Abstract
Inflammation can promote colon cancer. Mechanistic studies indicate that γ-tocopherol (γT), a major form of vitamin E in diets, has anti-inflammatory and anticancer properties. Here we investigated the effectiveness of γT and a mixture of tocopherols against colitis and colitis-promoted colon tumorigenesis in male BALB/c mice. γT or mixed tocopherols (at 0.1% diet) did not show any effect on colon tumorigenesis induced by azoxymethane (AOM, 10mg/kg) with three cycles of dextran sodium sulfate (DSS at 1.5–2.5%). γT failed to exhibit protection of severe colitis caused by three cycles of DSS at 2.5%. In contrast, when AOM-initiated carcinogenesis was promoted by relatively mild colitis induced by one-cycle DSS (1.5%), γT, but not mixed tocopherols, suppressed total multiplicity of macroscopic adenomas (P=0.06) and large adenomatous polyps (>2mm2, P<0.05) by 60% and 85%, respectively. γT also significantly decreased tumor multiplicity (>2mm2) induced by AOM with two cycles of 1.5% DSS even when dietary supplementation was started after AOM injection. Consistently, γT but not mixed tocopherols attenuated DSS (1.5%)-induced colon inflammation and damage as well as formation of atypical glandular hyperplasia. Mice supplemented with tocopherols had high fecal excretion of 13′-carboxychromanol, a long-chain vitamin E metabolite shown to have potent anti-inflammatory activities. Our study demonstrates that γT is able to alleviate moderate but not severe colitis and its promoted tumorigenesis, and indicates that inflammation severity should be considered in evaluating anticancer effectiveness of chemoprevention agents.
Keywords: vitamin E, tocopherol, carboxychromanol, inflammation, colon cancer, colitis
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in men and women combined in the United States. Due to the lack of effective treatment for the late-stage disease, chemoprevention represents a highly attractive strategy to reduce death from the cancer [1]. It has been recognized that inflammation contributes to cancer promotion and progression including colon cancer [2–5]. Inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease dramatically enhance the risk of colorectal cancer [3]. Proinflammatory pathways and mediators including eicosanoids catalyzed by cyclooxygenases (COXs)- or 5-lipoxygenase and cytokines/chemokines are shown to promote tumorigenesis [2, 4, 5]. Meanwhile, anti-inflammatory strategies such as inhibition of COXs by non-steroidal anti-inflammatory drugs have proven to be one of the most promising approaches for chemoprevention [2, 6]. In particular, COX inhibitors including aspirin have convincingly reduced the risk of colorectal cancer and recurrence of adenomatous polyps [2, 6].
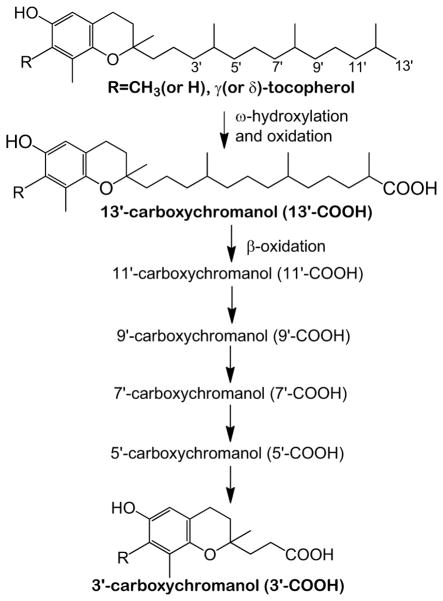
Natural forms of vitamin E have eight structurally related lipophilic antioxidants, including α, β, γ, δ-tocopherol (αT, βT, γT and δT) and four corresponding tocotrienols. Vitamin E forms such as γT and δT are metabolized via hydroxylation and oxidation of the side chain to 13′-hydroxychromanol and various carboxychromanols including 13′-carboxychromanol (13′-COOH) and the terminal metabolite 3′-carboxychromanol (3′-COOH) (Figure 1) as well as conjugated carboxychromanols [7–10]. Mechanistic studies indicate that γT, δT and their metabolites have anti-inflammatory properties. For instance, γT is effective in scavenging reactive nitrogen species [11, 12]. γT and δT, but not αT, inhibit COX- and 5-lipoxygenase -mediated eicosanoid formation in cellular environments [13–17]. Interestingly, 13′-COOHs derived from γT and δT are more potent than un-metabolized vitamin precursors in suppression of COX- and 5-lipoxygenase-catalyzed reactions [14, 17]. Consistent with these mechanistic studies, γT but not αT, when administered prior to induction of inflammation, decreases proinflammatory eicosanoids in an acute inflammation model in rats [18]. γT also attenuates peritonitis-caused ascorbate oxidation and protein nitration [19] and alleviates severe burn-related injury in sheep [20]. In addition to inflammation-attenuating effects, γT and 13′-COOH are shown to inhibit the growth and induce death of various types of cancer cells [21–24].
Figure 1.
γT and δT are metabolized via hydroxylation and oxidation of the side chain to form hydroxychromanol and carboxychromanols.
