Abstract
The ability of muscles to regenerate successfully following damage diminishes with age and this appears to be a major contributor to the development of muscle weakness and physical frailty. Successful muscle regeneration is dependent on appropriate re-innervation of regenerating muscle. Age-related changes in the interactions between nerve and muscle are poorly understood but may play a major role in the defective regeneration. During aging there is defective redox homeostasis and an accumulation of oxidative damage in nerve and muscle that may contribute to defective regeneration. The aim of this review is to summarise the evidence that abnormal Reactive Oxygen Species (ROS) generation in nerve and/or muscle may be responsible for the defective regeneration that contributes to the degeneration of skeletal muscle observed during aging. Identifying the importance of ROS generation in skeletal muscle during aging could have fundamental implications for interventions to prevent muscle degeneration and treatments to reverse the age-related decline in muscle mass and function.
Keywords: Reactive oxygen species, Aging, Skeletal Muscle, Regeneration, Innervation, Neuromuscular junction
Introduction
Physical frailty is a key aspect of aging
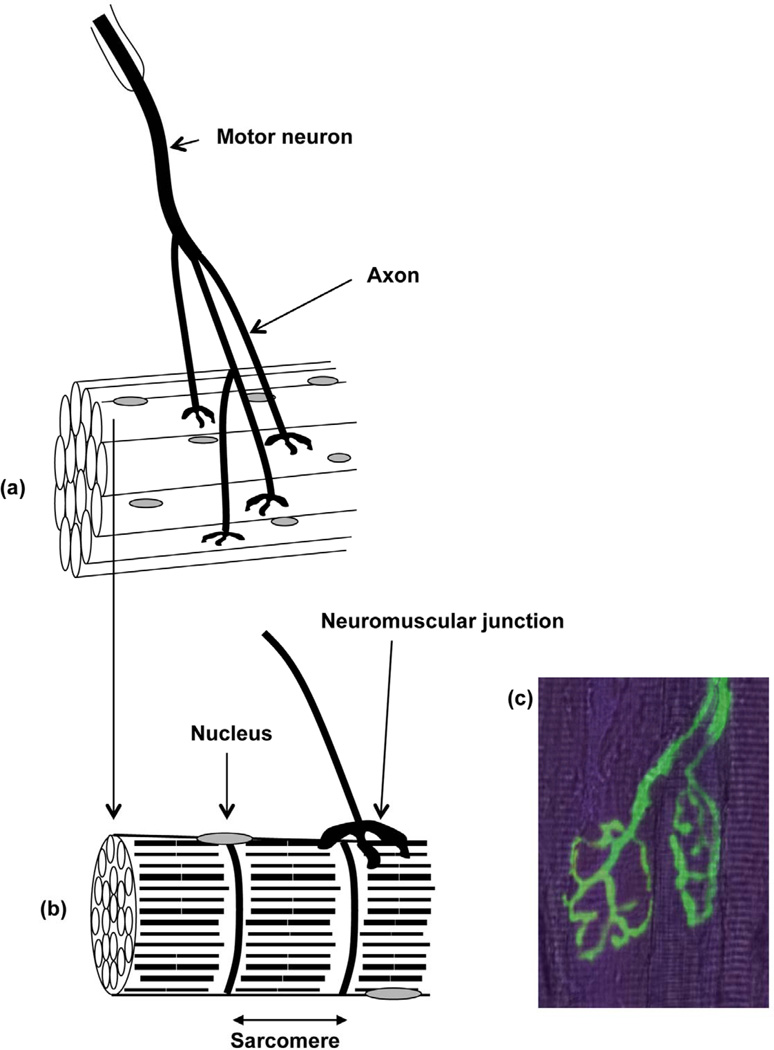
Sarcopenia is an age-related condition associated with a progressive decline of muscle mass, strength and quality [1]. Sarcopenia plays a major role in the pathogenesis of frailty. Increasing physical frailty results in a substantial reduction of the quality of life for older people. The motor unit is the functional unit for contraction of muscles and comprises the motor neuron, neuromuscular junctions and the muscle fibers innervated by the motor neuron (Figure 1). Age-related changes in nerves, muscle cells and the interactions between nerves and muscles are responsible for the loss of strength [2]. The reduction in muscle strength with age in mammals is due to a decrease in muscle cell (fiber) number and a reduction in the size and force generation of the remaining muscle fibers. The remaining muscle fibers of older individuals are further compromised showing an increased susceptibility to damage in comparison with muscles of younger adults and a reduced ability to fully regenerate following damage [3]. There is increasing evidence that the failure of old muscle to regenerate successfully following forms of damage that occur during routine use of muscles is an important mechanism by which weakness and further loss of muscle fibers develops [4, 5].
Fig. 1.
