Abstract
Alpha band (8–12 Hz) phase dynamics in the visual cortex are thought to reflect fluctuations in cortical excitability that influences perceptual processing. As such, visual stimuli are better detected when their onset is concurrent with specific phases of the alpha cycle. However, it is unclear whether alpha phase differentially influences cognitive performance at specific times relative to stimulus onset (i.e., is the influence of phase maximal before, at, or after stimulus onset?). To address this, participants performed a delayed-recognition, working memory (WM) task for visual motion direction during two separate visits. The first visit utilized functional magnetic resonance (fMRI) imaging to identify neural regions associated with task performance. Replicating previous studies, fMRI data showed enagement of visual cortical area V5, as well as a prefrontal cortical region, the inferior frontal junction (IFJ). During the second visit, transcranial magnetic stimulation (TMS) was applied separately to both the right IFJ and right V5 (with the vertex as a control region) while electroencephalography (EEG) was simultaneously recorded. During each trial, a single pulse of TMS (spTMS) was applied at one of six time points (−200, −100, −50, 0, 80, 160 ms) relative to the encoded stimulus onset. Results demonstrated a relationship between the phase of the posterior alpha signal prior to stimulus encoding and subsequent response times to the memory probe two seconds later. Specifically, spTMS to V5, and not the IFJ or vertex, yielded faster response times, indicating improved WM performance, when delivered during the peak, compared to the trough, of the alpha cycle, but only when spTMS was applied 100 ms prior to stimulus onset. These faster responses to the probe correlated with decreased early event related potential (ERP) amplitudes (i.e., P1) to the probe stimuli. Moreover, participants that were least affected by spTMS exhibited greater functional connectivity between V5 and fronto-parietal regions. These results suggest that posterior alpha phase indexes a critical time period for motion processing in the context of WM encoding goals, which occurs in anticipation of stimulus onset.
Keywords: transcranial magnetic stimulation, electroencephalography, alpha band, phase, working memory, motion
Introduction
Working memory (WM) for visual motion information is critical for every day activities, such as when trying to cross a busy street. This scenario requires maintaining memory traces of vehicular motion in one direction, while traffic in the other direction is assessed. Despite its important function, the neural basis of motion-based WM encoding is still unclear. To understand how the brain encodes visual motion into WM, two fundamental questions must be answered: which neural regions are involved and when is their involvement critical for performance? Important steps along this path have been accomplished by studies exploring the localization of cortical processing in response to viewing motion stimuli. Neuroimaging has revealed that area V5/hMT+, within the medial temporal lobe, shows a selective response to visual motion (Culham et al., 2001; Schoenfeld et al., 2007; Zeki et al., 1991). Although the location of V5 is well defined, the role of higher cognitive regions that influence V5 processing is still unclear, as well as when are the critical processing time periods relative to stimulus onset.
To assess the timing of visual cortical processes, studies using electroencephalography (EEG) and magnetoencephalography (MEG) have often focused on posterior alpha band oscillations between 8 and 12 Hz, which characterizes both suppression and timing of attentional processes involved in selection of stimulus representations (Freunberger et al., 2009; Klimesch, 2012). Interestingly, it has been found that the phase of ongoing alpha band oscillations reflects fluctuations in visual cortical excitability, such that stimuli presented during the peak (maximum amplitude) of the alpha oscillation are better detected than stimuli that appear during the trough (minimum amplitude) (Busch et al., 2009; Mathewson et al., 2009; Zauner et al., 2012). Consistent with this, the effects of transcranial magnetic stimulation (TMS) are known to be contingent on oscillatory parameters (Rubens and Zanto, 2012), including the alpha phase when a TMS pulse is applied. For example, a single pulse of TMS (spTMS) to the occipital cortex is more likely to evoke a phosphene when applied during peak alpha phase (Dugue et al., 2011) and neural entrainment to 10Hz-TMS (i.e., alpha-like) is highest when TMS onset coincides with peak alpha phase (Thut et al., 2011).
Importantly, these results indicate that perception may be oscillatory in nature, affording temporal windows of optimal opportunity to process information from the environment. Therefore, we hypothesized that perturbing visual cortical activity at specific phases of the ongoing alpha rhythm would differentially alter WM for motion direction.
Although it is reasonable to hypothesize that motion WM may be contingent upon the phase of ongoing alpha oscillations, a plethora of previous research has revealed the importance of the timing of spTMS relative to stimulus onset for motion processing. These studies utilized spTMS to transiently alter V5 activity at various times relative to motion stimuli onset and assess motion detection performance. Results indicated that spTMS to V5 can disrupt motion processing when applied prior to motion onset (−150 – 0 ms post onset) (Beckers and Zeki, 1995; Laycock et al., 2007; Maus et al., 2013; Sack et al., 2006; Stevens et al., 2009), near motion onset (0 – 50 ms post onset) (Beckers and Homberg, 1992; Beckers and Zeki, 1995; Laycock et al., 2007; Maus et al., 2013), and following motion onset (80–200 ms post onset) (Anand et al., 1998; Bosco et al., 2008; Laycock et al., 2007; Maus et al., 2013; Sack et al., 2006; Silvanto et al., 2005; Stevens et al., 2009; Walsh et al., 1998). These results suggest that multiple temporal windows are critical for motion processing. Thus, the effects of spTMS on motion WM performance may be contingent on both alpha phase at the time of the TMS pulse, as well as the specific time of the pulse relative to stimulus onset.
It has also been proposed that critical motion processing time periods arise not solely based on intrinsic V5 activity, but via interactions of distributed, fronto-parietal networks that transmit top-down signals to V5 (Laycock et al., 2007). This is highly plausible given the role of fronto-parietal regions both during periods of stimulus expectation (i.e., prior to the stimulus onset)(Bollinger et al., 2010; Carlsson et al., 2000; Coull and Nobre, 1998) and early sensory processing (Foxe and Simpson, 2002; Ruff et al., 2006; Silvanto et al., 2006; Zanto et al., 2011b). We have previously shown that WM for motion direction is associated with modulation of activity in V5 that is dependent on functional connections between V5 and fronto-parietal regions subserving attention and memory processes (Zanto et al., 2010a; Zanto et al., 2011b). Specifically, a region within the prefrontal cortex, the inferior frontal junction (IFJ), was shown to be consistently engaged across individuals and causally involved in modulating neural activity in visual cortex during WM encoding at the P1 (100 ms post stimulus onset) of the event related potential (ERP). In addition to the attention based, or top-down, modulation of the P1 to motion stimuli, it is interesting to note that the P1 may be generated, at least in part, by alpha band oscillations (Freunberger et al., 2008). Therefore, we hypothesized that spTMS targeting V5 and IFJ would impact WM performance, as well as the P1 amplitude, based on the posterior alpha phase at the time of TMS pulses, and that the time relative to stimulus onset would interact with this influence of phase.
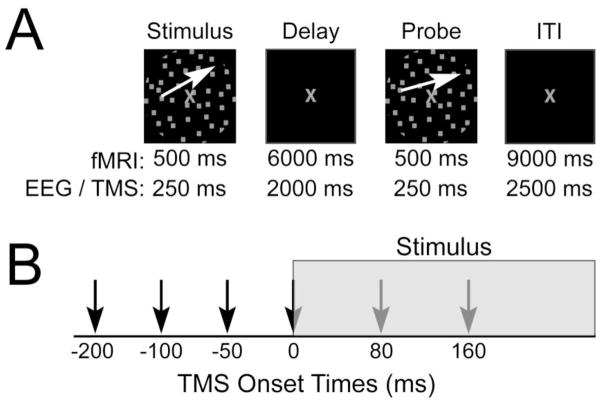
To address our hypothesis, a delayed recognition task for motion direction was used in two separate experimental sessions (Fig. 1A). Guided by the results from previous studies (Zanto et al., 2010a; Zanto et al., 2011b), the first session utilized functional magnetic resonance imaging (fMRI) to identify, on an individual participant level, task-related neural regions V5 and IFJ that serve as spTMS targets. The second session employed spTMS to stimulate neural activity in these regions at specific time points jittered around the onset of motion stimuli to be encoded, with a non-network region, vertex, serving as a control (Fig. 1B). Motion direction was maintained in mind over a delayed period and probed in a recognition test 2 seconds later. Additionally, during the second session, EEG was simultaneously recorded to assess spTMS effects based on the ongoing posterior alpha phase during TMS pulses, as well as to elucidate the neural basis of spTMS effects on WM performance.
