fold increase in serum ALT. Compared to their respective controls, liver triglyceride content was 36% higher in the ethanol animals a value that narrowly missed statistical significance (p=0.054). Chronic consumption of ethanol is known to reduce levels of glutathione via reactive aldehyde conjugation and to affect the overall activity of glutathione metabolizing enzymes [29]. Chronic ethanol consumption led to an overall decrease in the total level of GSH and in the ratio of GSH/GSSG (1.5-fold) suggesting that redox balance is impaired in this model of chronic ethanol consumption. To further characterize the effects of ethanol on the overall redox capacity of the liver, activity assays of GST and GSR and GPX were performed. From the data in Table 1, the activities of both GST (1.58-fold) and GSR (1.23-fold) were significantly increased in ethanol fed compared to control diet animals. Glutathione peroxidase significantly decreased by 1.24-fold following ethanol consumption.
Effects of chronic ethanol administration on PTEN and Akt phosphorylation and activity
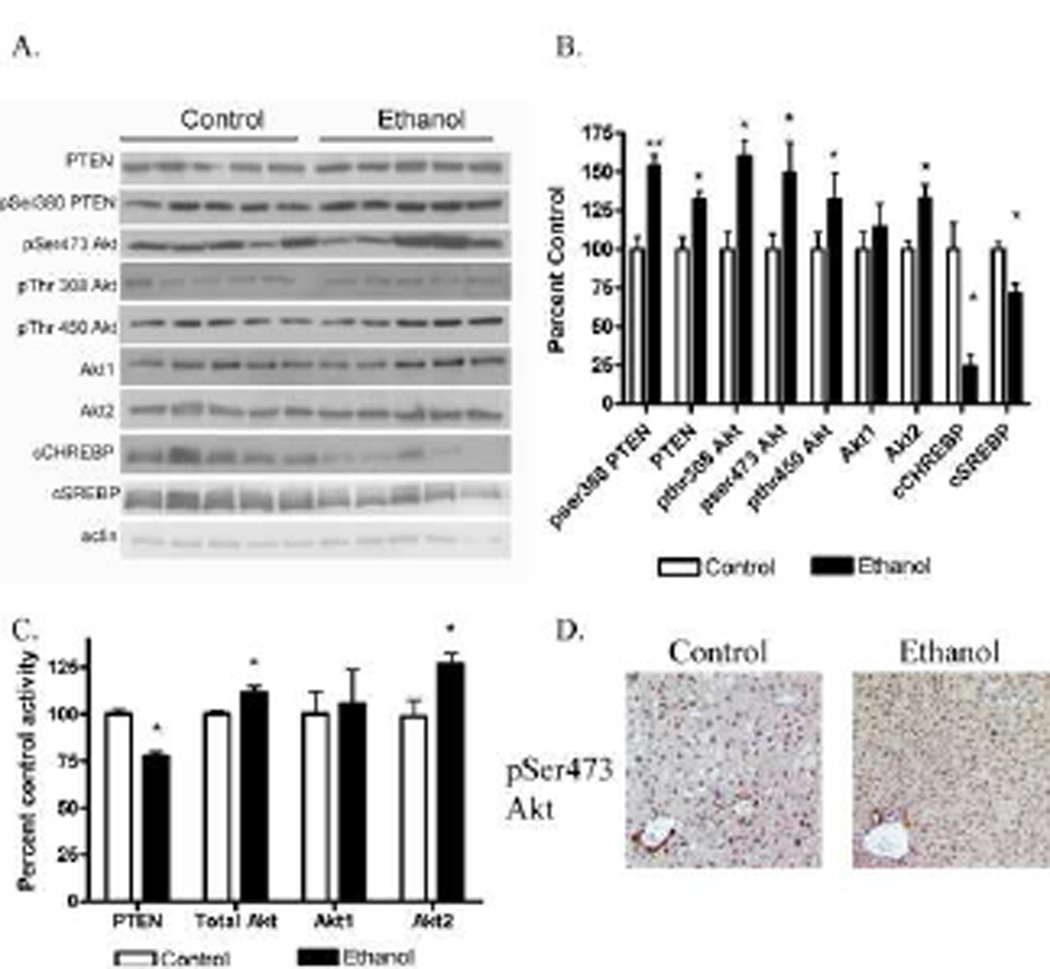
The PTEN/Akt pathway contributes to regulation of metabolic pathways in the liver and has previously been implicated in the formation of chronic ethanol mediated steatosis [30, 31]. As shown in Figure 2A and 2B, compared to isocaloric controls, Western blot analysis of whole cell lysates indicated a significant increase in both total PTEN and total Akt2 expression in ethanol treated animals while no significant change occurred in total Akt1 expression. Increased phosphorylation of PTEN on Ser380 contributes to downregulation of enzymatic activity [32] . In ethanol treated mice, PTEN phosphorylation was significantly increased compared to control animals. Examination of total Akt phosphorylation indicated a significant increase in pThr308Akt, pSer473Akt and pThr450Akt in ethanol treated animals compared to controls.
Figure 2. Chronic ethanol mediated changes in PTEN and Akt in mouse cytosolic liver fractions.
(A) Cytosolic fractions from both ethanol fed and control groups were analyzed via Western blotting and probed for total PTEN, phospho Ser380 PTEN, total Akt1, total Akt2, phosphoSer473 Akt, phosphoThr308 Akt and phosphoThr450 Akt, cytosolic ChREBP and cytosolic SREBP using rabbit polyclonal antibodies (B) Quantification of the Western blots (actin normalized). (C) Using mouse liver whole cell extracts, 150µg of total protein was examined for PTEN activity using a malachite green phosphate release assay and DiC8 PtdIns(3,4,5)P3. Total Akt, Akt1 and Akt2 activities were determined using an in vitro Akt kinase activity assay with a synthetic GSK3αβ peptide substrate and total immunoprecipitated total Akt, Akt1 and Akt2. (D) Increased pSer473Akt cytosolic and nuclear staining in liver sections isolated from ethanol fed mice. Data were analyzed using paired Students t-test (respective ethanol and control group) n=5 (*p<0.05, **p<0.01).
Both sterol response element binding protein (SREBP) and carbohydrate response element binding protein (ChREBP) are involved in regulation of genes involved in lipogenesis [33–36]. Akt activation contributes to the formation of steatosis by acting on downstream transcription factors including SREBP [37]. ChREBP has been proposed to link gluconeogenesis and lipogenesis via to Akt phosphorylation [38, 39]. In a high fat diet model, overexpression of ChREBP results in increased Akt phosphorylation and steatosis [38]. During lipogenesis, both ChREBP and SREBP translocate from the cytosol to the nucleus. To determine the effects of Akt activation on ChREBP and SREBP, cytosolic fractions were examined for ChREBP and SREBP expression. From Figure 2A and 2B, compared to pair-fed controls, cytosolic localization of both SREBP and ChREBP in the ethanol fed mice are significantly decreased.
The ethanol-induced decrease in cytosolic SREBP suggests that the change in total Akt phosphorylation leads to increased translocation of SREBP. Therefore the direct effects of ethanol on PTEN activity and total Akt, Akt1 and Akt2 activity were assessed. As presented in Figure 2C, PTEN activity decreased by 25% in whole cell liver lysates from ethanol animals compared to pair-fed controls. PTEN activity directly affects Akt activation via the elimination of PtdIns (3,4,5 P)3 lipids necessary for Akt recruitment to intracellular membranes and subsequent phosphorylation/activation. Given that PTEN activity is decreased, we therefore sought to determine the effects of ethanol on total Akt, Akt1 and Akt2 activity (Figure 2C). In the ethanol treated mice, there was a significant increase in both total Akt and Akt2 (30%) activity but not Akt1 activity suggesting that decreased PTEN activity is linked to Akt2 activation.
