Abstract
Neural oscillations in the theta band (4-8 Hz) are prominent in the human electroencephalogram (EEG), and many recent electrophysiological studies in animals and humans have implicated scalp-recorded frontal midline theta (FMT) in working memory and episodic memory encoding and retrieval processes. However, the functional significance of theta oscillations in human memory processes remains largely unknown. Here, we review studies in human and animals examining how scalp-recorded FMT relates to memory behaviors and also their possible neural generators. We also discuss models of the functional relevance of theta oscillations to memory processes and suggest promising directions for future research.
Introduction
Recent work in neuroscience has indicated that neural oscillations may play a fundamental role in human cognition (Fell and Axmacher, 2011; Siegel et al., 2012). In particular, theta oscillations have received considerable attention from researchers, including studies of hippocampal theta in rodents (for review, see Buzsáki, 2002) and cortical theta in humans (Kahana et al., 2001; Mitchell et al., 2008). Scalp electroencephalography (EEG) studies in humans have consistently reported prominent theta power enhancement over frontal regions during various working memory (WM) and episodic memory tasks (Klimesch, 1999), but the functional significance of these oscillations in memory processes remains unclear.
In this review, we aim to provide a concise overview of theta involvement in WM and episodic memory, with a particular emphasis on scalp-recorded theta oscillations that are prominent around Fz electrode site, also known as “frontal midline theta” (FMT)1. We begin by discussing the electrophysiological signatures of scalp-recorded FMT and its potential neural sources. Next, we summarize recent studies relating FMT modulations to working memory and episodic memory tasks. Finally, we conclude by reviewing current models regarding how FMT might contribute to memory.
What is frontal midline theta?
Electrophysiological features and measures of FMT
The observation of scalp-recorded theta oscillations can be dated back to at least Arellano and Schwab (1950), but the term “frontal midline theta” was not introduced until Ishihara and Yoshi (1972). Using a set of strenuous mental tests such as continuous arithmetic calculations, Ishihara and Yoshi (1972) reported increased occurrence of EEG fluctuations centering around 6.5 Hz that was maximal over frontal sites while participants were actively engaged in the tests. Since then, several studies have investigated the functional significance of scalp-recorded FMT (e.g., Mizuki et al., 1980; Sasaki et al., 1994; Laukka et al., 1995; Iramina et al., 1996; Sasaki et al., 1996a; 1996b; Gevins et al., 1997; Asada et al., 1999). Although there are slight variations in terms of the frequency range of FMT among scalp EEG studies, it is generally agreed that FMT is in the range of 4-8 Hz rhythmic activities and typically maximal around the Fz electrode site (Ishihara et al., 1981; Yamaguchi et al., 1990). In terms of power spectral profile, FMT is manifested as a prominent peak at theta frequency range on frontal EEG signals (see Figure 1). Numerous cognitive operations have been shown to modulate FMT power (Klimesch, 1999; Mitchell et al., 2008), but it is most readily observable during tasks that involve sustained, internally-directed cognition without external stimuli or responses (Gevins et al., 1997; Raghavachari et al., 2001; Jensen and Tesche, 2002; Tsujimoto et al., 2003; 2006; 2010; Meltzer et al., 2007; 2008; Scheeringa et al., 2009; Hsieh et al., 2011; Roberts et al., 2013).
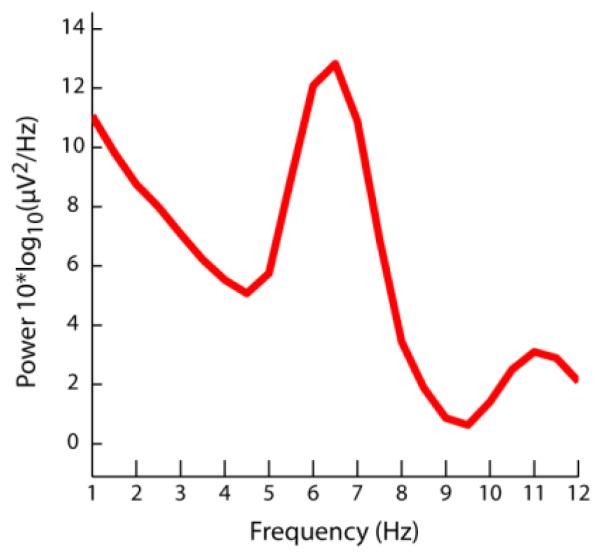
Figure 1.
Power spectrum showing prominent peak at theta (4-8Hz) frequency band. Frequency decomposition was computed on scalp-recorded EEG data from Fz channel during the maintenance of temporal order information in a working memory task (i.e., the “ORDER” trial condition in Figure 2A). The power spectrum was computed on data during the delay period of ORDER working memory task on trials in which temporal order information was correctly identified. Power spectrum was computed based on scalp-recorded EEG data collected from one example participant in Hsieh et al. (2011).
Putative neural generators of FMT
Source modeling based on scalp EEG and MEG data (Gevins et al., 1997; Asada et al., 1999; Ishii et al., 1999; Meltzer et al., 2007; Onton et al., 2005) suggests that FMT could be generated by sources in the anterior cingulate (ACC) and medial prefrontal cortex (mPFC). Consistent with these source-modeling results, direct electrophysiological recordings in monkeys have identified theta sources in BA32 (rostral ACC) and BA9 (dorsolateral PFC; dlPFC) (Tsujimoto et al., 2003; 2006; 2010). Using intracranial EEG (iEEG) recordings from humans, Raghavachari et al. (2001) also reported evidence for local generators of theta oscillations in the middle frontal gyrus during a WM task.
In addition to mPFC, several lines of evidence indicate a close relationship between FMT and activity in the “default mode network” (DMN; Raichle et al., 2001), a network of brain regions that is heavily interconnected with the mPFC (Kondo et al., 2005; Parvizi et al., 2006). For instance, Scheeringa et al. (2008) acquired simultaneous EEG and functional magnetic resonance imaging (fMRI) data during rest and showed that FMT power correlated negatively with blood oxygenation level-dependent (BOLD) signal in the ACC, mPFC, precuneus, posterior cingulate, and angular gyrus—all components of the DMN. The inverse relationship between FMT and DMN BOLD activity has since been replicated and further shown to be behaviorally relevant to both episodic memory and WM tasks (Scheeringa et al., 2009; Michels et al., 2010; White et al., 2012). Additionally, it has been shown that individuals who show greater modulation of FMT power with WM load exhibited larger task-evoked decreases in DMN BOLD signal (Meltzer et al., 2007).