Figure 1.
Experimental paradigm. (a) Delayed recognition task for motion direction. White arrows depict the direction of motion and were not present during the experiment. (b) Timing of spTMS onsets relative to the onset of the stimuli to be remembered. Note: only one TMS pulse was applied per trial.
1. Methods
2.1. Participants
Twenty-three healthy individuals (mean age = 25.7 years, 11 females) participated in the experiment. All participants gave informed consent to engage in the study according to procedures approved by the Committee for Human Research at the University of California. All participants had normal or corrected-to-normal vision. Three participants were excluded from analyses; one did not complete both sessions and two exhibited excessive artifacts in the EEG data.
2.2. Experimental Design
Visual stimuli were generated and presented using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). The stimuli consisted of a circular aperture of 300 dots (0.1° × 0.1° each) that subtended 9° of visual angle centered at the fovea. Dots were gray and moving with 100% coherence at 10° per second. Stimuli were presented with a gray fixation cross in the center of the circular aperture on a black background. The experimental paradigm was a delayed recognition task (Fig. 1A). Participants were required to remember the direction of motion of the primary stimulus and press one of two buttons to indicate whether the direction of motion of the probe stimulus matched the direction held in memory (match/no match). One half of the probe stimuli matched the primary stimulus. Participants were instructed to respond as quickly as possible without sacrificing accuracy. During the final second of the intertrial interval (ITI), the gray fixation cross turned green to indicate the start of the next trial. The task was divided into two blocks for the fMRI session and six blocks for the EEG/spTMS session. Prior to each block, participants were reminded of the task instructions. During the experiment, participants received 50 trials in the fMRI and 1260 trials in the EEG/spTMS, resulting in 14 and 105 minutes for the fMRI and EEG/spTMS sessions, respectively. The directions of motion for each trial were constrained to one quadrant and randomly selected. In the event of a “no match” trial, the directions of motion were separated by an angle determined through a pre-experiment thresholding procedure.
2.3. Thresholding Procedure
Prior to each experimental session, participants engaged in a motion thresholding procedure in order to minimize perceptual discriminability differences between participants, as previously described (Zanto et al., 2010b). Briefly, a staircase procedure required participants to determine whether two stimuli (directions of motion) were different from each other. The two stimuli were presented for 500 ms each and separated by 2000 ms. The procedure continued until “just 100%” level of performance was reached, meaning if the stimuli were any more similar, performance would drop below 100%. Thresholding determined the magnitude of deviation between two non-matching directions of motion that each participant received during the experiment.
2.4. Functional Magnetic Resonance Imaging
All fMRI data were collected on a Siemens 3T MAGNETOM Trio. Echo planar imaging data was acquired (flip angle = 90°, echo time = 25 ms, repetition time = 2 s) from 33 interleaved axial slices (0.5-mm gap) with a 1.8 × 1.8 × 3 mm voxel size (field of view = 23 cm, 128 × 128 matrix). All pre-preprocessing of the data was conducted in SPM5 (Wellcome Department of Imaging Neuroscience, London, England). Raw blood oxygen level–dependent (BOLD) data were corrected offline for slice-timing acquisition and motion artifacts. A 5-mm isotropic Gaussian smoothing kernel was applied before modeling the data. To aid in anatomical localizations of BOLD activity, we acquired high-resolution T1-MPRAGE images (1 × 1 × 1 mm voxel size, field of view = 160 × 240 × 256 mm, repetition time = 2300 ms, echo time = 3 ms, flip angle = 9°).
2.5. fMRI Region of Interest Localization
Following the thresholding procedure and prior to beginning the main fMRI experimental task, participants were presented a 1-back memory task for directions of motion that served as a localizer for a motion-sensitive region of interest (ROI): V5/hMT+. Motion stimuli (as described above) were presented in ten, 16 sec blocks interleaved with 16 sec of rest when participants passively viewed stationary gray dots. Within each block, stimuli were presented for 300 ms with a 500 ms interstimulus interval. Upon identifying a 1-back matched stimulus, participants were instructed to press the right-sided button. This occurred twice at random time points within each block (i.e., two 1-back matched stimuli for each block). BOLD data from the motion localizer were analyzed using a general linear model (GLM) and epochs spanning the duration of stimulus presentation were convolved with the SPM canonical hemodynamic response function. The motion sensitive ROI was selected in native space as the most significant cluster of activation (p < 0.01) in the right middle temporal gyrus (specifically, V5/hMT+).
2.6. fMRI Functional Connectivity Analysis
Functional connectivity network maps from the motion direction WM task were created for each participant as described previously using a beta series connectivity analysis approach (Gazzaley et al., 2004; Rissman et al., 2004). The encoding and retrieval stages from every trial were modeled with their own separate regressor within the GLM and a mean beta value was extracted for the V5 ROI (per trial). Although two stages were modeled during each trial, only the encoding period was subject to analysis for this study. Therefore, the ROI beta values from the encoding period were correlated across trials with every voxel in the brain to find regions with covariant activity. This procedure produced a whole brain Pearson’s r-value map for each participant and a Fisher’s r-to-z transformation was applied. The z-values were subsequently normalized to the Montreal Neurological Institute (MNI; 2 × 2 × 2 mm voxel size) template and Gaussian smoothed (5-mm full width at half maximum) for group level analysis. Effects on motion WM encoding were assessed via planned t-tests against the rest period baseline and cluster thresholding based on Monte Carlo simulations were used to correct for multiple comparisons, resulting in a corrected significance of p < 0.001.
2.7. Transcranial Magnetic Stimulation
A Magstim Standard Rapid TMS Unit (Jali Medical Inc) was used to generate TMS pulses with a 70 mm figure-of-eight induction coil. The magnetic stimulus had a biphasic waveform with a pulse width of about 300 μs. The Brainsight frameless stereotaxic software (Rogue Research, Montreal, Canada) was used to co-register the participant’s head, coil and high-resolution T1-weighted MRI images into a common digital workspace. Three neural regions were targeted for spTMS: right V5, right IFJ, and vertex (as control). The V5 target was identified using each individual’s motion localizer data, whereas the IFJ target for spTMS was identified using each individual’s functional connectivity data (with V5 ROI as seed-region). Although multiple neural regions exhibited significant functional connectivity to V5 during the delayed recognition task, the right IFJ was targeted based on its known involvement in WM encoding for motion stimuli and its between participant consistency (Zanto et al., 2010a; Zanto et al., 2011b). Data from V5 and IFJ were subsequently overlaid onto each participant’s T1-weighted MRI image for precise spTMS targeting.
During the second experimental session, for each trial, spTMS was applied at one of the six following time points: −200, −100, −50, 0, 80, or 160 ms relative to encoding stimulus onset (Fig. 1B), or not applied at all. The onset (or absence) of spTMS was randomized across trials. During the EEG/spTMS session, each participant engaged in 6 task blocks, two for each stimulation site (V5, IFJ, vertex). The order of stimulation site block was counterbalanced across participants. While engaged in the task, participants remain seated upright with the EEG cap on and spTMS was applied directly above the electrodes. Vertex was targeted between electrodes CZ and CPZ. The coil was held such that the handle protruded toward the back of the head. spTMS pulse intensity was held at 70% maximum stimulator output for each participant. This intensity was chosen based on pilot data that found it to be on average 120% the active motor threshold when applied directly above the EEG electrodes. All participants wore earplugs during both sessions of the experiment as protection from the fMRI noise (session 1) and spTMS clicking (session 2).
2.8. Electroencephalography
Electrophysiological signals were recorded at 1024 Hz through a 24-bit BioSemi ActiveTwo 64-channel Ag-AgCl active electrode EEG acquisition system (Cortech Solutions, LLC). Electrode offsets were maintained between +/− 20 mV. All data were processed via in-house MATLAB scripts. Raw EEG data were referenced to the average off-line. spTMS artifacts were removed by defining a 30 ms window beginning at spTMS onset and overwriting the artifact with Gaussian noise that has the same standard deviation as the 50 ms preceding the spTMS pulse. Any remaining capacitance effects were removed with an independent component analysis. Eye movements were monitored via an Eye-Trac 6000 (Applied Science Laboratories) to ensure the participants focused on the fixation cross and did not exhibit spTMS-related blinks. Data epochs were extracted beginning 400 ms pre-stimulus onset and ending 1000 ms post-stimulus onset. Sixty epochs were collected for each TMS onset time (−200, −100, −50, 0, 80, 160, no TMS) and TMS site (V5, IFJ, vertex). Epochs that contained an eye-related artifact during stimulus presentation were discarded from subsequent analysis.