Data from Figure 2A and 2C clearly show that chronic ethanol results in an increase in Akt phosphorylation. To further substantiate these data, immunohistochemical analysis of pSer473Akt was performed using liver sections from control and ethanol fed mice. From Figure 2D, in the pair fed mice, pSer473Akt is predominantly in the nucleus with strong staining of cholangiocytes around the biliary tract. Ethanol consumption however, resulted in a global increase in cytosolic as well as nuclear staining of pSer473Akt.
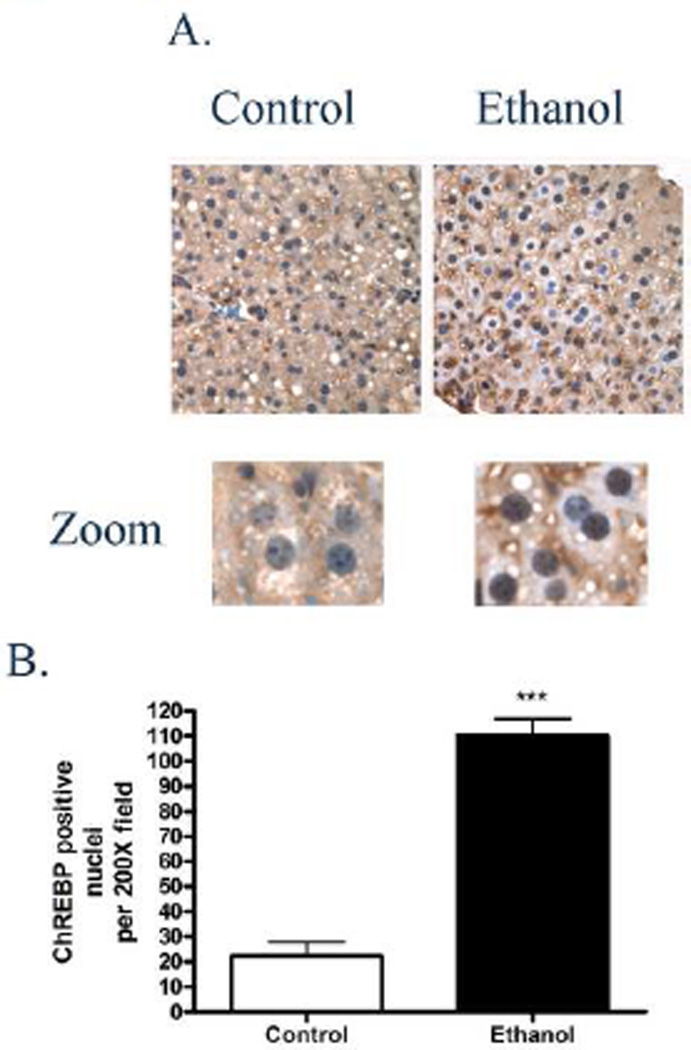
Chronic ethanol consumption decreases cytosolic SREBP [40]. In Figure 2A, decreased cytoplasmic localization of ChREBP occurs following chronic ethanol consumption. Ethanol-mediated changes in cytoplasmic localization of ChREBP have not been reported. To further characterize the decrease in cytoplasmic localization of ChREBP, localization was examined using immunohistochemistry on liver sections prepared from ethanol or pair-fed mice. From Figure 3A, in ethanol-fed animals, there is a marked clearing of ChREBP in the cytoplasm of hepatocytes in zones 1 and 2 that corresponds with an increase in nuclear ChREBP staining. As shown in the zoom panel, ChREBP staining is primarily cytosolic in the control-fed animals with mild nuclear staining. Ethanol consumption however significantly enhanced ChREBP nuclear staining. To quantify ethanol-induced increases in nuclear ChREBP, total number of ChREBP positive/200X field were counted. As shown in Figure 3B, nuclear localization of ChREBP significantly increased 5-fold (p<0.001) in ethanol-fed mice compared to pair fed control animals, further substantiating chronic ethanol-induced ChREBP translocation to the nucleus.
Figure 3. Effects of chronic ethanol consumption on ChREBP nuclear localization.
(A) Positive ChREBP nuclear staining was observed in the periportal region (zones 1–2) in liver sections from ethanol fed mice. (B) Digitally enhanced view of ChREBP stained nuclei. (C). Quantification of ChREBP positive nuclei per 400× field, N=3 (***p<0;001) (5 fields counted for each Con/ETOH fed animal). Original Magnification 400×.
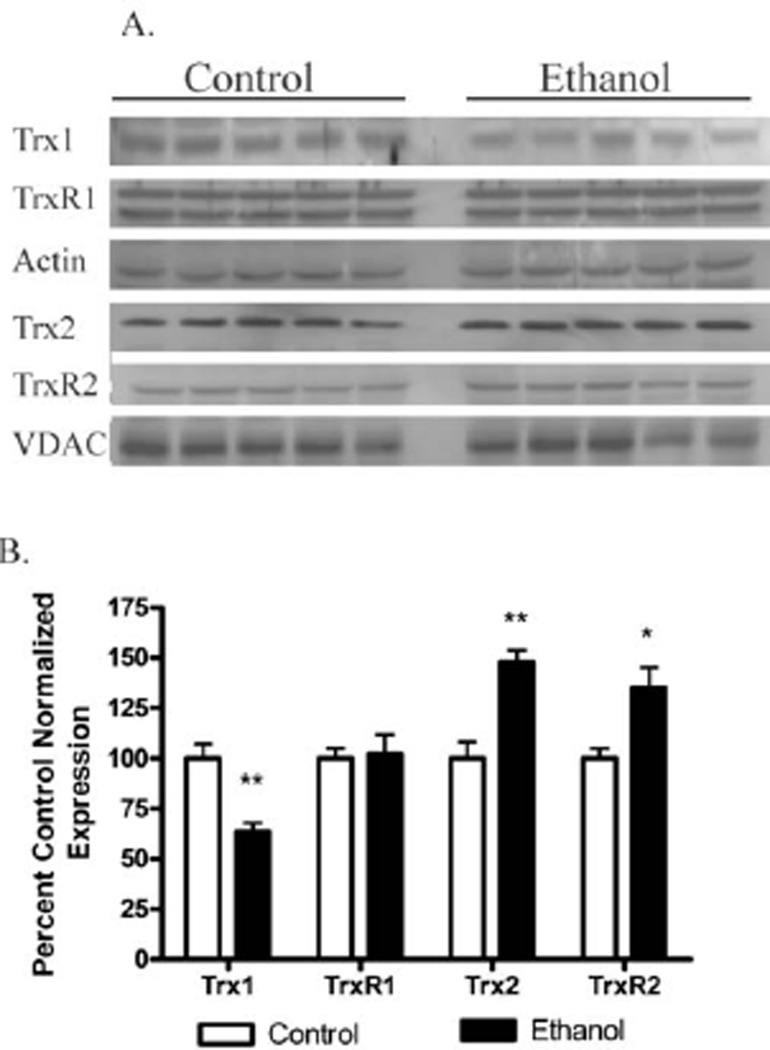
Effects of chronic ethanol administration on thioredoxin expression
Another key regulator of PTEN activity is the Thioredoxin system. Via their ability to reduce oxidized cysteine residues, thioredoxin’s (Trx’s) play a key role in mitigation of protein oxidative damage [41]. Thioredoxin has been demonstrated to reduce cysteine residues on PTEN following hydrogen peroxide treatment [17]. In addition, Trx1 has been demonstrated to physically interact with PTEN via the PTEN C2 domain [42]. Recently, relative expression of Trx1 was determined to be decreased following four weeks of ethanol treatment in female C57Bl6J mice [43]. Given the role that Trx’s play in PTEN activity, the effects of long term ethanol consumption on Trx expression was examined. As shown in Figure 4A and B, Trx1 expression significantly decreased by 35% following ethanol but no change was evident in TrxR1. In the mitochondrion, a distinct isoform of Trx is expressed (Trx2). Thioredoxin 2 has been demonstrated to contribute to mitochondrial resistance to oxidative stress promoting an increase in resistance to reactive aldehydes and cell survival [44]. Chronic ethanol consumption resulted in a 33% increase in Trx2 expression and a 30% increase in TrxR2 mitochondrial expression.