These results suggest a highly robust relationship between the DMN and FMT, although the interpretation of this relationship is not straightforward. One possibility is that the inverse relationship between DMN activity and FMT power is mediated by their differential response to tasks that require external attention. That is, the DMN is disengaged during attention-demanding tasks (Weissman et al., 2006), and FMT oscillations are increased during attention-demanding tasks (Ishihara and Yoshi, 1972; Asada et al., 1999; Ishii et al., 1999; Aftanas and Golocheikine, 2001; Kubota et al., 2001; Sauseng et al., 2007), so one might expect FMT power to be negatively correlated with DMN BOLD even if the two phenomena were not directly related to each other. This idea, however, is difficult to reconcile with evidence from iEEG studies. Evidence from several iEEG studies indicates that theta oscillations are likely generated in multiple DMN regions, including the mPFC, precuneus, retrosplenial, and posterior parahippocampal cortex (Tsujimoto et al., 2003; 2006; Ekstrom et al., 2008; Tsujimoto et al., 2010; Foster and Parvizi, 2012). The available evidence, therefore, is more consistent with the idea that FMT may reflect oscillatory activity that propagates throughout the DMN. In light of evidence reviewed below, indicating that FMT is related to the active engagement of working and episodic memory processes, it is reasonable to speculate that DMN deactivation during attention demanding tasks could be an indicator of theta-related processes.
An important, and currently unresolved question, however, is why theta activity is often associated with reduced BOLD signal. One possibility, suggested by Kilner et al. (2005), is that the metabolic activity related to BOLD responses could be driven by overall shifts in the EEG spectral profile between low and high frequencies (see also Scheeringa et al., 2011 and Burke et al., 2013). Electrophysiological observations in monkeys (Logothetis et al., 2001) and cats (Niessing et al., 2005) are consistent with this hypothesis, suggesting that enhanced hemodynamic responses are associated with a transition of oscillatory power from delta/theta frequency band to lower/upper gamma band. Accordingly, it is possible that during periods of increased theta power, there may be a drop in power in higher frequencies such as gamma, and a net reduction in metabolic demand within DMN regions. This idea is speculative, however, and further study will be required.
In addition to the DMN, the hippocampus is an obvious candidate for the generation of theta (Klimesch, 1999; Bastiaansen and Hagoort, 2003; Mitchell et al., 2008), given the vast literature on hippocampal theta oscillations in rats (for reviews, see (Vanderwolf, 1988; Vinogradova, 1995; Vertes and Kocsis, 1997; Buzsáki, 2002; 2005). It is unlikely that direct volume conduction of hippocampal activity contributes much to FMT recorded at the scalp (Nunez and Srinivasan, 2005), but it is possible that the hippocampus may interact with, and thereby contribute to, theta activity in cortical regions. The presence of strong connections from the hippocampus (CA1 and subiculum) to the mPFC and dlPFC (Rosene and Van Hoesen, 1977; Swanson, 1981; Goldman-Rakic et al., 1984; Ferino et al., 1987; Jay et al., 1989; Sesack et al., 1989; Barbas and Blatt, 1995) lends credence to this idea (Klimesch, 1999; Mitchell et al., 2008).
The strongest evidence for a relationship between cortical and hippocampal theta has come from studies that simultaneously recorded activity in the rodent mPFC and hippocampus. Several studies have demonstrated that mPFC neuronal spiking preferably occurs at specific phase of hippocampal theta oscillations (Hyman et al., 2005; Siapas et al., 2005; Jones and Wilson, 2005a; Sigurdsson et al., 2010; Gordon, 2011; Kim et al., 2011). Phase-locking of PFC spiking to hippocampal theta is behaviorally related to spatial memory performance (Jones and Wilson, 2005b; Sigurdsson et al., 2010; Kim et al., 2011) and it is most prominent after learning has occurred (Kim et al., 2011). Studies have additionally demonstrated that theta coherence in local field potentials (LFPs) recorded from rodent PFC and the hippocampus is particularly strong during spatial decisions that require expression of memory, and only after the rats have acquired the spatial memory tasks (Benchenane et al., 2010; Kim et al., 2011). Moreover, PFC spiking activity is best phase-locked to hippocampal theta oscillations that occurred in the past (approximately 50 ms before) (Siapas et al., 2005), suggesting a directionality in PFC-hippocampal communication, with hippocampal theta leading PFC activity (see also Anderson et al., 2010). Consistent with these findings, transgenic mice with disrupted PFC-hippocampal theta coherence showed impaired spatial memory (Sigurdsson et al., 2010).
Together, these findings are suggestive of a close link between hippocampal and PFC theta activity. It is possible that direct anatomical projections from the hippocampus to PFC interneurons (see Tierney et al., 2004) enable theta-modulated hippocampal neurons to drive PFC interneuron activity, which, in turn, modulate theta oscillations in the PFC (see Blatow et al., 2003). This speculation is supported by a recent study (Benchenane et al., 2010) showing that PFC interneurons, whose activity was phase-locked to hippocampal theta, inhibited PFC pyramidal cells when behaviorally-relevant information was processed. Benchenane et al. (2010) further hypothesized that PFC interneurons might act as “local theta oscillator” that modulates PFC cell dynamics. Although these studies demonstrate a close relationship between theta activity in the hippocampus and mPFC, most of them are correlational. A more refined animal model directly manipulating hippocampal theta oscillations (i.e., abolishing or restoring hippocampal theta, see also Givens and Olton, 1990; Leutgeb and Mizumori, 1999; McNaughton et al., 2006) is needed to help determine whether hippocampus theta plays a role in driving/initiating frontal theta in humans (Anderson et al., 2010), or whether frontal theta can be generated locally, independent of hippocampal activity (see also Cashdollar et al., 2009).
