Abstract
In this study, we reveal that a neutral polysaccharide isolated from a Chinese medicinal herb, named Ginseng (Panaxgiseng C.A. Meyer), promotes maturation of BMDCs via inducing changes both inside and outside BMDCs, as well as changes of functions. These affects of NGP on BMDCs were evaluated with use of conventional scanning electronic microscopy (SEM), transmission electronic microscopy (TEM) for morphology of BMDCs, flow cytometry (FCM) for key surface markers of BMDCs, cytochemistry assay, FITC-dextran, bio-assay for their phagocytosis and enzyme linked immunosorbent assay (ELISA) for cytokine production by BMDCs. Our results proved that NGP induced maturation of BMDCs as reflected by the downregulation of acid phosphatase (ACP) activity inside the BMDCs, which occurs when phagocytosis of BMDCs decreased, while antigen presentation increased upon maturation. These data also revealed higher expression of MHC II, CD80, CD86, CD83, CD40 and secretion of higher level of IL-12 and low level of TNF-α. Our approach suggests that NGP could therefore stimulate the maturation of murine BMDCs through a series of regulation to the BMDCs.
Keywords: neutral ginseng polysaccharides, dendritic cells, maturation, immunomodulation, phagocytosis
Introduction
Ginseng is a traditional Chinese herbal medicine with a pivotal role and a well-known plant medicine in the world, which has been used for centuries clinically for treatment of many diseases, such as cancer, hepatitis and other immune handicapped cases.1 Also it has been using as a major tonic for poor body health or recovery from illness. So far several components from different Ginseng species in America, Korea and China with broad medical functions have been identified.2 NGP, a key component of immunoregulation with C18H32O16 in formula, 504 in molecular weight, composed of O-α-(1→ 6)-D-Glucan is a purified compound isolated from Ginseng.3 The reports from early studies claimed that NGP was with many immune-enhancing properties, like activation of macrophage and NK cells, increasing secretion of cytokines such as IL-2, IL-12 and IL-6, production of antibody by B cell, regulating immune function, inflammatory or antiviral processes and response to stress and fatigue.4-7 In China NGP has become a commercial product for patients with various kinds of infections to improve the immune defense during infections, immune dysfunction and other immune boosting needed situation, such as cancers.8-12 However, so far there are no reports on either the concrete mechanisms or maturation changes of DCs post treatment with NGP and the precise mechanisms to regulate the cells in immune system through dendritic cells (DCs) by NGP remain unclear.
Dendritic cells (DCs), originally identified by Steinman (1972),represent the milestone of the cancer immunology.13,14 DCs are professional antigen presenting cells (APCs) that possess the ability to initiate naïve T cell response. Therefore they play key role in driving T cell responses that result in cell-mediated immunity.More recently increasing number of articles showed great potential of DCs in both cancer therapy and vaccine preparations.15-17
In this study, we performed the following studies to investigate and analyze the changes of murine BMDCs induced by NGP, in order to gain better understanding of the mechanisms through which NGP regulate immune cells. Based on these data broader clinical applications can be expected.
Results
The purity of BMDCs purified by MACS
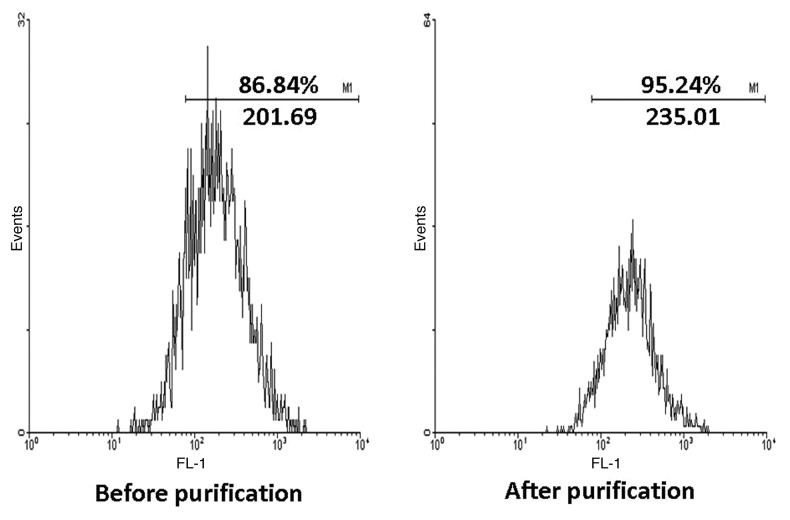
The cultured BMDCs were purified with MACS and lot of other cells, or debris, were removed. The final purity of BMDCs reached approximately 95% as shown in Figure 1 for further study.
Figure 1. After cultured with GM-CSF and IL-4 for 6 d, the purity of CD11c+ cells were examined by FACS and the percentage were over 86%. Then purified by MACS, the CD11c+ cells were enriched and the result of FACS approached 95%. FSC/SSC plot was shown in order to get an impression on the purity of the isolated cells.
Expansion of BMDCs induced by a range of NGP
The cultured BMDCs after induced by NGP expanded into different numbers with various degrees of maturity at different time point (Fig. 2A). With MTS method we determined that the most optimal concentration of NGP at which cells grew best was 200 μg/ml as shown in Figure 2B.