Organization of a motor unit (a) Bundle of muscle fibers innervated by a motor neuron (b) Neuromuscular junction on a single muscle fiber (c) Fluorescent image of neuromuscular junctions and peripheral axons from the Tibialis Anterior (TA) muscle of a young thy1-YFP mouse. The underlying muscle fibers are stained with phalloidin.
The fundamental processes leading to loss of muscle mass and function with age are still unclear, but in all species, tissues (including skeletal muscle) of aged organisms contain increased oxidative damage to lipids, DNA and proteins [6–8] and recent data from our laboratory has demonstrated that isolated skeletal muscle fibers from old mice at rest showed an increase in ROS activities compared with isolated skeletal muscle fibers from young mice at rest [9]. The hypothesis that a chronic increase in the generation of ROS plays a key role in the development of age-related muscle dysfunction has received considerable attention. Therefore, the purpose of this review is to summarise the roles of ROS in motor nerve function and muscle regeneration and how this is modified during aging.
Complete skeletal muscle regeneration depends on functional innervation
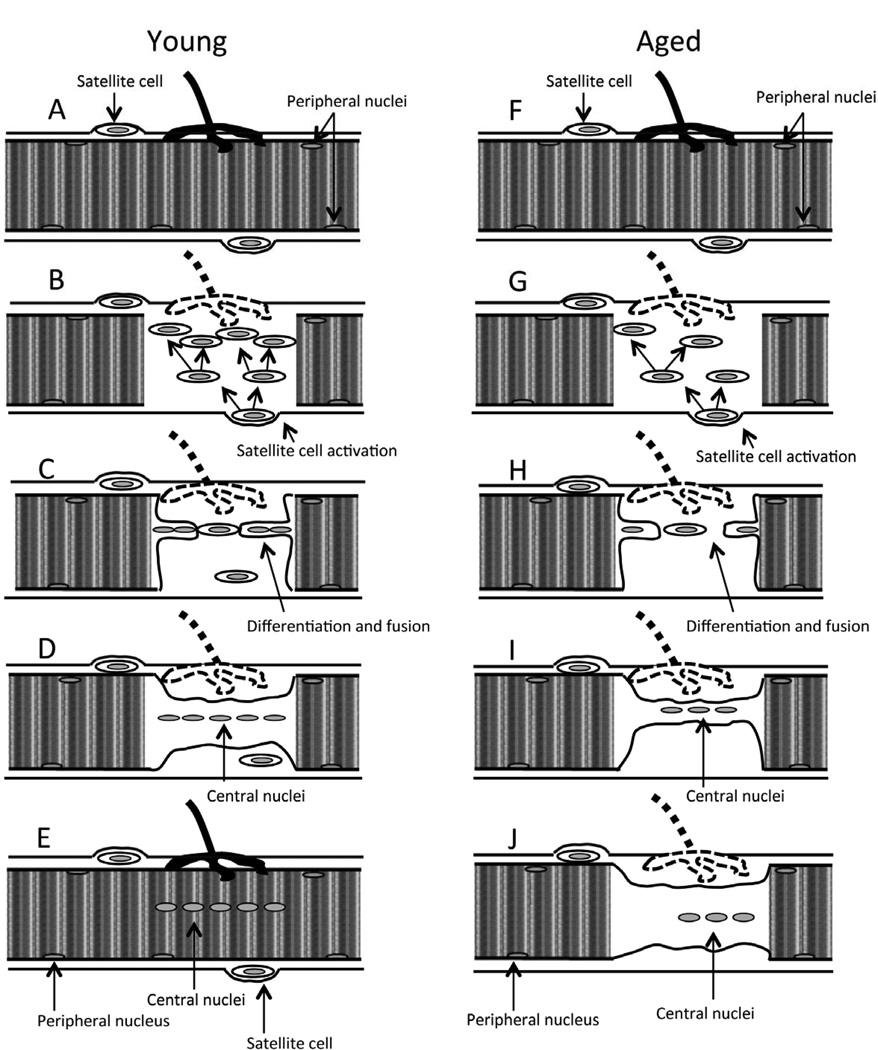
Skeletal muscle regenerates following damage due to the presence of undifferentiated, mononuclear satellite cells (tissue-specific stem cells) found under the basement membrane of muscle fibers (Figure 2A). These become activated and proliferate to become myogenic precursor cells (mpc’s otherwise known as myoblasts) following injury (Figure 2B; [10]). Proliferating myoblasts migrate to the damaged region of the muscle, fuse to form myotubes and differentiate to form muscle fibers (Figure 2C-D). Full regeneration and recovery of damaged muscle is dependent upon appropriate functional re-innervation of the regenerating muscle fiber and it appears that this occurs relatively late in the process of maturation of the regenerated fiber (Figure 2E). This requirement for reinnervation is apparent since denervated skeletal muscle is unable to regenerate fully following damage [11] and maturation of developing immature muscle fibers is critically dependent upon innervation [12].
