Abstract
Natural leaching processes and/or anthropogenic contamination can result in ground water concentrations of the essential metal manganese (Mn) that far exceed the current regulatory standards. Neurological consequences of Mn drinking water (DW) overexposure to experimental animals, i.e. mice, including its brain deposition/distribution and behavioral effects are understudied. Adult male C57BL/6 mice were exposed to Mn via the DW for 8 weeks. After 5 weeks of Mn exposure, magnetic resonance imaging revealed significant Mn deposition in all examined brain regions; the degree of Mn deposition did not increase further a week later. Behaviorally, early hyperactivity and more time spent in the center of the arenas in an open field test, decreased forelimb grip strength and less time swimming in a forced swim test were observed after 6 weeks of Mn DW exposure. Eight-week Mn DW exposure did not alter striatal dopamine, its metabolites, or the expression of key dopamine homeostatic proteins, but it significantly increased striatal 5-hydroxyindoleacetic acid (a serotonin metabolite) level, without affecting the levels of serotonin itself. Increased expression (mRNA) of glial fibrillary acidic protein (GFAP, an astrocyte activation marker), heme oxygenase-1 and inducible nitric oxide synthase (oxidative and nitrosative stress markers, respectively) were observed 8 weeks post Mn DW exposure in the substantia nigra. Besides mRNA increases, GFAP protein expression was increased in the substantia nigra pars reticulata. In summary, the neurobehavioral deficits, characterized by locomotor and emotional perturbations, and nigral glial activation associated with significant brain Mn deposition are among the early signs of Mn neurotoxicity caused by DW overexposure.
Keywords: Manganese, behavioral deficits, serotonin imbalance, astrocyte activation, T1 relaxation time
Introduction
Although manganese (Mn) is an essential transition metal (Anderson et al. 2008; Erikson et al. 2007; Talavera et al. 1999), exposure to excessive Mn levels in either occupational or environmental settings may cause neurological dysfunction of the basal ganglia (Aschner et al. 2009; Kinawy 2009; Takeda 2003). The major neurological disorder caused by excessive Mn exposure, manganism, consists of many symptoms that are similar to Parkinson’s disease (PD), i.e., postural instability, rigidity, and speech disturbances (Cersosimo and Koller 2006; Lucchini et al. 2009; Pal et al. 1999; Rajput et al. 1991).
Occupational exposure to Mn via inhalation remains a major concern (Dorman et al. 2006b; Erikson et al. 2007); however, exposure to Mn via contaminated drinking water (DW) is increasingly associated with adverse neurological outcomes (Ljung and Vahter 2007). For example, exposure to Mn-contaminated well water is associated with increased infant mortality, memory deficits and lower intelligence scores in children (Bouchard et al. 2011; Hafeman et al. 2007; Wasserman et al. 2006; Woolf et al. 2002). Moreover, the Bouchard study emphasized the increased risk of overexposure to Mn through DW rather than diet as the Mn intake from water, but not diet, correlated significantly with Mn deposition in children’s hair samples (Bouchard et al. 2011). Taken together, the epidemiological data suggest that ingestion of Mn through DW can be neurotoxic, especially to younger populations. However, neurological consequences of Mn overexposure via consumption of contaminated DW by adults remain unclear. Some early epidemiological studies (Kondakis et al. 1989), but not others (Vieregge et al. 1995), reported neurological impairment in elderly people after long-term exposure to Mn from DW. Even though the Kondakis et al. (1989) study lacked a detailed exposure scenario description, it certainly underscored the potential for adverse health effects associated with chronic consumption of Mn contaminated DW. In this regard, life-time exposure via multiple routes to moderately excessive Mn is considered a risk factor for the development of PD (Lucchini et al. 2009).
Multiple studies have reported Mn-induced locomotor deficits and emotional disturbances, such as anxiety and/or depression in humans (Bowler et al. 2003; Laohaudomchok et al. 2011) and laboratory animals (Dodd et al. 2005; Lazrishvili et al. 2011; Olanow et al. 1996; Witholt et al. 2000). Limited laboratory studies have focused on the neurobehavioral consequences of Mn DW exposure; locomotor deficits have been demonstrated in few rat studies (Avila et al. 2010; Fordahl et al. 2012) and in a single early study in mice (Chandra et al. 1979). It is also worth noting that there is very little information on the non-motor impairments (i.e., anxiety, depression) induced by Mn via DW exposure in rodents (Lazrishvili et al. 2011) as compared with other exposure routes (Hogas et al. 2011; Liu et al. 2006). Mn-induced neurochemical changes in the basal ganglia, mainly in the striatal dopamine (DA) and serotonin (5-HT) levels have been demonstrated by several studies (Hirata et al. 2001; Struve et al. 2007; Tran et al. 2002); the DA and 5-HT alterations in the striatum caused by Mn have been implicated in the development of motor and emotional impairments, respectively (Moreno et al. 2009). Of note, the rodent studies that examined Mn-induced alterations of DA and/or 5-HT homeostasis in a DW experimental paradigm were with rats (Eriksson et al. 1987; Subhash and Padmashree 1991); mouse data are non-existent.