Working memory and FMT
WM processes support the active maintenance of information so that it can be manipulated or quickly accessed at a later time (Baddeley, 1986; 2003). Several electrophysiological studies have also reported persistent FMT oscillations during WM tasks (Gevins et al., 1997; Raghavachari et al., 2001; Jensen and Tesche, 2002; Meltzer et al., 2007; 2008; Scheeringa et al., 2009; Hsieh et al., 2011). For instance, Gevins et al. (1997) used a n-back task that allowed them to examine changes in activity as a function of the amount of information maintained in WM. Gevins et al. found that FMT power directly increased with increasing WM load (i.e., number of items to be maintained in WM) in both verbal and spatial WM tasks. Complementing these findings, Jensen and Tesche (2002) conducted a magnetoencephalography (MEG) study of the Sternberg WM paradigm using digits as stimuli and found that the power of theta oscillations over frontal sensors not only increased during retention period, but was also parametrically modulated by the number of digits being maintained in WM - the amplitude of theta power increased as WM load increased (see also Meltzer et al., 2008, for similar findings in a human iEEG study). The finding of parametric modulation of FMT with WM load has since stimulated a series of studies (e.g., Klimesch et al., 1999; Meltzer et al., 2007; 2008; Scheeringa et al., 2009; Hsieh et al., 2011; Roberts et al., 2013) aiming to further characterize the functional significance of FMT enhancement during WM tasks.
In addition to increases of theta related to WM load, some results suggest “gating” of theta oscillations over several brain regions, including the frontal cortex, during performance of a WM task. In an iEEG study, Raghavachari et al. (2001) showed that the power of theta oscillations directly recorded from middle frontal gyrus drastically increased at the start of a WM trial, stayed elevated throughout the entire WM duration, and then sharply decreased as soon as a response decision had been reached.
Although it is clear that FMT is related to WM processes, at present, there is no clear interpretation of the functional significance of these oscillations. Some data suggest a general role for theta oscillations in coordinating the reactivation of information represented in posterior cortical areas. For instance work by Rainer and colleagues has shown that, theta activity in monkey visual area V4 was enhanced during performance of a visual WM task (Lee et al., 2005), and theta phase synchrony between V4 and dlPFC was predictive of accurate performance (Liebe et al., 2012). Single unit recordings from the same sites revealed that stimulus-selective activity of V4 neurons during the memory delay was phase-locked to the ongoing theta oscillation (Lee et al., 2005). These findings suggest that theta oscillations might regulate the activation of relevant visual object information that is maintained in WM.
An additional possibility is that theta oscillations may have a particular role in coordinating the sequential reactivation of neuronal ensembles that represent individual items in WM (Lisman and Idiart, 1995; Jensen and Lisman, 2005; Jensen, 2006; Lisman and Jensen, 2013). For instance, Lisman and colleagues proposed a computational model suggesting that individual items in WM could be maintained by activation of specific ensembles of neurons within a single gamma (30-80 Hz) cycle. The role of theta oscillations is to regulate activations of different item representations such that each item representation is sequentially activated at a different phase of a theta cycle based on the order in which it is perceived (Lisman and Idiart, 1995). Moreover, the number of gamma cycles that are nested within a theta cycle determines the number of multi-item representations that can be maintained in WM.
A recent iEEG study provided initial support for the model by demonstrating that theta-gamma cross frequency coupling2 supports multi-item WM maintenance (Axmacher et al., 2010). Consistent with the idea that theta frequency determines the number of items that can be maintained in WM buffer, Axmacher et al. (2010) showed that the theta frequency range (at which gamma oscillations was modulated) shifted toward lower frequencies with increasing WM load, suggesting that longer theta cycles (i.e., lower theta frequency) are conducive to the maintenance of increased number of item representations, as it allows for the accommodation of more gamma cycles within a single theta cycle.
One implication of the work described above is that theta may be disproportionately important for the maintenance of temporal order information, relative to conditions in which only item information must be maintained. According to the theta-gamma model, theta oscillations provide a mechanism though which sequence order between individual items is retained by representing each item at a different theta phase; the earlier the item in the sequence, the earlier the theta phase associated with it (Jensen and Lisman, 2005; Jensen, 2006; Lisman and Jensen, 2013). This idea is potentially consistent with the results of EEG studies of the n-back and Sternberg WM tasks. In such tasks, when memory load is relatively high, participants may automatically maintain the temporal order of the items (Hasher and Zacks, 1979; Mangels, 1997). Thus, FMT modulations with increasing WM load might relate to the maintenance of temporal order information.
We recently conducted a scalp EEG study to more directly investigate the relationship between FMT and temporal order maintenance (Hsieh et al., 2011). In this study, participants performed two types of WM tasks that required the participants to maintain either information about specific items (“ITEM” trials) or about the temporal order of the items (“ORDER” trials) (see Figure 2). ITEM and ORDER trials were carefully matched for task difficulty so that they would not differ in overall attentional demands. We hypothesized that, if FMT oscillations in WM are associated with the maintenance of temporal order information, ORDER trials should elicit stronger FMT enhancement as compared to ITEM trials. Consistent with this prediction, we found that FMT power was increased during temporal order maintenance as compared to during item maintenance. Moreover, FMT activity was related to behavioral performance – the FMT was evident for high performers on the order WM task but not for low performers. Importantly, the comparable behavioral performance between the two WM tasks suggested that the observed oscillatory effects could not be attributed to differential attentional demands between item and order WM tasks.
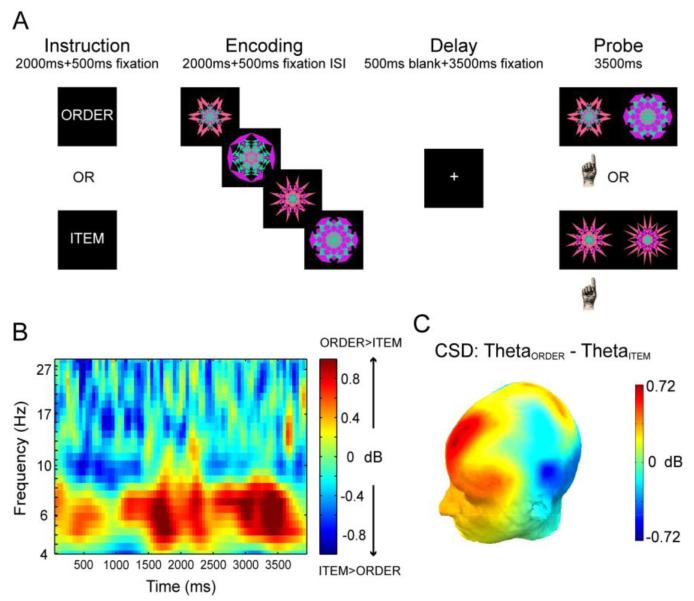
Figure 2.