To assess posterior phase during spTMS, a lateral-occipital region of interest (ROI) was created by averaging over 5 electrodes from right (P4, PO4, P6, P8, PO8) hemisphere, consistent with the topographical distribution of the visual event related potential (ERP). Epoched data from this ROI whose voltage exceeded a threshold of +/− 100 μV were rejected. Artifact free trials were then convolved with complex Morlet wavelets (family ratio: fO/σf = 7) to resolve frequencies from 4 to 70 Hz and served as a means to band pass filter the data into theta (4–8 Hz), alpha (8–12 Hz) and beta (12–20 Hz) frequency bands. Instantaneous phase was calculated via a Hilbert transform on data from each frequency band. Individual trial epochs were sorted into two categories based on the phase at spTMS onset: peak or trough. A peak epoch is defined as any epoch such that the phase is 0°+/− 90° at the TMS pulse, whereas a trough epoch is defined by a phase of 180°+/− 90° at the TMS pulse. Please note that the Hilbert transform utilized a cosine function, and so phase angles are reported accordingly (i.e., the maximal (peak) cosine value is at 0°, minimum (trough) value is at 180°). Although the phases at the TMS pulse exhibited a relatively even distribution about the phase circle (Supplementary Table 1), a bootstrapped random sample (N=5000) from the phase category with more epochs was used for analysis to ensure equal sample sizes between peak and trough epochs for each participant, TMS time, and stimulation site.
To calculate event-related potentials (ERPs) to the probe stimulus, epochs (both peak and trough) were band-pass filtered from 1–30 Hz and those that exceeded a voltage threshold of +/− 50 μV were rejected. A 200 ms pre-stimulus baseline was subtracted from each epoch prior to calculating the ERP. P1 values were chosen as the average amplitude between 80–100 ms, whereas the N1 was identified as the average amplitude between 155–175 ms post-stimulus onset. These windows were selected as they encompassed the maximal P1 and N1 amplitudes when averaged over all subjects and conditions (Supplementary Figure 1). Latency of the P1 and N1 were assessed by identifying the largest peak in a window between 50–150 ms post-stimulus onset for the P1 or 120–220 ms for the N1. Amplitude and latency data were averaged across electrodes (P4, PO4, P6, P8, PO8) to be consistent with the ROI used to identify the phase categories (peak and trough). Additionally, a contralateral ROI was created to assess laterality effects in the ERP (P3, PO3, P5, P7, PO7). Statistical analysis of EEG data as well as behavioral data utilized a repeated measures analysis of variance (ANOVA). If sphericity was violated, a Greenhouse-Geisser correction was used. Main effects and interactions were assessed via planned paired two-tailed t-tests and a false discovery rate correction was applied to control for multiple comparisons (Benjamini and Hochberg, 1995) to yield a corrected p < 0.05.
2. Results
3.1. fMRI Session: Functional Connectivity
fMRI data from the first experimental session were analyzed to identify two specific neural regions, V5 and IFJ, on an individual participant level, which have previously been found to be involved in encoding motion direction into WM (Zanto et al., 2010a; Zanto et al., 2011b). These regions then served as spTMS targets in the second experimental session. Using a 1-back motion direction task (localizer), motion sensitive cortical region V5 was identified in each participant (Schoenfeld et al., 2007; Zeki et al., 1991), which at the group level exhibited stronger activity in the right, compared to left hemisphere. In order to identify neural networks involved in encoding motion features into WM during the experimental paradigm, the right V5 region served as a seed in a functional connectivity analysis using the beta series correlation approach (Gazzaley et al., 2004; Rissman et al., 2004). Results showed widespread functional connectivity of many cortical regions with V5 during WM encoding, which encompassed occipital, parietal, temporal, and frontal cortical regions (Supplementary Figure 2). Importantly, we replicated previous work indicating an involvement of the inferior frontal junction (IFJ) in the V5 network while encoding motion features into WM (Zanto et al., 2010a; Zanto et al., 2011b). Although bilateral IFJ connectivity to right V5 was observed, the magnitude of connectivity was stronger in the right hemisphere (Supplementary Figure 1). Thus, the right IFJ, right V5 and vertex (as control) were selected for spTMS in the second experimental session (Fig. 2).
Figure 2.
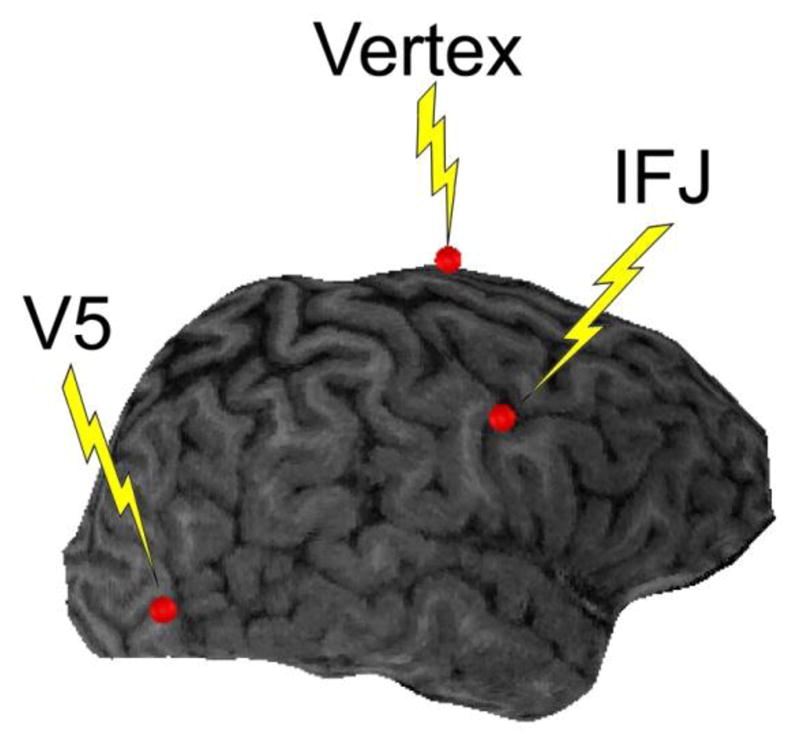
Targets for spTMS. Targets (red dots) shown are for one representative participant superimposed on their brain image.
3.2. EEG/spTMS Session: Memory Performance
3.2.1. Comparisons between spTMS and no spTMS
Behavioral data from the second experimental session were analyzed to assess whether spTMS during encoding affected subsequent memory performance. First, accuracy and response time (RT) data were compared to the trials in which no TMS pulse occurred to assess spTMS differences from baseline. Baseline (i.e., no spTMS) and spTMS data was categorized based on the phase (i.e., peaks or troughs) from a right posterior region of interest (ROI, see methods) as informed by the phase at the time of the TMS pulse (or where the pulse would have been for baseline data comparisons). Paired t-tests (corrected for multiple comparisons, see methods) on trials categorized by posterior alpha (8–12 Hz) phase indicated no accuracy differences. However, RT was observed to significantly decrease compared to baseline (i.e., no spTMS) only when spTMS was applied during an alpha peak 100 ms prior to stimulus onset (t(19) = 4.18, p < 0.01). This indicates that spTMS effects on WM performance may be contingent on both the time of stimulation as well as the phase of the ongoing posterior alpha oscillation. When trials were categorized based on theta (4–8 Hz) or beta (12–20 Hz) phase at the time of spTMS, no differences were observed compared to baseline for either accuracy or RT data at any phase, time point, or stimulation site. As hypothesized, this suggests that WM performance may be selectively altered by spTMS based on alpha phase. Therefore, subsequent analyses will focus on data categorized by posterior alpha phase oscillations.