In addition to the direct, oxidative stress-dependent toxic effect of Mn on neurons, (Milatovic et al. 2009; Zhang et al. 2004), recent evidence has highlighted the ability of Mn to damage neurons indirectly by causing and/or enhancing glial cell activation (Filipov et al. 2005; Filipov and Dodd 2012; Zhao et al. 2009). In vivo, several researchers have reported Mn-induced reactive gliosis and elevated expression of inflammatory cytokines following inhalation (Antonini et al. 2009), intragastric gavage (Moreno et al. 2009) and intravenous injection (Verina et al. 2011) of Mn; none of these studies used the increasingly relevant DW exposure route. Additionally, data regarding the basal ganglia expression of neuronal/glial-derived oxidative and nitrosative stress markers, such as heme oxygenase-1 (HO-1) and inducible nitric oxide synthase (NOS2), which are affected by Mn in vivo (Verina et al. 2011) and in vitro (Dodd and Filipov 2011), in a DW exposure paradigm are lacking.
Increased brain Mn deposition is considered a hallmark feature and a prerequisite for Mn neurotoxicity (Aschner et al. 2005) and is often measured via magnetic resonance imaging (MRI; Fitsanakis et al. 2006). Hyperintense signals in T1 weighted brain MRI images, indicative of increased Mn deposition, have been reported in humans (Josephs et al. 2005), non-human primates (Park et al. 2007) and rodents (Kim et al. 2012; Lee et al. 2005). MRI studies in Mn-exposed humans and non-human primates have used the pallidal index % (PI %; the ratio of the globus pallidus to frontal white matter or neck muscle signal intensity multiplied by a factor of 100) as an indicator of brain Mn deposition (Fitsanakis et al. 2006; Guilarte et al. 2006b) because of the notable T1-weighted hyperintense signals in the globus pallidus and substantia nigra (Dorman et al. 2006b; Shinotoh et al. 1995; Uchino et al. 2007). However, PI’s utility may be less than previously thought as evidence from recent human and primate studies with relatively low Mn exposure levels revealed a wide-spread pattern of Mn distribution similar to that seen in rodent brains (Chaki et al. 2000; Dorman et al. 2006b; Fitsanakis et al. 2008; Guilarte et al. 2006b; Sen et al. 2011).
Compared to humans and non-human primates, there are limited rodent studies which have exploited MRI to determine brain Mn deposition; instead, most studies have relied on post-mortem analysis of brain Mn levels (Avila et al. 2010; Dodd et al. 2005; Kontur and Fechter 1988). Of note, majority of the Mn neurotoxicity rodent studies that have employed MRI are in rats (Cross et al. 2004; Finkelstein et al. 2008; Kim et al. 2012); in a single study, where high levels of Mn were tested for its ability as a contrast agent, mice were used (Lee et al. 2005). More importantly, there are no MRI reports where the Mn deposition and neurotoxic effects of Mn via DW exposure were studied in mice. It is also noteworthy that there is limited information on the brain Mn deposition over multiple time points in any rodent studies, including the C57BL/6 mouse strain, which is a model mouse strain for neurotoxicity studies (Jiao et al. 2012; McLaughlin et al. 2006; Messiha 1990; Sedelis et al. 2000).
Hence, in the current study, the main objectives were to monitor the brain Mn deposition following subchronic DW exposure in C57BL/6 mice and to determine the neurotoxic effects of Mn DW exposure on selected behavioral, neurochemical and molecular parameters. T1 weighted MRI was used to characterize the brain deposition/regional distribution of Mn in the mouse brain following DW exposure. To assess the potential neurobehavioral consequences of Mn DW exposure, we employed a collection of behavioral tests that could effectively assess both the locomotor as well as emotional changes induced by Mn DW exposure. To investigate the neurochemical alterations induced by Mn, striatal concentrations of DA, 5-HT and their metabolites were determined. Additionally, to gain a mechanistic insight, the activation of glial cells and the expression of oxidative/nitrosative stress markers in the basal ganglia (striatum, substantia nigra) of mice exposed to Mn via the DW were examined.
Materials and methods
Reagents
Unless otherwise stated, all chemicals including manganese (II) chloride (as MnCl2.4H2O) were purchased from Sigma (St. Louis, MO).
Animals
Male C57BL/6 mice (4–5 months old) weighing 28.51 ± 3.51 g (mean ± SEM) were procured from Taconic (Hudson, NY) and housed (five/cage) with food available ad libitum on a 12-h light/dark cycle in an AAALAC accredited facility throughout the study. All procedures involving animal handling in this study were carried out according to the latest NIH guidelines (8th edition, NRC, 2010) and were approved in advance by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia.
Animal treatment
Control and Mn-treated groups (n = 9–10/group) were exposed to vehicle (NaCl; 0.4 g Na/l) or MnCl2 (0.4 g Mn/l) in deionized water for 8 weeks. The control and Mn solutions were freshly prepared and changed weekly. Body weight (BW) and water intakes were also recorded weekly. The Mn dose and dosing route used in this study were modeled based on several reports wherein a significant increase in brain Mn, altered DA homeostasis, as well as locomotor deficits were exhibited by animals after Mn exposure for periods and different DW Mn concentrations ranging from 10 to 51 weeks and from 1 g/l to as high as 20 g/l, respectively (Calabresi et al. 2001; Chandra and Shukla 1981; Lai et al. 1999). The concentration used in the current study was selected to be lower and it closely relates to the one used by Avila et al. that resulted in a significant, human exposure-relevant, two-fold increase in brain Mn level and subsequent locomotor deficits in rats following chronic (4 months) exposure to Mn (as MnCl2) via DW (Avila et al. 2010).