FMT power increases during maintenance of temporal order information. (A) Schematic diagram of the working memory tasks. (B) Time-frequency spectrogram illustrates the difference in oscillatory power between correct order and correct item trials during working memory delay. The x-axis represents time relative to the onset of the 4s delay period and the y-axis represents logarithmically spaced frequencies. The shown spectrogram is the average of three time-frequency spectrograms from Fz, F1, and F2 channels. (C) Topographic map of the difference in oscillatory power between correct order and correct item trials in theta frequency band during working memory delay. Oscillatory power is computed from current source density (CSD) estimates of scalp-recorded EEG that can more sharply localize EEG activities from superficial neocortical sources. Figures are adapted from Hsieh et al. (2011).
The relationship between FMT and the maintenance of temporal order information was further corroborated by another study in which maintenance of temporal order information was directly compared with the maintenance of spatial information (Roberts et al., 2013). Sequentially presenting a set of four visual objects that each occupied a different location on the screen in a WM trial, Roberts et al. (2013) reported FMT increases in WM trials that required maintenance of temporal order information (the temporal order at which visual objects were presented) as compared to WM trials that required maintenance of spatial information (the locations at which visual objects occupied). The striking convergence between the results of Roberts et al. (2013) and Hsieh et al. (2012) suggests that FMT oscillations are preferentially involved in the maintenance of temporal order information.
In summary, FMT oscillations are increased during WM tasks and are parametrically modulated by the number of items maintained in WM. Our recent scalp EEG results further demonstrated that FMT may play a special role in the maintenance of temporal order information, which is consistent with computational models suggesting that theta oscillations could coordinate the sequential activation of multiple items in WM. Further work needs to be done in order to determine whether items maintained in WM become activated at different gamma cycles that are nested within the slower theta oscillation. This idea is reasonable in light of the finding that single neurons exhibit object-selective activity that is aligned to a particular phase angle of the theta oscillation (Lee et al., 2005).
Recent advances in multivariate pattern classification (MVPC) techniques (Haxby et al., 2001; Norman et al., 2006b; Hanson and Halchenko, 2008; Shinkareva et al., 2008; Simanova et al., 2010; Mansfield et al., 2012; Jafarpour et al., 2013) present an opportunity to further test Lisman’s model by testing whether the replay of individual items is associated with a distinct theta phase during WM maintenance. A recent MEG has provided promising results along these lines. Using a delayed matched-to-sample task in which participants were required to maintain either indoor or outdoor scene information in each WM trial, Fuentemilla et al. (2010) reported reactivation of content-specific information (i.e., based on MVPC output) during the WM delay. Furthermore, reactivation of maintained information was phase locked to theta oscillations, and theta phase-locking of replays was positively correlated with behavioral performance on the WM task. These results suggest the importance of theta oscillations in coordinating periodic replay of item representations during WM maintenance (Jensen and Lisman, 2005).
Episodic memory and FMT
Episodic memory refers to the ability to remember the details of past events (Tulving, 2002). It is well established that the hippocampus, parahippocampal cortex, and PFC play important roles in episodic memory (Eichenbaum et al., 2007; Wheeler et al., 1997), and as noted above, theta oscillations are evident in these regions. Accordingly, many studies have sought to find links between FMT and episodic memory by comparing theta power elicited on trials that were associated with successful memory performance against trials that were not associated with successful memory performance.
A number of scalp EEG studies have reported that FMT during memory encoding is enhanced for items that are subsequently recollected (Klimesch et al., 1997; Mölle et al., 2002; Summerfield and Mangels, 2005; Hanslmayr et al., 2009; White et al., 2012; see also Klimesch, 1999; Nyhus and Curran, 2010). Convergent results were reported in an iEEG study, which showed that theta oscillations generated from within the PFC predicted episodic encoding success (Sederberg et al., 2003; 2007). Although it might be argued that the FMT subsequent memory effect simply reflects differential mental effort or attentional resources favoring the subsequently remembered items (Ishihara and Yoshi, 1972; Asada et al., 1999; Ishii et al., 1999; Kubota et al., 2001; Sauseng et al., 2007; Aftanas and Golocheikine, 2001), a study by Klimesch et al. (1996) suggested that this may not be the case. Klimesch et al. (1996) found that, even with relatively similar levels of attention, items that were subsequently remembered still elicited stronger FMT power as compared to items that were subsequently forgotten, suggesting that FMT oscillations are related to intrinsic episodic memory processes and are unlikely to reflect global attentional fluctuations.
A recent study demonstrated that the relationship between theta activity and memory encoding might depend on connectivity between the hippocampus and PFC (Cohen, 2011). By using diffusion tensor imaging (DTI), Cohen (2011) quantified the integrity of white matter tracts connecting the hippocampus and PFC and found that participants with higher hippocampal-PFC connectivity tended to have better long-term memory (LTM) performance. Moreover, participants with higher hippocampal-PFC connectivity exhibited slower frequency oscillations at theta/delta frequency bands (i.e., the peak of oscillatory power shifted to lower frequencies) over frontal regions in scalp-recorded EEG. These findings demonstrate the interplay between the hippocampus and PFC during LTM in humans.
In addition to encoding, some studies have also reported links between theta power and retrieval of episodic memories (Burgess and Gruzelier, 1997; Klimesch et al., 2000; Guderian and Düzel, 2005; Klimesch et al., 2006; Osipova et al., 2006; Gruber et al., 2008; Addante et al., 2011). Studies have shown that FMT power is higher during correct recognition of studied items than during correct rejection of non-studied items (Burgess and Gruzelier, 1997; Klimesch et al., 2000; Guderian and Düzel, 2005; Gruber et al., 2008), a comparison that is similar to the old-new effects traditionally examined in event-related potential (ERP) studies (Friedman and Johnson, 2000). Results from Klimesch et al. (2006) suggest that these memory-related effects on FMT oscillations do not simply reflect a general processing demand. Using a continuous recognition task, Klimesch et al. (2006) showed that early (~200-500 ms after stimulus onset) FMT power decreased with increasing temporal lag between study and test items. This is the opposite of what would be expected if FMT reflected nonspecific effort or task difficulty, as task difficulty increases as the study-test lag increases.