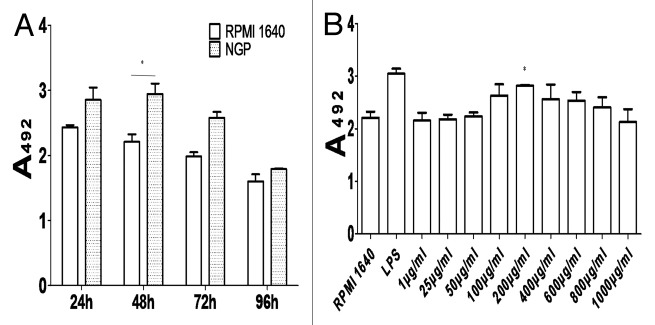
Figure 2. Determination of BMDCs proliferation at different time point (A) and at different concentration of NGP (B) by MTS method.
General morphology of the BMDCs treated with NGP
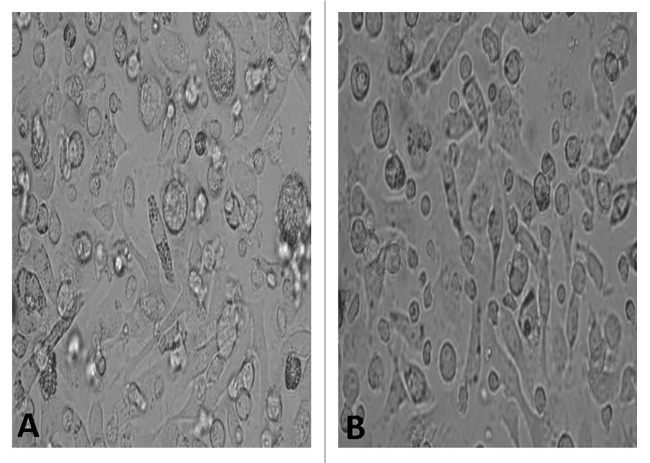
The BMDCs were cultured under the condition of GM-CSF and IL-4 for 6 d and then the cells were observed under conventional light microscope. The picture in Figure 3 showed the BMDCs were large with oval or irregularly shaped nuclei and many long or short dendrites. These phenomena were found more in the NGP group compared with those in the control group.
Figure 3. The morphology(×200) under light microscope showed the change of BMDCs. (A) RPMI 1640, (B) NGP.
SEM for fine morphology of BMDCs
Mature DC usually expresses more complicated surface molecules, which include receptors for uptaking antigens and binding with other cell to trigger an immune response. Great changes on surface of DC will happen while DC goes into maturation. The pictures of secured SEM were compared before and post treatment with NGP. The BMDCs post treatment with NGP exhibited more matured shape with more long protrusions and more cascading folds than that untreated DCs did as shown in Figure 4.
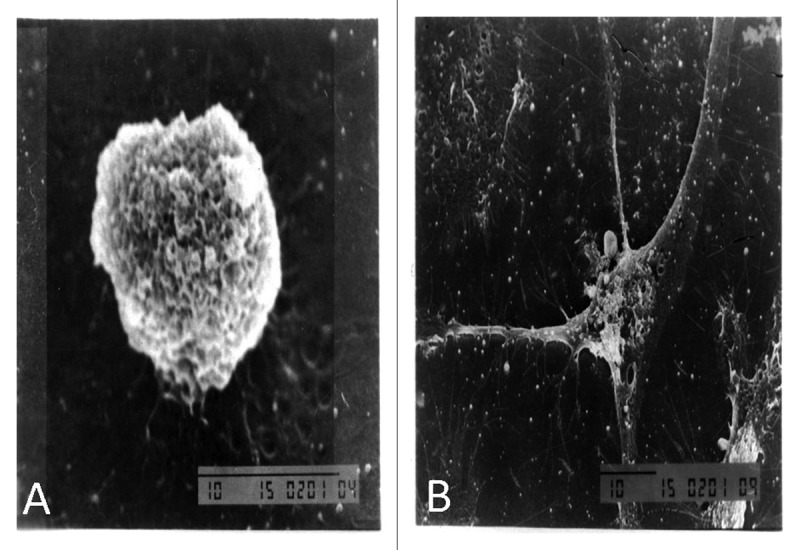
Figure 4. Scanning electron microscopy showed BMDCs morphology. Most of the BMDCs treated with RPMI 1640 were small and round with smooth rim shown in Figure 4A (×5000). However, BMDCs treated with NGP have rough cell surface with many folds and different types of protrusions shown in Figure 4B (×2000).
TEM for intracellular changes inside the BMDCs
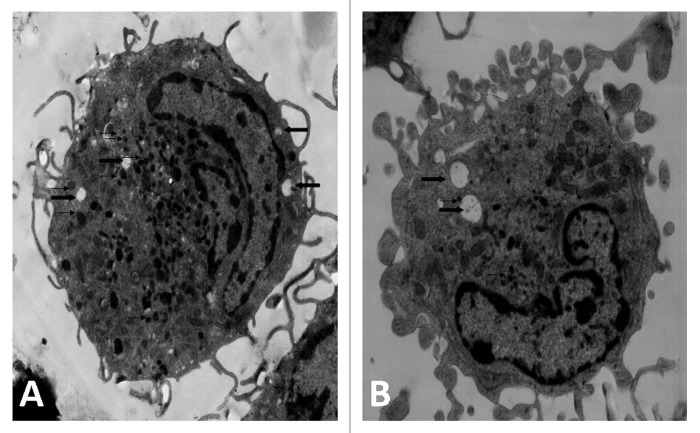
Generally immature DC is stronger in phagocytosis and contains more phagosomes. When DC becomes matured the numbers of phagosomes inside the DC will reduce with increased expression of key surface markers .The pictures in Figure 5 from TEM demonstrated that there was greater reduction for phagosomes inside the mature BMDC than those inside the immature BMDC.