Behavioral tests were carried after 6 weeks (n = 8/group); T1 weighted MRI was conducted after 5 and 6 weeks of Mn treatment on subsets of the mice (n = 4/group). As a positive control, mice (n = 2) were given a single subcutaneous (s.c.) injection of 50 mg/kg Mn (Eschenko et al. 2010; Malheiros et al. 2012) and were subjected to MRI prior to and 24 h after Mn injection. Behavioral tests and MRI analysis are described in detail below. Mice were sacrificed and organs (brain, liver, spleen, and thymus) were harvested at the end of the exposure period (8 weeks). One-half of the brain was fixed in 4 % paraformaldehyde and flash frozen for immunohistochemistry analysis, while the other half was frozen on dry ice for neurochemistry, qPCR and western blot analyses as described below.
MRI studies
MRI was conducted using a 7 Tesla (Agilent, Santa Clara, CA) magnet, with a quadrature birdcage volume coil for excitation. Acquisition parameters of the two dimensional (2D) T1 weighted images obtained using spin echo multislice sequence were TR 500 ms, TE 17 ms, averages 8, data matrix 256 × 256, orientation coronal, slices 17, thickness 1.00 mm with no gap. T1 relaxation time measurements were performed with a fast spin-echo based inversion recovery method with the following parameters (TR 5000 ms, effective TE 48 ms, ETL 8, signal averages 1, data matrix 256 × 256, orientation coronal, slices 17, slice thickness 1.00 mm with no gap and 10 inversion recovery times, TI 10, 25, 50,100, 200, 500, 1000, 1500, 2000, 3000 ms; Lee et al. 2005).
Prior to imaging, mice were anesthetized with 1.0 % isoflurane in a 30 %:70 % O2:N2 gas mixture with a flow rate 0.8–1.0 l/min. Anesthesia was maintained by a nose cone supplying 0.75–3.0 % isoflurane delivered in medical air at 1 l/min as necessary. Physiological parameters (respiratory rate and rectal temperature) were monitored using a small animal monitoring system (Small Animal Instruments, Inc., Stony Brook, NY) throughout the imaging period.
Defined regions of interest (ROIs) included olfactory bulb (Olf), cortex (Cor), striatum (Str), globus pallidus (Gp), hippocampus (Hip), hypothalamus (Hyp), substantia nigra (Sn), pituitary gland (Pit), cerebellum (Cbl), pons (Pon) and medulla (Med). To determine the best method for assessing Mn deposition in the mouse brain following DW exposure, signal-to-noise ratio (SNR), T1 values and PI % were the three parameters derived from the image analyses that were compared. Signal intensity from each ROIs and standard deviation (SD) of noise/background were measured using NIH Image J software (Image J 1.42). Using these values, SNR was calculated as described previously (Lee et al. 2005). T1 maps were generated using in-house graphical user interface (GUI) that runs on Matlab and T1 values across different brain regions were quantified using Matlab software (Matlab 7.9). PI % was defined as the signal intensity ratio of the Gp relative to the neck muscle multiplied by a factor of 100 in axial T1-weighted MRI images (Guilarte et al. 2006b). For the positive control group, both SNR and T1 values were collected from all slices that represented a particular ROI (refer to supplementary Fig. S1) within an animal and these data were used for statistical analysis.
Behavior
Behavioral measures were assessed at 6 weeks post Mn DW exposure (30 min open field, pole test, grip strength, and forced swim test; the 4 tests performed in succession). Behavioral tests chosen have been used extensively in rodents as measures of locomotor and emotional function (Perona et al. 2008; Prut and Belzung 2003; Sedelis et al. 2000), alteration of which is common in Mn-exposed humans (Bouchard et al. 2007; Josephs et al. 2005). Some of these tests have also documented behavioral impairments in rodents exposed to Mn via different (non-DW) routes (Cordova et al. 2012; Dodd et al. 2005; Torrente et al. 2002). All animals were naïve to behavioral apparatuses prior to initiation of testing and all tests were performed in a behavioral testing designated room located nearby, but separate from that in which animals were housed with the experimenter blinded to the animal treatments.
Open field
Mouse activity was monitored for a period of 30 min in an open field arena (25 cm × 25 cm ×40 cm; Coulbourn Instruments, Whitehall, PA). Total distance traveled (cm) and number of crossings in a 16 square grid area were recorded as a measure of locomotor activity (Lim et al. 2001; Takeuchi et al. 2011) using Limelight video tracking software (Actimetrics, Wilmette, IL) and analyzed per 5 min intervals. Additionally, level of anxiety was determined by measuring the time spent in defined regions, namely the center versus perimeter of the square arena (Ageta et al. 2008).
Grip strength
This test was performed using a strength gauge with an attached mouse specific square wire grid (6 cm × 6 cm; Bioseb, France), similar to Miller et al. (2010). Mice were carefully placed in front of the wire grid and allowed to grab hold with both forepaws. Once grip was established, mice were gently lifted to induce a gripping reflex. The maximum grip force per trial was recorded in newtons (N) for a series of four trials (Dodd et al. 2005) with 1 min inter-trial interval. The average maximum grip force of the four trials was used for statistical analysis.
Pole test
Mice were placed upright on a gauze-wrapped pole (1 cm in diameter and 55 cm in height). Turning criteria included a full body turn with the head facing down the pole. The maximum time allowed for turning was 60 s and the total time per trial was 120 s (Staropoli et al. 2012). If a mouse did not turn within the first 60 s, it was gently guided and a maximum measurement (60 s) was recorded. A total of four trials were completed with a 3–5 min resting period between each trial. The average time to turn, time to descend, and total time spent on the pole from the four trials was used for statistical analysis (Royl et al. 2009).