3.2.1. Consequences of alpha phase and time of spTMS
Next, baseline (no spTMS) data was assessed for differences based on the posterior alpha phase (i.e., peaks compared to troughs) at each of the time points assessed by spTMS (−200, −100, −50, 0, 80, 160 ms). Paired t-tests were conducted for each of the three sites where spTMS was otherwise applied (V5, IFJ, vertex) and each of the six time points. Results indicated that both accuracy and RT were unchanged based on the posterior alpha phase in the absence of spTMS (each comparison p > 0.18). This indicates that any phase-based differences in performance following spTMS may be attributed to the perturbation of neural activity from the spTMS and not due to ongoing cortical oscillations.
Accuracy and RT data from the spTMS conditions were then submitted to separate repeated measures ANOVAs of Time X Phase X Site; i.e. 1) Time of TMS pulse relative to stimulus onset (−200, −100, −50, 0, 80, 160 ms), 2) Phase of ongoing oscillation during TMS pulse (peak, trough), and 3) Site of spTMS stimulation (V5, IFJ, vertex) as factors. To be clear, the factor ‘Phase’ refers to whether the TMS pulse occurred closer to a peak or trough of the ongoing oscillation within the alpha (8–12 Hz) frequency band from a posterior electrode ROI composed of right lateral-occipital electrodes.
When trials were categorized based on alpha phase at the time of the TMS pulse, WM accuracy for the probe (Supplementary Table 2) displayed a significant Time X Phase interaction (F(5,95) = 2.88, p < 0.05) and no other interactions or main effects were observed. To explore this interaction, paired t-tests were used for each time point to compare accuracy when spTMS was applied at an alpha peak relative to accuracy when spTMS was applied at an alpha trough. Results indicated no significant differences at any time point (each comparison, p > 0.14). Additional post-hoc analysis indicated that accuracy decreased when spTMS was applied during an alpha peak 200 ms prior or 80 ms post stimulus onset compared to when spTMS was applied during an alpha peak 100 ms prior to stimulus onset (both comparisons, p < 0.05). Although the three-way interaction was not significant, accuracy data was compared between each spTMS test site (IFJ/V5) and control (vertex) to assess whether this result was contingent on the site of stimulation. Paired t-tests indicated no accuracy differences between IFJ/V5 and vertex when spTMS was applied during an alpha peak 200 and 100 ms prior or 80 ms post stimulus onset (each comparison, p > 0.6). Therefore, as the spTMS effects are not site-specific, these accuracy changes may reflect a general distraction effect to the TMS pulse that is both time and phase dependent. To assess whether this distraction effect differs from baseline, accuracy was compared between “no spTMS” trials (averaged over stimulation sites) and trials where the TMS pulse occurred during an alpha peak 200 ms prior, 100 ms prior, and 80 ms post stimulus onset (also averaged over stimulation sites). Results indicated that accuracy declined when spTMS was applied 200 ms prior to stimulus onset (t(19) = 2.76, p < 0.05). Nonetheless, as described in the first analysis, spTMS to each of the stimulation sites did not yield significant differences from baseline, thus supporting the interpretation of this as a non-specific distraction effect.
RT data also yielded no main effects, but there was a significant interaction for Site X Phase (F(2,38) = 4.93, p < 0.05) and Site X Phase X Time (F(10,190) = 2.29, p < 0.05). Planned comparisons of the three-way interaction indicated that RT was faster following spTMS applied to V5 during an alpha peak compared to an alpha trough 100 ms prior to stimulus onset (t(19) = 4.86, p < 0.01; Table 1, Fig. 3). This RT dependency on phase was not observed when spTMS was applied to any other time point or site of stimulation (p > 0.5, each comparison). Additionally, spTMS to V5, compared to vertex control, resulted in speeded RT when the TMS pulse occurred during an alpha peak 100 ms prior to stimulus (t(19) = 2.97, p < 0.01), whereas slowed RT, compared to vertex control, was observed when the V5 spTMS occurred during an alpha trough 100 ms prior to stimulus (t(19) = 2.72, p < 0.05). Accordingly, the magnitude of RT sensitivity to phase (peak–trough) revealed a larger RT difference when spTMS was applied to V5 compared to vertex control (t(19) = 4.64, p < 0.01; Fig. 3). Together, this suggests that visual WM performance may be differentially modulated by spTMS to V5 based on alpha phase 100 ms prior to stimulus onset.
Table 1.
Mean (SE) RT data (in ms) per condition.
| Time of TMS Pulse (ms) | |||||||
|---|---|---|---|---|---|---|---|
| Site | Alpha Phase | −200 | −100 | −50 | 0 | 80 | 160 |
| IFJ | Peak | 806 (29) | 830 (33) | 836 (27) | 833 (32) | 818 (31) | 829 (28) |
| Trough | 813 (30) | 810 (29) | 820 (27) | 823 (31) | 833 (31) | 847 (32) | |
| Vertex | Peak | 805 (33) | 827 (31) | 821 (36) | 803 (27) | 823 (35) | 803 (27) |
| Trough | 799 (30) | 795 (34) | 823 (31) | 805 (32) | 816 (31) | 811 (32) | |
| V5 | Peak | 838 (36) | 782 (28)* | 810 (30) | 813 (27) | 814 (28) | 816 (30) |
| Trough | 832 (28) | 852 (32) | 821 (34) | 822 (32) | 839 (31) | 841 (32) | |
Numbers in bold font indicate significant differences at that TMS site between peak and trough data (p < 0.05).
Asterisk indicates significant difference between the data and the “no spTMS” baseline (p < 0.05).
Figure 3.
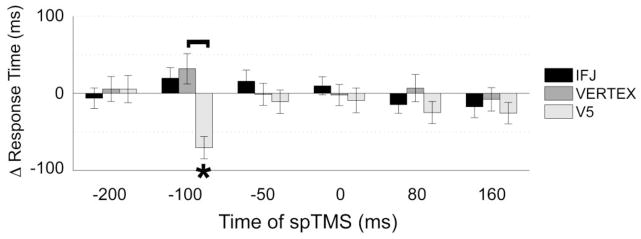
Phase-based effects of spTMS on RT data. Following V5 spTMS, response times were differentiated based on the phase of the alpha cycle at spTMS onset. Error bars represent SE, Δ = alpha peak–alpha trough, brackets indicate significant differences between stimulation sites (p < 0.05), asterisks indicate significant differences based on whether spTMS occurred during an alpha peak or alpha trough (p < 0.05). Thus, a significant negative Δ RT at −100ms for V5 indicates a faster response (lower RT) following peak vs trough spTMS.
3.2.3. Specificity of spTMS effects
The previous analysis revealed that spTMS to V5 exhibited differential effects on WM performance as indicated by an influence on RT that was based on the alpha phase in the right lateral-occipital region. To assess the site specificity of the alpha phase based effects, the previous analysis was conducted two more times using phase information from electrode ROIs around vertex (CZ, CP1, CP2, CPZ, PZ) and the IFJ (F4, FC2, FC4, FC6, C4). When using an ROI around vertex, a main effect of Phase was observed such that spTMS elicited faster RT when applied during an alpha peak compared to a trough (F(1,19) = 7.40, p < 0.05). Importantly, no other main effects or interactions were observed for either the vertex or IFJ ROIs. Thus, V5 spTMS effects on WM processes that are selective to site, time and phase are best indexed by alpha phase in occipital cortex ipsilateral to the TMS pulse.
3.3. Event Related Potentials
As just shown, the alpha phase, time and site dependent influence of spTMS on WM performance was revealed as an influence on RT to the probe stimuli presented approximately two seconds after spTMS. To investigate the neural underpinnings of this behavioral effect, ERP data time-locked to the onset of probe stimuli were analyzed. Amplitude and latency measures of early visual processing signatures that have been shown to be modulated by attention, the P1 (~100 ms after stimulus onset) and N1 (~170ms after stimulus onset), were submitted to separate repeated measures ANOVAs with Time (−200, −100, −50, 0, 80, 160 ms), Phase (peak, trough), stimulation Site (V5, IFJ, vertex) and Hemisphere (left, right) as factors. Of note, the topographies of the P1 and N1 (Supplementary Figure 1), when averaged over stimulation sites and times, verified our selection of lateralized posterior electrodes for analysis. Because spTMS effects on RT were modulated exclusively by posterior alpha phase, and not theta or beta band phase, nor in any other spatial ROI, ERP data were only analyzed based on posterior alpha phase during spTMS. Given the effects of spTMS on RT, we expected a similar Site X Phase X Time interaction in the ERP, such that spTMS applied during an alpha peak 100 ms prior to the encoded stimulus onset would exhibit the greatest change in the ERP to the probe.