Figure 5. Transmission electron microscopy revealed BMDCs morphology, surface, cytoplasm and organelles. After cultured with NGP for 48 h, the majority of cells had a more irregular surface with more cytoplasmic projections, less vacuoles (heavy arrow) and lysosomes (thin arrow). (A) RPMI1640, (B) NGP (×5,000).
ACP activity
The activity of ACP represents DC’s ability to digest antigens and its number reflect the maturity of DC via an inverse relationship. That means after the BMDCs were treated with 200μg/ml NGP in the culture for 48h, immature BMDCs became matured with a marked decrease in ACP activity, accompanying a gradual termination of phagocytosis or antigen ingestion, and simultaneously, an increased antigen presentation. This also led to the production of IL-12, which would work as an intensified signal to initiate CD4+T cells response.
The ACP activity of the BMDCs was measured by the method illustrated above and yielded the following activity number: 48.032 ± 1.381 U/gprot in the LPS group and 57.850 ± 3.760 U/gprot in the NGP group (p < 0.01) vs. 66.912 ± 2.324 U/gprot in the RPMI 1640 group, as shown in Figure 6. This is consistent with the number of phagosomes inside the BMDC.
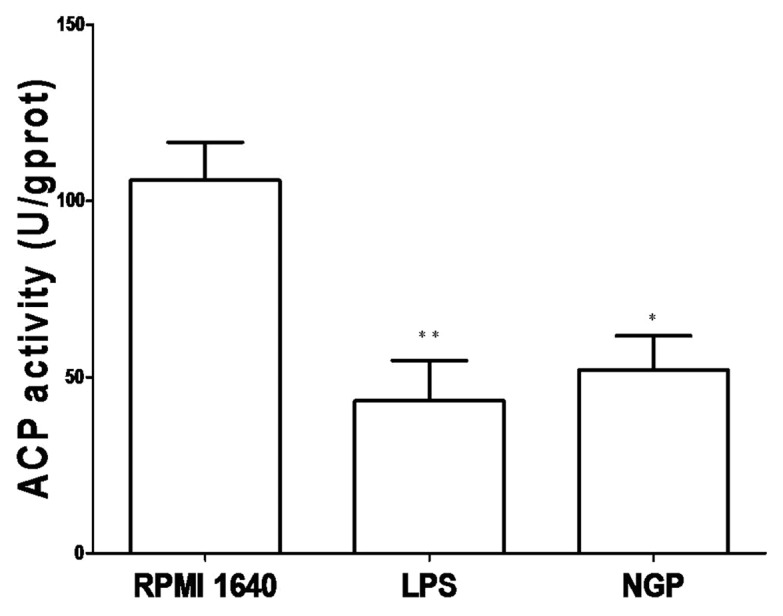
Figure 6. NGP decreased ACP activity inside the BMDCs. All data of ACP activity were shown as means ± S.E.M. (n = 3).
Confirmation of the BMDCs’ phagocytosis by FCM
Immature DC is stronger in phagocytosis and contains more phagosomes. When phagocytosis inside BMDCs took place the BMDCs phagocyted FITC labeled Dextran with higher G means. When the BMDCs were cultured in 200 μg/ml NGP for 48 h, they became matured with decreased phagocytosis as reflected in correspondingly reduced G means, shown in Figure 7. Mean ± sd from three sample per group were shown.
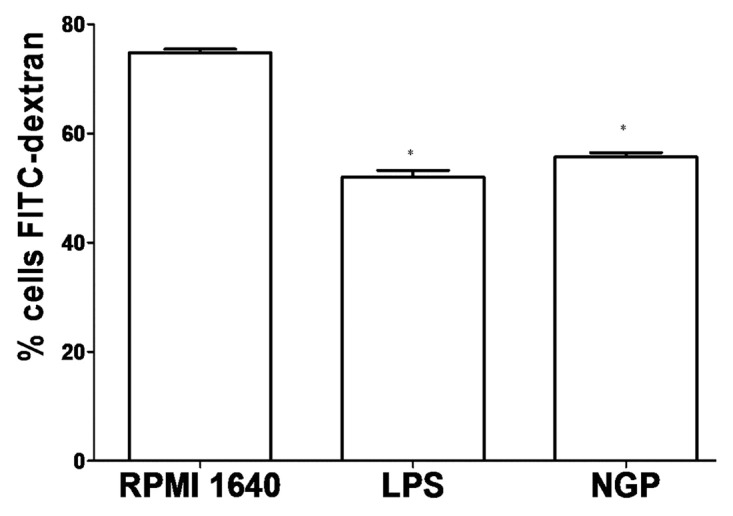
Figure 7. Cellular immunohistochemistry of phagocytosis by BMDC. The BMDCs in different groups were stained with DAB kit. The BMMCs were observed with an inverted phase contrast microscope (CMS GmbH Light Microscopes, Leica Microsystems) (×400).The immature BMDC filled with DAB precipitate could be seen clearly.