Figure 4. Chronic ethanol induced changes in mouse cytosolic/mitochondrial liver thioredoxins.
(A) Cytosolic /mitochondrial fractions from pair fed and ethanol fed groups were analyzed via Western blotting and probed for Trx1, TrxR1, Trx2 and TrxR2. (B) Quantification of the Western blots (actin/VDAC normalized). Data were analyzed using paired Students t-test (respective ethanol and control group) n=5 (*p<0.05, **p<0.01).
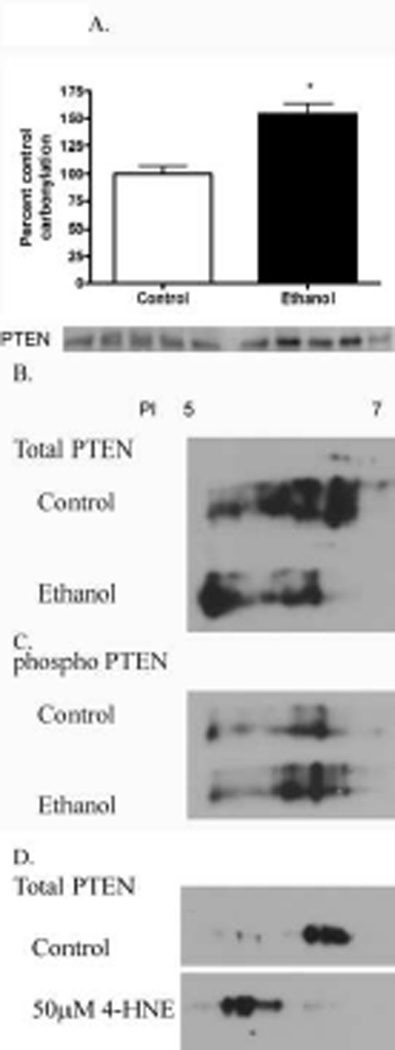
PTEN is carbonylated in livers of mice chronically treated with ethanol
Both carbonylation and phosphorylation of PTEN are known to decrease enzymatic activity [7, 8, 45]. Previously, we have demonstrated that in HepG2 cells, PTEN is a target of carbonylation which contributes to a decrease in enzymatic activity [8]. To determine if there is an effect of chronic ethanol ingestion on intracellular PTEN carbonylation in mice, a method of biotin hydrazide modification of protein bound reactive aldehydes was employed [7]. From Figure 5A, overall carbonylation of PTEN is significantly increased by 1.5-fold in chronic ethanol treated mice compared to control animals. To provide additional insight into the specific amino acid residue modified by reactive aldehydes, whole cell lysates were first treated with iodoacetic acid (IAA) followed by 2-dimensional SDS-PAGE/Western blotting. From Figure 5B, compared to control-fed animals, treatment with IAA induced a decrease in the isoelectric point of PTEN in ethanol-fed animals.
Figure 5. Effects of chronic ethanol consumption of carbonylation of PTEN.
(A) 150 µg of cytosolic protein from ethanol or control fed mice was incubated for 2 h with 2.5 mM biotin hydrazide and purified using streptavidin pulldown. Samples were subsequently analyzed using reducing SDS-PAGE/Western blotting with rabbit polyclonal anti-PTEN. (B) Two-dimensional SDS-PAGE Western blotting of PTEN from whole cell extracts (50mM IAA treatment 30min) isolated from control and ethanol-fed mice. (C) Two-dimensional Western analysis of phosphorylated PTEN from whole cell extracts (50mM IAA 30min) isolated from control and ethanol-fed mice. (D) Two-dimensional Western analysis of PTEN from lysates isolated from HepG2 cells treated with 50µM 4-HNE (60min) followed by 50mM IAA 30 min. Data were analyzed using paired Students t-test (respective ethanol and control group) n=5 (*p<0.05)
In Figure 2A and 2B there is an increase in PTEN phosphorylation in the ethanol-fed animals. Phosphorylation also affects protein isoelectric point. To determine the isoelectric point of differentially phosphorylated species of PTEN, samples were treated as described in Figure 5B followed by Western blotting for PTEN phosphorylation. From Figure 5C, PTEN phosphorylation is increased in the ethanol fed animals but the shift in isoelectric point is not as evident as in Figure 5A. Previously, we reported the PTEN was a target of reactive aldehydes in HepG2 cells [8]. To further substantiate our data, HepG2 cells we treated with 50µM 4-HNE followed by IAA and 2D analysis. As shown in Figure 5D, treatment of HepG2 cells with 4-HNE results in a decrease in the isoelectric point of PTEN. Overall, this data demonstrates, for the first time, carbonylation of PTEN occurs in an animal model of ethanol-induced oxidative stress.
Based on the increase in PTEN carbonylation we repeatedly attempted to identify intracellular sites of modification using immunoprecipitations of total PTEN from 4mg of total hepatic protein and a LTQ Orbitrap mass spectrometer. As shown in Supplemental Figure 1A, Western blotting analysis clearly indicates PTEN immunoprecipitation. Immunoprecipitates from control and ethanol-treated mice were run on a standard SDS-PAGE followed by Coomassie staining. As shown in Supplemental Figure 1B, there were no significant differences in the staining intensity between control and ethanol immunoprecipitates. From the gel, 6 unique regions were excised followed by trypsin digestion and LC/MS analysis. Surprisingly, although PTEN was detectable in the Western blot, no detectable peptides from PTEN were identified via LC/MS/MS (Supplemental Figures 1C, D, E, F, G, H). The lack of identification of PTEN from cellular lysates is not surprising. To our knowledge, PTEN has not been identified from animals using proteomic screens. This suggests that although PTEN is identifiable as carbonylated via Western blotting techniques, the total protein levels prepared for analysis are not sufficient for detection by LC/MS.