Fig. 2.
Muscle cell regeneration following damage. (A) Quiescent young muscle cell with peripheral nuclei and satellite cells. (B) Following damage, satellite cells are activated and start to proliferate. Satellite cells differentiation and fusion with (C) each other and (D) with the damaged muscle fiber. (E) Newly regenerated muscle fiber with central nuclei and renewed satellite cells. (F) Quiescent aged muscle cell with peripheral nuclei and satellite cells. (G) Following damage, satellite cells are activated to proliferate but proliferation is slower. Satellite cell differentiation and fusion with (H) each other and (I) with the damaged muscle fiber happens at a slower rate. (J) Incomplete regeneration of aged muscle fiber.
Defective regeneration of damaged muscle contributes to loss of muscle mass and may be caused by defective re-innervation
In muscle from old rodents and humans, the process of regeneration is defective and this inability to fully recover from damage appears to play a major role in age-related loss of muscle mass [13–15]. The mechanisms underlying the failure of recovery from damage in muscles of old animals have been the subject of previous studies (for reviews see [4, 5]). Conboy and Rando [13] concluded that ‘failure of muscle maintenance and repair in aged rodents may be caused by an age-related decline in environmental cues rather than a decline in satellite cell function or potential’. Data from our laboratory examining in vivo models of muscle regeneration indicate that muscles of old mice do not regenerate fully following damage caused by lengthening contractions [16], a form of damage that is frequently observed during day-to-day use of some muscles. Muscles of old mice initially showed the same pattern of force loss and recovery as adult mice following the damaging contraction protocol, but did not achieve a full recovery (Figure 2F-J), maintaining a 40–50% deficit in maximum force generation at 28 days following damage [16], a deficit which appears to be permanent [3]. Data also indicate that the age-related defect predominately affects the latter phases of the processes of muscle regeneration, when successful innervation is critical [16].
The potential role of defective re-innervation of the regenerating muscle fibers in the incomplete recovery seen following muscle damage in old mice has not been extensively examined, although the aging process clearly influences motor nerve function and plasticity. Several studies have reported both atrophy and loss of axons in older individuals and a loss of myelination of some axons in motor nerves. Skeletal neuromusclular junctions (NMJs) are also extensively affected during aging. For example, data from Valdez et al [17] has demonstrated that NMJs in aged mice show a variety of age-related structural alterations, including axonal swellings, sprouting, synaptic detachment, partial or complete withdrawal of axons from some postsynaptic sites, and fragmentation of the postsynaptic specialization. The function of the terminal Schwann cells that produce myelin, cover the neuromuscular junctions and act as a guide for regenerating axons during reinnervation has also been reported to be reduced leading to reduced myelin production (for a review see [18]. Most data on the effect of aging on regeneration and repair of damaged motor neurons has been obtained from studies of the processes following direct nerve injury which have shown that the capacity for axonal regeneration and re-innervation is reduced in nerves from older rodents. Kawabushi et al [19] examined motor neuron function and repair following experimental sciatic nerve section in both young and old rats and found a greater incidence of immature axonal terminals in muscles of old compared with young rats. In addition, the rate of re-innervation of muscle was reduced in the muscles of old rats and the axonal terminals were not associated with Schwann cells. In other studies, the same group demonstrated a reduced rate of re-growth of axons and formation of new functional neuromuscular junctions following crush injury to peripheral nerves of old compared with young rodents [20].
In summary, although age-related changes in the interactions between nerve and muscle are still poorly understood, they appear to play a major role in the defective regeneration of skeletal muscle during aging.
ROS generation by skeletal muscle
Muscle generates superoxide and nitric oxide (NO) with the secondary formation of peroxynitrite, hydrogen peroxide and other ROS [21]. Physical exercise is associated with a dramatic increase in oxygen consumption in the working muscles. Most of the oxygen is utilised in the mitochondrial electron transport chain, where it is reduced to water [22]. Early studies suggested that in the mitochondrial electron transport chain, 2–5% of oxygen is converted to ROS, primarily superoxide radical and for that reason mitochondria are commonly considered as the predominant source of ROS in skeletal muscle cells. However, more recent data by several groups (for example see [23, 24]) argue against this and recent assessments of the rate of superoxide production by mitochondria indicate that only approximately 0.15% of the total oxygen consumed is reduced to superoxide. There is now considerable data demonstrating that mitochondria are not the only source of ROS production in skeletal muscle and that are other sources of ROS production within muscle cells including nicotinamide adenine dinucleotide phosphate (NADPH) oxidases [25] as well as Phospholipase A2 (PLA2) dependent processes (For an extensive review see [26]). In addition, data from Viña’s laboratory has demonstrated that superoxide anion is also generated by xanthine oxidase (XO) in contracting rat skeletal muscles cells [27]. In skeletal muscle, superoxide radicals are also found in the extracellular fluid and are produced at a relatively low rate with a significant increase during contractile activity [28–30].