Analysis of the P1 amplitude to the probe revealed no significant main effects, but showed a trend towards a significant Phase X Time X Hemisphere (F(5,95) = 2.55, p < 0.05) interaction as well as a Site X Phase X Time interaction (F(10,190) = 2.96, p < 0.01). Planned comparisons of the Site X Phase X Time interaction focused on the data when spTMS was applied 100 ms prior to the encoded stimulus onset (Fig. 4). This analysis indicated that spTMS to V5, compared to vertex control, resulted in increased P1 amplitude to the probe when spTMS was applied during an alpha trough 100 ms prior to the encoded stimulus onset (t(19) = 1.94, p = 0.07; Table 2). Direct comparisons between peak and trough data (Fig. 5), within each TMS site, showed phase-based differences when spTMS was applied to V5 (t(19) = 2.77, p < 0.05), but not IFJ or vertex control (each comparison p > 0.19). Accordingly, the magnitude of the P1 phase sensitivity (peak–trough) revealed a larger amplitude difference when spTMS was applied to V5 compared to vertex control (t(19) = 2.76, p < 0.05). To assess whether any of these effects exceeded baseline values, additional planned comparisons vs baseline were conducted on the P1 amplitude following spTMS 100 ms prior to stimulus onset. Results indicated that the P1 amplitude decreased, relative to baseline (i.e., no spTMS), when spTMS was applied to V5 during an alpha peak (t(19) = 2.55, p < 0.05; Table 2). Interestingly, no differences were observed, compared to baseline, at any other site of stimulation or alpha phase when spTMS was applied 100 ms prior to stimulus onset.
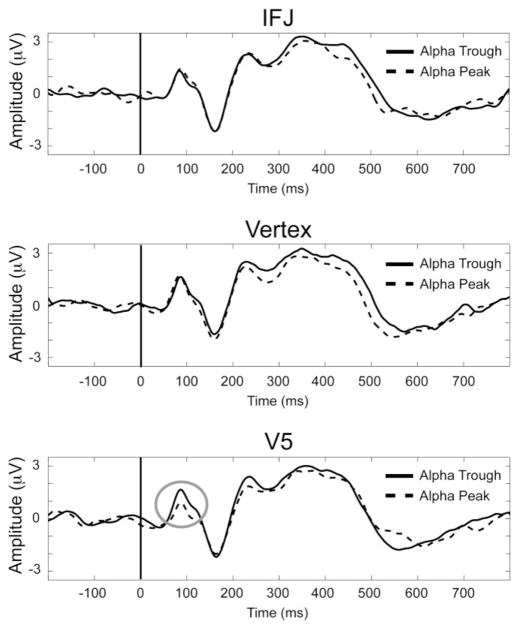
Figure 4.
ERPs to probe stimuli. Data shown are from trials where spTMS was applied 100 ms prior to encoded stimulus onset and averaged over left and right hemisphere regions of interest (see Fig. 4) from each stimulation site: IFJ (top), vertex (middle), and V5 (bottom). Effects of spTMS to V5 100 ms prior to WM encoding resulted in differential P1 amplitudes to probe stimuli (gray circle) based on the ongoing alpha phase during the TMS pulse.
Table 2.
Mean (SE) P1 amplitude data per condition.
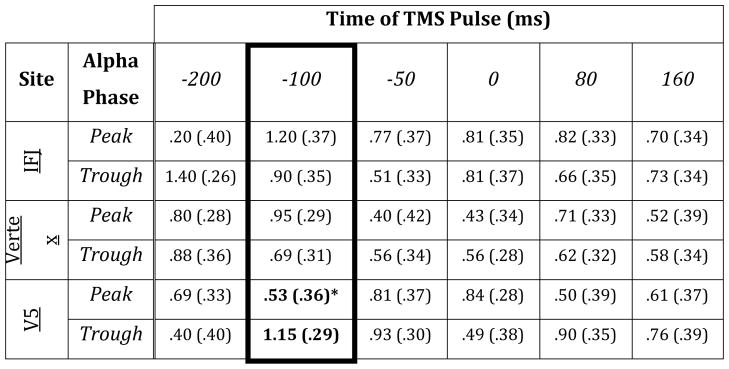
|
Planned comparisons were conducted on data when spTMS was applied 100 ms prior to stimulus onset (highlighted in black frame).
Numbers in bold font indicate significant differences at that TMS site between peak and trough data (p < 0.05).
Figure 5.
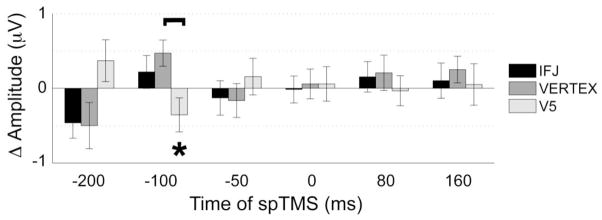
Phase based effects of spTMS on P1 amplitude to probe stimuli. Similar to spTMS effects on RT (see Fig. 3), following V5 spTMS, the P1 amplitude was differentiated based on the phase of the alpha cycle at spTMS onset. Error bars represent SE, Δ = alpha peak – alpha trough, brackets indicate significant differences between stimulation sites (p < 0.05), asterisks indicate significant differences based on whether spTMS occurred during an alpha peak or alpha trough (p < 0.05).
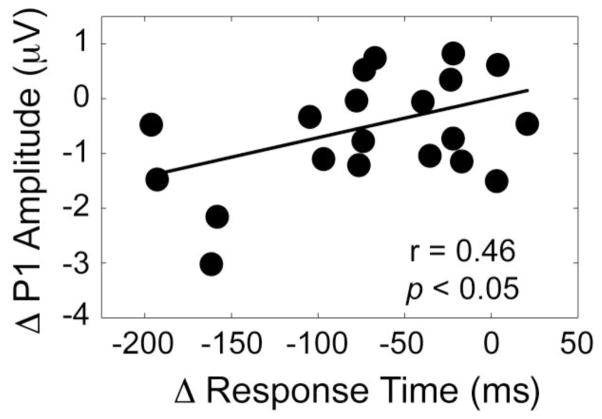
To explore a direct relationship between the P1 amplitude and RT, a regression analysis was conducted between the magnitudes of the phase effect (peaks–troughs) when spTMS was applied to V5 100 ms prior to stimulus onset. This resulted in a significant correlation between RT and the P1 amplitude to the probe (r = 0.46, p < 0.05; Fig. 6), such that participants whose P1 amplitude displayed the largest alpha phase-based impact by spTMS were the same participants who exhibited the largest changes in RT due to spTMS. Together, this suggests that early measures of visual processing of the probe, as indexed by the P1 amplitude, may affect visual WM performance, and that these neural and behavioral measures may be differentially modulated by spTMS to V5 based on alpha phase 100 ms prior to the encoded stimulus onset.
Figure 6.
Correlation between RT and the P1 amplitude to the probe following spTMS 100 ms prior to encoded stimulus onset. Participants who exhibited the greatest phase-based spTMS (peak – trough) induced change in P1 amplitude displayed the largest WM RT difference. Δ = alpha peak – alpha trough.
The P1 latency exhibited no main effects, but a Phase X Hemisphere interaction was observed (F(1,19) = 14.59, p < 0.01). Post hoc analysis indicated that the probe P1 peaked slower in the left hemisphere ROI when spTMS was applied during an alpha trough when compared to an alpha peak or when compared to the right hemisphere ROI regardless of phase during spTMS (each comparison, p < 0.05). Nonetheless, the P1 latency was not differentially modulated from vertex control, nor was it affected by the time of spTMS. As such, this latency shift does not seem to be involved in the WM performance changes induced by spTMS.
