Abstract
Introduction: Older age has been associated to serious adverse events (AE) following yellow fever (YF) vaccination in passive surveillance studies, but few prospective studies involving seniors have been published.
Results: A total of 906 persons were evaluated; 78 were not vaccinated and 828 received the vaccine; 700 (84.7%) were interviewed after vaccination: 593 (84.7%) did not report any symptoms or signs following YF vaccine; 107 (15.3%) reported at least one AE temporally associated to YF vaccination: 97 (13.9%) had systemic AE and 17 (2.4%) reported AE at the injection site (7 had both systemic and local AE). Data regarding previous vaccination was available for 655 subjects. Statistically significant higher rates of systemic AE were observed among subjects who received the first YF vaccination (17.5%) in comparison to persons who had been previously vaccinated (9.5%).
Methods: This observational prospective study aimed to describe AE following YF vaccination in persons aged ≥ 60 y. From March 2009 to April 2010, seniors who sought YF vaccination at a reference Immunization Center in São Paulo city, Brazil, were included. Demographic and clinical data, previous YF vaccination, travel destination and the final decision regarding YF vaccination or not were collected from standardized medical records. Active AE assessment was done through telephone or electronic mail interview performed approximately 14 d after immunization.
Conclusion: Most persons aged ≥ 60 y may be safely vaccinated against YF. Before vaccination, they must be carefully screened for conditions associated to altered immunocompetence and for risk of exposure to YF.
Keywords: yellow fever vaccine, adverse events following immunization, seniors
Introduction
Yellow fever (YF) is a viral hemorrhagic fever endemic in tropical areas of Africa and South America, where it causes outbreaks at irregular intervals.1 A live attenuated vaccine (17D strain), available for more than 70 y, is the most effective way to protect against the disease. The vaccine is highly efficacious: 30 d after immunization, neutralizing antibodies are detected in 99% of vaccinees, and provides long-lasting protection (≥ 10 y). It is recommended for all persons aged ≥ 9 mo living in or traveling to endemic areas. Booster doses should be administered every 10 y.2 Contraindications of YF vaccine includes: children aged < 6 mo, immunocompromised hosts and persons with a history of anaphylaxis after a previous dose of the vaccine or after ingestion of egg. Pregnant women should not be vaccinated, unless at high-risk.2 Adverse events (AE) following YF vaccination are usually mild: fever, malaise, headache and myalgia may be observed, 5 to 10 d after vaccination, in 10 to 30% of vaccinees.3 Severe AE, such as hypersensitivity reactions, neurologic and viscerotropic disease may rarely occur.4
In Brazil, during the 2000s, the endemic area for YF has expanded from the Northern and Central-Western regions to Southeastern and Southern regions, highly populated areas without prior recommendation for YF vaccination. From December 2008 to April 2009, the expanding area of YF cases occurrence in Rio Grande do Sul and São Paulo states exceeded those classified as at risk, and the situation was characterized as “Public Health Emergency of National Concern”: 51 confirmed cases and 21 deaths for YF (case-fatality rate, 41.2%) were reported in these states. The area with recommendation for YF vaccination has been expanded and the vaccination was intensified all over the country. From October 2008 to August 2009, 22,452,800 doses of YF vaccine were distributed (> 10 million doses in areas previously without vaccine recommendation). In the same period, there were 56 confirmed cases of severe AE following YF vaccine: 47 neurotropic disease (no deaths) and 9 viscerotropic disease (9 deaths).5
In São Paulo city, the most populous city in the Southeast of the country, outside the area with new recommendation for universal YF vaccination (Fig. 1), there was an increase in the demand for YF vaccine by travelers. Due to concerns or misinformation regarding the AE following YF vaccine and little experience in travelers’ vaccination, in some vaccination clinics, persons aged 60 y and more who would travel to endemic areas were not vaccinated and were referred to a reference Immunization Center, leading to increased anxiety among seniors regarding YF vaccination.
Figure 1. Area of recommended yellow fever vaccination in Brazil, 2010.
This study aimed to describe the AE temporally associated to YF vaccination among persons aged 60 y and more who were vaccinated in a reference immunization center in São Paulo city, Brazil.