Immunohistochemical staining of the BMDCs cells after treatment with NGP
After staining with DAB kit, and restaining with hematoxylin, the DC under light microscope displayed phagocytosing. The pictures showed phagocytosing horseradish peroxidase was in process, and showed more phagocytosis in the immature BMDCs cells than in the mature BMDCs as shown in Figure 8.This is consistent with the change of ACP activity and also with phagocytic effect by DC.
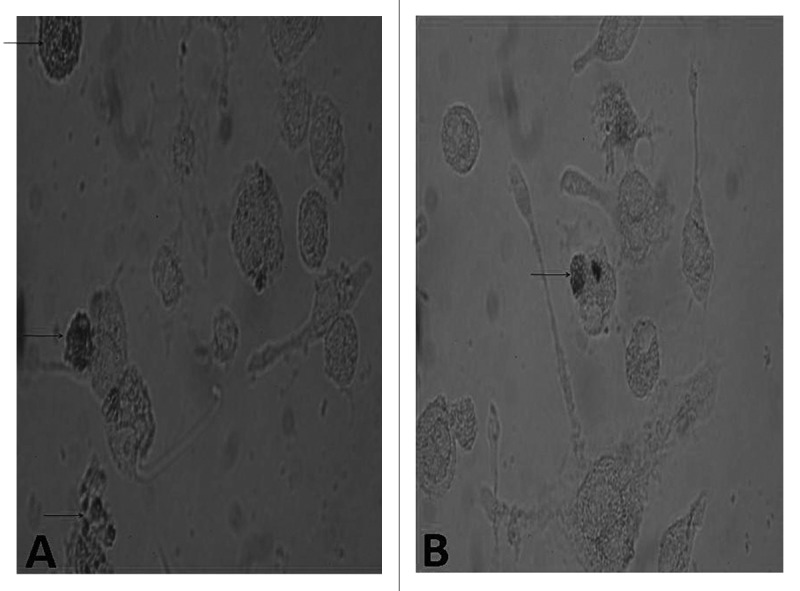
Figure 8. Comparison of FITC-dextran uptaking by immature and mature BMDCs. The BMDCs in different groups were prepared and compared in their ability to uptake FITC-dextran. A representative of three independent experiments was shown. The cells with lower FITC-dextran uptake were preincubated with LPS or NGP. For the analysis of dextran uptaking, the data for controls incubated at 4°C should be subtracted from the 37°C data. All data are represented as means ± S.E.M.(n = 3). *p < 0.05 vs. these in RPMI 1640. **p < 0.01 vs. those in RPMI 1640.
Analysis of the BMDCs ' key surface markers by FCM
The cultured BMDCs were stained with mAbs following steps mentioned above and underwent FCM analysis. The comparison between testing group and control group showed that the percentage of MHC II expression on NGP-treated BMDCs were 2-fold more than those on the control. Accordingly, there were increased percentages of CD80, CD86, CD83 and CD40 on the BMDCs treated with NGP. The data shown in Figure 9 indicated the distribution of the expression of the key surface markers.
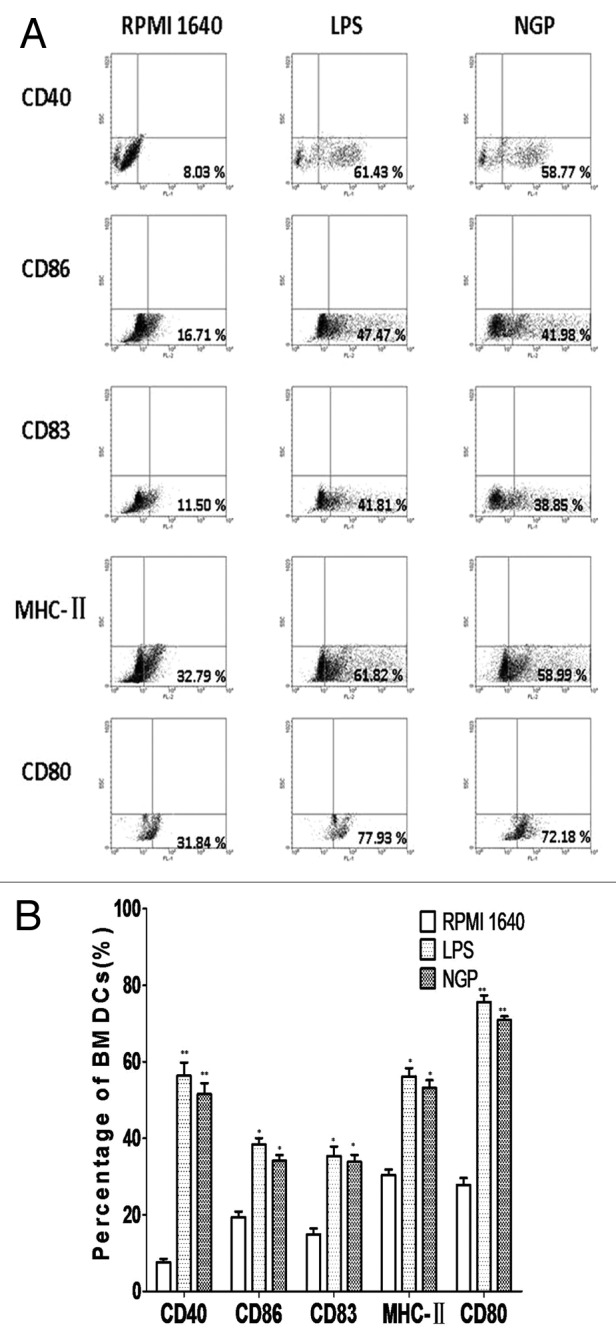
Figure 9. (A) NGP enhanced the phenotypic maturation of BMDC. The BMDC were phenotypically assessed using FACS analyses to determine the expression levels of specific cell surface molecules, as shown in the percentage of expression. NGP clearly increased the expression of CD40, CD80, CD83, CD86 and MHC-II (a representative of three independent experiments). (B) NGP enhanced the phenotypic maturation of murine DCs. All data were shown as means ± S.E.M.(n = 3). *p < 0.05 vs. these in RPMI 1640. **p < 0.01 vs. those in RPMI 1640.