The N1 amplitude exhibited no main effects or interactions. However, analysis of the N1 latency revealed a main effect of Site (F(2,38) = 4.22, p < 0.05), but no other main effects or interactions. Post hoc analysis showed that the N1 latency was slowed when spTMS was applied to V5 (M = 164 ms, SE = 1) compared to vertex (M = 162, SE = 1; t(19) = 3.29, p < 0.01), whereas the N1 latency to the probe did exhibit any differences when spTMS was applied to the IFJ (M = 162, SE = 1) compared to V5 or vertex (each comparison, p > 0.10). Similar to the P1 latency, the N1 latency was not altered by spTMS in a similar time-dependent manner and did not exhibit any alpha phase sensitivity as WM performance.
Together, these results are similar to the spTMS effects on WM RT, such that the P1 amplitude to the probe stimulus is differentially affected by V5 spTMS based on the posterior alpha phase of the spTMS pulse immediately prior to encoding, which is dependent on the precise time relative to encoding stimulus onset. This is particularly striking in that spTMS occurred prior to WM encoding, whereas these P1 differences were observed approximately 2 seconds later, during the probe stimulus. Given that these effects were only found for V5 stimulation 100 ms prior to the encoded stimulus onset, this indicates that early motion processing during WM recognition was selectively affected by spTMS to a motion sensitive visual region prior to stimulus encoding.
3.4. Individual Variability in TMS Effects
Although it is known that the effects of TMS are variable (Wassermann, 2002), the source of this individual variability is often unclear. We have recently provided evidence that TMS effects on motion direction WM are less in participants who display greater functional connectivity between V5 and frontal cortex (Zanto et al., 2011b). Thus, here we hypothesized that those participants who exhibited the smallest phase-based TMS effects in the current experiment, might have differential functional connectivity between right V5 and frontal cortical regions. To address this, participants were categorized into two groups based on a median split of their RT sensitivity to alpha phase-based spTMS effects (i.e., peak – trough RT following V5 spTMS 100 ms prior to stimulus onset). V5 functional connectivity network maps from the first experimental session were contrasted between these two groups via unpaired t-tests and corrected for multiple comparisons (as detailed in the Methods section).
As hypothesized, participants who exhibited the least phase-based TMS effects on WM performance showed greater functional connectivity between right V5 and multiple frontal regions (Fig. 7; Table 3), including the IFJ. To verify whether this increased V5-IFJ connectivity was related to the IFJ spTMS target (which was defined by V5-IFJ connectivity), ROIs were created for each participant based on their IFJ spTMS target and an unpaired t-test was conducted on the connectivity values at the IFJ spTMS target between the two groups. Results confirmed that those participants who exhibited the least phase-based TMS effects on WM performance showed the greatest V5-IFJ functional connectivity (t(18) = 4.66, p < 0.01). Additionally, greater connectivity was observed with a parietal and temporal region as well, in these individuals. Interestingly, participants who displayed the greatest phase-based spTMS effects exhibited greater functional connectivity in only one region (right temporal pole, (x=54,y=6,z=−24), 360 mm3) compared to participants who were least affected. Overall, these results support previous findings that suggest TMS effects are influenced by the magnitude of functional connectivity (Zanto et al., 2011b).
Figure 7.
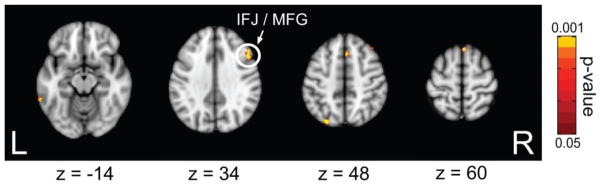
Contrasts of functional connectivity between participants who were least and most affected by phase-based spTMS (peak – trough) to V5 100 ms prior to stimulus onset. Greater functional connectivity between right V5 and frontal (including IFJ), parietal, and temporal regions was observed in participants who were least affected by spTMS.
Table 3.
Regions that exhibited increased functional connectivity to V5 in participants whose performance were least affected, compared to participants whose performance was most affected, by alpha phase-based spTMS (peak – trough) to V5 100 ms prior to stimulus onset.
| Center of Mass (MNI) | |||||
|---|---|---|---|---|---|
| Lobe | Region | X | Y | Z | Volume (mm3) |
| Frontal | Middle Frontal Gyrus (R) | 40 | 28 | 42 | 1,118 |
| Superior Frontal Gyrus (R) | 2 | 24 | 54 | 855 | |
| Inferior Frontal Junction (R) | 50 | 20 | 32 | 379 | |
| Temporal | Middle Temporal Gyrus (L) | −64 | −46 | −14 | 350 |
| Parietal | Superior Parietal Lobule (L) | −26 | −78 | 48 | 340 |
3. Discussion
This study used spTMS to assess the causal influence of both time relative to stimulus onset and ongoing lateral-occipital alpha phase on motion direction processing in visual area V5 on subsequent WM performance. Results showed that spTMS to right V5 immediately prior to motion direction encoding modulated both RT and the P1 amplitude to a subsequent memory probe based on the phase of the alpha oscillation. Interestingly, this was related to the specific timing of spTMS, such that the phase-based effects were present only at 100 ms prior to the onset of the encoded stimuli. It was further shown that participants with the smallest phase-based sensitivity to spTMS exhibited the greatest functional connectivity between right V5 and frontal, parietal, and temporal regions. Together, these results suggest that lateral-occipital alpha phase serves as an index of critical stages of motion processing that occur in anticipation of WM encoding.
When spTMS was applied to V5 during an alpha peak compared to a trough, 100 ms prior to encoding stimulus presentation, subsequent RTs to a WM probe were faster and the P1 amplitude generated by the probe were smaller. Importantly, the alpha phase-based sensitivity (peak – trough) of the P1 amplitude to spTMS predicted subsequent changes in WM performance. This corroborates our previous report suggesting that TMS induced alterations of early sensory processes bias the performance of higher cognitive processes such as WM (Zanto et al., 2011b). Interestingly, RT following V5 spTMS compared to vertex spTMS were faster when stimulated at a peak, and slower when stimulated at a trough, suggesting differential TMS effects that were both enhancing and suppressive based on alpha phase. This interpretation is consistent with recent reports that alpha phase represents alternating microstates of inhibition and excitation (reviewed in Mathewson et al., 2011), and suggest that the effects of spTMS may reflect either constructive or destructive interference with neural activity based on the peak or trough of the alpha cycle, respectively. This is also reminiscent of other studies that stress the importance of cognitive state during TMS, which may result in differential outcomes (e.g., Pasley et al., 2009).
It was expected that phase-based effects would be observed at different time points relative to stimulus presentation in accordance with critical time windows of motion processing. Interestingly, only one time point elicited differential spTMS responses for both RT and P1 amplitude, 100 ms prior to stimulus onset. This result is somewhat in conflict with research indicating that multiple time points exist pre and post stimulus onset that are important for motion processing (Anand et al., 1998; Beckers and Homberg, 1992; Beckers and Zeki, 1995; Bosco et al., 2008; Laycock et al., 2007; Maus et al., 2013; Sack et al., 2006; Silvanto et al., 2005; Stevens et al., 2009; Walsh et al., 1998). However, key methodological differences may account for this divergence. First, it should be noted that the duration of the stimulus (250 ms) in the current study was longer than many motion detection tasks (50 – 100 ms). This additional time may benefit WM processes that were transiently perturbed by spTMS, leading to null findings. Second, the time at which performance was assessed in the current study is dramatically different. The effects of a single TMS pulse may last tens to hundreds of milliseconds (Thut and Pascual-Leone, 2010), and motion detection tasks often assess performance while the neural consequences of the TMS are still occurring. Here, we assessed performance more than two seconds after the TMS pulse, which is well after the direct effects subsided. Furthermore, it may be that WM processes, compared to detection, utilize a more complex network of neural activity that may not be as sensitive to some time points. We have provided some evidence in support of this; e.g., when TMS is pulsed during different periods of neural excitability (i.e., alpha peaks and troughs), the effects of TMS are dampened in participants who exhibit greater engagement of fronto-parietal networks. Indeed, the effects of TMS are contingent on cognitive state (Pasley et al., 2009), which provides a reasonable account for the differences in spTMS effects between the current WM task and previous perceptual detection tasks.