Results
Nine hundred and six persons aged 60 y and older sought the Immunization Center to be vaccinated: 828 (91.4%) received the YF vaccine and 78 (8.6%) were not vaccinated. The subjects’ demographic data, chronic conditions, medications used, previous YF vaccination and travel destination are presented in Table 1. Most subjects (546/906, 60.3%) had active chronic conditions, of whom 35 (3.9%) had conditions that lead to altered immunocompetence (13 were vaccinated and 22 were not vaccinated); other 45 (5%) had previous malignancies (defined as those whose treatment had been ended more than one year before the visit). Most subjects were taking drugs for chronic conditions and eight (0.9%) were taking immunosuppressive drugs (one was vaccinated and seven were not vaccinated). Use of immunosuppressive therapy and autoimmune disease were more common in women (1.3% and 2.1%, respectively) as compared with men (0.5% and 1.3% respectively). Most (54.3%) had not been previously vaccinated against YF (58.4% of women and 48.5% of men). The majority was traveling to areas at risk of YF transmission (51.6% of all, 56.5% of men and 48% of women) or without risk of YF transmission but with vaccination requirement for travelers from Brazil (37.6%). Seventy seven persons had received the YF vaccine within the past 10 y but they did not have documentation of the previous vaccination and were traveling to countries that require international certificate of YF vaccination. Among the 78 persons who were not vaccinated, 44 (56,4%) were traveling to areas without risk of YF, 16 (20.5%) had contraindication for YF vaccination due to altered immunocompetence and were instructed to use barrier measures; 7 (9%) had been vaccinated within the past 10 y; 6 (7.7%) refused vaccination or decided to reconsider the travel after being informed about the AE associated to YF vaccine; and for 5 (6.4%) subjects, the reason for not administering the vaccine was not clear.
Table 1. Demographic characteristics, chronic conditions, medications used, previous yellow fever (YF) vaccination and travel destination, according to final decision of vaccinating or not, for 906 persons aged 60 y and older who sought YF vaccination in a reference Immunization Center, in São Paulo, Brazil, from March 2009 to April 2010.
| Vaccinated (828) n (%) |
Not-vaccinated (78) n (%) |
All (906) n (%) |
|
|---|---|---|---|
|
Gender Female Male |
467 (56.4) 361 (43.6) |
60 (76.9) 18 (23.1) |
527 (58.2) 379 (41.8) |
|
Age Min–Max Mean Median Standard deviation |
60–94 68 67 6.4 |
60–96 70 67 7.7 |
60–96 68 67 6.6 |
|
Chronic conditions Chronic condition with altered immunocompetence Other chronic conditions* Previous malignancies None |
13 (1.6) 465 (56.1) 42 (5.1) 308 (37.2) |
22 (28.2) 46 (59.0) 3 (3.8) 7 (9.0) |
35 (3.9) 511 (56.4) 45 (5.0) 315 (34.8) |
|
Medications being used Immunosuppressive drugs** or corticosteroids in immunosuppressive doses# Corticosteroids in lower doses or topical Other drugs None Not reported |
1 (0.1) 12 (1.5) 491 (59.3) 296 (35.7) 28 (3.4) |
7 (9.0) 11 (14.1) 44 (56.4) 7 (9.0) 9 (11.5) |
8 (0.9) 23 (2.5) 535 (59.1) 303 (33.4) 37 (4.1) |
|
Previous YF vaccination No < 10 y ≥ 10 y Not known / reported |
452 (54.6) 77 (9.3) 249 (30.1) 50 (6) |
40 (51.3) 12 (15.4) 21 (26.9) 5 (6.4) |
492 (54.3) 89 (9.8) 270 (29.8) 55 (6.1) |
|
Travel destination Area with risk of YF transmission Area without risk of YF transmission, but with vaccination requirement for travelers from Brazil Area without risk of YF transmission or vaccination requirement Not reported |