Cytokine assay of the IL-12p70 and TNF-α by ELISA
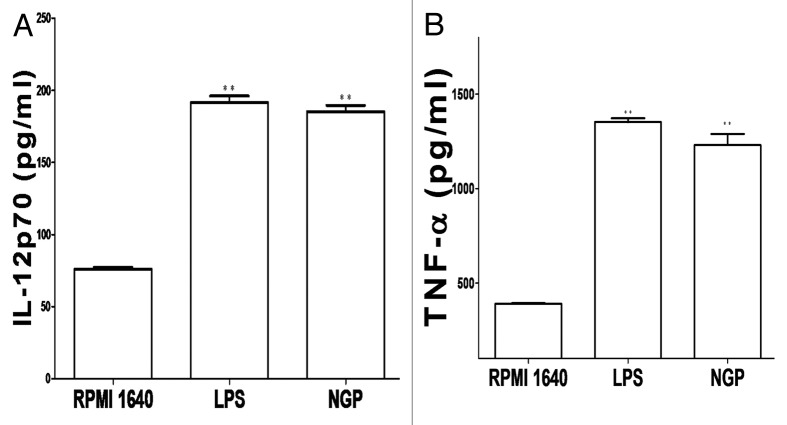
After the immature BMDCs were exposed to 200 μg/ml for 96h, the cultured BMDCs became matured both morphologically and functionally. Besides the higher expression of key surface markers the matured BMDCs also produced higher levels of IL-12p70, as shown in Figure 10A. Similarly, the production of TNF-α was shown in Figure 10B.
Figure 10. The BMDCs matured in the presence of LPS or NGP produce more IL-12p70 and TNF-α. Cytokines IL-12p70 and TNF-α secretion were tested by ELISA from cultured supernatants of 5 × 104 cells/well of immature and mature BMDC. Results were expressed as the mean ± SEM of triplicate samples. NGP led to an approximate twice of production in IL-12p70 with *p < 0.05 vs. these in RPMI 1640 and TNF-α with **p < 0.01 vs.those in RPMI 1640.
Obviously, these results shown in Figure 10 were corresponding with those obtained in morphological maturation of the BMDCs and data from ACP activity above.
Mixed lymphocyte reaction assay.
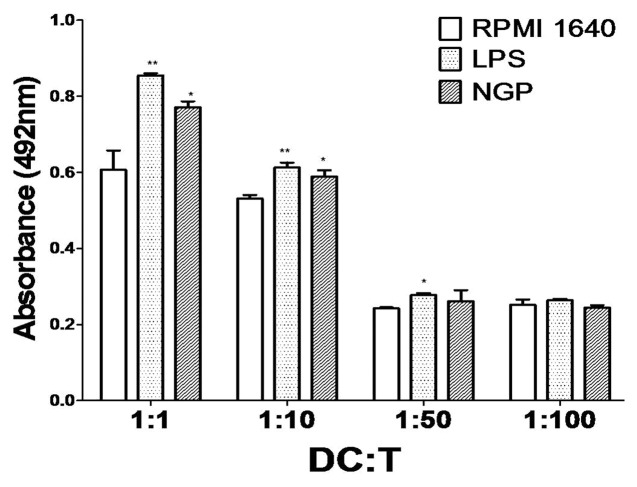
The T cell proliferation was measured by the MTS assay. The T cell proliferation indirectly reflects the ability of DCs to stimulate the proliferation of T cell. The results showed that, as compared with the control group, the BMDCs from NGP-treated group or LPS-treated group could dramatically increased the proliferation of T cell .(Fig. 11) When the ratio of dendritic cells to T cells was at 1:1, there was a significant increase in T cell activation and proliferation.
Figure 11. Mixed lymphocyte reaction assay. Dendritic cells derived from bone marrow were co-cultured with the lymphocytes for 3 d at the ratios of 1:1, 1:10, 1:50, 1:100 and proliferation of lymphocytes was measured by MTS assay. All data were shown as means ± S.E.M. (n = 3). *p < 0.05 vs. these in RPMI 1640. **p < 0.01 vs. those in RPMI 1640.
Discussion
There are many reports on the biological functions and clinical application of Ginseng or its fractions of various components. Also there are a few approaches on either properties or clinical usage with different Ginseng polysacharisdes. NGP, being an purified component of Ginseng extract and an effective immune modulator, plays a very important role in both immune modulation and immune boosting based clinical applicatiom.19 In fact, NGP’s general function of upregulating immune system has been recorded previously.1,20-22 The data of their findings indicated that NGP could upregulate the functions of cells in both innate immune and adaptive immune systems. These included: significantly increasing phagocytosis as well as production of cytokines of macrophage,3 improving B lymphocyte proliferation induced by LPS, enhancing the activity of monocyte by upregulating the expression of HLA-DR and other application for various cases clinically.23-25
Recently, accumulated data on NGP elucidated that NGP alone or when combined with other agents could retard tumor growth, resulting in marked regression of tumor . In China, actually this clinical application has been an very important alternative for terminal cancer patients or other immune dysfunction cases. .