Based on these anti-inflammatory and anticancer properties, γT has been proposed to be potentially useful in chemoprevention against cancer [13, 15, 21]. To this end, tumor suppression effects of γT on prostate and breast cancer have been reported in preclinical animal models [25–27]. As to colon cancer, Ju et al [28] showed that γT-rich mixed tocopherols at 0.17% and 0.3% diet suppressed tumorigenesis induced by azoxymethane (AOM) and promoted by dextran sodium sulfate (DSS)-induced colitis in CF-1 mice. It is also reported that γT and mixed tocopherols inhibited AOM-induced aberrant crypt foci (ACF), a surrogate marker for colon cancer, in F344 rats [29, 30]. Despite these in vivo studies, the role of γT in colon cancer prevention remains unclear because the effect of this vitamin E form on tumor endpoint has not been studied. In addition, how inflammation influences anticancer effectiveness (by γT and mixed tocopherols), a clinically important question, has not been addressed. Here we investigated and compared the effect of γT and a mixture of tocopherols (containing γT, δT and αT at 45, 45 and 10%) at 0.1% diet on DSS-caused colon inflammation and AOM-DSS-induced tumorigenesis, which respectively mimic colitis and colitis-promoted colon cancer, in male Balb/c mice. We also examined how inflammation severity may have impact on the relative anticancer and anti-colitis efficacy.
MATERIALS AND METHODS
Materials and diets
γT (~95%) and δT (>90%) was obtained from Yasoo Health Inc (Johnson City, TN) and Sigma (St Louis, MO), respectively. The purity of both tocopherols was confirmed by us using HPLC. Azoxymethane (AOM) was purchased from Sigma and dextran sodium sulfate (DSS, Mw 36,000–50,000) was from MP Biochemicals (Solon, Ohio). Vitamin E metabolites including γ-, α-CEHC (2-(β-carboxyethyl)-6-hydroxychroman) were from Cayman Chemicals (Ann Arbor, MI).
Mice in the control group were fed AIN-93G diet, and those in γT- or mixed tocopherol-group were fed γT- and mixed tocopherol-supplemented AIN-93G diet at 0.1% diet, respectively. Mixed tocopherols were made by mixing 45% γT, 45% δT and 10% (+)-α-tocopherol acetate (Sigma). This composition was based on the fact that γT and δT often coexist in various food sources and δT appears to be as effective as γT in anti-inflammatory activities [14, 17]. To minimize potential oxidation of vitamin E, diets were stored in 4C and food given to mice was changed once a week.
AOM-DSS-induced colon cancer model
The animal use protocols for all the studies were approved by the Purdue animal care and use committee. Male Balb/c mice (5–6 weeks) from Harlan (Indianapolis, IN) were single-housed under controlled temperature with unrestricted access to diets and water. After a week of acclimatization, mice were randomly divided into control and supplement groups.
To induce colon cancer, animals were given an intraperitoneal injection with 10 mg/kg BW AOM in 0.1mL phosphate buffered saline (PBS). Seven days later, DSS was administrated in drinking water for 1–3 cycles at indicated concentrations to induce colitis. In AOM-DSS studies 1 and 2, mice were given diets supplemented with tocopherols a week before AOM was injected. In the AOM-DSS study 3, mice were given γT-supplemented diet three days after AOM was injected. The duration of each study and dietary supplementation are specified in figures 2A, 3A and 3F. In the all studies, animals were observed daily and weighed weekly. Food intake was recorded once a week.
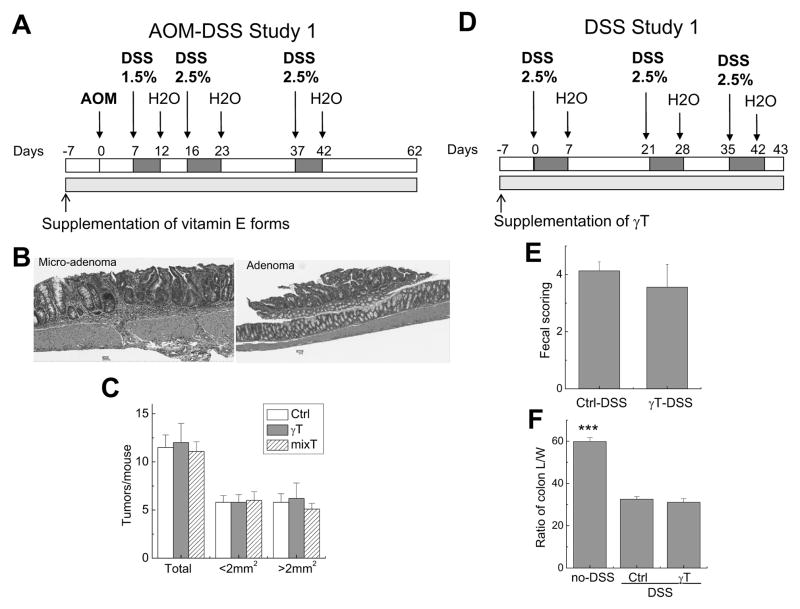
Figure 2. Effects of γT and mixed tocopherols on AOM and three cycles of DSS (1.5–2.5%)-induced colon carcinogenesis (A–C) or colitis induced by three cycles of DSS (2.5%) (D–F).
A: The design of the AOM-DSS study 1; B: Polyps in AOM-DSS study 1 were histopathologically identified to have microadenomas and adenomas; C: γT or mixed tocopherols did not affect tumor multiplicity in the AOM-DSS study 1 (Means ± SEM, n=14–15); D: The design of DSS study 1; E and F: γT supplementation did not show protection of DSS-induced colitis as indicated by fecal scorings (E) and the ratio of colon length and weight (L/W) (F) in DSS study 1 (means ± SEM, n=6 per group). ***P < 0.001 indicates differences between non-DSS controls vs. DSS-treated animals.