In addition to memory processes that ensue following processing of items to be encoded or retrieved, some recent studies have suggested that the cognitive state or mode (Rugg and Wilding, 2000) before an event can also determine the success of memory formation and retrieval (Guderian et al., 2009; Rutishauser et al., 2010; Addante et al., 2011; Fell et al., 2011; Gruber et al., 2013). In a study in which each study item was preceded by a reward cue indicating the amount of monetary reward if the study item was subsequently remembered, Gruber et al. (2013) reported that FMT power following a reward cue, but before the to-be-encoded word, exhibited a robust subsequent memory effect: FMT between the presentation of the reward cue and the onset of a word was predictive of later memory for the word. Moreover, the subsequent memory effect was only evident for cues that signified high reward but not low reward. Gruber et al. (2013) speculated that the theta effect might be mediated by the dopaminergic midbrain system that could potentially modulate hippocampal-PFC theta coherence (Benchenane et al., 2010). In a MEG study in which subsequently-recalled studied items were directly compared with studied items that were not later recalled, Guderian et al. (2009) used source modeling and showed that theta power localized to medial temporal regions was enhanced prior to the onset of items that were subsequently recalled. Using iEEG in epilepsy patients, it has been reported that pre-stimulus hippocampal theta power is related to encoding success (Fell et al., 2011). Additionally, coherent timing of hippocampal spikes relative to ongoing theta oscillations prior to an encoding event is predictive of subsequent memory for that event (Rutishauser et al., 2010).
With respect to episodic retrieval, Addante et al. (2011) recorded scalp EEG while participants were performing a source memory retrieval task in which participants were required to identify presented old items along with their associated encoding task (i.e., living/nonliving or pleasant/unpleasant task). They found that FMT power before the stimulus event is specifically predictive of accurate retrieval of source details (i.e., which encoding task) but not item information (see Figure 3). Moreover, the pre-stimulus FMT enhancement was positively correlated with post-stimulus parietal theta activity that was related to successful source memory retrieval. Based on these findings, Addante et al. (2011) argued that FMT before a stimulus event might be related to a preparatory process that facilitates the retrieval of episodic details upon the presentation of a retrieval cue. Whether the preparatory process reflects a reinstantiation of the contextual state at encoding or a general neurocognitive state that facilitates retrieval of contextual details remains to be explored.
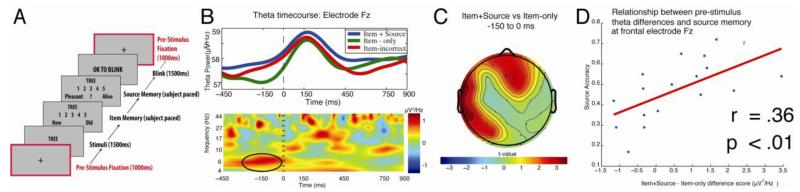
Figure 3.
Pre-stimulus FMT enhancement before successful episodic retrieval. (A) Schematic diagram of the episodic retrieval task. (B) (Upper) time course of theta power for item+source, item-only, and item-incorrect trials at Fz electrode site. (Lower) Time-frequency spectrogram illustrates the difference in oscillatory power between item+source versus item-only retrieval at Fz electrode site. The zero time point on the x-axis represents the onset of test stimulus. (C) Topographic map of the difference in oscillatory power between item+source and item-only in theta frequency band during the pre-stimulus period (i.e., −150 to 0 ms before test stimulus onset). (D) Pre-stimulus theta power differences at Fz were positively correlated with individual differences in source memory accuracy. Figures are adapted from Addante et al. (2011).
The results described above all suggest positive associations between theta oscillations and memory performance. However, it should be noted that theta oscillations can be associated with forgetting under certain circumstances (Hanslmayr et al., 2010; Staudigl et al., 2010). For instance, using a retrieval-induced forgetting paradigm in which selective retrieval of some studied items leads to forgetting of closely related studied items (Anderson et al., 1994), Staudigl et al. (2010) found that FMT power during selective retrieval was related to later forgetting of items that did not receive selective retrieval. Source estimation procedures implicated the ACC as the source of this theta effect. The results were interpreted as suggesting that theta oscillations play a role in resolving competition during selective retrieval by inhibiting representations of interfering items. As we will discuss below, these findings are in close agreement with a computational model proposed by Norman and colleagues (Norman et al., 2005; 2006a; 2007), suggesting that theta oscillations may play a role in suppressing representations of competing associations (i.e., items closely related to selectively-retrieved items) while also strengthening representations of target memories (i.e., items that received selective retrieval).
Functional interpretations of FMT
Thus far, we have reviewed evidence demonstrating the relationship between FMT oscillations and WM, episodic encoding and retrieval. The evidence strongly suggests that FMT oscillations are meaningfully related to cognition. The wide range of phenomena to which theta has been linked make it challenging to ascribe any simple functional interpretation. Adding further complexity to this issue is the fact that FMT oscillations are probably not exclusively related to performance on traditional memory paradigms. Indeed, numerous studies have shown that FMT oscillations are also modulated by error-related signals (e.g., Luu et al., 2004; Trujillo and Allen, 2007) and reward expectation (Cohen et al., 2007; Gruber et al., 2013), even though these tasks do not seem to have significant memory demands. If FMT oscillations are not exclusive for memory, then it is logical to ask whether FMT oscillations play any particular role in memory processing.
We suspect that it may be overly simplistic to assume a single role for FMT oscillations. To the extent that theta oscillations can be observed in multiple, functionally distinct regions, the specific cognitive functions associated with FMT may depend on the particular neural sources that generate theta oscillations during the task in question (Kahana et al., 2001). That said, we think that the intrinsic rhythmicity of neural oscillations that are associated with scalp-recorded FMT may be leveraged to optimize particular kinds of computations that are relevant to memory.
For example, as described earlier, computational simulations (Lisman and Idiart, 1995; Jensen and Lisman, 2005; Jensen, 2006) have demonstrated that theta-gamma phase coupling could provide a mechanism by which multiple item representations can be sequentially activated in each theta cycle. This view is compatible with evidence implicating FMT in multi-item WM (Gevins et al., 1997; Jensen and Tesche, 2002), as well as studies suggesting that FMT might be particularly relevant to maintenance of temporal sequence information (Hsieh et al., 2011; Roberts et al., 2013). Moreover, the temporal distance between activation of successive item representations is substantially compressed to ~20ms, which corresponds roughly to the duration of one cycle of gamma oscillation, and is well within a time frame to allow these items to be associated via spike timing dependent plasticity (i.e., long-term potentiation; LTP) (Markram et al., 1997; Bi and Poo, 1998). Thus, the sequential activation of items in a theta cycle could allow for the item representations to become directly linked through Hebbian plasticity mechanisms and, as a result, support the storage of long-term episodic memories (Jensen and Lisman, 1996), which are unique sequences of briefly occurring events. Jensen and Lisman (1996) hypothesized that the relative long time constant of slow NMDA channels (~150 ms, which is about one cycle of theta oscillations) provides a plausible neural mechanism to link/encode together (through LTP) unique sequence of events (each associated with a gamma subcycle nested within a theta cycle) constituting an episodic memory. Furthermore, properties of slow NMDA channels also allow retrieval of sequence memory by presenting initial events of the memory (Jensen and Lisman, 1996).