From our results above, we found that NGP’s immunomodulating effects on DCs were present and functional, as reflected by following findings: (1) NGP shows positive regulation on DCs at a range of concentration and the best concentration is 200 μg/ml (Fig. 2), (2) the clear changes of matured DC in morphology are with more protrusions and rougher surface (Fig. 3), which usually indicate increased expression of key surface markers or other receptors, which are related to phagocytosis, (3) the number of phagosomes reduction inside the DC (Fig. 4) and ACP activity (Fig. 5) are usually, parameters indicating maturation of DC, and an indicators of phagocytosis reduction (Fig. 7), with an simultaneous increase in antigen presentation, corresponding to the higher expression of MHC class II, CD86 and CD40 molecules (Fig. 8), (signals between the T cell receptor and co-receptor paths) and (4) higher production of IL-12 levels (Fig. 9) would intensify DC-CD4+T cell pathway, resulting in increased Th1 responses, coordinating other immune cells such as macrophage involved in immune responses. Also the presence of IL-12 is necessary for CD4+T cell activation which in turn secretes other cytokines such as IL-2, IFN-γ and TNF-β, coordinating cellular immune responses. Meanwhile, IL-12 is also responsible for triggering a chain of immune responses in the immune network. Simultaneously, proinflammatory TNF-αsecretion is downregulated in the ongoing process of the DCs’ maturation. Usually at early stage of infection DCs secret higher amount of TNF-αto be involved in initiating innate immunity. However, with the infection going on DCs become matured and begin to trigger adaptive immunity though mounting antigen presentation and accordingly, followed by the decreased production of TNF-α.
The morphology study by SEM, TEM for sub-cellular study and histochemistry study above, plus ACP test after treatment with 200μg/ml NGP confirmed and reconfirmed the boosting effect to DC maturation by NGP consistently.
Besides these, we also need to notice that the increased IL-12 production not only acts on DCs themselves as an autocrine and paracrine signal molecule, resulting in DCs expansion further, but also would augment CD4+T cell population, and increase stability of the pathway between the DCs and CD4+T cells, which, in turn, will result in more secretion of cytokines like IL-12 and TNF by the DCs and IFN-γ by the CD4+T cells to be involved in initiatinga chain of immune responses in body.22
The immune system is a diverse and very complicated entity, in which numerous immune cells as well as molecules are interconnected, forming a coordinated interaction between innate and adaptive immune systems. Moreover, internal interactions among the immune cells are done through a variety of signal cytokines that regulate or even police each other as a part of dynamic immune system. When NGP works, it modulates the functions of DCs, not only via a direct binding to the receptors on DCs, but also via an indirect route by regulating other immune cells such as macrophages, which can secrete high levels of IL-12, IL-6 and TNF-α, adding a synergistic effect to DCs and NK cells, which can also secrete IFN-γ, inducing the maturation of DCs. As a result of this chain of effects, there is then an intensified immune network maintaining the balance of the body.
Because DCs have emerged as the most potent and professional antigen presenting cells to stimulate naïve T cells and initiate T cell responses, functioning as messengers between the innate and adaptive immunities. We may think about potential application of NGP as an adjuvantin therapeutic vaccines in cancer immunotherapy and for other immune handicapped diseases.
Our contribution could therefore help further understanding of NGP’s immunoregulating impact on immune system via modulating DC, and activation of chain responses among immune cells, through which NGP would be functioning. At last, this inestigation also provides a practicable mode of action for NGP and highlights its clinical potential as an immune remedy for for cancer patients with damaged immune system after chemotherapy. As far as we know this is the first time to present data about detailed analysis of morphology, cellular and sub-cellular structure of BMDC under influence of NGP and this approach will help us with better understanding positive modulation by NGP to BMDC via the mechanisms elucidated above and to whole immune system as well. NGP can markedly enhance BMDC’s maturation and functions with supplying MHC class II molecules, plus other co-stimulatory molecules like CD80 (86), CD83 and CD40, accompanying higher production of IL-12, and this chain activation resulted in upregulation of antigen presentation and thus effectively activating CD4+T cell, strengthening the DC-CD4+T cell pathway and IFN-γ secreted by CD4+T cell will activate macrophage, so that whole immune system is intensified.
Materials and methods
Major reagents
NGP (> 99% purity, 3 mg/ml) was bought from Pude Pharmaceutical Company. The affect of NGP at a range of concentrations from1 μg/ml to 1000 μg/ml on the proliferation of DCs in vitro was tested, and the optimal concentration was found out. Based on these approaches, the optimal concentration of 200 μg/ml was used in current study. Interleukin-4 (IL-4) and recombinant murine granulocyte macrophage colony stimulating factor (GM-CSF) were obtained from PeproTech Inc. The mAbs used in this study include FITC-conjugated anti-CD40, PE-anti-MHC-IIand PE-anti-CD83, PE-anti-MHC-II and PE-anti-CD86from eBioscience and BD PharMinge. The ELISA assay kits for IL-12p70 and TNF-αwere purchased from eBioscience. Lipopolysaccharide (LPS) was a product of Sigma-Aldrich. Other chemicals frequently used in our laboratory were all products from Sigma-Aldrich or BD PharMingen.