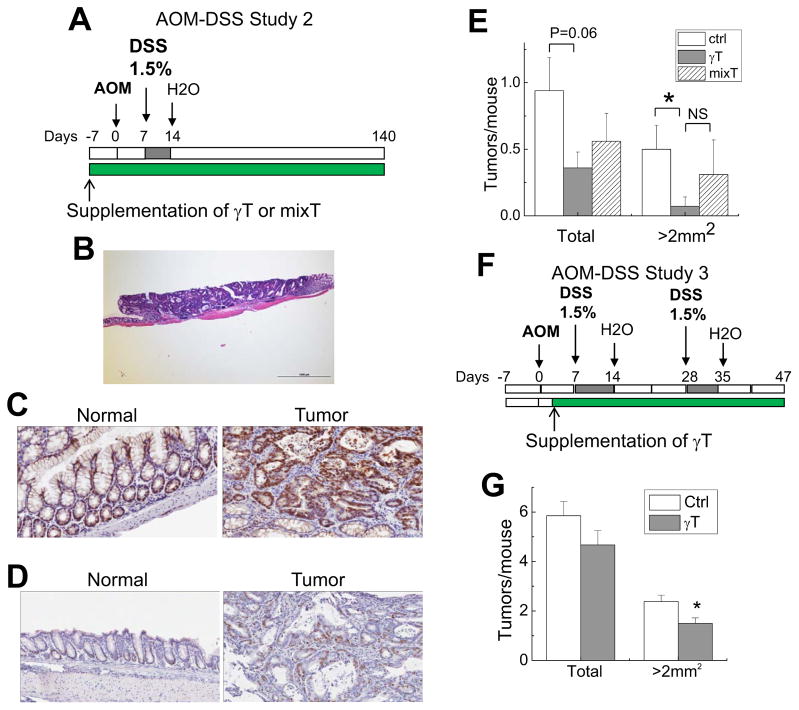
Figure 3. The effect of γT and mixed tocopherols on colon tumorigenesis induced by AOM with one cycle (A–E) or 2 cycles of DSS (1.5%) (F, G).
A: The design of AOM-DSS study 2; B: Macroscopic tumors were histopathologically identified as adenomas in the AOM-DSS 2; C and D: Immunohistological analysis of beta-Catenin (C) and Ki67 (D) in normal and tumor tissues in the AOM-DSS study 2; E: Effects of γT and mixed-tocopherols on tumor multiplicity and polyps with size of >2 mm2 in the AOM-DSS study 2 (means ± SEM, n=14–15). *P < 0.05 indicates difference between control and γT group. F: The design of AOM-DSS study 3; G: γT supplementation on tumor multiplicity in AOM-DSS study 3 (means ± SEM, n=12–13). *P < 0.05 difference between control vs. γT-supplement group.
DSS-induced colon inflammation and evaluation of fecal scoring
Colon inflammation was induced by feeding mice with different cycles and concentrations of DSS in drinking water for indicated days (details indicated in figures 2D and 4A). During the studies, body weight and food intake were monitored every other day. Rectal bleeding and stool consistency were evaluated daily as an indicator of colitis and were scored as following (fecal scoring, 0–6): bleeding: 0 = no blood, 1 = feces with blood less than 50%, 2 = feces with blood more than 50% but less than 100% and 3 = feces with full blood; stool softness, 0 = hard, 1 = a bit soft, 2 = soft and 3 = very soft.
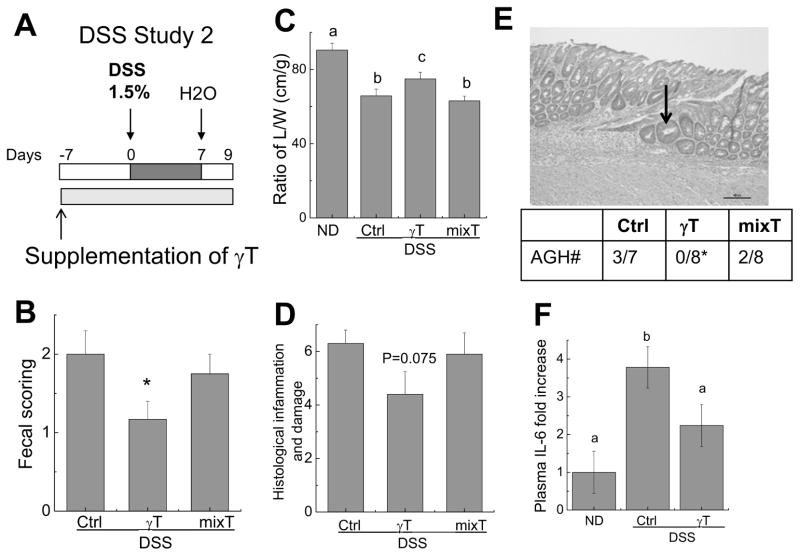
Figure 4. The effect of γT or mixed tocopherols on one cycle of 1.5% DSS-induced colon inflammation.
A: The design of DSS study 2; B–D: Effects of γT or mixed tocopherols on fecal scoring (B), the ratio of colon L/W (C), and histopathological scores for inflammation and damage in the colon (D); E: Histopathological identification of atypical glandular hyperplasia (AGH, indicated by an arrow). The table shows the incidence of AGH in each group. F: The effect on plasma IL-6. All results are means ± SEM (n = 7–12). *P < 0.05 indicates differences between control and γT group (B, E); Means without a common letter differ, P < 0.05 (C) and (F).
Tissue harvest and tumor analysis
During tissue harvest, colons were removed, rinsed with cold PBS, cut open longitudinally and macroscopically examined. The size and number of macroscopic tumors were measured and recorded. Colons were fixed flat in 4% formaldehyde at 4C overnight. Fixed colons were cut longitudinally in half and routinely processed, embedded in paraffin and sectioned at 5 μm. Five sections (5μm) were cut stepwise (at approximately 150 μm intervals) through each half colon block (total 10 slides from each colon) and stained with H&E. Histopathological assessment of tumors was conducted in a blind fashion by board certified veterinary pathologists.