Forced swim test
This test was performed as described previously (Perona et al. 2008; Petit-Demouliere et al. 2005), but with minor modifications. Mice were gently placed in a large cylindrical container (18 cm in diameter and 25 cm in height) filled approximately one third from the edge with tap water (27 ± 1° C) and swimming behavior was recorded for a period of 15 min. Upon test completion, mice were given a 5–10 min recovery period underneath a heated lamp before returning them to their home cages. Swimming was defined by vigorous tail, forelimb, or hindlimb movement required to propel the mouse forward, climbing was defined as pawing at the sides of the container, and immobility was defined as minimal tail, forelimb, or hindlimb movement required to keep the mouse afloat but not resulting in a forward movement (Deak et al. 2005). Limelight video tracking software (Actimetrics) was utilized to score the total time spent swimming, climbing, or immobile by an experimenter blinded to treatment group and analyzed per 5 min intervals.
Neurochemistry
Neurochemical analysis to determine striatal concentration of DA, 5-HT and their metabolites (homovanillic acid, HVA; 3,4-dihydroxyphenylacetic acid, DOPAC; 5-hydroxyindoleacetic acid, 5-HIAA) was performed using HPLC with electrochemical detection as we have described it previously (Coban and Filipov 2007). Prior to statistical analysis, all neurochemistry data were normalized on per mg of tissue basis. Tissue protein concentration was determined after sample digestion in NaOH; final protein (µg/ml) concentration was determined using the method of Bradford (Biorad, Hercules, CA, USA).
Immunohistochemistry (IHC)
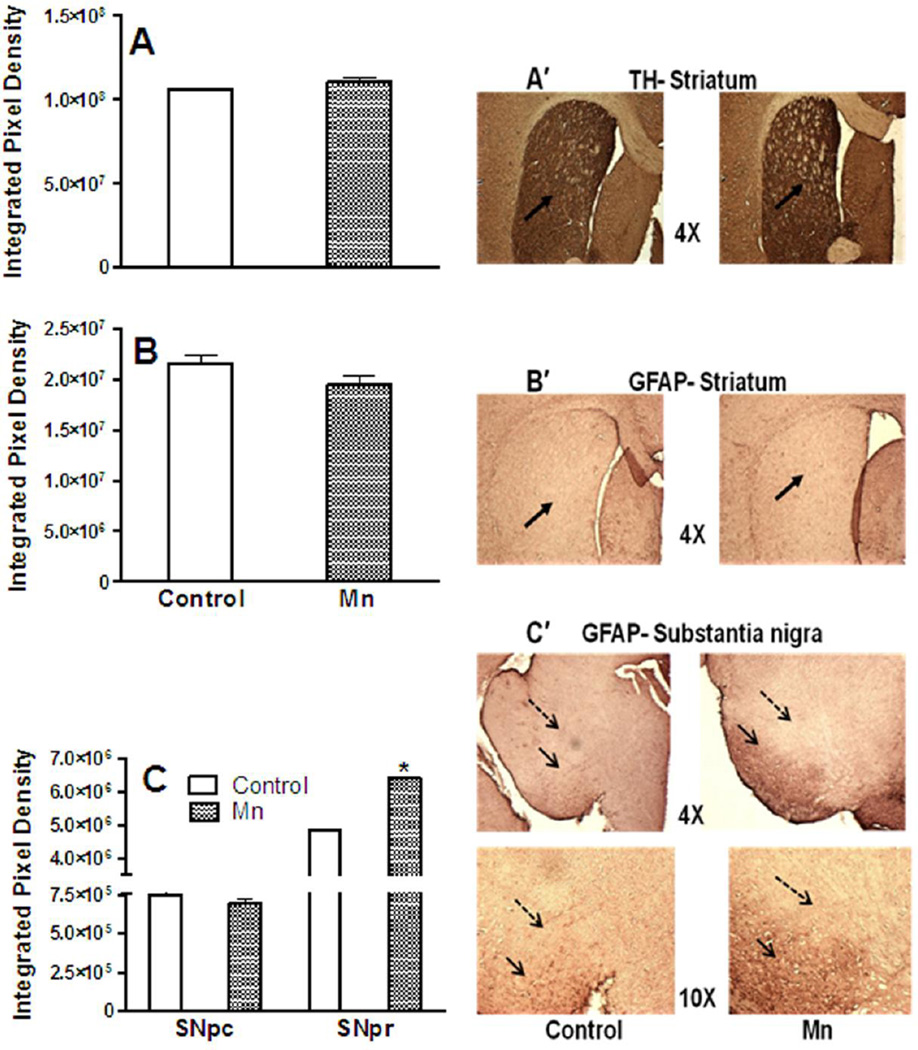
Coronal sections (40-µm thick) were used for immunohistochemical analysis to examine tyrosine hydroxylase (TH; EMD Millipore, Billerica, MA, 1:2,000 dilution) immunoreactivity in the striatum and glial fibrillary acidic protein (GFAP; EMD Millipore, 1:150 dilution) immunoreactivity in the striatum, substantia nigra pars compacta (Snpc) and substantia nigra pars reticulata (Snpr) as published previously (Coban and Filipov 2007), but with few modifications. First, following substrate (3, 3’ diaminobenzidine; DAB for TH staining; Novared for GFAP staining) addition, the respective sections were mounted on poly-L-lysine coated slides, washed in tap water for 5 min and placed on a slide warmer for 3 h. Afterwards, slides were transferred consecutively into 95 % and 100 % dehydrants (Histogene Inc, Lake Forest Park, WA) and cleared in Xylene (Decon Labs Inc, Bryn Mawr, PA). The mean integrated pixel density measured using NIH Image J software (Image J 1.42) from random medial sections (n = 2/brain; all brain sections anatomically matched) was used to analyze the intensity of TH and GFAP staining in striatum. GFAP staining intensity was also assessed from randomly selected medial sections (n = 2/brain; all brain sections anatomically matched) of the substantia nigra with staining in the Snpc and Snpr analyzed separately.