Extending the argument described above, one could speculate that theta-gamma coupling could be critical for supporting the representation of temporal context information inherent to all episodic memories. This idea was proposed by Hasselmo and Eichenbaum (2005). Inspired by the temporal context model (TCM, Howard and Kahana, 2002; Howard et al., 2005) and observation of context-sensitive firing properties of hippocampal neurons (Wood et al., 2000), Hasselmo and Eichenbaum (2005) proposed that hippocampal theta might play a role in the formation of temporal context that facilitates subsequent retrieval of recent episodes based on the temporal context. This idea is consistent with results demonstrating that simulated lesions of the anterior thalamic-hippocampal axis, which reduce hippocampal theta (see also Winson, 1978; Lee et al., 1994), impair context-dependent memory retrieval. Consistent with context sensitivity of hippocampal theta, Guderian et al. (2009) reported pre-stimulus theta subsequent memory effects (which were source localized to the medial temporal lobe) and argued that they might reflect pre-activation of a mnemonic context that promotes memory encoding. Based on these results, it seems that hippocampal theta might play a special role in context-guided memory behaviors. Interestingly, the recent finding of pre-stimulus FMT and its correlation with source memory accuracy during episodic retrieval (Addante et al., 2011) indicates that FMT might also relate to reinstantiation of contexts that facilitate memory retrieval. One question that follows is that whether the context effects associated with hippocampal theta and FMT reflect qualitatively different or similar underlying neural mechanisms. Further research is needed to distinguish the functional significance of hippocampal-based and frontal-based theta oscillations.
Staudigl and Hanslmayr (2013) also advanced the idea that FMT may be related to context representation, speculating that theta power will be closely associated with successful memory performance only when encoding and retrieval contexts are similar to each other (Hanslmayr and Staudigl, 2013; Staudigl and Hanslmayr, 2013). In their study, participants studied words superimposed on movie clips and their memories for words were later tested by presenting the word with either the same or a different movie clip. They found that FMT power during encoding was enhanced for subsequently remembered items than for subsequently forgotten items that were tested in the same context (i.e., the same movie clip). In contrast, when a studied item was tested under a context that was different from its encoding context, negative theta subsequent memory effects were observed (Staudigl and Hanslmayr, 2013).
Another account of FMT is that theta oscillations might have systematic effects on strong and weak representations in a neural network (Norman et al., 2005; 2006a; 2007). Inspired by the close link between inhibitory interneurons and theta oscillations (Buzsaki, 2002) and findings that LTP and long-term depression (LTD) operate at different phases of theta oscillations (Huerta & Lisman, 1996; Hyman et al., 2003), Norman et al. (2005; 2006a; 2007) developed a learning algorithm in which the strength of inhibition oscillates at theta rhythm such that weak target memories are strengthened and strong competitors are suppressed. According to the model, theta oscillations reflect oscillating levels of inhibition that bias the competition between representations in a network. As inhibition levels rise during a theta cycle, strong target memories are selectively activated, and as inhibition levels fall, competing representations are moderately activated as well. The difference between the high and low inhibition states leads to the strengthening of the targeted memories (through LTP) and weakening of the competitors (through LTD).
As briefly mentioned earlier, the oscillating inhibition model has been used to explain the relationship between theta oscillations and retrieval-induced forgetting: theta oscillations suppress competing memories that interfere with selective retrieval of target memories and strengthen weak parts of selectively retrieved memories (Norman et al., 2007). The inhibition model could also be extended to explain general relationships between theta activity and memory in terms of reducing the effects of interference. For instance, FMT oscillations during WM maintenance (Gevins et al., 1997; Jensen and Tesche, 2002; Meltzer et al., 2007, 2008) could facilitate the activation of currently relevant items on a given WM trial and the inhibition of competing representations of (now-irrelevant) items from previous WM trials.
General Conclusions and Future Directions
Our goal in the present review was to provide a brief synopsis of the available evidence regarding the neural origins of scalp-recorded theta and on the relationship between theta activity and memory processes. The literature in this area is rapidly expanding, and we are now at the point at which the field can move beyond basic questions about whether there is a relationship between theta and memory, and move towards testing of different models to account for how theta activity influences memory. The models discussed here represent just a few possible explanations for how theta oscillations might influence neural computations and thereby influence memory processes. However, more detailed modeling of cortical theta is clearly necessary in order to understand the range of phenomena that have been linked to theta oscillations.
We anticipate that the use of pattern classification techniques will help examine the validity of existing models of theta oscillations. For instance, the theta-gamma model predicts that each item representation is associated with a distinct theta phase and that the theta phase with which an item is associated reflects the temporal order in which it was encoded. On the other hand, the oscillating inhibition account hypothesizes that target and competitor memory representations are associated with distinct theta phases. By applying trained pattern classifiers to decode momentary neural representation (on the order of milliseconds) at different theta phases, researchers may be able to test the predictions of these models.
We also anticipate that further insights will come from studies that can directly manipulate theta activity (e.g., Sigurdsson et al., 2010). For instance, transgenic mice with risk genes for schizophrenia have been reported to show compromised PFC-hippocampal theta synchrony during a WM task. Future studies using pharmacological, electrophysiological, or optogenetic techniques to experimentally manipulate cortical theta activity in animal models could allow for precise investigations of the effects of theta oscillations on local circuit computations. In humans, methods to entrain (Nitsche et al., 2008) or perturb (Hamidi et al., 2009; Johnson et al., 2010) brain oscillations could be used to test whether FMT oscillations are critical or necessary for memory functions. By using transcranial magnetic stimulation (TMS) and transcranial alternating/direct current stimulation (tACS, tDCS), researchers have been able to causally manipulate neural oscillations (e.g., Hamidi et al., 2009; Sauseng et al., 2009; Sela et al., 2012). These brain stimulation techniques may lead to important answers about the functions of FMT and possibly even lead to new approaches to the treatment of memory disorders that are associated with theta dysfunction in patients who suffer from epilepsy (Chauvière et al., 2009) and traumatic brain injuries (Fedor et al., 2010; Lee et al., 2013).
Highlights.