Characterization of PTEN modification by 4-HNE in vitro
We have previously reported that recombinant human PTEN (rhuPTEN) was modified by 4-HNE at a molar ratio of 1:1 and that IC50 of 4-HNE towards PTEN was approximately 1.5µM [8]. The locations of the 4-HNE-mediated modifications of PTEN however, were not identified. To identify the residues of PTEN modified in response to treatment with 4-HNE, recombinant protein was incubated with increasing molar ratios of 4-HNE followed by SDS-PAGE, excision and trypsin digestion. Peptides were extracted and analyzed by LC/MS. Concurrently a small sample of treated and untreated protein was run on SDS-PAGE and analyzed by Western blotting for 4-HNE modification. As illustrated in Supplemental Figure 2, treatment of rhuPTEN with 4-HNE led to the formation of multimeric forms of PTEN at approximate molecular weights of 55–60, 120 and 180 kDa via protein crosslinking (Bands 1, 2, 3).
MS Identification of 4-HNE modified rhuPTEN
Previously, rhuPTEN has been demonstrated to be inhibited by 4-HNE at a molar ratio of 1:1, which corresponded to a molar concentration of 1.5µM. To identify the amino acids modified by 4-HNE, rhuPTEN was treated in vitro with increasing molar concentrations of 4-HNE. 4-HNE modifications were stabilized via sodium borohydride reduction followed by SDS-PAGE purification and trypsin digestion. Analysis of tryptic digests from each band resulted in an average MS peptide sequence coverage of 55– 65%. Surprisingly, no peptides containing the active site Cys124 were identified. The resulting mass spectra data presented in Table 2 represents a summary of peptides and amino acids identified from rhuPTEN treated at a 1:1 molar ratio (1µM) or from each band (bands 1, 2, 3) excised at a 10:1 molar ratio (10µM). From Table 2 and Supplemental Table 1, using Mascot and mass shifts of (Michael addition 156.22, 158.24 reduced, Schiff Base 138.104, 140.12 reduced), Cys71, Cys136, Lys147, Lys223, Cys250, Lys254, Lys313, Lys327 and Lys344 were adducted by 4-HNE at the 1:1 molar ratio condition with an ions scores greater than 32. Electron Transfer Dissociation (ETD) is an alternative method to Collision Induced Dissociation (CID) to identify post-translational modifications. Recombinant huPTEN was treated with 4-HNE, digested and analyzed via ETD. Not surprisingly, similar modifications were identified using Mascot searches of tryptic peptides isolated from each multimeric band (Supplemental Table 2). From the resulting mass spectra no new amino acids were identified to be modified (Data not Shown). In addition, the percent peptide sequence coverage did not decrease significantly following multimerization. This suggests that other residues may be modified resulting in formation of protein crosslinks. To verify each site of modification, each peptide was subjected to MS/MS fragmentation and the resulting distribution of b/y ions examined. As presented in Figure 6A, 6B and Supplemental Figure 3A–F, the residues that we identified to be 4-HNE adducted were confirmed via b/y ion distributions, with the exception of Cys136 which could only be confirmed using b ions. It should be noted that although its role with respect to enzymatic activity is not clear, Cys136 has been shown to be mutated in some Cowden’s disease patients [46].
Table 2. Mascot results from LC/MS/MS analysis of rhuPTEN: UCntreated and 4-HNE modified (NaBH4 reduced).
Amino acid number (AA), Experimental (expt), calculated (calc).
| StartEnd | Observed | Mr(expt) | Mr (calc) | Ions Score | Sequence | Modification |
|---|---|---|---|---|---|---|
| 16 – 41 | 1023.1533 | 3066.4381 | 3066.4321 | 60 | YQEDGFDLDLTYIYPNIIAMGFPAER | Oxidation (M) |
| 42 – 47 | 368.7033 | 735.3920 | 735.3915 | 50 | LEGVYR | |
| 48 – 55 | 472.7441 | 943.4736 | 943.4723 | 62 | NNIDDVVR | |
| 67 – 74 | 570.3118 | 1138.6090 | 1138.6056 | 33 | IYNLCAER | HNE+Delta:H(2) (C) |
| 75 – 80 | 367.6782 | 733.3418 | 733.3395 | 40 | HYDTAK | |
| 131 –142 | 517.6209 | 1549.8409 | 1549.8360 | 33 | TGVMICAYLLHR | Oxidation (M); HNE+Delta: H(2)(C) |
| 145 –159 | 893.4635 | 1784.9124 | 1734.9097 | 104 | FLKAQEALDFYGEVR | |
| 145 –159 | 643.6920 | 1943.0542 | 1943.0404 | 42 | FLKAQEALDFYGEVR | HNE+Delta:H(2)(K) |
| 148 – 159 | 699.3386 | 1396.6626 | 1396.6623 | 90 | AQEALDFYGEVR | |
| 148 –161 | 552.2785 | 1653.8137 | 1653.8111 | 38 | AQEALDFYGEVRTR | |
| 164 –172 | 493.2924 | 984.5702 | 934.5716 | 67 | KGVTIPSQR | |
| 165 –172 | 429.2441 | 856.4736 | 856.4767 | 41 | GVTIPSQR | |
| 173 –183 | 765.9042 | 1529.7938 | 1529.7918 | 64 | RYVYYYSYLLK | |
| 174 –183 | 687.8534 | 1373.6922 | 1373.6907 | 52 | YVYYYSYLLK | |
| 184 –197 | 861.9746 | 1721.9346 | 1721.9365 | 57 | NHLDYRPVALLFHK | |
| 222 –233 | 654.8484 | 1307.6822 | 1307.6834 | 71 | VKIYSSNSGPTR | |
| 222 –233 | 733.9147 | 1465.8148 | 1465.8140 | 45 | VKIYSSNSGPTR | HNE+Delta:H(2) (K) |
| 224 –233 | 541.2672 | 1080.5198 | 1080.5200 | 72 | IYSSNSGPTR | |
| 235 –254 | 859.7638 | 2576.2696 | 2576.2583 | 49 | EDKFMYFEFPQPLPVCGDIK | Oxidation (M); HNE+Delta: H(2) (K) |
| 255 –260 | 403.7126 | 805.4106 | 805.4123 | 36 | VEFFHK | |
| 255 –263 | 588.8116 | 1175.6086 | 1175.6087 | 47 | VEFFHKQNK | |
| 268 –289 | 901.4262 | 2701.2568 | 2701.2523 | 69 | DKMFHFWVNTFFIPGPEETSEK | Oxidation (M) |
| 270 –289 | 820.3848 | 2458.1326 | 2458.1304 | 34 | MFHFWVNTFFIPGPEETSEK | Oxidation (M) |
| 309 –322 | 811.9340 | 1621.8534 | 1621.8563 | 84 | ADNDKEYLVLTLTK | |
| 309 –322 | 594.3384 | 1779.9934 | 1779.9870 | 51 | ADNDKEYLVLTLTK | HNE+Delta:H(2) (K) |
| 323 –330 | 538.3031 | 1074.5916 | 1074.5921 | 46 | NDLDKANK | HNE+Delta:H(2) (K) |
| 336 –342 | 451.7245 | 901.4344 | 901.4334 | 44 | YFSPNFK | |
| 343 –349 | 449.7740 | 897.5334 | 897.5324 | 39 | VKLYFTK | |
| 343 –349 | 528.8394 | 1055.6642 | 1055.6631 | 37 | VKLYFTK | HNE+Delta: H(2) (K) |
| 350 –378 | 1049.4603 | 3145.3591 | 3145.3596 | 120 | TVEEPSNPEASSSTSVTPDVSDNEPDHYR | |
| 379 –403 | 971.0820 | 2910.2242 | 2910.2316 | 61 | YSDTTDSDPENEPFDEDQHTQITKV |
Figure 6. 4-HNE modification of rhuPTEN using LC/MS/MS analysis.