Western blotting
Effects of Mn on the expression of key striatal proteins, including TH, dopamine receptor-2 (D2DR), NOS2, GFAP, glutamate decarboxylase 1 (GAD1) and HO-1 were determined by western blot analysis according to a previously described procedure (Coban and Filipov 2007), with minor modifications. After determining the protein concentration in the tissue lysate using the Bradford method (Bio-Rad), 10 µg of protein per sample was loaded onto10 % bis-acrylamide gels. The PVDF (polyvinylidene difluoride) membranes (EMD Millipore) on to which protein was transferred (semi-dry transfer) were blocked with 5 % milk, incubated overnight at 4 °C with primary antibodies namely anti-TH (EMD Millipore; 1:3,000 dilution), anti-D2DR, anti-HO-1, anti-GAD1, anti-NOS2 (Santa Cruz Biotechnology, Santa Cruz, CA; all 1:500) and anti-GFAP (Biomeda; 1:1,000). Following incubation with appropriate secondary antibody conjugated with horseradish peroxidase (1: 10,000 to 1: 100,000), bands of interest were identified with chemiluminescence substrate (Supersignal West Pico substrate; Thermo Fisher Scientific, Rockford, IL); membranes were stripped using Gentle Review buffer (Amresco, Solon, OH) and were reprobed for β-actin (1:500; Santa Cruz). Quantity One software (Bio-Rad) was used to determine the pixel density of the bands of interest which was normalized to β-actin prior to statistical analysis.
Real-time quantitative PCR (qPCR)
Total RNA from substantia nigra samples was isolated using E.Z.N.A. microelute total RNA kit (Omega Bio-Tek, Inc., Norcross, GA) and quantified using Take 3 plate and Epoch microplate spectrophotometer (Biotek, Winooski, VT). One µg RNA was used to synthesize the first strand cDNA using qScript cDNA SuperMix (Quanta Bioscience, Gaithersburg, MD) and a peltier thermal cycler (Bio-Rad; 5 min 25 °C, 30 min 42 °C, and 5 min 85 °C). Using 10 ng of cDNA per sample, expression of TH, D2DR, HO-1, NOS2, GAD1, and GFAP were determined by qPCR using mouse-specific, certified primers and SYBR Green (Qiagen, Valencia, CA). Amplifications were performed in a Mx3005P qPCR machine (Stratagene) programmed for an initial warming (10 min, 95 °C) followed by 45 cycles (30 s, 95 °C, 1 min, 60 °C) with each sample run in triplicate. Data were analyzed using the ΔΔCt method and are presented as relative induction (fold-changes) of TH, D2DR, HO-1, NOS2, GAD1 and GFAP normalized to β-actin.
Statistical analysis
Two-way analysis of variance (ANOVA) was conducted to analyze MRI data (Mn × time) between the two imaging time points (5 and 6 weeks) and the open field (Mn treatment × interval) data. T-test was used to analyze change in BW, organ weight (g/kg BW) and water intake (ml/kg BW), MRI parameters within a time point, immunohistochemistry, neurochemistry, western blot, qPCR and the other behavior endpoints. If ANOVA’s Mn effect or interaction was found significant, treatment means were separated by Student Newman-Keuls post hoc test. All results (except for the positive control group for the MRI component of the study) are presented as mean ± SEM and are considered significant at p ≤ 0.05. For the positive control group, paired T-test was used to compare MRI data from the different brain ROIs before and 24 h after Mn s.c. injection. The positive control MRI data are presented as mean ± SD and considered significant at p ≤ 0.05. Following statistical analysis, all non-tabular and non-image data were presented in a graphical format with graphs created using the GraphPad Prism software (GraphPad Prism 5.01; GraphPad software Inc., San Diego, CA).