Another reason as to why the current study did not reveal a spTMS effect post-stimulus onset may have to do with a differential magnitude of V5 spTMS effects before and after stimulus presentation. It has been noted that spTMS prior to stimulus onset is the most critical time point for functional activation of V5 (Laycock et al., 2007), such that spTMS prior to stimulus onset, compared to spTMS post stimulus onset, results in twice as large of a behavioral effect (Stevens et al., 2009). Here, we corroborated previous reports indicating V5 spTMS alters motion processing prior to stimulus onset (Beckers and Zeki, 1995; Laycock et al., 2007; Maus et al., 2013; Sack et al., 2006; Stevens et al., 2009). The current results extend the previous research in motion detection to WM for motion direction, and suggest that the effects of spTMS post-stimulus onset may not reach the magnitude of impact critical for neural processes that are engaged in a more complex cognitive task.
Although the pre-stimulus effects of spTMS are in accordance with previous work, the specific functional significance of neural activity 100 ms prior to the stimulus onset is unclear. It is intriguing to note that this time corresponds to one cycle of the alpha band. As such, it could be argued that the TMS pulse induced a phase reset, which would decrease phase variability at stimulus onset and place the alpha activity in an optimal phase to receive the visual stimulus. However, a phase reset would align the phase at stimulus onset, regardless of the phase at the TMS pulse, thereby nullifying any phase-based effects. Another possibility is that the TMS pulse enhanced the ongoing alpha oscillation, leading to larger peak and trough alpha amplitudes at stimulus onset when spTMS was applied at a peak or trough, respectively. However, this would also mean that spTMS at 50 ms prior to stimulus onset would exhibit similar (if not greater) phase based effects on performance and neural activity to the probe, and this was not observed. An alternative account as to why pre-stimulus spTMS affected performance suggests that backward propagation may alter V1 responsivity at early stages of visual processing (Stevens et al, 2009). This would suggest that spTMS does not alter anticipatory processes, but rather, reflects lingering TMS effects on bottom up processes. However, this would indicate that spTMS at 50 ms prior to stimulus onset would also alter subsequent WM performance, but this was not observed.
A possible role for V5 engagement 100 ms prior to stimulus onset is its involvement in anticipatory neural processes. The importance of anticipatory processes is well established and rests on extensive findings of an increase in neural activity prior to stimulus onset in cortical regions that selectively process the expected stimuli, such as a spatial location (Kastner et al., 1999), feature (Chawla et al., 1999; Shulman et al., 1999), or object category (Puri et al., 2009; Bollinger et al., 2010). This anticipatory increase in neural activity serves to enhance performance, for example, in target detection (Miniussi et al., 1999; Rohenkohl and Nobre, 2011), discrimination (Praamstra et al., 2006; Zanto et al., 2011a), and working memory (Bollinger et al., 2010). This anticipatory top-down modulation is an important aspect of the mechanistic overlap that bridges selective attention and working memory (Gazzaley and Nobre, 2012), and so although the behavioral effects were observed on WM performance, they likely reflect an impact on anticipatory process that is common across these cognitive operations.
Whereas the idea that perception and attention may be oscillatory has been around for years (e.g., Large and Jones, 1999), only recently has a surge of research provided strong evidence for this hypothesis, as reflected in alpha oscillations (reviewed in Hanslmayr et al., 2011; Thut et al., 2012; VanRullen et al., 2011). A single TMS pulse to the occipital lobe elicits alpha band oscillations (Rosanova et al., 2009), which may reflect engagement of fronto-parietal alpha band neural networks (Garcia et al., 2011). These fronto-parietal networks are used in a top-down fashion to modulate neural activity in the visual cortex based on attentional goals (Ruff et al., 2006; Silvanto et al., 2006; Silvanto et al., 2009; Taylor et al., 2007; Zanto et al., 2011b), and may occur in anticipation of a stimulus (Coull and Nobre, 1998; Taylor et al., 2007; Zanto et al., 2011b). This could help explain why participants who were least affected by spTMS displayed more fronto-parietal functional connectivity. It is possible that these participants relied more heavily on top-down influences as opposed to intrinsic sensory processes that may be indexed by alpha phase in the lateral-occipital cortex. This is in line with previous observations that increased functional connectivity reduces the effects of TMS to the IFJ (Zanto et al., 2011b), and that the influence of phase on task performance may be lessened by tasks with higher cognitive demands (Drewes and VanRullen, 2011).
V5-spTMS during an alpha peak, relative to trough, showed reduced P1 amplitude to probe stimuli, which preceded speeded response times. We have previously shown that the P1 amplitude is larger when motion stimuli are attended and smaller when ignored (Zanto and Gazzaley, 2009; Zanto et al., 2010b). Thus, one interpretation for the reduced P1 amplitude to probe stimuli is that less attention was required for WM performance to be retained. This may be because V5-spTMS at an alpha peak resulted in an enhanced representation of the encoded stimulus relative to alpha trough spTMS, rendering it easier to maintain a high fidelity representation and thus less attentionally demanding to perform the subsequent memory test. Another interpretation, although not necessarily distinct, comes from the literature on cognitive training showing a reduced visual ERP amplitude concomitant with improved WM performance following training (Berry et al., 2010) or practice (Berry et al., 2009). Although these studies assessed P1 to encoded stimuli, their results suggest that training and practice results in efficient WM processes such that less neural activity may be used to attain improved performance. This would suggest that spTMS engaged the optimal neural mechanisms for encoding motion into WM, which led to efficient neural processing during probe recognition. Whereas interpretations of neural efficiency do not always invoke the concept of attention, the notion of efficiency may be characterized as the utilization of fewer attentional and processing resources to achieve the same goal.
The fact that spTMS to the right IFJ did not affect performance measures may be attributed to the modulatory role of functional connectivity between right V5 and the contralateral IFJ (Zanto et al., 2011b). Although the current data showed that left IFJ functional connectivity to V5 was not as strong as right IFJ functional connectivity, it may have played a role in maintaining neural and performance measures. An alternative explanation for the null IFJ spTMS effect may be that the previously identified critical time windows for motion processing reflect feedforward and feedback signals between V1 and V5, and not fronto-parietal regions (Stevens et al., 2009). Thus, stimulating IFJ would have no bearing on visual processes. However, given the extent of research indicating fronto-parietal involvement in expectation (Bollinger et al., 2010; Carlsson et al., 2000; Coull and Nobre, 1998) and early sensory processes (Foxe and Simpson, 2002; Ruff et al., 2006; Silvanto et al., 2006; Zanto et al., 2011b), it seems likely that frontal and parietal regions are involved in WM encoding during these time periods.
4. Conclusions
We have shown that alpha phase indexes cortical excitability in the occipital lobe, corroborating previous reports, and extend upon this by showing that perturbing neural activity in a phase-dependent manner alters subsequent WM performance selectively at 100 ms prior to stimulus onset. Furthermore, we demonstrated that participants least affected by spTMS exhibited greater functional connectivity between V5 and multiple prefrontal cortical regions. This suggests that the role of alpha phase on visual cortex excitability is contingent upon the engagement of neural networks involving higher cognitive control areas. These results will help guide future TMS studies that may be hindered by individual variability of TMS effects by encouraging data collection of the phasic state of cortical excitability during a TMS pulse and the magnitude of functional connectivity with other brain regions. Overall, this data contributes to a growing literature that describes the oscillatory timing of information processing and its role in cognition.
Supplementary Material
Highlights.