Nitric oxide is also generated continuously in skeletal muscle. Resting muscles produce low levels of NO and the production of NO is increased during contractile activity [31, 32]. In contrast to the harmful effects of other ROS, generation of nitric oxide is thought to be useful (see section below). However, excess levels of nitric oxide can be cytotoxic and overproduction is thought to be involved in several inflammatory diseases [33]. Nitric oxide can also react with superoxide to form peroxynitrite (ONOO−) that is far more reactive than either of the parent radicals [21].
Due to the complex methods necessary for measuring ROS directly, only very few studies have attempted to monitor ROS activity in intact skeletal muscle during contractions and aging. We have previously demonstrated that contractile activity increased extracellular skeletal muscle ROS generation in both adult and old mice with no evidence for an age-related exacerbation of ROS generation [30]. More recently, we have demonstrated that isometric contractions to isolated skeletal muscle fibers from old mice resulted in no increase in oxidation of the non-specific, fluorescent probe for reactive oxygen and nitrogen species, dichlorodihydrofluorescein (CM-DCFH) compared with isolated skeletal muscle fibers from old mice at rest [9]. However, in the same study we demonstrated that skeletal muscle fibers from old mice have increased DCFH oxidation compared with skeletal muscle fibers from young mice at rest. This is in agreement with previous studies showing that the production of ROS is elevated is skeletal muscle of old rodents [34]. These changes impact negatively on muscle fiber homeostasis [35] and some genetic manipulations that reduce ROS activities preserve muscle function during aging (see below). Chronic inactivity of muscle is also associated with increased ROS generation [36]. It is therefore possible that the increased ROS production seen in muscles during aging may be related to the decreased physical activity of older individuals and may contribute to the age-related increase in redox imbalance within the muscle fibers.
ROS play a fundamental signalling role during muscle regeneration and re-innervation
Regulated changes in ROS formation are important in maintaining the normal sequence and development of myogenic processes. For example, changes in nitric oxide (NO) production facilitate fusion of cultured single myoblasts to myotubes and NO is a messenger in synaptic signalling in nerve-myotube co-cultures. Crucially, myotubes respond to nerve-muscle contact by localisation of the muscle isoform of neuronal nitric oxide synthetase (nNOS) to the acetylcholine receptor with an increase in NO formation that may act to prevent unwanted duplication of innervation of the myotube. There is less information on the effects of other ROS on myogenesis and regeneration although ROS activity due to NAD(P)H oxidase activity increases dramatically (~8 fold) in myoblasts from adult mice during differentiation [37]. Both excessive formation or a reduction in ROS during differentiation have also been shown to prevent formation of mature myotubes [37, 38]. In addition, data by Hansen et al indicated that excessive ROS activities inhibited myogenesis in muscle cultures, while treatment of the muscle cells with a ROS scavenger produced a more reductive cellular redox potential and enhanced myoblast differentiation [39]. We have demonstrated that myoblasts derived from glutathione peroxidase 1 knockout (Gpx1−/−) mice had decreased proliferation and increased apoptosis compared with wild-type cells and differentiated poorly with many residual mononuclear cells and the formation of only few, immature myotubes [40].
ROS play a key role in the loss of skeletal muscle mass observed during aging
ROS activities in many tissues increase with age and there is evidence that increased ROS generation may be the underlying reason for several age-related pathologies. Skeletal muscle has a substantial protective system and can enhance this system by increasing the production of cytoprotective proteins such as Heat Shock Proteins (HSPs) and antioxidant defence enzymes such as the superoxide dismutase enzymes, CuZnSOD and MnSOD, via activation of the transcription factors NFκB and AP-1 [28, 41]. Although it was originally reported that CuZnSOD is only expressed in the cytosol of cells, there is now evidence that a substantial part of cellular CuZnSOD is localized to the mitochondrial inter-membrane space (IMS) [42]. During aging, there is an accumulation of oxidative damage in muscle and an inability to activate these cytoprotective responses particularly following contractile activity [8, 41]. Interventions that maintain adaptive responses prevent the accumulation of oxidative damage and preserve some aspects of age-related muscle dysfunction [8, 16].