Results
Body weight, Organ weight and Water intake
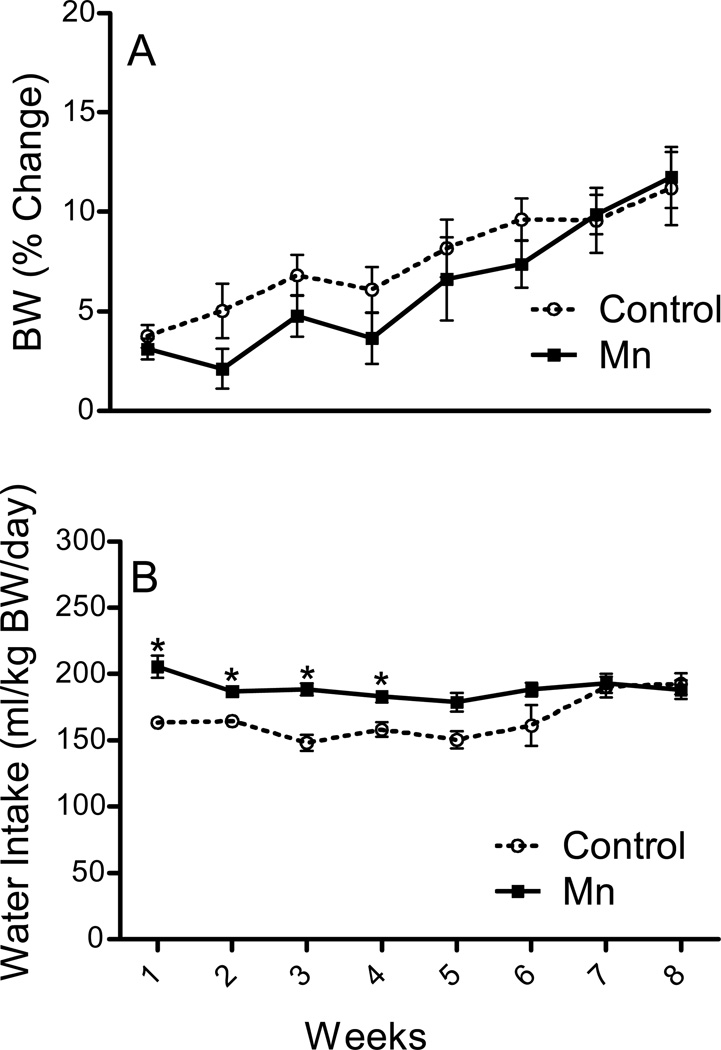
BW was not affected by Mn (p ≥ 0.1) throughout the study; both groups gained about 10 % weight by the end of the 8-week experimental period (Fig. 1a). Similarly, 8 weeks of Mn exposure did not affect brain, liver, spleen and thymic weights (p ≥ 0.25; data not shown). With respect to water consumption, Mn-exposed mice showed a constant intake throughout the entire experimental period (Fig. 1b). Saline-treated control group exhibited lesser (p ≤ 0.05) water intake than the Mn-treated mice for the first four weeks, which afterwards returned to the level of water intake observed in the Mn-treated group (Fig. 1b).
Fig. 1. Effect of Mn DW (0.4 g/l) exposure on percentage (%) change in body weight (BW; a) and water intake (ml/kg BW/day; b) of adult male C57BL/6 mice during the 8 weeks of treatment duration.
BW and water intake are presented as mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
MRI
Compared to T1 weighted images acquired from control mice after 5 weeks of Mn DW exposure, images from Mn treated mice showed bilaterally widespread increased intensities, eg., in the Olf, Str, Gp, Hip, Hyp, Sn and Pit ROIs (supplementary Fig. S1). T1 weighted images of the positive control brains (imaged 24 h post 50 mg/kg Mn s.c.) also displayed signal enhancement in all ROIs evaluated; the signal was more prominent than the one from DW exposed mice (supplementary Fig. S1).
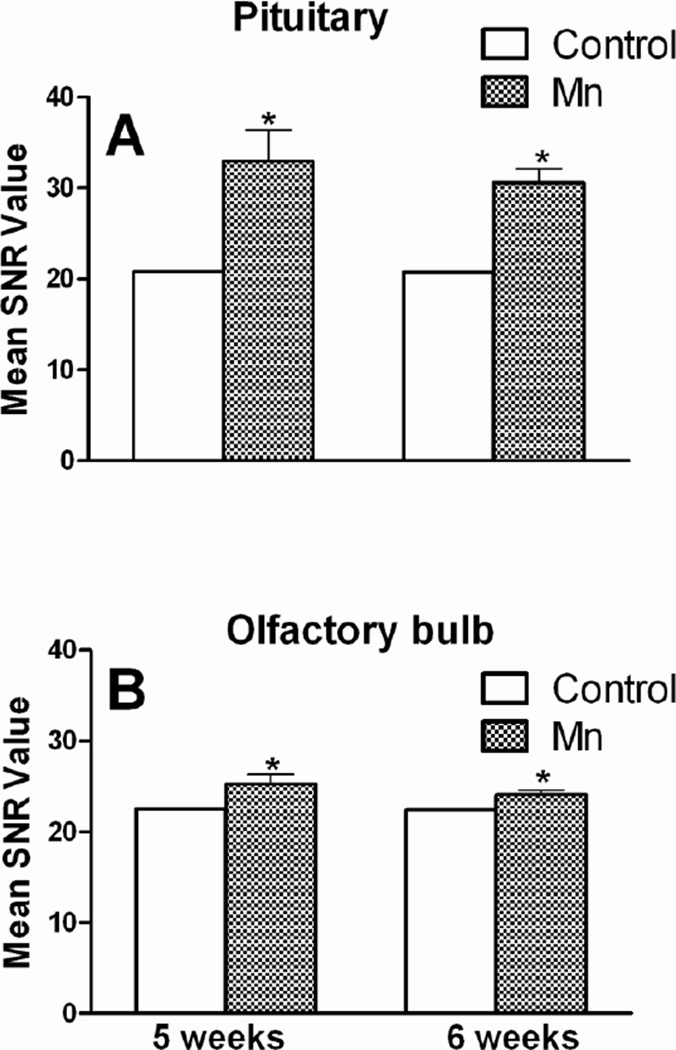
Two-way ANOVA was conducted to compare the effect of Mn across the two imaging time points (5 and 6 weeks) and did not reveal any significant effect of time of imaging (p ≥ 0.30). To illustrate this, representative SNR data from Olf and Pit ROIs showing no significant difference between 5 and 6 weeks data, but significantly increased SNR at both time points by DW Mn are presented in Fig. 2. For all subsequent analyses, 5 and 6 weeks MRI data were combined. Change in SNR (Table 1) and T1 values (Fig. 3) were calculated for each selected region of the brain. On comparison, Pit (the region deficient in blood brain barrier [BBB]) showed the highest increase (53 %), Olf and Med showed only modest increase (8 % and 9 %, respectively) in SNR following Mn DW exposure (Table 1). The increase in SNR in the cortex was 19%. Acute s.c. administration of Mn (50 mg/kg; used as a positive control) produced a significant increase in SNR values in all selected ROIs. Compared to the Mn DW group, short of Cor, Cbl and Pon, the increase in SNR value in the positive control group was substantially greater in all evaluated regions (Table 1).