435 (52.5) 327 (39.5) 62 (7.5) 4 (0.5) |
32 (41) 14 (18) 32 (41) 0 |
467 (51.6) 341 (37.6) 94 (10.4) 4 (0.4) |
Note: *Cardiovascular diseases, diabetes mellitus, lung diseases, liver diseases (not cirrhosis), non-dialysis kidney diseases, neurologic diseases, hypertyroidism, hypotyroidism, prostatic hyperplasia, glaucoma, depression, polycythemia, pancreatic insuficiency, chronic urticaria, erythema nodosum, arthritis, dyspepsia. **Methotrexate (2), hydroxychloroquine (2), Azathioprine (1), IL-6 Blocker (1). #Prednisone ≥ 20 mg/day for > 14 d
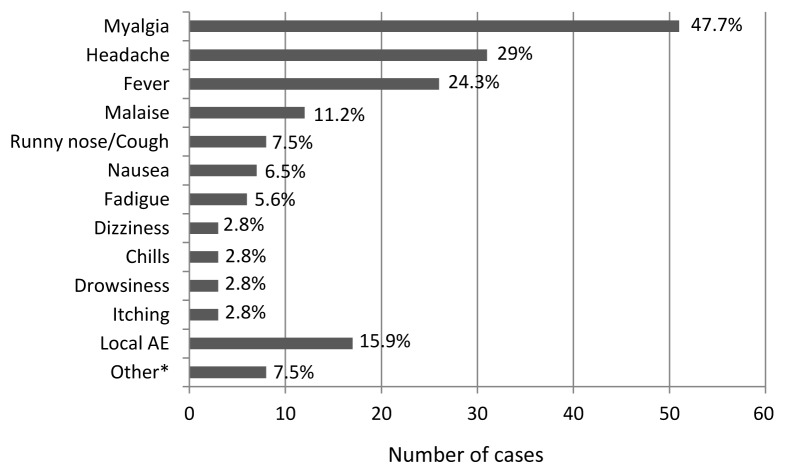
Seven hundred of the 828 (84.5%) vaccinees were successfully contacted after immunization; three of them spontaneously returned to the reference center due to AE. The interval between vaccination and the interview ranged from 6 to 155 d (median 28 d, standard deviation = 29). No symptoms or signs associated to YF vaccination was reported by 593 (84.7%) subjects; the other 107 (15.3%) reported at least one adverse event temporally associated to YF vaccination: 97 (13.9%) had systemic AE (mainly myalgia, headache and fever) and 17 (2.4%) reported AE at the injection site (7 had both systemic and injection site AE) (Fig. 2). Injection site AE included local pain (6), erythema (5), pruritus (4) and ecchymosis (2). The mean interval between YF vaccination and the beginning of symptoms was 4.2 d (ranging from 0 to 15 d) and the mean duration of symptoms was 2.7 d (ranging from 0.5 to 10 d). Among those 107 subjects who presented at least one AE temporally associated to YF vaccination, 69 (64.5%) had spontaneous resolution of symptoms, 32 (30.5%) used symptomatic drugs (analgesic and antipyretic), five (4.8%) sought medical care and four (3.8%) had been submitted to laboratory tests. No serious AE attributable to YF vaccination (anaphylaxis, neurologic or viscerotropic disease) were observed. Two subjects have been hospitalized for myocardial infarction within two months of vaccination; one died and there is no information on the outcome of the other. Thirteen persons with altered immunocompetence who were traveling to endemic area were vaccinated since it was considered that the risk of YF transmission was higher than vaccination risks. Twelve of them were successfully contacted—ten reported no symptoms or signs following YF vaccination and two presented mild AE. A woman with discoid lupus without systemic immunosuppressive drugs presented myalgia seven days after vaccination. The symptom had spontaneously resolved within four days. Another woman with rheumatoid arthritis without systemic immunosuppression had malaise and pruritus at the injection site one day after vaccination. The symptoms resolved spontaneously within 1 d (malaise) and 7 d (pruritus).
Figure 2. Categorization of adverse events (AE) among the 107 persons aged ≥ 60 y that reported AE following YF vaccination. Sao Paulo, Brazil, 2009–2010. *Flushing (1), weakness (2), diarrhea (2), abdominal pain (1), anorexia (1), feeling of heaviness in legs (1).
Table 2 presents the statistical analysis of the frequency of AE following YF vaccination according to age, gender, immunocompetence and previous YF vaccination. Significantly higher rates of systemic AE were observed among persons who received the first YF vaccination in comparison to persons who had previously received one or more doses of the vaccine. There was also higher rate of local AE among female subjects.