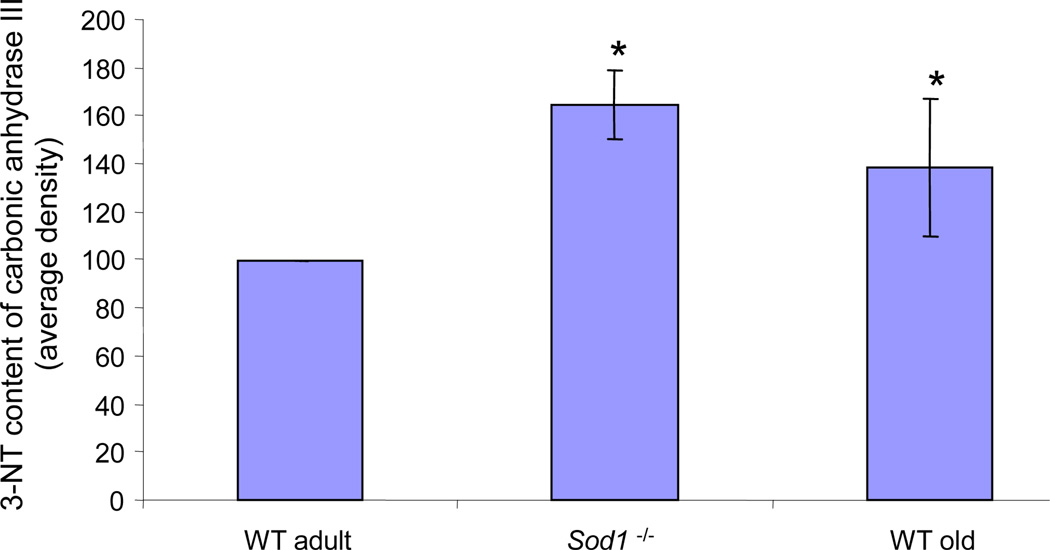
A further clear link between age-related muscle loss and increased ROS production has been indicated by studies of mice lacking CuZnSOD (Sod1−/− mice). These mice have an accelerated decline in muscle mass and function [43]. A major effect of aberrant ROS generation is oxidative damage to proteins [44–46] and previous work from our laboratory has shown that muscles of old wild-type mice and adult mice lacking CuZnSOD have an elevated content of 3-nitrotyrosine residues in the major cytosolic protein, carbonic anhydrase III in comparison with muscles from adult wild type mice (Figure 3; [7]). In our laboratory we have also examined whether mice lacking SOD1 show the lack of adaptation to contractile responses that is seen in old WT mice. Data indicate that, in contrast to adult WT mice, but in common with old WT mice, Sod1−/− mice demonstrated a constitutive activation of NFκB with increased production of pro-inflammatory cytokines and a constitutive increase in the content of HSPs in muscle at rest and failed to activate cytoprotective adaptive responses to contractile activity [47]. In addition, adult Sod1−/− mice show loss of motor function and contractility, decline in the number of motor units, partial denervation, degeneration of NMJs and mitochondrial dysfunction [47–51].
Fig. 3.
Relative 3-nitrotyrosine content of carbonic anhydrase III in skeletal muscle of adult wild type, old wild type and adult Sod1−/− mice (redrawn from [7]). Muscles of old wild type mice and adult mice lacking SOD1 have an elevated content of 3-nitrotyrosine residues in carbonic anhydrase III in comparison with muscles from adult wild type mice. Data are presented as a percentage of values from muscles of adult wild-type mice. *P<0.05 cf. adult wild type.
There is little direct data on the roles of ROS or effects of ROS on the structure and function of peripheral motor nerves, although aging leads to an increase in oxidative damage to proteins, lipids and nucleic acids in the central nervous system [52] and the age-related decrease in CNS function has been associated with an increase in markers of oxidative stress [53]. Schwann cells are rich in fatty acids and serve as a major substrate for ROS, therefore they can be particularly vulnerable to the accumulation of oxidative damage seen during aging [54]. Data from Opalach et al [55] demonstrated that aged animals had an accumulation of ubiquitinated and oxidatively damaged proteins within myelinated peripheral nerves and that this pronounced age-related oxidative damage is associated with the activation of proinflammatory events. Increased production of ROS are also thought also play an important role in age-related neurodegenerative diseases such as Alzheimer’s disease, motor neurone disease and Parkinson’s disease by damaging macromolecules within the nervous system (see [56] for a review).
Implications of defective ROS and oxidative damage for regeneration in skeletal muscle
There is increasing evidence that ROS are actively involved in controlling muscle differentiation. In previous collaborative studies we have demonstrated that when myoblasts from Gpx1−/− mice are induced to differentiate into fused multinucleated myotubes, they show significant impairment and form only a few immature myotubes compared to wild-type cells. In vivo, muscle fiber areas are also decreased in Gpx1−/− mice compared with wild-type mice [40]. These data strongly support the idea that ROS are crucial during myogenic differentiation and play a key role during muscle regeneration and repair.