Histological analysis of colon inflammation
The colon sections from DSS study 2 were assessed in a blind fashion by a board certified veterinary pathologist for inflammation and tissue damage using a semiquantitative scoring system (0–12) [31]: Severity of inflammation: 0 = none, 1 = mild, 2 = moderate and 3 = severe; Extent of inflammation: 0 = none, 1 = focal/locally extensive, 2 = multifocal and 3 = diffuse; Ulceration: 0 = none, 1 = focal/locally extensive, 2 = multifocal and 3 = diffuse; Necrosis: 0 = none, 1 = mild, 2 =moderate and 3 = severe. If atypical glandular hyperplasia (AGH) was observed, AGH is indicated.
Immunohistochemistry for detecting β-catenin and Ki67
Immunohistochemical staining was performed on formalin-fixed, paraffin sections of colon tissues using rat monoclonal anti-Ki67 (1:50, Dako; M7249) and rabbit monoclonal anti-beta catenin (1:500, Abcam, ab32572). The corresponding secondary antibodies were obtained from Dako. Staining was visualized with 3, 3′-diaminobenzidine (DAB) as the chromogen. Glass slides were scanned and digitized at 20x magnification with an Aperio ScanScope slide scanner (Aperio, Vista, CA).
Multiplex-based immunoassay for plasma cytokines
Cytokines and chemokines in the plasma samples from DSS study 2 were analyzed by Procarta immunoassay via Affymetrix service. Polystyrene beads were used in the assay. Seven cytokines (7-plex) were analyzed including IFN-γ, IL-1β, IL-6, IL-12p40, Gro-alpha (KC), MIP-2, and TNFα.
Analysis of tocopherols in the plasma and feces
Vitamin E forms were analyzed as previously described [18, 32]. Briefly, plasma tocopherols were extracted using a mixture of methanol/hexane (2:5, v/v) in the presence of 0.8 mM butylated hydroxytoluene [9, 19]. For fecal samples, ~30mg feces were smashed and homogenized in 2mL methanol with ascorbate (0.2 mg/mL). After a brief centrifugation, 1.5 mL methanol layer was obtained and added with 200 μL of PBS, and extracted by 5mL hexane. Hexane layer was dried and reconstituted into ethanol for HPLC analysis. Tocopherols were separated on a 150 × 4.6mm, 5μm Supelcosil™ LC-18-DB column (Supelco, Bellefonte, PA), and eluted with 95:5 (v/v) methanol/0.1M lithium acetate (final 25mM, pH 4.75) at a flow rate of 1.3 ml/min [18, 32]. Tocopherols were monitored by coulometric detection (Model Coulochem II, ESA Inc., Chelmsford, MA) at 300 (upstream) and 500mV (downstream electrode) using a Model 5011 analytical cell.
Analysis of metabolites in the feces by liquid chromatograph tandom mass spectrometry (LC-MS/MS)
The detailed LC-MS/MS method for analyzing vitamin E metabolites are published elsewhere (manuscript in preparation). Briefly, 28–30 mg fecal samples were homogenized in methanol with ascorbate (0.2 mg/mL). After centrifugation, 1.5ml of methanol layer was dried and resuspended into 200 μL methanol, which was then diluted ten times with addition of synthesized δ-tocotrienol-13′-carboxychromanol (1 μM) as an internal standard prior to injection into LC-MS/MS. A mixture of vitamin E metabolites was run as external standards. The LC-MS/MS analysis was done with an Agilent 1200 liquid chromatography system coupled to an Agilent 6460 QQQ mass spectrometer equipped with a jet stream electrospray ionization (ESI) source (Santa Clara, CA). The chromatography utilized an Atlantis dC18 column (2.1 × 150 mm, 3 μm) from Water’s Corporation (Milford, MA). Buffer A and B consisted of acetonitrile/ethanol/water (165/135/700, v/v/v) and acetonitrile/ethanol/water (539/441/20, v/v/v), respectively. The LC gradient ran at 0.3mL/min as follows: 0–1 min, 0 % B; at 30min, 99 % B; at 35min, 99 % B; at 37min, 0 % B. Negative polarity ESI was used with gas temperature of 325°C, gas flow at 10 L/minute, nebulizer pressure at 30 psi, sheath gas temperature of 250°C, sheath gas flow at 7 L/minute, capillary voltage at 4000V, nozzle voltage at 1500V, and an electron multiplier voltage of −300V. All data were evaluated with Agilent MassHunter Qualitative Analysis software, version B.01.04.
Statistical analysis
Data were analyzed by one-way ANOVA and post hoc Tukey’s multiple comparisons or student t-test when overall group effects were significant. P < 0.05 was considered significant.
RESULTS
γT or mixed tocopherols showed no protective effects on tumorigenesis induced by AOM with three cycles of DSS (1.5–2.5%)
In the AOM-DSS study 1, colon tumorigenesis was initiated by injection of AOM (10 mg/kg bw) and promoted by three cycles of DSS at 1.5–2.5% in drinking water (Figure 2A). After 62 days, AOM-DSS treatment led to marked induction of tumorigenesis as indicated by high incidence of tumors with average of >11 polyps per mouse (Figure 2C). These tumors were primarily found in the middle to distal colon, an observation similar to previous studies in this model [33, 34]. Histopathological analyses indicated that these tumors were adenomas or microadenomas (Figure 2B).