Table 2. Statistic analysis of the frequency of adverse events (AE) following yellow fever immunization in persons aged ≥ 60 y, in São Paulo, Brazil, 2009–2010.
| Systemic AE | n/total (%) | Statistic test | p | |
|---|---|---|---|---|
| Age (years) |
60–69 |
69/437 (15.8) |
χ2 = 3.638 |
0.056 |
| > 70 |
28/263 (10.6) |
|||
| Sex |
Female |
52/393 (13.2) |
χ2 = 0.294 |
0.588 |
| Male |
45/307 (14.7) |
|||
| Altered immunocompetence |
Yes |
2/8 (25) |
Fisher = 0.841 |
0.359 |
| No |
95/692 (13.7) |
|||
| 1rst YF vaccine dose* |
Yes |
67/382 (17.5) |
χ2 = 8.397 |
0.004 |
| No |
26/273 (9.5) |
|||
|
Local AE |
|
|
|
|
| Age (years) |
60–69 |
10/437 (2.1) |
χ2 = 0.97 |
0.756 |
| > 70 |
7/263 (2.7) |
|||
| Sex |
Female |
15/393 (3.8) |
χ2 = 7.288 |
0.007 |
| Male |
2/307 (0.7) |
|||
| Altered immunocompetence |
Yes |
1/8 (12.5) |
Fisher = 3.459 |
0.063 |
| No |
16/692 (2.3) |
|||
| 1rst YF vaccine dose* |
Yes |
9/382 (2.4) |
χ2 = 0.029 |
0.865 |
| No |
7/273 (2.6) |
|||
|
Total AE |
|
|
|
|
| Age (years) |
60–69 |
74/437 (16.9) |
χ2 = 2.439 |
0.118 |
| > 70 |
33/263 (12.5) |
|||
| Sex |
Female |
62/393 (15.8) |
χ2 = 0.166 |
0.683 |
| Male |
45/307 (14.7) |
|||
| Altered immunocompetence |
Yes |
2/8 (25) |
Fisher = 0.589 |
0.443 |
| No |
105/692 (15.2) |
|||
| 1rst YF vaccine dose* | Yes |
73/382 (19.1) |
χ2 = 7.923 | 0.005 |
| No | 30/273 (11) |
Note: *Information on previous YF vaccination was available for only 655 of 700 subjects who were sucessfully contacted for assessment of AE following vaccination.
Discussion
In this study, most (84.7%) vaccinees did not present any AE following routine YF vaccination. Mild systemic AE, particularly myalgia, headache and fever, were most frequently reported by those 107 (15.3%) subjects who reported an AE. No serious AE was reported and the great majority of subjects who presented an AE did not require medical attention. Significantly higher rates of systemic AE were observed among persons who received the first YF vaccination (17.5%) in comparison to persons who had previously received one or more doses of the vaccine (9.5%). Not including a concurrent comparative younger age group was a limitation of our study, but the rates of mild to moderate AE following YF were similar to the rates reported in a clinical trial of three YF vaccines (17D or 17DD) conducted in Rio de Janeiro, involving 1,087 adults,11 in which local AE were observed in 3.3 to 5.1% of subjects who received the vaccine and in 2.6% of subjects in the placebo group, whereas systemic AE (mainly fever, headache and myalgia) were observed in 17.8 to 21.7% of vaccinees and in 14.3% of the placebo group.11 In other clinical trials, the rates of AE following YF vaccination varied widely (from 4% to 83%).3 Lower rates of AE were found by Monath et al. in subjects aged ≥ 65 y (74.4%) than in subjects aged 18 to 44 y (83.3%).12 The lower frequency of AE observed in our study may be due to the fact that many subjects had received a previous dose of the YF vaccine (41.7% of the subjects interviewed about AE and for whom there are information regarding a previous dose of the vaccine).
Altered immunocompetence and use of immunossupressive drugs were more frequent in the not-vaccinated group, as show in Table 1. This was expected since they are contraindications to YF vaccination. Nevertheless, we vaccinated some people with those conditions, considering their high risk of YF exposure and mild immunocompromise, and no serious adverse events were observed among then.
Cases of neurotropic and viscerotropic disease associated to YF vaccination in persons aged ≥ 60 y have been reported13,14 and several studies based on passive surveillance reported greater frequency of serious AE following YF vaccination in the elderly.15 In one study, the estimated rates of serious AE reported were 6.3 and 12.6/100,000 administered doses for persons aged 60–69 and ≥ 70 y, respectively, in comparison to 2.7 to 4.6/100,000 among those aged ≤ 59 y.16 The occurrence of such serious AE has not been fully understood.17 In Brazil, a study based on the passive National Surveillance System of AE following immunizations estimated overall rates of serious AE as: hypersensitivity, 0.9/100,000 vaccine doses; anaphylactic shock, 0.023/100,000; neurotropic disease, 0.084/100,000; and viscerotropic disease, 0.019/100,000.18 Brazilian data also suggest an association between older age and higher rates of serious AE following YF vaccination. The reported rates of viscerotropic disease were 0.019/100,000 administered doses of vaccine among persons aged 15 to 59 y and 0.047/100,000 among persons aged 60 y and more.18 Immunosenescence, greater frequency of chronic conditions and use of immunosuppressive drugs is expected in the elderly and may concur to the higher frequency of serious AE observed in this age group.