Fig. 2. Time-independency of Mn deposition in the brain.
Pituitary gland (a) and olfactory bulb (b) signal-to-noise ratio (SNR) from mice exposed to Mn DW (0.4 g/l) for 5 or 6 weeks. SNR data are presented as mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Table 1. Region-independency of Mn deposition in the brain.
Effect on signal-to-noise ratio (SNR) in the brains of mice exposed to Mn via DW (0.4 g/l) for 5 or 6 weeks or to 50 mg/kg BW Mn s.c. (images were collected from the same mice before and 24 h post Mn). Data are represented as a mean change in SNR ± SEM in Mn DW group compared to control (n = 4/group) with percentage (%) change in parenthesis and a mean change in SNR ± SD from Mn s.c. (pre- and 24 hour post) group (n = 2) with % change in parenthesis.
| Regions of Interest (ROIs) | Mn DW Group | Mn s.c. Group |
|---|---|---|
| Mean Change in SNR (%) | Mean Change in SNR (%) | |
| Olfactory bulb (Olf) | 1.77 ± 0.38* (8) | 8.88 ± 3.40* (48) |
| Cortex (Cor) | 3.33 ± 0.88* (19) | 2.14 ± 1.40* (12) |
| Striatum (Str) | 2.50 ± 0.81*(17) | 3.85 ± 2.51* (21) |
| Globus pallidus (Gp) | 1.91 ± 0.27* (12) | 3.97 ± 2.13* (23) |
| Hippocampus (Hip) | 2.75 ± 0.61* (15) | 4.11 ± 1.25* (21) |
| Substantia nigra (Sn) | 2.88 ± 0.70* (15) | 4.73 ± 0.70* (24) |
| Hypothalamus (Hyp) | 4.01 ± 0.89* (21) | 5.00 ± 0.06* (25) |
| Pituitary (Pit) | 11.02 ± 1.68* (53) | 15.39 ± 6.00* (73) |
| Cerebellum (Cbl) | 2.38 ± 0.90* (14) | 2.4 ± 0.85* (13) |
| Pons (Pon) | 2.10 ± 0.74* (12) | 2.36 ± 1.07* (13) |
| Medulla (Med) | 2.06 ± 0.62* (9) | 3.48 ± 1.09* (20) |
Indicates a significant effect of Mn (p ≤ 0.05).
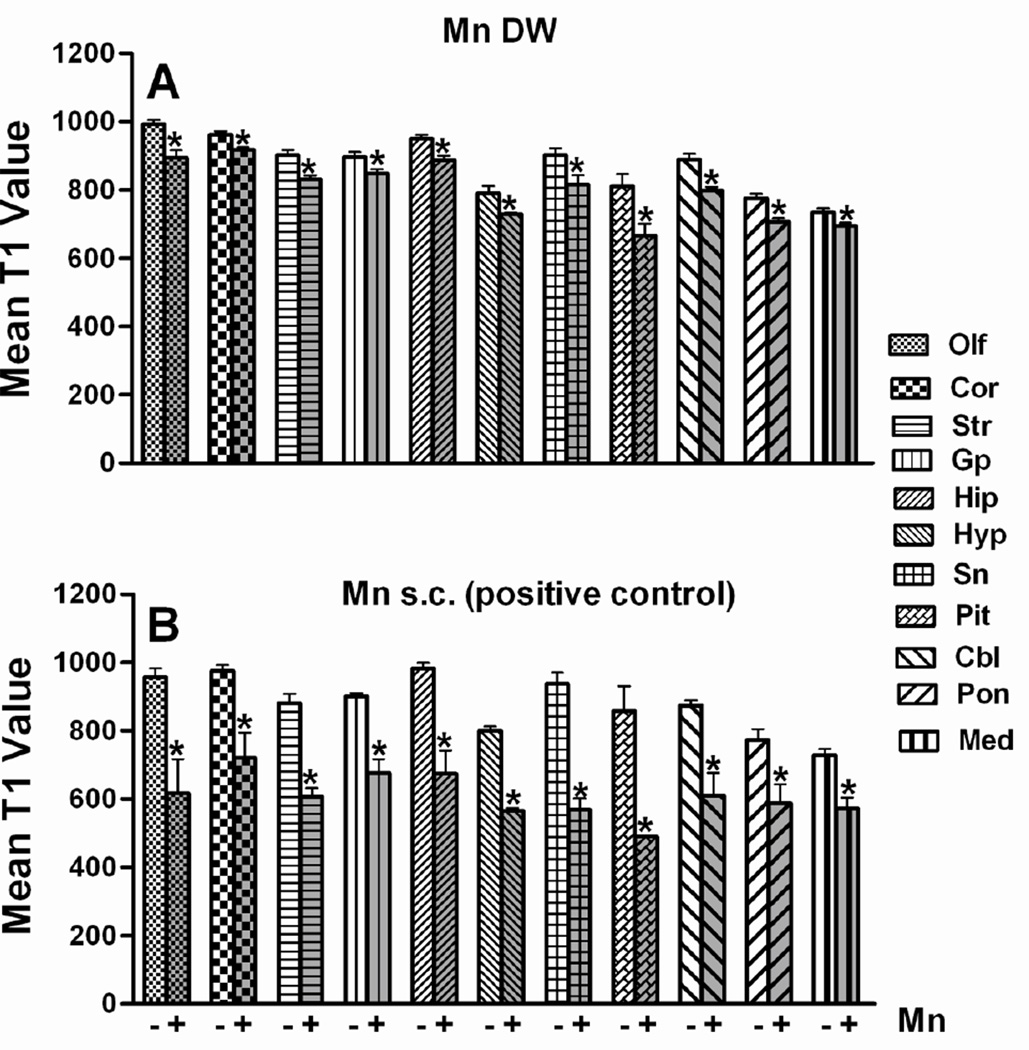
Fig. 3. T1 relaxation time (milliseconds, ms) in different brain regions of mice exposed to Mn via DW (0.4 g/l; panel a) or via a single s.c. injection (panel b; 50 mg/kg; positive control) 24 h prior to imaging.