(A) 4-HNE modified peptide containing Cys71 (+158), MS/MS analysis was performed and the resulting b/y ion fragmentation confirmed peptide identity (IYNLc*AER). (B) 4-HNE modified peptide containing Lys 327 (+158), MS/MS analysis was performed and the resulting b/y ion fragmentation confirmed peptide identity (NDLDk*ANK).
Although we have identified amino acids in PTEN modified by 4-HNE, the data shown in Figure 5 does not provide evidence concerning the susceptibility of each modified residue to adduction. Therefore, we performed a time course using rhuPTEN and 10-fold molar excess of 4-HNE. A trypsin/chymotrypsin double digest followed by analysis by LC/MS/MS was performed in an attempt to increase our coverage of the active site. As shown in Supplemental Figure 4A, at the two minute time point, 4-HNE treatment resulted in modification and crosslinking of rhuPTEN. A summary of peptide coverage is presented in Supplemental Figures 4B, C and D. Compared to our previous trypsin digestion, the combination of trypsin and chymotrypsin did not result in an increase in peptide coverage (63%-control, 63% 4-HNE treatment) but active site cysteine was identified in the untreated samples (LSEDDNHVAAIHCK mascot score 39). This peptide was not present in the 4-HNE modified digest. In addition, peptides containing Cys71 or Lys327 were not identified under either condition. The fact that a peptide containing the active site Cys124 was not identified suggests that Cys124 may be post-translationally modified resulting in a different mass.
In Silico molecular modeling of 4-HNE modified PTEN
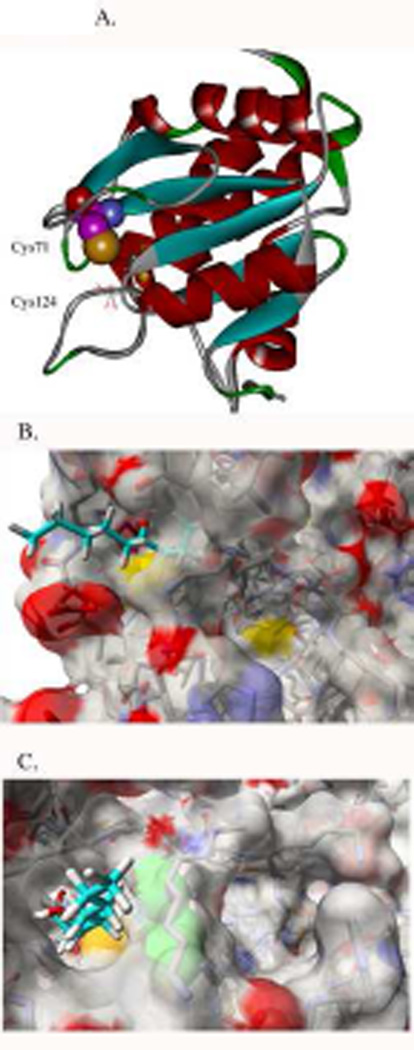
Computational-based minimization simulations were performed using the crystal structure of human PTEN bound to L(+)-tartrate (Figures 7, 8 and 9) [25]. Each amino acid identified in at the 1:1 molar ratio in table 3 (Cys71, Cys136, Lys147, Lys223, Cys250, Lys254, Lys313, Lys327 and Lys344) was modified by 4-HNE and subjected to computer based minimization (Supplemental Figure 5). To determine the effects of 4-HNE modification on overall PTEN structure, minimized structures of untreated and 4-HNE treated were overlaid. From Supplemental Figure 6, no significant structural changes were evident. Of all the residues identified, two were determined to be of particular relevance to PTEN catalytic activity and function: Cys71 (Figure 7A, B, C, D) and Lys327 (Figure 8A, B). In Figure 7A, the addition of 4-HNE on Cys71 via a Michael addition leads to a stable 4-HNE adduct adjacent to the active site of PTEN. From Figure 7B, the 4-HNE modification of Cys71 is not adjacent to Cys124 in the tartrate bound state. The only crystal structure of PTEN is in the tartrate-bound state. In addition, Cys71 is known to form a disulfide with Cys124, however it was determined that in the tartrate bound state Cys124 was not sufficiently close enough to form a disulfide bond. Therefore, the structure of unbound PTEN was minimized to allow for the formation of a disulfide and Cys71 subsequently modified with 4-HNE. In the new unbound state, the side chain of Cys124 is flipped away from the active site and located adjacent to Cys71 (Figure 7C). Thus, 4-HNE modification of Cys71 in the disulfide form of PTEN has the potential to hinder substrate access to Cys124.
Figure 7. In silico molecular modeling of 4-HNE modified Cys71 on huPTEN [60].
(A) Ribbon diagram demonstrating location of Cys71 and Cys124 (B) Electron density map demonstrating charge polarity (blue +, red −) and relationship of 4-HNE (slight blue stick) modified Cys71(yellow) and Cys124 (colored yellow-lower). (C) Electron density map demonstrating relative location of Cys124 following movement into the inactive state (4-HNE light blue stick, Cys71 is colored yellow CPK, Cys124 is colored green CPK)
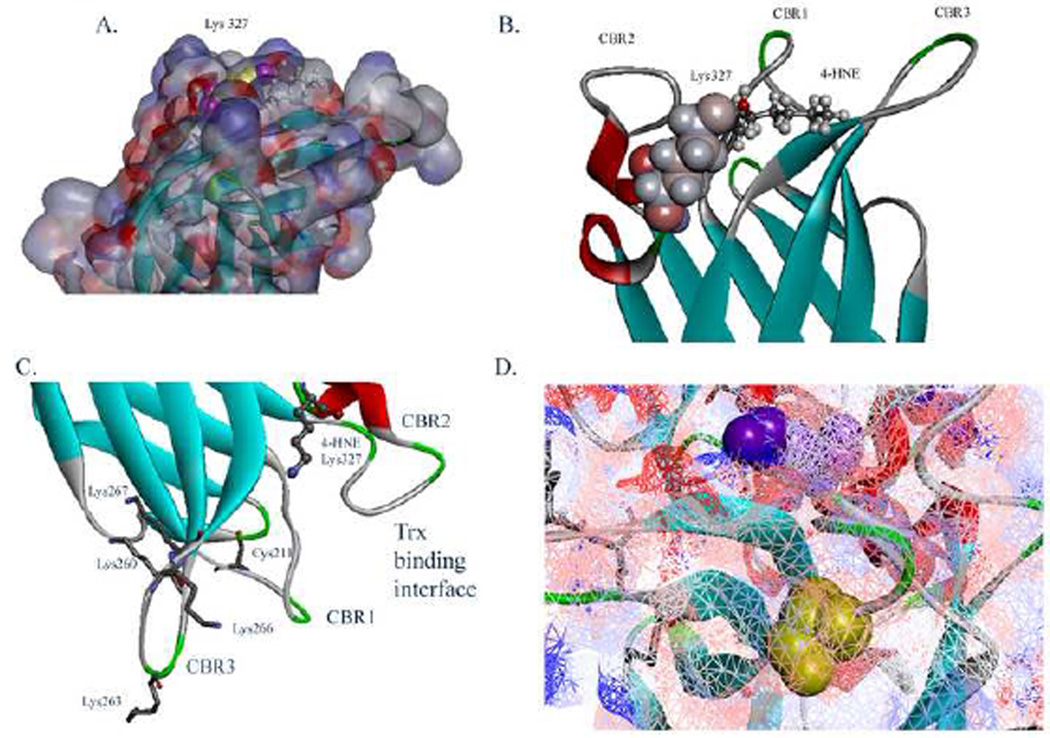
Figure 8. In silico molecular modeling of 4-HNE modified Lys327 on the C2 domain of huPTEN.