ROS are also known to play a central role in a variety of muscle pathologies including muscle cachexia [57] and Duchenne muscular dystrophy [58, 59]. In addition, increased ROS production in skeletal muscles significantly contributes to inactivity-induced muscle atrophy (for an extensive review see [60]). However, there is little data on ROS and regeneration during aging. Studies from our laboratory have demonstrated that the inability to produce HSPs plays a critical role in the development of functional deficits that occur with aging in skeletal muscle. Studies using transgenic mice overexpressing HSP70 have shown that increased muscle content of HSP70 provided protection against the fall in specific force associated with aging, and facilitated rapid and successful regeneration following contraction-induced damage in muscles of old HSP70 overexpressor mice compared with the impaired regeneration and recovery normally observed in old wild type mice [16]. More recently, we have also demonstrated that overexpression of HSP10, a mitochondrial chaperone protein, can also preserve muscle function during aging in mice [61].
The redox sensitive transcription factor NFκB also plays a crucial role in the proliferation, differentiation and regeneration of muscle cells. Results obtained using C2C12 myoblast cell line have demonstrated that NFκB is constitutively active in proliferating myoblasts and that NFκB can inhibit myogenesis by inhibiting the synthesis of MyoD, which is a muscle-specific transcription factor involved in muscle development and repair or via transcriptional regulation of cyclin D1 which is an important regulator of cell cycle progression [62, 63]. In addition, it has been shown that systemic administration of curcumin, a pharmacological inhibitor of NFκB, stimulates muscle regeneration after traumatic injury [64]. Moreover, muscle specific NFκB inhibition in mice, through targeted deletion of the activating kinase inhibitor of NFκB kinase 2 (IKK2) has also been shown to facilitate skeletal muscle regeneration through enhanced satellite cell activation and reduced fibrosis in response to muscle damage [65]. We have previously demonstrated that the NFκB DNA binding activity is increased in quiescent muscles from old wild-type mice compared with adult mice [41] suggesting that the constitutive activation of NFκB may be functioning as an inhibitor of myogenic differentiation and regeneration during aging.
Since ROS have been found to be involved in controlling muscle regeneration, several studies have been focused on the properties and efficacy of antioxidants such as vitamin C and vitamin E to scavenge ROS and therefore to decrease oxidative damage in muscle. However, these studies, the majority of which were exercise related, investigated indirect indicators of muscle damage (for example plasma creatine kinase [66]) rather than specific markers of muscle regeneration. In addition, due to the variable study designs and differences in the type and amounts of the antioxidants, results from supplementation studies are highly inconsistent and/or not adequate, especially in human models. However, there is some evidence suggesting that vitamin E supplementation can promote myocyte plasma membrane repair in culture and in intact muscles in situ when exposed to an oxidant challenge suggesting that vitamin E is essential for maintenance of skeletal muscle homeostasis [67].
Several studies have also been conducted on the effectiveness of different polyphenols. For example dietary apple polyphenols have been shown to enhance muscle function in rats [68] and have preventive effects against lengthening contraction-induced muscle injuries [69]. Regarding muscle regeneration, treatment of C2C12 myoblasts with resveratrol, which is a unique grape wine polyphenol, results in initiation of differentiation [70]. The beneficial effects of polyphenols have also been demonstrated in vivo in studies using grape seed-derived proanthocyanidolic oligomer (PCO) supplementation which had the ability to accelerate muscle regeneration when taken as a preventative strategy before injury [71] or short-term post-injury [72]. Red wine phenolic extract intake has also been shown to prevent age-related impairment in skeletal muscle mitochondrial function through decreased ROS production [73]. However, further studies are needed to determine whether other polyphenols might exert the same protective effects particularly in regeneration during aging and whether these might be translated to human physiology.
Conclusions and Future directions
Identifying the importance of ROS generation during muscle re-innervation and regeneration could have fundamental implications for interventions to prevent muscle degeneration during aging and treatments to reverse the age-related decline in muscle mass and function. More specifically, by identifying which particular ROS disrupt the normal processes of re-innervation and muscle regeneration, it will be possible to identify targeted treatments to minimise these deleterious effects. These interventions will be aimed at decreasing the loss of skeletal muscle that occurs with age and ultimately improving the quality of life of older individuals.
Highlights.
Muscle regeneration following damage diminishes with age.
Successful muscle regeneration is dependant on appropriate re-innervation.
Age-related changes in nerve and muscle play a role in defective regeneration.
During aging there is an accumulation of oxidative damage in nerve and muscle.
Abnormal reactive oxygen species activities play a role in defective muscle re-innervation during aging.
Acknowledgements
The authors would like to thank their present and previous collaborators and colleagues and to acknowledge generous financial support from the BBSRC, MRC, Wellcome Trust, United States National Institute on Aging and Research into Ageing/Age UK.