To evaluate potential chemoprevention activity, mice were fed γT- or mixed tocopherol-supplemented diet (at 0.1% diet) one week prior to AOM injection and the supplementation continued until the end of the study. Supplementation of vitamin E forms had no effect on body weight or food intake (data not shown). Neither γT nor mixed tocopherols showed significant impact on the incidence of macroscopic polyps (Figure 2C). When tumors were categorized as small (< 2 mm2) and large (> 2 mm2) sizes, no obvious effects were observed from either supplement diet.
γT supplementation did not show protection of colon inflammation induced by three cycles of 2.5% DSS
Because mixed tocopherols at 0.3% diet did not show beneficial effects on DSS-induced colon inflammation [28], here in a study paralleling to the AOM-DSS study 1, we asked whether γT can be protective to colon inflammation in mice treated with 3 cycles of DSS at 2.5% (DSS study 1, Figure 2D). As expected, DSS treatment caused colon bleeding and diarrhea (shown as fecal scoring) as well as decreased ratio between the length and weight of the colons (ratio of colon L/W) (Figure 2E and 2F). We found that γT supplementation showed no protection of colon inflammation, due to the lack of impact on the clinical scores of fecal bleeding and stool consistency (Figure 2E) and inability of reversing colitis-caused decrease of colon L/W ratio (Figure 2F). These results are in agreement with those observed in the AOM-DSS Study 1 where γT was not beneficial to tumorigenesis induced by AOM with three cycles of 1.5–2.5% DSS.
γT is better than mixed tocopherols in reducing tumor incidence when tumorigenesis was induced by AOM and one cycle of 1.5% DSS
Considering the high tumor incidence from AOM-DSS study 1, we reasoned that the lack of protective effects from tocopherols may be because colon inflammation and turmorigenesis promoted by 3 cycles of DSS at 1.5–2.5% were too severe to be protected by tocopherol supplements. Therefore, in the AOM-DSS study 2, we examined whether γT is preventative against colon tumorigenesis promoted by one cycle of DSS at 1.5% (Figure 3A). AOM with one-cycle 1.5% DSS led to markedly fewer tumor incidence, i.e., ~1 tumor per mouse (Figure 3E), than that induced by AOM with 3 cycles of 1.5–2.5% DSS where each mouse had >11 tumors (Figure 2C). All the macroscopic tumors were found in the middle to distal colon and were histologically identified as adenomas (Figure 3B), an observation similar to a previous study in the same model [34]. Immunohistochemistry analyses revealed that these adenomas had increased beta-catenin immunoreactivity in the cytosol or nucleus, whereas beta-catenin immunereactivity was exclusively located in the cytoplasmic membrane in normal tissues (Figure 3C). In addition, increased Ki67 (a proliferation marker) staining was also co-localized with adenoma polyps, indicating abnormal growth rate (Figure 3D).
In contrast to the AOM-DSS study 1, γT supplementation decreased tumor multiplicity by 60% (P=0.06) when tumorigenesis was promoted by one-cycle DSS (figure 3E). Interestingly, γT treatment suppressed the number of relatively large tumors with sizes >2mm2 by 85% (P<0.05, Figure 3E). These observations indicate that γT supplementation inhibited tumor multiplicity and growth. In contrast, mixed tocopherols showed non-significant inhibitory effects on total tumor multiplicity, and did not have any effect on large tumors (>2mm2) due to one mouse bearing three large-size tumors in this group (Figure 3E). Nevertheless, there is no statistical difference between γT- and mixed tocopherol group in the effect on tumor multiplicity (Figure 3E).
γT supplementation inhibited tumorigenesis induced by AOM with two cycles of 1.5% DSS even when the supplementation was started after AOM injection
To examine whether γT can delay tumor progression when supplementation starts after initiation of carcinogenesis, we carried out the AOM-DSS study 3 where γT supplementation was started after AOM injection (Figure 3F). In this study, a new batch of AOM was purchased from Sigma and appeared to be more potent than the previous batch used in the AOM-DSS studies 1 and 2, as AOM (10 mg/kg) injection alone caused 15% mortality compared with none in the previous studies. This is likely due to higher purity of new AOM than the previous batch according to the manufacture. AOM combined with two cycles of DSS (1.5%) led to development of average six macroscopic polyps per mice, which was fewer than that in the AOM-DSS study 1 (three cycle of 1.5–2.5% DSS) and more than AOM-DSS study 2 (one cycle of 1.5%DSS). Supplementation of γT after AOM injection significantly decreased the number of tumors with a size larger than 2 mm2 by 36% (P<0.05), although it did not significantly decrease the total tumor multiplicity (Figure 3G).
γT but not mixed tocopherols attenuated colon inflammation induced by one cycle of 1.5% DSS
We next examined whether γT has any effect on moderate colon inflammation caused by one cycle of 1.5% DSS as designed in DSS study 2 (Figure 4A). Treatment with one cycle of 1.5% DSS resulted in colon bleeding and diarrhea (fecal scoring) and decrease of colon L/W, which was milder than that by 3 cycles of 2.5% DSS (DSS study 1) (comp. Fig. 4B&C vs. Fig. 2E&F). Unlike observations with three cycles of 2.5% DSS in DSS study 1, γT supplementation alleviated colon inflammation induced by one-cycle 1.5% DSS as indicated by significantly decreased fecal scoring compared with control-diet group (Figure 4B). γT also significantly attenuated DSS-caused reduction of colon L/W ratio (Figure 4C). On the other hand, mixed tocopherols showed no beneficial effects. Consistently, histological analyses indicated a trend in decreasing inflammation severity scores and tissue necrosis in γT-fed animals (Figure 4D). In addition, atypical glandular hyperplasia (AGH) was observed in control (3 out of 7) and mixed tocopherol-fed mice (2 out of 8), whereas none was found in the γT group (P<0.05, Figure 4E), consistent with superior protection from γT supplement.