The sample size of our study was not sufficient to detect serious rare AE but it was fairly large, and sufficient to detect most frequent, mild to moderate AE. Additionally, the active assessment of the AE following YF vaccination, through phone calls or electronic mail, allowed higher sensitivity to detect mild and self-limited AE. This approach was well accepted by the subjects—only two persons refused to be called by the researchers. A previous study assessed AE following YF vaccination through phone call and internet, but the subjects, aged from 1 to 83 y (mean 44.4 y), should call the researchers to report any AE.19 The most frequent AE were fever, myalgia and headache, similarly to the observed in our study, but Durbin et al. also detected a case of jaundice.17
Another limitation of our study was the short interval between YF vaccination and traveling, which hampered the contact with the subjects within the programmed time. This was the main reason for the lack of information on AE following YF vaccination in 15.4% of the participants. In addition, part of the subjects was contacted only after their trip, which may have led to memory bias, especially for mild AE. However, it is unlikely that moderate to serious AE have been missed.
In our study, the frequency of systemic AE was significantly higher in seniors who received their first YF vaccination. Such association would be expected for live attenuated vaccines, as the case of YF vaccine. Camacho et al. reported significantly higher viremia in subjects who were seronegative before vaccination and also higher, although not statistically significant, frequency of systemic AE following YF vaccination in persons with detectable viremia.11 Roukens et al. found higher and long-lasting viremia in the elderly.20
Most persons aged ≥ 60 y may be safely vaccinated against YF, however they must be carefully screened for conditions associated to altered immunocompetence and for risk of exposure to YF.
Further studies evaluating the safety, immunogenicity of YF vaccine, as well as the duration of protection in the elderly are necessary.
Patients and Methods
This is an observational prospective study conducted at a reference Immunization Center (Centro de Referência para Imunobiológicos Especiais, CRIE) of the Hospital das Clínicas da Universidade de São Paulo (HC-FMUSP), from March 2009 to April 2010.
Persons aged 60 y and older who sought the Immunization Center to receive the YF vaccine were invited to participate and enrolled in the study after signing an informed consent. Demographic data, chronic conditions, medications being used, previous YF vaccination, travel destination and the final decision regarding YF vaccination or not were collected from a standardized form filled in by the attendant physician. Decision regarding YF vaccination or not were individually taken by the attendant physician based on travelers’ destination and risk of AE related to YF vaccination. All subjects were given information regarding the YF vaccine-associated AE and asked to return to the service in case of any AE temporally associated to vaccination. AE active assessment was done through a standardized telephone or electronic mail interview performed about 14 d after YF vaccination. The interview included questions regarding local and systemic AE. Solicited AE included fever, headache, myalgia, jaundice, pain, edema and erythema. The date of AE onset, duration of symptoms, medical care and medications used were also actively asked. Fever, fatigue, diarrhea, pain and local AE were defined according to the Brighton Collaboration Group.6-10
The 17DD Yellow Fever Vaccine from Bio-Manguinhos/Fiocruz was used for all vaccinees. The rates of systemic and local AE following YF vaccination are described. The rates of AE were analyzed by age (60–69 y and 70 and more), sex, presence of altered immunocompetence and dose of vaccine (first and others) using Pearson chi-square or Fisher’s exact test, as appropriate. Statistical analysis was performed using SPSS 11.0. The project was approved by the Ethical Committee of the HC-FMUSP.
Acknowledgment
An abstract of this paper was presented as a poster at the 15th Annual Conference on Vaccine Research, in Baltimore, MD USA, May 7–9, 2012.