Preparation of murine BMDCs
A modified protocol from a previously described method18 was used for preparation of BMDCs as follow:The bone marrow cells from the femurs and tibias of female C57BL/6 mice (4–6 weeks old from pathogen free animal house, China Medical University) were depleted of red cells with lyses buffer. Approximately, a total 106 cells were placed in 24-well plates fulfilled with 3 ml of RPMI 1640 supplemented with 10% fetal bovine serum, GM-CSF (10 ng/ml), IL-4 (10 ng/ml), 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. The cells incubated for 4 h in plates were then gently swirled and the medium containing non-adherent cells was replaced with fresh medium as described above. Supplemented medium was replaced every 3d and on 6d of culture, all the cells expressing CD11c in all wells were sorted out respectively using MACS (MiltenyiBiotec) per manufacturer’s instruction and seeded them into new wells with fresh medium. The purity of the sorted cells was determined by FCM analysis (Fig. 1). Finally, the CD11c-positive cells (BMDCs) were treated with or without NGP at 200 μg/ml for another 48h for the phenotypic studies.
MTS assay to determine expansion of BMDCs treated with a range of concentrations of NGP
The BMDCs were digested into cell suspension and the number of the BMDCs was adjusted to 105/ml. Subsequently the BMDCs were seeded to 96 well microplates, 100 μl/well, triplicate/well.
NGP was dissolved in RPMI1640 medium, filtrated and diluted at range of concentrations from 1 μg/ml to 1000 μg/ml. LPS (10 ng/ml) was used as positive control in this study. The OD number of each well was measured at 570 nm (A570) with use of bichomaticmicroplate reader post treatment with NGP for 48 h by established method of MTS for cell expansion.
Morphological study of the BMDCs with SEM
The BMDCs post treatment with 200 μg/ml NGP for 48 h, were collected and checked for morphological study by SEM (JEOL, JSM-T300). The cell preparation steps included glutaraldehyde fixation, cleaning, osmium tetroxide post-fixation, cleaning, replacement, critical point drying and spraying gold. Finally the prepared samples underwent check with SEM.
Observation of intracellular phagosomes inside the BMDCs with TEM
Another portion of BMDCs post treatment with 200 μg/ml NGP for 48 h, were collected and observed for intracellular phagosomes with TEM (JEOL JEM-1200EX). The sample preparation steps included cultured cell digestion by trypsin, centrifugation at 1,000 RPM for 10 min, glutaraldehyde fixation, slicing. Finally the prepared samples were analyzed by TEM.
ACP activity detection
The concentration of the BMDCs was adjusted to106/ml. The ACP activity inside the DCs post treatment with 200 μg/ml NGP for 48 h was measured at OD 520 nm by the phenol-4-AAP (amino anti-pyrine) method with ACP testing kit (Jiancheng Bio-engineering Institute of South).
BMDCs’ phagocytosis by FCM
Another portion of BMDCs post treatment with 200 μg/ml NGP for 48 h, were collected and tested for phagocytic effect.100 μl FITC-Dextran was added to the BMDC culture, incubated at 4°Cfor 2 h, subsequently for 1 h at 37°C. After incubation, cells were harvested and re-suspended in PBS, and analyzed in a flow cytometer.
Reconfirmation of BMDCs’ phagocytosis by cellular immunohistochemistry
Another portion of BMDCs post treatment with 200 μg/ml NGP for 48 h, were collected for immunohistochemistry. The cell preparation steps included 0.08 mg/ml horseradish peroxidase was added to the cells, followed by fixation with methanol for 4 h, staining with DAB kit, restaining with hematoxylin. Finally, the sample was observed under conventional light microscope.
Analysis for key surface markers by FCM
The cultured BMDCs served as both testing groups (post treatment with 200 μg/ml NGP for 48 h) and control groups were collected respectively and stained with anti-CD40, anti-CD-83, anti-CD86 and anti-MHC II antibodies for 30 min, and then washed twice with PBS/2% FACS. The cells were harvested and immediately fixed in 4% paraformaldehyde, followed by being collected using FACS Calibur (Becton Dickinson). The data, obtained from the analysis of the fixed cells by FCM, were then analyzed using WinMDI 2.9 (Joseph Trotter, BD Biosciences).
Cytokine assay
The culture supernatant of the BMDCs post treatment with 200 μg/ml NGP for 96 h was collected for IL-12p70 and TNF-αassaying per the instruction included in the ELISA kit. The absorbance at 450 nm (A450) was measured using a bichomaticmicroplate reader.
Mixed lymphocyte reaction assay (MLR)
The BMDCs from C57BL/6 mice were treated with LPS, NGP or left untreated for 48 h. At 3d, the BMDCs were collected and washed three times with RPMI 1640 culture medium, and finally the cells concentration was adjusted to 3 × 105/ml to serve as stimulator cells. The lymphocytes were isolated from the spleen of C57BL/6 mice to serve as responder cells. The lymphocytes (3 × 107/ml) were incubated in 96-well cell-culture plates (Corning-Costar) with graded numbers of BMDCs (at a ratio of 1:1, 1:10, 1:50 and 1:100) for 3d. The cell activation and proliferation was monitored by measuring the absorbance in each well at 492 nm (A492) using a bichromaticmicroplate reader. Results were expressed as the mean ± sd from triplicate wells.