To further characterize the mechanism underlying the observed benefits, we analyzed plasma cytokines and chemokines using a multiplex-based immunoassay. Among 7 cytokines analyzed, IL-6 and KC were significantly elevated in DSS-treated mice compared with non-DSS controls. γT supplementation significantly attenuated IL-6 enhancement (Figure 4F), and non-significantly decrease KC (not shown), which is consistent with histological data.
Concentrations of tocopherols and metabolites in the plasma and feces
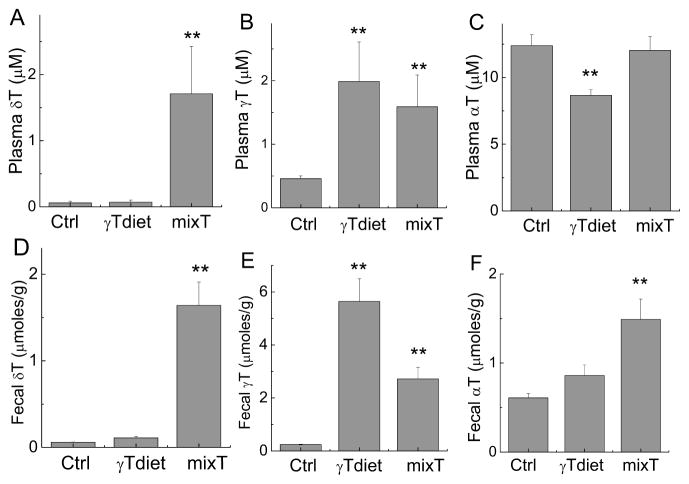
To evaluate the bioavailability of tocopherols and their metabolites, we measured plasma and fecal concentrations of tocopherols and metabolites. Supplementation with γT and mixed tocopherol led to significant increase of γT or both γT and δT in the plasma and feces, respectively (Figure 5). Although γT diet contained >2-fold γT than mixed tocopherol diet, plasma γT levels were similar in both γT and mixed tocopherol supplemented mice (Figure 5B). This suggests that γT may reach a plateau in the plasma due to excretion and metabolism. Consistently, fecal excretion of γT in mice fed γT-supplemented diet was twofold higher than that in those fed mixed tocopherols (Figure 5E). We noticed greater excretion of γT than δT from mixed tocopherols despite equal amounts of these two in this diet (comp. Fig. 5D vs. 5E). In addition, γT supplementation significantly decreased αT in the plasma, probably due to increased αT fecal excretion (Figure 5F). The mice supplemented with mixed tocophoerols, which have 0.01% αT, had no increased plasma αT compared to the control group (Figure 5C). This may be explained by 2-fold enhanced fecal excretion of αT (Figure 5F).
Figure 5. Tocopherols in the plasma and feces from mice in AOM-DSS study 2.
Plasma and fecal samples were extracted and tocopherols were measured by HPLC as described in the Materials and Methods section. **P < 0.01 indicates differences between mice fed with control diet and diets supplemented with γT or mixed tocopherols. Results are means ± SEM (n=5 per group).
Because vitamin E metabolites, especially 13′-COOHs, have been shown to have anti-inflammatory activities [14, 17], we analyzed these metabolites in feces. Supplementation of γT or mixed tocopherols led to marked increases of fecal excretion of all carboxychromanols (Table 1). Among these metabolites, 13′-COOHs were the highest and accounted for >60% of total metabolites (Table 1). Interestingly, mice fed mixed tocopherols had non-significantly higher 13′-COOHs combined from γT and δT than those from γT alone diet. On the other hand, mice on the mixed tocopherol diet had greater variance of fecal excretion of 13′-COOHs (0.37–1.4 μmol/g) than those fed γT alone diet (0.6–0.9 μmol/g).
Table 1.
Major vitamin E metabolites in feces (μmoles/g) from mice fed control, γT- or mixed tocopherol (mixT)-supplemented diet in the AOM-DSS study 2.
| 3′-COOH | 5′-COOH | 7′-COOH | 9′-COOH | 11′-COOH | 13′-COOH | 13′-OH | Total | |
|---|---|---|---|---|---|---|---|---|
| Ctrl diet | ||||||||
| γ+δ series | 0.002±0.001 | n.d | 0.001±0.000 | 0.002±0.002 | 0.005±0.004 | 0.028±0.016 | 0.023±0.014 | 0.061±0.035 |
| γT-diet | ||||||||
| γ-series | 0.058±0.02 | 0.023±0.02 | 0.017±0.01 | 0.066±0.03 | 0.10±0.03 | 0.73±0.17 | 0.22±0.06 | 1.22±0.26 |
| fold↑ | 27 | - | 17 | 33 | 20 | 26 | 10 | 20 |
| mixT-diet | ||||||||
| γ-series | 0.017±0.01 | 0.006±0.01 | 0.007±0.01 | 0.043±0.04 | 0.067±0.04 | 0.55±0.31 | 0.16±0.1 | 0.84±0.46 |
| δ-series | n.d. | 0.008±0.01 | 0.005±0.004 | 0.015±0.01 | 0.048±0.03 | 0.55±0.28 | 0.25±0.15 | 0.88±0.46 |
| fold↑ | 34 | - | 12 | 29 | 23 | 39 | 17 | 28 |
Fecal samples were extracted as described in Methods and Materials. Metabolites of vitamin E were analyzed using LC-MS/MS (Means ± STD, n=5/group). Fold↑ refers to the ratio between metabolites derived γT (or γT and δT combined) from γT-diet (or mixT diet) to those in the control diet, respectively. For fecal samples from γT-supplemented group, metabolites from γT are shown as those from δT were mostly undetectable.