Abbreviations
- AP-1
activator protein-1
- CNS
central nervous system
- CuZnSOD
copper, zinc superoxide dismutase GPx1, glutathione peroxidase 1
- GSH
reduced glutathione
- HSPs
heat shock proteins
- MnSOD
manganese superoxide dismutase
- mpc’s
myogenic precursor cells
- NAD(P)H
reduced nicotine adenine dinucleotide phosphate
- NFκB
nuclear transcription factor κappa B
- NMJs
neuromuscular junctions
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SOD1
copper, zinc superoxide dismutase
- WT
wild type
- XO
xanthine oxidase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in neurobiology. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. The American journal of physiology. 1990;258:C436–C442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- 4.Ehrhardt J, Morgan J. Regenerative capacity of skeletal muscle. Current opinion in neurology. 2005;18:548–553. doi: 10.1097/01.wco.0000177382.62156.82. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes & development. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 6.Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free radical biology & medicine. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 7.Vasilaki A, Simpson D, McArdle F, McLean L, Beynon RJ, Van Remmen H, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Formation of 3-nitrotyrosines in carbonic anhydrase III is a sensitive marker of oxidative stress in skeletal muscle. Proteomics. Clinical applications. 2007;1:362–372. doi: 10.1002/prca.200600702. [DOI] [PubMed] [Google Scholar]
- 8.Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- 9.Palomero J, Vasilaki A, Pye D, McArdle A, Jackson MJ. Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibers at rest, but not during contractions. American journal of physiology. Regulatory, integrative and comparative physiology. 2013 doi: 10.1152/ajpregu.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiological reviews. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 11.Borisov AB, Dedkov EI, Carlson BM. Abortive myogenesis in denervated skeletal muscle: differentiative properties of satellite cells, their migration, and block of terminal differentiation. Anatomy and embryology. 2005;209:269–279. doi: 10.1007/s00429-004-0429-7. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Askanas V, Engel WK. Human muscle cultured in monolayer and cocultured with fetal rat spinal cord: importance of dorsal root ganglia for achieving successful functional innervation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:3131–3141. doi: 10.1523/JNEUROSCI.07-10-03131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 14.Carlson ME, Silva HS, Conboy IM. Aging of signal transduction pathways, and pathology. Experimental cell research. 2008;314:1951–1961. doi: 10.1016/j.yexcr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degens H. Age-related skeletal muscle dysfunction: causes and mechanisms. Journal of musculoskeletal & neuronal interactions. 2007;7:246–252. [PubMed] [Google Scholar]
- 16.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 17.Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. Journal of the peripheral nervous system : JPNS. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawabuchi M, Chongjian Z, Islam AT, Hirata K, Nada O. The effect of aging on the morphological nerve changes during muscle reinnervation after nerve crush. Restorative neurology and neuroscience. 1998;13:117–127. [PubMed] [Google Scholar]
- 20.Kawabuchi M, Zhou CJ, Wang S, Nakamura K, Liu WT, Hirata K. The spatiotemporal relationship among Schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. The Anatomical record. 2001;264:183–202. doi: 10.1002/ar.1159. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MJ. Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:2285–2291. doi: 10.1098/rstb.2005.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free radical biology & medicine. 1995;18:1079–1086. doi: 10.1016/0891-5849(94)00212-3. [DOI] [PubMed] [Google Scholar]
- 23.Aydin J, Andersson DC, Hanninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Human molecular genetics. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- 24.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. The Journal of biological chemistry. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 25.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of Mitochondrial and Nonmitochondrial Sources Implicate Nicotinamide Adenine Dinucleotide Phosphate Oxidase(s) in the Increased Skeletal Muscle Superoxide Generation That Occurs During Contractile Activity. Antioxidants & redox signaling. 2013;18:603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological reviews. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. The Journal of physiology. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. American journal of physiology. Cell physiology. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 29.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 30.Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 31.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 32.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B. Oxidative stress in skeletal muscle. Basel, Switzerland: Birkhauser Verlag; 1998. [Google Scholar]
- 34.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. Journal of applied physiology (Bethesda, Md : 1985) 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 35.Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. The Journal of physiology. 2011;589:2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talbert EE, Smuder AJ, Min K, Kwon OS, Szeto HH, Powers SK. Immobilization-Induced Activation of Key Proteolytic Systems in Skeletal Muscles Is Prevented by a Mitochondria-Targeted Antioxidant. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00471.2013. [DOI] [PubMed] [Google Scholar]