V5 spTMS prior to encoding affected neural measures and WM performance at probe
spTMS effects were dependent on posterior alpha phase
spTMS effects were dependent on the time relative to stimulus onset
Participants least affected by TMS exhibited increased V5 functional connectivity
Acknowledgments
This work was supported by NIH grant R01MH096861. We would like to thank Joaquin Anguera and Peter Wais for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand S, Olson JD, Hotson JR. Tracing the timing of human analysis of motion and chromatic signals from occipital to temporo-parieto-occipital cortex: A transcranial magnetic stimulation study. Vision Research. 1998;38:2619–2627. doi: 10.1016/s0042-6989(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Beckers G, Homberg V. Cerebral visual-motion blindness - transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proceedings of the Royal Society of London Series B-Biological Sciences. 1992;249:173–178. doi: 10.1098/rspb.1992.0100. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zeki S. The consequences of inactivating areas V1 and V5 on visual-motion perception. Brain. 1995;118:49–60. doi: 10.1093/brain/118.1.49. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Berman RA, Wurtz RH. Functional Identification of a Pulvinar Path from Superior Colliculus to Cortical Area MT. Journal of Neuroscience. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy J, Delahunt P, Mahncke H, Gazzaley A. The influence of perceptual training on working memory in older adults. Public Library of Science One. 2010:5. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Rutman AM, Clapp WC, Gazzaley A. Practice-Related Improvement in Working Memory is Modulated by Changes in Processing External Interference. Journal of Neurophysiology. 2009;102:1779–1789. doi: 10.1152/jn.00179.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working-memory and long-term memory performance. Journal of Neuroscience. 2010;30:14399–14410. doi: 10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F. Contributions of the Human Temporoparietal Junction and MT/V5+to the Timing of Interception Revealed by Transcranial Magnetic Stimulation. Journal of Neuroscience. 2008;28:12071–12084. doi: 10.1523/JNEUROSCI.2869-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The Phase of Ongoing EEG Oscillations Predicts Visual Perception. Journal of Neuroscience. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Petrovic P, Skare S, Petersson KM, Ingvar M. Tickling expectations: Neural processing in anticipation of a sensory stimulus. Journal of Cognitive Neuroscience. 2000;12:691–703. doi: 10.1162/089892900562318. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nature Neuroscience. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham J, He S, Dukelow S, Verstraten FAJ. Visual motion and the human brain: what has neuroimaging told us? Acta Psychologica. 2001;107:69–94. doi: 10.1016/s0001-6918(01)00022-1. [DOI] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. This Is the Rhythm of Your Eyes: The Phase of Ongoing Electroencephalogram Oscillations Modulates Saccadic Reaction Time. Journal of Neuroscience. 2011;31:4698–4708. doi: 10.1523/JNEUROSCI.4795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugue L, Marque P, VanRullen R. The Phase of Ongoing Oscillations Mediates the Causal Relation between Brain Excitation and Visual Perception. Journal of Neuroscience. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Fellinger R, Sauseng P, Gruber W, Klimesch W. Dissociation Between Phase-Locked and Nonphase-Locked Alpha Oscillations in a Working Memory Task. Human Brain Mapping. 2009;30:3417–3425. doi: 10.1002/hbm.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freunberger R, Holler Y, Griesmayr B, Gruber W, Sauseng P, Klimesch W. Functional similarities between the P1 component and alpha oscillations. European Journal of Neuroscience. 2008;27:2330–2340. doi: 10.1111/j.1460-9568.2008.06190.x. [DOI] [PubMed] [Google Scholar]
- Garcia JO, Grossman ED, Srinivasan R. Evoked potentials in large-scale cortical networks elicited by TMS of the visual cortex. Journal of Neurophysiology. 2011;106:1734–1746. doi: 10.1152/jn.00739.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: Bridging selective attention and working memory. Trends in Cognitive Sciences. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Desposito M. Functional connectivity during working memory maintenance. Cognitive Affective and Behavioral Neuroscience. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Gross J, Klimesch W, Shapiro KL. The role of alpha oscillations in temporal attention. Brain Research Reviews. 2011;67:331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large EW, Jones MR. The dynamics of attending: How people track time-varying events. Psychological Review. 1999;106:119–159. [Google Scholar]
- Laycock R, Crewther DP, Fitzgerald PB, Crewther SG. Evidence for fast signals and later processing in human V1/V2 and V5/MT+: A TMS study of motion perception. Journal of Neurophysiology. 2007;98:1253–1262. doi: 10.1152/jn.00416.2007. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. A Disynaptic Relay from Superior Colliculus to Dorsal Stream Visual Cortex in Macaque Monkey. Neuron. 2010;65:270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To See or Not to See: Prestimulus alpha Phase Predicts Visual Awareness. Journal of Neuroscience. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent pulsed-inhibition of ongoing cortical processing. Frontiers in Psychology. 2011;2:1–15. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus GW, Ward J, Nijhawan R, Whitney D. The Perceived Position of Moving Objects: Transcranial Magnetic Stimulation of Area MT+ Reduces the Flash-Lag Effect. Cerebral Cortex. 2013;23:241–247. doi: 10.1093/cercor/bhs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Wilding EL, Coull JT, Nobre AC. Orienting attention in time - Modulation of brain potentials. Brain. 1999;122:1507–1518. doi: 10.1093/brain/122.8.1507. [DOI] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention witnout moving the eyes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI. A shared inhibitory circuit for both exogenous and endogenous control of stimulus selection. Nature Neuroscience. 2013;16:473–U143. doi: 10.1038/nn.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-Dependent Variability of Neuronal Responses to Transcranial Magnetic Stimulation of the Visual Cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Kwok HF, Oostenveld R. Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci. 2006;26:5448–5455. doi: 10.1523/JNEUROSCI.0440-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri AM, Wojciulik E, Ranganath C. Category expectation modulates baseline and stimulus-evoked activity in human inferotemporal cortex. Brain Research. 2009;1301:89–99. doi: 10.1016/j.brainres.2009.08.085. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rohenkohl G, Nobre AC. Fluctuations of anticipatory neural oscillations according to temporal expectations. Journal of Neuroscience. 2011;31:14076–14084. doi: 10.1523/JNEUROSCI.3387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural Frequencies of Human Corticothalamic Circuits. Journal of Neuroscience. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens MT, Zanto TP. Parameterization of transcranial magnetic stimulation. Journal of Neurophysiology. 2012;107:1257–1259. doi: 10.1152/jn.00716.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Linden DE, Goebel R, Muckli L. The temporal characteristics of motion processing in hMT/V5+: combining fMRI and neuronavigated TMS. Neuroimage. 2006;29:1326–1335. doi: 10.1016/j.neuroimage.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, Heinze HJ, Hillyard SA. Spatio-temporal analysis of feature-based attention. Cerebral Cortex. 2007;17:2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. Journal of Neuroscience. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cerebral Cortex. 2005;15:1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Stimulation of the human frontal eye fields modulates sensitivity of extrastriate visual cortex. Journal of Neurophysiology. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Lavie N, Walsh V. The Perceptual and Functional Consequences of Parietal Top-Down Modulation on the Visual Cortex. Cerebral Cortex. 2009;19:327–330. doi: 10.1093/cercor/bhn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nature Neuroscience. 2004;7:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Smith AT, Cotton PL, Bruno A, Moutsiana C. Dissociating Vision and Visual Attention in the Human Pulvinar. Journal of Neurophysiology. 2009;101:917–925. doi: 10.1152/jn.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LK, McGraw PV, Ledgeway T, Schluppeck D. Temporal characteristics of global motion processing revealed by transcranial magnetic stimulation. European Journal of Neuroscience. 2009;30:2415–2426. doi: 10.1111/j.1460-9568.2009.07034.x. [DOI] [PubMed] [Google Scholar]
- Taylor PCJ, Nobre AC, Rushworth MFS. FEF TMS affects visual cortical activity. Cerebral Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J. The Functional Importance of Rhythmic Activity in the Brain. Current Biology. 2012;22:R658–R663. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topography. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS Causes Local Entrainment of Natural Oscillatory Signatures. Current Biology. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Busch NA, Drewes J, Dubois J. Ongoing EEG phase as a trial-by-trial predictor of perceptual and attentional variability. Frontiers in Psychology. 2011;2:1–9. doi: 10.3389/fpsyg.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proceedings of the Royal Society B-Biological Sciences. 1998;265:537–543. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clinical Neurophysiology. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Pan P, Liu H, Bollinger J, Nobre AC, Gazzaley A. Age-Related Changes in Orienting Attention in Time. Journal of Neuroscience. 2011a;31:12461–12470. doi: 10.1523/JNEUROSCI.1149-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Bollinger J, Gazzaley A. Top-down modulation of visual feature processing: The role of the inferior frontal junction. Neuroimage. 2010a;53:736–745. doi: 10.1016/j.neuroimage.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011b;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010b;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauner A, Fellinger R, Gross J, Hanslmayr S, Shapiro K, Gruber W, Muller S, Klimesch W. Alpha entrainment is responsible for the attentional blink phenomenon. Neuroimage. 2012;63:674–686. doi: 10.1016/j.neuroimage.2012.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JDG, Lueck CJ, Friston KJ, Kennard C, Frackowiak RSJ. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–U124. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.