(A) Surface electron density map of the C2 of PTEN protein surface with 4-HNE modified Lys327(Red- negative charged residues, blue-positively charged residues, 4-HNE-stick, Lys327-yellow/magenta). Note additional hydrophobic surface formed by 4-HNE addition. (B) Ribbon diagrams demonstrating location of calcium binding regions 1,2,3 and 4-HNE modified Lys327 . (C) Ribbon diagram depicting relative location of 4-HNE-Lys327 and the Trx1 binding site. (D) Surface electron density map depicting top view of relative location of Cys211 (Yellow) and Lys327 (Magenta).
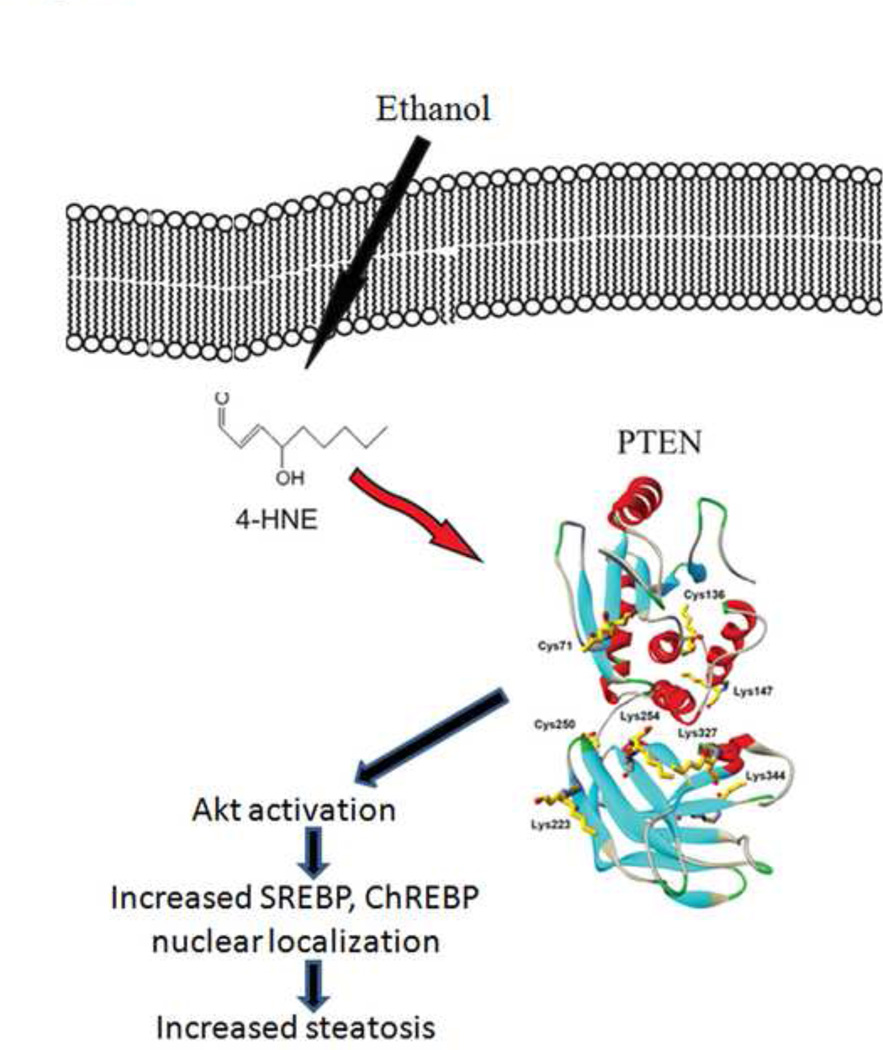
Figure 9. Model of ethanol induced carbonylation in mice.
During chronic ethanol consumption, production of reactive aldehydes such as 4-HNE leads to increased carbonylation and inhibition of PTEN. The resulting decrease in PTEN activity corresponds to increased activation of Akt2 and increased steatosis.
The effect of 4-HNE adduction of Lys327 in the PTEN C2 domain was then examined using minimization studies. From Figure 8A, 4-HNE adduction of Lys327 creates a hydrophobic surface that perturbs the positively charged surface formed by the calcium binding regions (CBR’s 1,2,3) on the C2 domain of PTEN. Another perspective is shown in Figure 8B, 4-HNE modification forms an additional hydrophobic surface laterally along the membrane interacting surface of the PTEN C2 domain. Thioredoxins bind PTEN via interactions with Cys211 in the PTEN C2 domain, therefore the effects of Lys327 adduction on the Trx1 binding interface was examined [47]. From Figure 8C, D, adduction of Lys327 creates a bulky hydrophobic group that alters the Trx1/PTENC2 interface. Therefore 4-HNE modification of Lys327 has the potential to alter not only membrane association but Trx1 association as well.
Discussion
An important consequence of chronic ethanol ingestion is the development of steatosis and hepatocellular injury. Accompanying these responses is an increased level of oxidative stress and production of lipid aldehydes, foremost of which is 4-HNE [48]. We have previously identified PTEN as an intracellular target of carbonylation by 4-HNE in a hepatic cell culture model [8]. Treatment of HepG2 cells with 4-HNE led to increased carbonylation of PTEN and an inhibition of enzyme activity resulting in an increase in Akt phosphorylation and contributing to increased formation of neutral lipids [8]. Thus, a primary objective of the present study was to determine the effects of increased oxidative stress and carbonylation on PTEN signaling in an in vivo murine model of ethanol toxicity. In addition we sought to identify the mechanism of inhibition of PTEN activity by the reactive aldehyde 4-HNE.
A key regulator of the PI3K pathway is the lipid phosphatase PTEN. PTEN has previously been demonstrated to regulate both cell survival (through Akt1) and hepatic lipid accumulation (via Akt2) [22, 49]. In a PTEN hepatospecific deletion model, mice exhibit a 5-fold increase in liver triglycerides. Concurrent deletion of Akt2 ameliorates this effect indicating that Akt2 regulates hepatic lipid accumulation [22]. Increased phosphorylation of the C-terminus of PTEN corresponds to a decrease in activity [32]. However, published reports examining regulation of the PI3K pathway in ethanol models are not consistent. In one report, PI3K activity is increased following ethanol feeding [50]. In another study, using female Long Evans rats, PTEN was identified as a potential contributor in ethanol-mediated toxicity with respect to decreased cell survival [30]. In that second report, the investigators found decreased PI3K activity and PTEN phosphorylation accompanied by increased PTEN expression and activity. The authors concluded that this contributed to a decrease in Akt signaling and the subsequent increase in hepatocyte apoptosis in ethanol treated animals. Interestingly, in another study using male rats, no change in PTEN expression or phosphorylation was evident but Akt phosphorylation of Thr308 decreased and phosphorylation of Ser473 was increased. Phosphorylation of both Thr308 and Ser473 is necessary for full Akt activation [51]. As expected based on Akt phosphorylation, the authors found overall Akt activity decreased in ethanol-fed compared to control animals. In the current study using mice and 9 weeks of ethanol feeding, we find that while there is a significant increase in PTEN expression, PTEN phosphorylation is also significantly increased. Concurrently, ethanol administration further decreases PTEN activity compared to high fat diet alone. Consistent with our results, a recent study indicated decreased PTEN activity in response to high fat diet-induced hepatosteatosis [18].