- 37.Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS. Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free radical biology & medicine. 2005;38:989–1001. doi: 10.1016/j.freeradbiomed.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hong F, Lee J, Song JW, Lee SJ, Ahn H, Cho JJ, Ha J, Kim SS. Cyclosporin A blocks muscle differentiation by inducing oxidative stress and inhibiting the peptidyl-prolyl-cis-trans isomerase activity of cyclophilin A: cyclophilin A protects myoblasts from cyclosporin A-induced cytotoxicity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1633–1635. doi: 10.1096/fj.02-0060fje. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JM, Klass M, Harris C, Csete M. A reducing redox environment promotes C2C12 myogenesis: implications for regeneration in aged muscle. Cell biology international. 2007;31:546–553. doi: 10.1016/j.cellbi.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Shin HS, Shireman PK, Vasilaki A, Van Remmen H, Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free radical biology & medicine. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age. Mechanisms of ageing and development. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kawamata H, Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxidants & redox signaling. 2010;13:1375–1384. doi: 10.1089/ars.2010.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free radical biology & medicine. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 44.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica chimica acta; international journal of clinical chemistry. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 45.Ghezzi P, Bonetto V. Redox proteomics: identification of oxidatively modified proteins. Proteomics. 2003;3:1145–1153. doi: 10.1002/pmic.200300435. [DOI] [PubMed] [Google Scholar]
- 46.Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Experimental gerontology. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 47.Vasilaki A, van der Meulen JH, Larkin L, Harrison DC, Pearson T, Van Remmen H, Richardson A, Brooks SV, Jackson MJ, McArdle A. The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu,Zn superoxide dismutase. Aging cell. 2010;9:979–990. doi: 10.1111/j.1474-9726.2010.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flood DG, Reaume AG, Gruner JA, Hoffman EK, Hirsch JD, Lin YG, Dorfman KS, Scott RW. Hindlimb motor neurons require Cu/Zn superoxide dismutase for maintenance of neuromuscular junctions. The American journal of pathology. 1999;155:663–672. doi: 10.1016/S0002-9440(10)65162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Remmen HV, Richardson A, Feldman EL, Brooks SV. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301:R1400–1407. doi: 10.1152/ajpregu.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, Siwek DF, Upton-Rice M, Brown RH., Jr Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53:1239–1246. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- 52.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navarro A, Sanchez Del Pino MJ, Gomez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;282:R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 54.Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron's perspective. Current opinion in clinical nutrition and metabolic care. 2013;16:21–26. doi: 10.1097/MCO.0b013e32835b5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation research. 2010;13:65–74. doi: 10.1089/rej.2009.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 57.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. The EMBO journal. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 58.Allen DG, Whitehead NP. Duchenne muscular dystrophy--what causes the increased membrane permeability in skeletal muscle? The international journal of biochemistry & cell biology. 2011;43:290–294. doi: 10.1016/j.biocel.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clinical and experimental pharmacology & physiology. 2006;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- 60.Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Current opinion in clinical nutrition and metabolic care. 2012;15:240–245. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kayani AC, Close GL, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle Cross-Sectional Area. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;299:R268–R276. doi: 10.1152/ajpregu.00334.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Molecular and cellular biology. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 64.Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. The American journal of physiology. 1999;277:C320–C329. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 65.Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. The Journal of clinical investigation. 2006;116:2945–2954. doi: 10.1172/JCI28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer- Kristensen J, Pedersen BK. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. American journal of physiology. Cell physiology. 2001;280:C1570–C1575. doi: 10.1152/ajpcell.2001.280.6.C1570. [DOI] [PubMed] [Google Scholar]
- 67.Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nature communications. 2011;2:597. doi: 10.1038/ncomms1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazato K, Song H, Waga T. Dietary apple polyphenols enhance gastrocnemius function in Wistar rats. Medicine and science in sports and exercise. 2007;39:934–940. doi: 10.1249/mss.0b013e31803df4bc. [DOI] [PubMed] [Google Scholar]
- 69.Nakazato K, Ochi E, Waga T. Dietary apple polyphenols have preventive effects against lengthening contraction-induced muscle injuries. Molecular nutrition & food research. 2010;54:364–372. doi: 10.1002/mnfr.200900145. [DOI] [PubMed] [Google Scholar]
- 70.Kaminski J, Lancon A, Aires V, Limagne E, Tili E, Michaille JJ, Latruffe N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochemical pharmacology. 2012;84:1251–1259. doi: 10.1016/j.bcp.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 71.Myburgh KH, Kruger MJ, Smith C. Accelerated skeletal muscle recovery after in vivo polyphenol administration. The Journal of nutritional biochemistry. 2012;23:1072–1079. doi: 10.1016/j.jnutbio.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Kruger MJ, Smith C. Postcontusion polyphenol treatment alters inflammation and muscle regeneration. Medicine and science in sports and exercise. 2012;44:872–880. doi: 10.1249/MSS.0b013e31823dbff3. [DOI] [PubMed] [Google Scholar]
- 73.Charles AL, Meyer A, Dal-Ros S, Auger C, Keller N, Ramamoorthy TG, Zoll J, Metzger D, Schini-Kerth V, Geny B. Polyphenols prevent ageing-related impairment in skeletal muscle mitochondrial function through decreased reactive oxygen species production. Experimental physiology. 2013;98:536–545. doi: 10.1113/expphysiol.2012.067496. [DOI] [PubMed] [Google Scholar]