Submitted
08/03/12
Revised
10/22/12
Accepted
10/30/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22714
References
- 1.Câmara FP, Gomes AL, Carvalho LM, Castello LG. Dynamic behavior of sylvatic yellow fever in Brazil (1954-2008) Rev Soc Bras Med Trop. 2011;44:297–9. doi: 10.1590/S0037-86822011005000024. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Yellow fever vaccine. WHO position paper. Wkly Epidemiol Rec. 2003;78:349–59. [PubMed] [Google Scholar]
- 3.Monath T, Cetron M, Teuwen D. Yellow fever vaccine In: SA; P, WA; O, P O, editors. Vaccines. 5th ed 2008. p. 959-1055. [Google Scholar]
- 4.Vasconcelos PF, Luna EJ, Galler R, Silva LJ, Coimbra TL, Barros VL, et al. Brazilian Yellow Fever Vaccine Evaluation Group Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358:91–7. doi: 10.1016/S0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- 5.Ministério da Saúde / Secretaria de Vigilância em Saúde. Emergências em Saúde Pública de Importância Nacional (ESPIN) de Febre Amarela Silvestre em São Paulo e no Rio Grande do Sul e a Situação Epidemiológica Atual no Brasil (2008/2009). Febre Amarela Silvestre, Brasil, 2009. Boletim de Atualização. Dezembro 2009. Available at: http://portal.saude.gov.br/portal/arquivos/pdf/boletim_febre_amarela_09_12_09.pdf Accessed in Aug 6, 2011.
- 6.Michael Marcy S, Kohl KS, Dagan R, Nalin D, Blum M, Jones MC, et al. Brighton Collaboration Fever Working Group Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:551–6. doi: 10.1016/j.vaccine.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones JF, Kohl KS, Ahmadipour N, Bleijenberg G, Buchwald D, Evengard B, et al. Brighton Collaboration Fatigue Working Group Fatigue: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5685–96. doi: 10.1016/j.vaccine.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 8.Gidudu J, Sack DA, Pina M, Hudson MJ, Kohl KS, Bishop P, et al. Brighton Collaboration Diarrhea Working Group Diarrhea: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:1053–71. doi: 10.1016/j.vaccine.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 9.Gidudu J, Kohl KS, Halperin S, Hammer SJ, Heath PT, Hennig R, et al. Brighton Collaboration Local Reactions Working Group for a Local Reaction at or near Injection Site A local reaction at or near injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2008;26:6800–13. doi: 10.1016/j.vaccine.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Gidudu JF, Walco GA, Taddio A, Zempsky WT, Halperin SA, Calugar A, et al. Brighton Immunization Site Pain Working Group Immunization site pain: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2012;30:4558–77. doi: 10.1016/j.vaccine.2012.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Camacho LA, de Aguiar SG, Freire MdaS, Leal MdaL, do Nascimento JP, Iguchi T, et al. Collaborative Group for the Study of Yellow Fever Vaccines Reactogenicity of yellow fever vaccines in a randomized, placebo-controlled trial. Rev Saude Publica. 2005;39:413–20. doi: 10.1590/S0034-89102005000300012. [DOI] [PubMed] [Google Scholar]
- 12.Monath TP, Cetron MS, McCarthy K, Nichols R, Archambault WT, Weld L, et al. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum Vaccin. 2005;1:207–14. doi: 10.4161/hv.1.5.2221. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J, et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet. 2001;358:98–104. doi: 10.1016/S0140-6736(01)05327-2. [DOI] [PubMed] [Google Scholar]
- 14.Monath TP. Suspected yellow fever vaccine-associated viscerotropic adverse events (1973 and 1978), United States. Am J Trop Med Hyg. 2010;82:919–21. doi: 10.4269/ajtmh.2010.10-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hyg. 2012;86:359–72. doi: 10.4269/ajtmh.2012.11-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, et al. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–82. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308–13. doi: 10.1016/j.coi.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Menezes Martins RM. MLM; Santos, EM; Cruz, RLS; Santos, PRS; Carvalho, SMD; Sato, HK; Schermann, MT; Mohrdieck, R; Leal, MLF; Homma, A. Yellow fever vaccine post-marketing surveillance in Brazil. Procedia in Vaccinology. 2010;2:178–83. [Google Scholar]
- 19.Durbin AP, Setse R, Omer SB, Palmer JG, Spaeder JA, Baker J, et al. Monitoring adverse events following yellow fever vaccination using an integrated telephone and Internet-based system. Vaccine. 2009;27:6143–7. doi: 10.1016/j.vaccine.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Roukens AH, Soonawala D, Joosten SA, de Visser AW, Jiang X, Dirksen K, et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS ONE. 2011;6:e27753. doi: 10.1371/journal.pone.0027753. [DOI] [PMC free article] [PubMed] [Google Scholar]