Statistical analysis
Mean, standard deviation and statistical significance were calculated using the SPSS application software and Prism (Statistical Package for Social Sciences, Version 16.0 for Windows). The results were expressed as mean ± sd of three or more independent experiments. The differences were evaluated by ANOVA for multiple groups and by the student t-test for two groups using the Prism (Graph Pad Software). The turkey test was used to compare testing group with control group. Statistical results used for post hoc analysis when p < 0.05 indicated significance. The results will be discussed later.
Acknowledgements
This work was supported financially by China Liaoning provincial foundation (2009225008-7 to Fengping Shan). We apologize to the researchers whose work could not be discussed here, due to space limitations.
Glossary
Abbreviations:
- BMDCs
bone marrow dendritic cells
- NGP
Neutral Ginseng polysaccharides
- ACP
acid phosphatase
- LPS
lipopolysaccharides
- SEM
scanning electronic microscopy
- TEM
transmission electronic microscopy
- FCM
flow cytometry
- ELISA
enzyme linked immunosorbent assay
- MTS
5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazoly)-3-(4-sulfophenyl)tetrazolium
- SEM
scanning electronic microscopy
- TEM
transmitted electron microscopy
- DAB
3,3′-diaminobenzidine
- FACS
fetal calf serum
- G mean
Geometric mean
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22612
References
- 1.Lemmon HR, Sham J, Chau LA, Madrenas J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. J Ethnopharmacol. 2012;142:1–13. doi: 10.1016/j.jep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Peng X, Sun P, Shi J, Yuan X, Zhu J, et al. Structural characterization and immunostimulatory activity of a novel linear α-(1→6)-D-glucan isolated from Panax ginseng C. A. Meyer. Glycoconj J. 2012;29:357–64. doi: 10.1007/s10719-012-9403-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS, Kim SY, et al. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson SA, Wong MH, Stryjecki C, De Boer A, Lui EM, Mutch DM. Unraveling the adipocyte inflammomodulatory pathways activated by North American ginseng. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.50. [DOI] [PubMed] [Google Scholar]
- 5.Yoo DG, Kim MC, Park MK, Park KM, Quan FS, Song JM, et al. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS ONE. 2012;7:e33678. doi: 10.1371/journal.pone.0033678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang CC, Zhao HX, Jiang MJ, Wang HW, He YM, Zeng X, et al. [Effects of polysaccharides isolated from Panax japonicus on immunosuppression mice] Zhong Yao Cai. 2011;34:91–4. [Article in Chinese] [PubMed] [Google Scholar]
- 7.Tung NH, Quang TH, Son JH, Koo JE, Hong HJ, Koh YS, et al. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–5. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]
- 8.Han H, Chen Y, Bi H, Yu L, Sun C, Li S, et al. In vivo antimalarial activity of ginseng extracts. Pharm Biol. 2011;49:283–9. doi: 10.3109/13880209.2010.511235. [DOI] [PubMed] [Google Scholar]
- 9.Jin Y, Hofseth AB, Cui X, Windust AJ, Poudyal D, Chumanevich AA, et al. American ginseng suppresses colitis through p53-mediated apoptosis of inflammatory cells. Cancer Prev Res (Phila) 2010;3:339–47. doi: 10.1158/1940-6207.CAPR-09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W, Zhang X, Wang B, Chen Y, Han H, Fan Y, et al. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J Med Food. 2010;13:270–7. doi: 10.1089/jmf.2009.1119. [DOI] [PubMed] [Google Scholar]
- 11.Du Xiao F, Jiang CZ, Wu CF, Won EK, Choung SY. Synergistic immunostimulatory effect of pidotimod and red ginseng acidic polysaccharide on humoral immunity of immunosuppressed mice. Pharmazie. 2008;63:904–8. [PubMed] [Google Scholar]
- 12.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–18. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 14.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–34. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 15.Shan FP, Xia YJ, Wang N, Meng JJ, Lu CL, Meng YM, et al. Functional modulation of the pathway between dendritic cells (DCs) and CD4+T cells by the neuropeptide: methionine enkephalin (MENK) Peptides. 2011;32:929–37. doi: 10.1016/j.peptides.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
- 17.Meng JJ, Hu XF, Shan FP, Hua H, Lu CL, Wang EH, et al. Analysis of maturation of murine dendritic cells (DCs) induced by purified Ganoderma lucidum polysaccharides (GLPs) Int J Biol Macromol. 2011;49:693–9. doi: 10.1016/j.ijbiomac.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biondo PD, Goruk S, Ruth MR, O’Connell E, Field CJ. Effect of CVT-E002 (COLD-fX) versus a ginsenoside extract on systemic and gut-associated immune function. Int Immunopharmacol. 2008;8:1134–42. doi: 10.1016/j.intimp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, et al. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int Immunopharmacol. 2008;8:1023–32. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007;8:39–44. doi: 10.4142/jvs.2007.8.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Predy GN, Goel V, Lovlin R, Donner A, Stitt L, Basu TK. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173:1043–8. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han SK, Song JY, Yun YS, Yi SY. Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm Res. 2005;28:343–50. doi: 10.1007/BF02977803. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Guilbert LJ, Li J, Wu Y, Pang P, Basu TK, et al. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4:311–5. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997;17(1A):323–31. [PubMed] [Google Scholar]