Neural oscillations
Frontal theta oscillations
Working memory
Episodic memory encoding
Episodic memory retrieval
Acknowledgements
We thank anonymous reviewers for their helpful comments and suggestions. This work was supported by an NIMH Grant awarded to C.R. (RO1MH0068721). Liang-Tien Hsieh is a Howard Hughes Medical Institute International Student Research fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that our use of “frontal” here refers to the scalp topography of FMT, not to underlying neural sources that contribute to scalp-recorded theta.
There are many cross-frequency coupling (CFC) measures (e.g., phase-amplitude CFC, phase-phase CFC, amplitude-amplitude CFC) that seem to be associated with different aspects of neural computations (for review, see Canolty and Knight, 2010). The cross-frequency mentioned here is specific to phase-amplitude CFC, which refers to the observation that power at higher-frequencies (e.g., gamma) is modulated by the phase of lower-frequency signals (e.g., theta).
References
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proc Natl Acad Sci USA. 2011;108:10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neuroscience Letters. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bjork R, Bjork EL. Remembering can cause forgetting: Retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex. 2010;20:1604–12. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Arellano AP, Schwab RS. Scalp and basal recording during mental activity. Proceedings of the 1st International Congress of Psychiatry. 1950;1:216–219. [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neuroscience Letters. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci USA. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford UP: 1986. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent Theta Oscillations and Reorganization of Spike Timing in the Hippocampal-Prefrontal Network upon Learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Brown J. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10:12–21. [Google Scholar]
- Burgess AP, Gruzelier JH. Short duration synchronization of human theta rhythm during recognition memory. NeuroReport. 1997;8:1039–1042. doi: 10.1097/00001756-199703030-00044. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar N, Malecki U, Rugg-Gunn FJ, Duncan JS, Lavie N, Duzel E. Hippocampus-dependent and -independent theta-networks of active maintenance. Proc Natl Acad Sci U S A. 2009;106:20493–20498. doi: 10.1073/pnas.0904823106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvière L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29:5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Hippocampal-prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr Biol. 2011;21:1900–1905. doi: 10.1016/j.cub.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation Between BOLD fMRI and Theta-Band Local Field Potentials in the Human Hippocampal Area. Journal of Neurophysiology. 2008;101:2668–2678. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M, Berman RF, Muizelaar JP, Lyeth BG. Hippocampal θ dysfunction after lateral fluid percussion injury. J Neurotrauma. 2010;27:1605–1615. doi: 10.1089/neu.2010.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG. J Neurosci. 2011;31:5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry A, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage. 2012;60:384–391. doi: 10.1016/j.neuroimage.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc. Res. Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Duzel E. Thetacoupled periodic replay in working memory. Curr Biol. 2010;20:606–612. doi: 10.1016/j.cub.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behavioral Neuroscience. 1990;104:849–855. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M, Watrous A, Ekstrom A, Ranganath C. Expected reward modulates encoding-related theta activity before an event. Neuroimage. 2013;64:68–74. doi: 10.1016/j.neuroimage.2012.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Giabbiconi C-M, Müller MM. Induced electroencephalogram oscillations during source memory: familiarity is reflected in the gamma band, recollection in the theta band. J Cogn Neurosci. 2008;20:1043–1053. doi: 10.1162/jocn.2008.20068. [DOI] [PubMed] [Google Scholar]
- Guderian S, Düzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15:901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Front Integr Neurosci. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bäuml K-H. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cerebral Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Aslan A, Bäuml K-H. Theta oscillations predict the detrimental effects of memory retrieval. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:329–338. doi: 10.3758/CABN.10.3.329. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T. How brain oscillations form memories — A processing based perspective on oscillatory subsequent memory effects. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.05.121. http://dx.doi.org/10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- Hanson SJ, Halchenko YO. Brain reading using full brain support vector machines for object recognition: there is no “face” identification area. Neural Comput. 2008;20:486–503. doi: 10.1162/neco.2007.09-06-340. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen. 1979;108:356–388. [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AS, Hasselmo ME. The temporal context model in spatial navigation and relational learning: Explaining medial temporal lobe function across domains. Psychol Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–299. [Google Scholar]
- Hsieh L-T, Ekstrom AD, Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. J Neurosci. 2011;31:10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Synaptic plasticity during the cholinergic theta-frequency oscillation in vitro. Hippocampus. 1996;49:58–61. doi: 10.1002/(SICI)1098-1063(1996)6:1<58::AID-HIPO10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Iramina K, Ueno S, Matsuoka S. MEG and EEG topography of frontal midline theta rhythm and source localization. Brain Topogr. 1996;8:329–331. doi: 10.1007/BF01184793. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hayashi H, Hishikawa Y. Distribution of frontal midline theta rhythm (FmO) on the scalp in different states (mental calculation, resting and drowsiness) Electroencephalogr. Clin. Neurophysiol. 1981;52:S19. [Google Scholar]
- Ishihara T, Yoshi N. Multivariate analytic study of EEG and mental activity in juvenile delinquents. Electroencephalography and Clinical Neurophysiology. 1972;33:71–80. doi: 10.1016/0013-4694(72)90026-0. [DOI] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. NeuroReport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505:337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jensen O. Maintenance of multiple working memory items by temporal segmentation. Neuroscience. 2006;139:237–249. doi: 10.1016/j.neuroscience.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Theta/gamma networks with slow NMDA channels learn sequences and encode episodic memory: role of NMDA channels in recall. Learn Mem. 1996;3:264–278. doi: 10.1101/lm.3.2-3.264. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends in Neurosciences. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Hamidi M, Postle BR. Using EEG to explore how rTMS produces its effects on behavior. Brain Topogr. 2010;22:281–93. doi: 10.1007/s10548-009-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Wilson M. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005a;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta Rhythms Coordinate Hippocampal-Prefrontal Interactions in a Spatial Memory Task. PLoS Biol. 2005b;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Mattout J, Henson R, Friston KJ. Hemodynamic correlates of EEG: A heuristic. Neuroimage. 2005;28:280–286. doi: 10.1016/j.neuroimage.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kim J, Delcasso S, Lee I. Neural Correlates of Object-in-Place Learning in Hippocampus and Prefrontal Cortex. J Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. NeuroReport. 1996;7:1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J. Paradoxical’alpha synchronization in a memory task. Cognitive Brain Research. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol. 2000;111:781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NEA, Yonelinas AP, Doppelmayr M. Oscillatory EEG correlates of episodic trace decay. Cerebral Cortex. 2006;16:280–290. doi: 10.1093/cercor/bhi107. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res. 2001;11:281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Laukka SJ, Järvilehto T, Alexandrov YuI, Lindqvist J. Frontal midline theta related to learning in a simulated driving task. Biological Psychology. 1995;40:313–320. doi: 10.1016/0301-0511(95)05122-q. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsáki G. Hippocampal theta following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Gurkoff GG, Izadi A, Berman RF, Ekstrom AD, Muizelaar JP, Lyeth BG, Shahlaie K. Medial septal nucleus theta frequency deep brain stimulation improves spatial working memory after traumatic brain injury. J Neurotrauma. 2013;30:131–139. doi: 10.1089/neu.2012.2646. [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase Locking of Single Neuron Activity to Theta Oscillations during Working Memory in Monkey Extrastriate Visual Cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Mizumori SJ. Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J Neurosci. 1999;19:6661–6672. doi: 10.1523/JNEUROSCI.19-15-06661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebe S, Hoerzer GM, Logothetis NK, Rainer G. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci. 2012;15:456–462. doi: 10.1038/nn.3038. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart M. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Mansfield EL, Karayanidis F, Cohen MX. Switch-Related and General Preparation Processes in Task-Switching: Evidence from Multivariate Pattern Classification of EEG Data. J Neurosci. 2012;32:18253–18258. doi: 10.1523/JNEUROSCI.0737-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Ruan M, Woodnorth M-A. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clinical Neurophysiology. 2007;118:2419–2436. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT. Effects of Working Memory Load on Oscillatory Power in Human Intracranial EEG. Cerebral Cortex. 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Bucher K, Lüchinger R, Klaver P, Martin E. Simultaneous EEG-fMRI during a working memory task: modulations in low and high frequency bands. PLoS ONE. 2010;7:e39447. doi: 10.1371/journal.pone.0010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta.”. Progress in Neurobiology. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Mizuki Y, Tanaka M, Isozaki H, Nishijima H, Inanaga K. Periodic appearance of theta rhythm in the frontal midline area during performance of a mental task. Electroencephalography and Clinical Neurophysiology. 1980;49:345–351. doi: 10.1016/0013-4694(80)90229-1. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Fehm HL, Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur J Neurosci. 2002;15:923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Cohen L, Wassermann E, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman E, Detre G, Polyn S. How inhibitory oscillations can train neural networks and punish competitors. Neural Comput. 2006a;18:1577–1610. doi: 10.1162/neco.2006.18.7.1577. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the complementary learning systems model: Oscillating inhibition and autonomous memory rehearsal. Neural Networks. 2005;18:1212–1228. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Detre G. A neural network model of retrieval-induced forgetting. Psychol Rev. 2007;114:887–953. doi: 10.1037/0033-295X.114.4.887. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci (Regul Ed) 2006b;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electrical fields of the brain. New York: Oxford UP: 2006. [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O. Theta and Gamma Oscillations Predict Encoding and Retrieval of Declarative Memory. J Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BM, Hsieh L-T, Ranganath C. Oscillatory activity during maintenance of spatial and temporal information in working memory. Neuropsychologia. 2013;51:349–357. doi: 10.1016/j.neuropsychologia.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rugg M, Wilding E. Retrieval processing and episodic memory. Trends Cogn Sci (Regul Ed) 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Nambu A, Kyuhou S, Matsuzaki R, Tsujimoto T. Studies on integrative functions of the human frontal association cortex by use of MEG. Electroencephalogr Clin Neurophysiol Suppl. 1996a;47:181–190. [PubMed] [Google Scholar]
- Sasaki K, Nambu A, Tsujimoto T, Matsuzaki R, Kyuhou S, Gemba H. Studies on integrative functions of the human frontal association cortex with MEG. Cogn Brain Res. 1996b;5:165–174. doi: 10.1016/s0926-6410(96)00053-5. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Tsujimoto T, Nambu A, Matsuzaki R, Kyuhou S. Dynamic activities of the frontal association cortex in calculating and thinking. Neuroscience Research. 1994;19:229–233. doi: 10.1016/0168-0102(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain Oscillatory Substrates of Visual Short-Term Memory Capacity. Current Biology. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MCM. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44:1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and Neocortical Gamma Oscillations Predict Memory Formation in Humans. Cerebral Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Sela T, Kilim A, Lavidor M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci. 2012;6:22. doi: 10.3389/fnins.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shinkareva SV, Mason RA, Malave VL, Wang W, Mitchell TM, Just MA. Using fMRI Brain Activation to Identify Cognitive States Associated with Perception of Tools and Dwellings. PLoS ONE. 2008;3:e1394. doi: 10.1371/journal.pone.0001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal Phase Locking to Hippocampal Theta Oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanova I, van Gerven M, Oostenveld R, Hagoort P. Identifying object categories from event-related EEG: toward decoding of conceptual representations. PLoS ONE. 2010;5:e14465. doi: 10.1371/journal.pone.0014465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S. Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Curr Biol. 2013;23:1101–1106. doi: 10.1016/j.cub.2013.04.074. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bauml KHT. Theta Oscillations Reflect the Dynamics of Interference in Episodic Memory Retrieval. J Neurosci. 2010;30:11356–11362. doi: 10.1523/JNEUROSCI.0637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Mangels JA. Coherent theta-band EEG activity predicts item-context binding during encoding. Neuroimage. 2005;24:692–703. doi: 10.1016/j.neuroimage.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Swanson L. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Degenetais E, Thierry A-M, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Trujillo L, Allen J. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. Journal of Neurophysiology. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, Sasaki K. Prefrontal theta oscillations associated with hand movements triggered by warning and imperative stimuli in the monkey. Neuroscience Letters. 2003;351:103–106. doi: 10.1016/j.neulet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y, Sasaki K. Theta Oscillations in Primate Prefrontal and Anterior Cingulate Cortices in Forewarned Reaction Time Tasks. Journal of Neurophysiology. 2010;103:827–843. doi: 10.1152/jn.00358.2009. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic Memory: From Mind to Brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Expression, control, and probable functional significance of the neuronal theta-rhythm. Progress in Neurobiology. 1995;45:523–583. doi: 10.1016/0301-0082(94)00051-i. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- White T, Jansen M, Doege K, Mullinger KJ, Park SB, Liddle EB, Gowland PA, Francis ST, Bowtell R, Liddle PF. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22114. doi:10.1002/hbm.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Tsuda K, Asada H. Topography of frontal midline theta rhythms during serial calculation. Electroencephalogr. Clin. Neurophysiol. 1990;75:S163. [Google Scholar]