DISCUSSION
We have demonstrated that γT significantly attenuates colon inflammation induced by one cycle of 1.5% DSS and suppresses inflammation-promoted colon tumorigenesis, but does not provide protection against colitis induced by three cycles of 2.5% DSS or its promoted colon tumor development. γT accomplishes these beneficial effects at 0.1% which is equivalent to a daily supplement of 900 mg γT by a human adult with body weight of 70 kg (calculation was based on [35]). On the other hand, mixed tocopherols at this dose were less effective toward attenuation of colitis or tumorigenesis. To our knowledge, this is the first report showing that γT at a supplemental dose is protective to moderate but not severe colon inflammation and inflammation- promoted carcinogenesis. Our study therefore indicates that the anticancer efficacy of γT closely correlates with its anti-inflammation effectiveness and is partially determined by the severity of inflammation.
It has been recognized that inflammation contributes to cancer promotion and progression [3, 5]. This notion is strongly supported by the current study where more severe inflammation correlates with higher tumor multiplicity and incidence. Specifically, three cycles of 2.5% DSS, which resulted in more severe colitis as indicated by higher fecal scoring and a greater decrease in colon L/W ratio (comp. Fig. 2E&F vs. Fig. 4B&C), led to higher tumor multiplicity than those induced by 1 cycle or 2 cycles of 1.5% DSS (comp. Fig. 2C vs. Fig. 3E & G). The observation that γT failed to be protective toward severe inflammation (induced by 3 cycles of 2.5% DSS) or its promoted tumorigenesis also accentuates a critical role of inflammation in cancer promotion. It is interesting to note that γT supplementation particularly decreased the number of large-size tumors, supporting the idea that this vitamin E likely inhibits tumor promotion/progression [3, 36]. Consistently, γT shows effective anticancer activities even when its supplementation starts after AOM injection. In line with the current observations, suppression of inflammation via deleting IκB kinase β (IKKβ) in myeloid cells resulted in a significant decrease in tumor growth [34].
As previously reported [34], we found that all the macroscopic tumors induced by AOM-DSS were adenomas. Adenomas are recognized as important pre-malignant lesions and clinically used for screening the risk of colorectal cancer in humans [37]. Removal of adenomatous polyps during colonoscopic examination is considered an effective practice to prevent colorectal cancer [38]. We observed that adenomas induced by AOM-DSS have increased β-catenin immunoreactivity in the cytosol and nucleus, in contrast to its localization to the cytoplasmic membrane in normal tissues. Translocation of β-catenin from the membrane to cytoplasm/nucleus has been reported in human sporadic colorectal adenomas and carcinomas and is an indication of activation of β-catenin-T cell transcription factor 4 (TCF-4) signaling, which is critical for colorectal tumorigenesis [37, 39]. Consistently, increased Ki67 staining is also observed in adenomatous polyps. These results therefore confirm that AOM-DSS induced colon tumorigenesis mimics the human disease and suggest that the anticancer activity by γT observed in this model may be clinically relevant. The fact that γT decreases the incidence of large-size adenomas is particularly intriguing, as large-size adenomas are likely to develop into malignancy and associated with high recurrence of colorectal neoplasms [40, 41].
Although recent publications have reported chemoprevention activities of a γT-rich tocopherol mixture or γT in the AOM-DSS [28] or AOM alone [30] models, respectively, our study is unique as we focused on how inflammation severity has an impact on anticancer and anti-colitis efficiency by γT and mixed tocopherols. In addition, we have compared anticancer effectiveness between γT and mixed tocopherols by evaluating tumor endpoint, whereas a previous study reported similar suppression of AOM-induced altered crypt foci (ACF) by γT or a γT-rich tocopherol mixture (at 0.2%) in F344 rats [30]. Furthermore, in the current study, mixed tocopherols at 0.1% diet were ineffective against tumor development, but they at 0.17–0.3% suppressed tumor incidence in another study with the same model [28]. These results seem to suggest that compared with γT, higher doses of mixed tocopherols may be necessary for achieving the same efficacy as γT especially under inflammation-involved conditions, which requires further evaluation.
We found that γT but not mixed tocopherols was effective in attenuating moderate colon inflammation induced by one cycle of 1.5% DSS. In a previous study, mixed tocopherols at even higher doses (0.17–0.3%) failed to show protection against1.5% DSS-induced colon inflammation in CF-1 mice [28]. Interestingly, Li et al [42] recently reported that mixed tocopherols attenuated 1% DSS-induced colitis and the protective effect was independent of antioxidant stress response factor Nrf2-mediated mechanism. In light of our current study, the inconsistent effect of mixed tocopherols on colon inflammation may be rooted in differences in inflammation severity. As to the underlying mechanisms, Li et al [42] showed that mixed tocopherols decreased DSS-enhanced serum prostaglandin E2 and oxidative stress markers. Here we observed suppression of IL-6 by γT supplementation, which is consistent with the histological analysis showing attenuated inflammation by γT. Since temporal changes in inflammatory mediators were observed in DSS-induced colitis [43], chronological studies are needed to further characterize anti-inflammatory mechanisms.