Carbonylation of PTEN has not been documented in an animal model. Here, we demonstrate for the first time that a significant increase in carbonylation of PTEN occurs in the ethanol-fed mice. In several studies, alkylation/carbonylation decreases PTEN activity leading to increased Akt activity [8, 52, 53]. None of these studies examined individual isoforms. However results of the present study show a significant increase in Akt2 expression but not Akt1 expression occurs in the ethanol treated animals potentially contributing to the increase in Akt2 activity exhibited in this model. No increase in Akt1 activity was evident. In addition, we observed a significant increase in both pThr308 Akt, and pSer473 Akt. Akt activation has also been shown to be regulated via a growth factor-independent, PI3K–independent mechanism. Phosphorylation of Ser124 and Thr450 on Akt is thought to prime the protein for further phosphorylation on Thr308 and Ser473 [54]. Compared to pair-fed control animals, we find a significant increase in phosphoThr450 suggesting that ethanol may regulate Akt phosphorylation via a yet unknown mechanism. As a result of Akt activation we find decreased cytosolic localization of both SREBP and ChREBP and an increase in nuclear localization of ChREBP which may contribute to the increase in liver triglycerides and steatosis in our model.
In vitro modification of recombinant PTEN by incubation with 4-HNE at a molar ratio of 1:1 resulted in a number of aldehyde-adducted amino acid residues. Foremost amongst these are Cys71 and Lys327 . Due to the presence of a reactive cysteine within its active site, PTEN is known to be susceptible to regulation by oxidative modification [15, 17, 42]. Regulation occurs via a redox sensitive mechanism involving the active site cysteine (Cys124) and an adjacent cysteine (Cys71). Treatment of PTEN with hydrogen peroxide leads to the formation of a disulfide between these two residues. This has been shown both in cell culture experiments and with recombinant protein in vitro. To verify this we used molecular modeling methods with the only existing crystal structure of PTEN (1D5R) to form a disulfide bond between the two residues. We found this required “flipping” the side chain of Cys124 nearly 180 degrees to a location adjacent to Cys71 . This suggests that the crystal structure is not indicative of the structure of PTEN upon formation of the disulfide bond. The actual conformation of the active site of PTEN in the unbound state or during disulfide bond formation is not known. It may be that the addition of a synthetic substrate (L(+)-tartrate) prevents the interaction between Cys71 and Cys124. The fact that we find Cys71 to be modified by 4-HNE is intriguing. Molecular modeling of Cys71 suggests that in the inactive state, Cys71 is adjacent to Cys124 and will inhibit substrate binding. Although we identified the active site cysteine following trypsin/chymotrypsin cleavage in untreated samples (LSEDDNHVAAIHCK), we did not observe the peptide containing Cys124 following 4-HNE treatment. This may be due to several reasons. First, Cys124 or Lys125 or both may be sites for 4-HNE modification. As shown in Supplemental Figure 3B, 4-HNE results in chemical crosslinking of rhuPTEN. Cross-linking has been demonstrated to occur between 4-HNE modified lysine residues in other proteins such as tubulin [55]. If the active site peptide is crosslinked, a crosslinked peptide may be created that is too large to be detected by LC/MS/MS. Second, there are several lysine residues immediately adjacent to Cys124 (HCKAGKGRT) that may also be modified, subsequently leading to altered and unrecognizable cleavage sites and the production of an even larger peptide. Within the active site of PTEN, Cys124 has a calculated pka of approximately 4.5. This produces a nucleophilic center that is sensitive to both peroxidation and nitrosylation [15, 56]. Thus, based on our data, we hypothesize that although not identified, the active site cysteine is modified by 4-HNE.
Membrane association of PTEN is in part regulated by its C2 domain with a preference towards polar membrane lipids such as phosphatidylserine and PtdIns (3,4,5) P3 [25]. The mechanism of membrane association is primarily via 3 loops on the end of a (β-barrel (CBR’s 1,2,3). In addition, there is a helix (Cα2) that results in an additional basic patch that has been identified in other C2 domains to contribute to membrane association [57, 58]. Following treatment of 4-HNE, we identified Lys327 as a modified residue of PTEN. Based on the crystal structure, Lys327 is in a position to contribute to membrane association. The addition of 4-HNE creates a hydrophobic surface instead of a cationic surface. Preventing membrane association would thereby inhibit the ability of PTEN to bind to its substrate and be reflected in a decrease in enzymatic activity. Furthermore, adduction of Lys327 may alter the ability of Trx to interact with PTEN further altering activity. Research has suggested that Trx both restores as well as inhibits PTEN activity [15, 17, 41, 42, 47]. Trx1 has also been shown to be a target of reactive aldehydes in other systems [59]. By not reducing oxidized cysteine residues on PTEN, ethanol-dependent decreases in Trx1 expression may contribute to PTEN inhibition. Thus, alteration of the Trx1 binding site combined with overall decreased Trx1 expression/altered glutathione homeostasis reflects an environment of increased oxidative stress contributing to decreased PTEN activity. Overall however, this supports a second plausible mechanism of enzyme inhibition.
In conclusion, we have identified for the first time, an increase in carbonylation of the lipid phosphatase PTEN in an animal model of ethanol-induced hepatic damage. The increased carbonylation of PTEN corresponds to decreased phosphatase activity and an increase in Akt2 activation (Figure 9). Furthermore, we have determined that carbonylation of both Cys71 near the active site of PTEN and Lys327 in the lipid binding C2 domain of PTEN contributes to the decrease in PTEN activity in vitro. Last, we hypothesize that the observed increased activation of Akt2 may stimulate increased lipid accumulation via activation of SREBP and ChREBP during chronic ethanol consumption. Future studies will examine the relative contribution of Akt activation on nuclear transcription factors in relation to lipid accumulation in chronic ethanol treated mice.
Supplementary Material
Acknowledgements
We wish to thank the Erin Genova at the University of Colorado Anschutz Medical Campus Pathology Core for assistance with immunohistochemical staining and Brittany Hodges at the University of Colorado Anschutz Medical Campus Proteomic Mass Spectrometry Facility for assistance in LC/MS analysis of PTEN. The Proteomic Mass Spectrometry Facility is supported in part by the Colorado Clinical Translational Science Institute and the University of Colorado Cancer Center CTSA Grant (UL1 RR025780) and the University of Colorado Cancer Center Grant (P30 CA046934).
Funding Sources:
This work was supported by NIH 5F32 AA018613-03 (C.T.S.), 5R37 AA009300-16 (D.R.P.), 5RO1 DK074487-04 (D.R.P.)
This work was supported by the National Institutes of Health (Institute of Alcohol Abuse and Alcoholism)[ F32 AA018613-01A1 CTS], [R37AA009300-14 DRP]. We would also like to acknowledge that data obtained from the LC/MS/MS analysis of PTEN was funded in part by a CTSA Grant [UL1-RR025780] and a University of Colorado Cancer Center Grant [P30 CA04934] to the University of Colorado Cancer Center Proteomic Mass Spectrometry Facility.