We observed high levels of vitamin E metabolites in the feces of mice from AOM-DSS study 2 and that 13′-COOHs accounted for >60% of total metabolites in supplemented animals. These data are supported by two recent publications where 13′-COOHs, measured by a sensitive GC-MS method, are the most abundant among metabolites in feces from mice supplemented with γT or δT [44, 45]. Although 13′-COOHs exist at high levels in fecal samples and are shown to have anti-inflammatory effects [14, 17], whether these metabolites directly contribute to the anticancer effects from γT supplementation remains to be determined. We did not observe significant difference in total fecal excretion of 13′-COOHs between supplementation of γT and mixed tocopherols. However, we noticed greater variances of metabolite excretion from mixed tocopherol-fed mice than that from γT-fed mice. It is tempting to speculate that the greater variance may result in more variation in anti-tumor effect by mixed tocopherols. This possibility warrants further study. In addition, future studies are needed to investigate whether metabolites are differentially generated from γT and mixed tocopherols in DSS-induced colitis, which may help explain their difference in the anti-colitis effect.
Our current study was inspired by the anti-inflammatory properties of γT, δT and their long-chain metabolites. The fact that γT supplementation exerted protective effects on moderate but not severe colon inflammation and its promoted carcinogenesis is consistent with the moderate anti-inflammatory activities from vitamin E forms observed in mechanistic studies [13, 14, 17]. In the future, it may be useful to identify biomarkers that indicate inflammation severity to help predict potential outcomes from dietary intervention.
γ-tocopherol or mixed tocopherols (at 0.1% diet) did not show beneficial effect on severe colitis or severe colitis-promoted colon tumorigenesis in male BALB/c mice.
γT, but not mixed tocopherols, attenuated moderate DSS-induced colitis and its promoted tumorogenesis in the colon.
Mice supplemented with tocopherols had high fecal excretion of 13′-carboxychromanol, a long-chain vitamin E metabolite shown to have potent anti-inflammatory activities.
Our study demonstrates that γT is able to alleviate moderate but not severe colitis and its promoted tumorigenesis.
Acknowledgments
FUNDINGS
This work was supported in part by R21CA152588, R21CA133651 and R01AT006882 from NCI and NCCAM of National Institutes of Health.
The authors would like to thank Tracy Wiegand at Purdue University Histology and Phenotyping Laboratory for her assistance in sample preparation for histopathological analyses.
ABBREVIATIONS
- AOM
azoxymethane
- DSS
dextran sodium sulfate
- α-T, β-T, γ-T or δ-T
α, β, γ, or δ-tocopherol
- 13′-COOH
13′-carboxychromanol
- AGH
atypical glandular hyperplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–543. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Dubois RN. Eicosanoids and cancer. Nature reviews. Cancer. 10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature immunology. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nature reviews. Clinical oncology. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 7.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. The Journal of nutrition. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 8.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. Faseb J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 9.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. Journal of lipid research. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. The Journal of biological chemistry. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 11.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha- tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. The American journal of clinical nutrition. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 16.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Molecular aspects of medicine. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunology. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free radical biology & medicine. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 20.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free radical biology & medicine. 2008;45:425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. {gamma}-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell SE, Stone WL, Lee S, Whaley S, Yang H, Qui M, Goforth P, Sherman D, McHaffie D, Krishnan K. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC cancer. 2006;6:13. doi: 10.1186/1471-2407-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. Faseb J. 2002;16:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 24.Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free radical biology & medicine. 2010;49:1315–1322. doi: 10.1016/j.freeradbiomed.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q, Rao X, Kim CY, Freiser H, Zhang Q, Jiang Z, Li G. Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide. International journal of cancer. Journal international du cancer. 2012;130:685–693. doi: 10.1002/ijc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi S, Takeshita K, Seeni A, Sugiura S, Tang M, Sato SY, Kuriyama H, Nakadate M, Abe K, Maeno Y, Nagao M, Shirai T. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate. 2009;69:644–651. doi: 10.1002/pros.20915. [DOI] [PubMed] [Google Scholar]
- 27.Yu W, Jia L, Wang P, Lawson KA, Simmons-Menchaca M, Park SK, Sun L, Sanders BG, Kline K. In vitro and in vivo evaluation of anticancer actions of natural and synthetic vitamin E forms. Mol Nutr Food Res. 2008;52:447–456. doi: 10.1002/mnfr.200700254. [DOI] [PubMed] [Google Scholar]
- 28.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutrition and cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 30.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5:644–654. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’HUigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. The Journal of experimental medicine. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Moreland M, Ames BN, Yin X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. The Journal of nutritional biochemistry. 2009;20:894–900. doi: 10.1016/j.jnutbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 34.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services, F., Center for Drug Evaluation and Research. Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. [Google Scholar]
- 36.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nature protocols. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 37.Fearon ER. Molecular genetics of colorectal cancer. Annual review of pathology. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 38.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. The New England journal of medicine. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 39.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 40.Yamaji Y, Mitsushima T, Ikuma H, Watabe H, Okamoto M, Kawabe T, Wada R, Doi H, Omata M. Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese. Gut. 2004;53:568–572. doi: 10.1136/gut.2003.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. Journal of carcinogenesis. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free radical biology & medicine. 2012;52:1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PloS one. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bardowell SA, Ding X, Parker RS. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. Journal of lipid research. 2012;53:2667–2676. doi: 10.1194/jlr.M030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. The Journal of biological chemistry. 2012;287:26077–26086. doi: 10.1074/jbc.M112.373597. [DOI] [PMC free article] [PubMed] [Google Scholar]