Abbreviations
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- ChREBP
carbohydrate response element binding protein
- Cyp2E1
Cytochrome P4502E1
- 4-HNE
4-hydroxy-2-nonenal
- NASH
non-alcoholic steatohepatitis
- PDK-1
phosphoinositide-dependent kinase 1
- PI3K
phosphatidylinositol 3-kinase
- PtdIns (3,4,5) P3
phosphatidylinositol 3,4,5 tris-phosphate
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PTP
protein tyrosine phosphatase
- SREBP
sterol response element binding protein
- Trx
Thioredoxin
- TrxR
Thioredoxin reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement: No competing financial interests exist for any of the authors.
References
- 1.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 2.Seki S, Kitada T, Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res. 2005;33:132–134. doi: 10.1016/j.hepres.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4- hydroxynonenal-modified proteins in the livers of rats with alcoholic liver disease. Hepatology. 1997;26:650–657. doi: 10.1002/hep.510260317. [DOI] [PubMed] [Google Scholar]
- 4.Hartley DP, Petersen DR. Co-metabolism of ethanol, ethanol-derived acetaldehyde, and 4-hydroxynonenal in isolated rat hepatocytes. Alcohol Clin Exp Res. 1997;21:298–304. [PubMed] [Google Scholar]
- 5.Sampey BP, Stewart BJ, Petersen DR. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J Biol Chem. 2007;282:1925–1937. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 7.Shearn CT, Fritz KS, Reigan P, Petersen DR. Modification of Akt2 by 4- hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 8.Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N, Jr, Petersen DR. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol Pharmacol. 2011;79:941–952. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 10.Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol. 2005;18:1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- 11.Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal. 2007 doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 13.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 14.Di Cristofano A, Pandolfi PP. The Multiple Roles of PTEN in tumor Suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 15.Cho SH, Lee CH, Ahn Y, Kim H, Ahn CY, Yang KS, Lee SR. Redox regulation of PTEN and protein tyrosine phosphatases in H(2)O(2) mediated cell signaling. FEBS Lett. 2004;560:7–13. doi: 10.1016/s0014-5793(04)00112-7. [DOI] [PubMed] [Google Scholar]
- 16.Yu CX, Li S, Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 17.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 18.Alisi A, Bruscalupi G, Pastore A, Petrini S, Panera N, Massimi M, Tozzi G, Leoni S, Piemonte F, Nobili V. Redox homeostasis and posttranslational modifications/activity of phosphatase and tensin homolog in hepatocytes from rats with diet-induced hepatosteatosis. J Nutr Biochem. 2011;23:169–178. doi: 10.1016/j.jnutbio.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe S, Horie Y, Suzuki A. Hepatocyte-specific Pten-deficient mice as a novel model for nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatol Res. 2005;33:161–166. doi: 10.1016/j.hepres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Hou X, Kanel G, Zeng N, Galicia V, Wang Y, Yang J, Wu H, Birnbaum MJ, Stiles BL. The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am J Pathol. 2010;176:2302–2308. doi: 10.2353/ajpath.2010.090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 24.Shearn CT, Fritz KS, Thompson JA. Protein damage from electrophiles and oxidants in lungs of mice chronically exposed to the tumor promoter butylated hydroxytoluene. Chem Biol Interact. 2011;192:278–286. doi: 10.1016/j.cbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 26.Brooks BR, Brooks CL, 3rd, Mackerell AD, Jr, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M. CHARMM: the biomolecular simulation program. J Comput Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onufriev A, Case DA, Bashford D. Effective Born radii in the generalized Born approximation: The importance of being perfect. Journal of Computational Chemistry. 2002;23:1297–1304. doi: 10.1002/jcc.10126. [DOI] [PubMed] [Google Scholar]
- 28.Orlicky DJ, Roede JR, Bales E, Greenwood C, Greenberg A, Petersen D, McManaman JL. Chronic ethanol consumption in mice alters hepatocyte lipid droplet properties. Alcohol Clin Exp Res. 2011;35:1020–1033. doi: 10.1111/j.1530-0277.2011.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roede JR, Orlicky DJ, Fisher AB, Petersen DR. Overexpression of peroxiredoxin 6 does not prevent ethanol-mediated oxidative stress and may play a role in hepatic lipid accumulation. J Pharmacol Exp Ther. 2009;330:79–88. doi: 10.1124/jpet.109.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- 31.Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011;35:2139–2151. doi: 10.1111/j.1530-0277.2011.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 33.Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63:2075–2080. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 36.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 37.Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab. 2010;21:268–276. doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand- Michel J, Ratziu V, Serfaty L, Housset C, Capeau J, Girard J, Guillou H, Postic C. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dentin R, Tomas-Cobos L, Foufelle F, Leopold J, Girard J, Postic C, Ferre P. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J Hepatol. 2012;56:199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 40.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 41.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology. 2009;49:1709–1717. doi: 10.1002/hep.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugano E, Isago H, Murayama N, Tamai M, Tomita H. Different anti-oxidant effects of thioredoxin 1 and thioredoxin 2 in retinal epithelial cells. Cell Struct Funct. 2013;38:81–88. doi: 10.1247/csf.12025. [DOI] [PubMed] [Google Scholar]
- 45.Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- 46.Scala S, Bruni P, Lo Muzio L, Mignogna M, Viglietto G, Fusco A. Novel mutation of the PTEN gene in an Italian Cowden’s disease kindred. Int J Oncol. 1998;13:665–668. doi: 10.3892/ijo.13.4.665. [DOI] [PubMed] [Google Scholar]
- 47.Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- 49.Yun SJ, Tucker DF, Kim EK, Kim MS, Do KH, Ha JM, Lee SY, Yun J, Kim CD, Birnbaum MJ, Bae SS. Differential regulation of Akt/protein kinase B isoforms during cell cycle progression. FEBS Lett. 2009;583:685–690. doi: 10.1016/j.febslet.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Onishi Y, Honda M, Ogihara T, Sakoda H, Anai M, Fujishiro M, Ono H, Shojima N, Fukushima Y, Inukai K, Katagiri H, Kikuchi M, Oka Y, Asano T. Ethanol feeding induces insulin resistance with enhanced PI 3-kinase activation. Biochem Biophys Res Commun. 2003;303:788–794. doi: 10.1016/s0006-291x(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- 52.Covey TM, Edes K, Fitzpatrick FA. Akt tactivation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 53.Covey TM, Edes K, Coombs GS, Virshup DM, Fitzpatrick FA. Alkylation of the tumor suppressor PTEN activates Akt and beta-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS One. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 55.Stewart BJ, Doorn JA, Petersen DR. Residue-specific adduction of tubulin by 4- hydroxynonenal and 4-oxononenal causes cross-linking and inhibits polymerization. Chem Res Toxicol. 2007;20:1111–1119. doi: 10.1021/tx700106v. [DOI] [PubMed] [Google Scholar]
- 56.Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, Lipton SA, Masliah E, Xu H, Liao FF. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J Biol Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 58.Perisic O, Paterson HF, Mosedale G, Lara-Gonzalez S, Williams RL. Mapping the phospholipid-binding surface translocation determinants of the C2 domain from cytosolic phospholipase A2. J BiolChem. 1999;274:14979–14987. doi: 10.1074/jbc.274.21.14979. [DOI] [PubMed] [Google Scholar]
- 59.Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy- 2-nonenal in vitro and in vivo. J Am Chem Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- 60.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP- PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.