A standard inversion-recovery, coronal T1-weighted fast spin-echo sequence with 10 inversion times (TR/TE/T1= 5000/48/10, 25, 50, 100, 200, 500, 1000, 1500, 2000 and 3000 ms, slice thickness: 1mm) was used to measure T1 relaxation time. ROIs were placed in the olfactory bulb (Olf), cortex (Cor), striatum (Str), globus pallidus (Gp), hippocampus (Hip), hypothalamus (Hyp), substantia nigra (Sn), pituitary (Pit), cerebellum (Cbl), pons (Pon) and medulla (Med). T1 values from Mn DW group (n = 4) are represented as mean ± SEM and T1 values from Mn s.c. group (n = 2) are presented as mean ± SD.* Indicates a significant effect of Mn (p ≤ 0.05).
Brains of Mn DW-exposed mice exhibited a significant reduction in the T1 relaxation time across all evaluated regions; the largest decrease (17 %) was observed in Pit (Fig. 3). The positive control group also showed a significant decrease in T1 value 24 h after Mn s.c. administration in all selected ROIs. Analogous to the findings from Mn DW group, 24 h after acute administration of a larger amount of Mn s.c., Pit showed the highest reduction (42 %) in T1 value (Fig. 3). T1 maps illustrate the significant effect of Mn on T1 relaxation time in Mn DW and Mn s.c. groups (Fig. 4). Statistical analysis of mean PI %, indicative of Mn accumulation in the Gp, revealed a significant (p ≤ 0.05) increase in PI in both the Mn DW and the positive control groups (9 % and 6 %, respectively; data not shown).
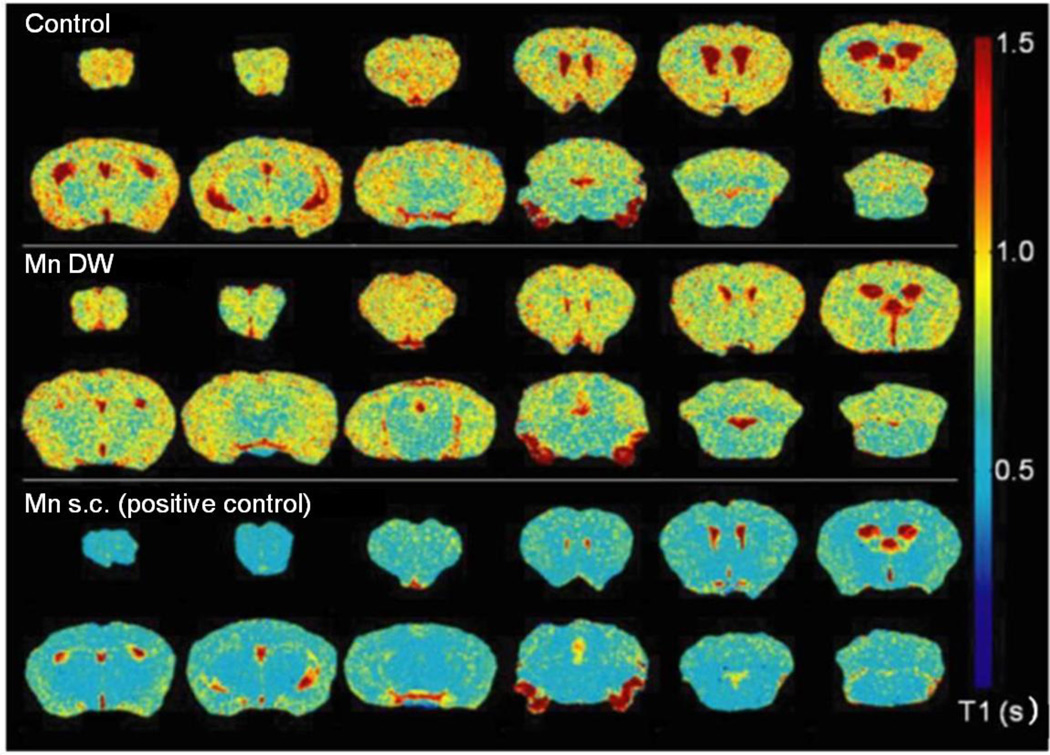
Fig. 4. Representative T1 map generated using Matlab software visually displaying Mn effects on T1 relaxation time.
Color transition from yellow to blue indicates a reduction in T1 (scale to the right). The T1 map from Mn DW group was from a mouse exposed to Mn via DW (0.4 g/l) for 5 weeks; the Mn s.c. (positive control) T1 map was from a mouse exposed to 50 mg/kg Mn s.c. 24 h prior to imaging.
Behavior
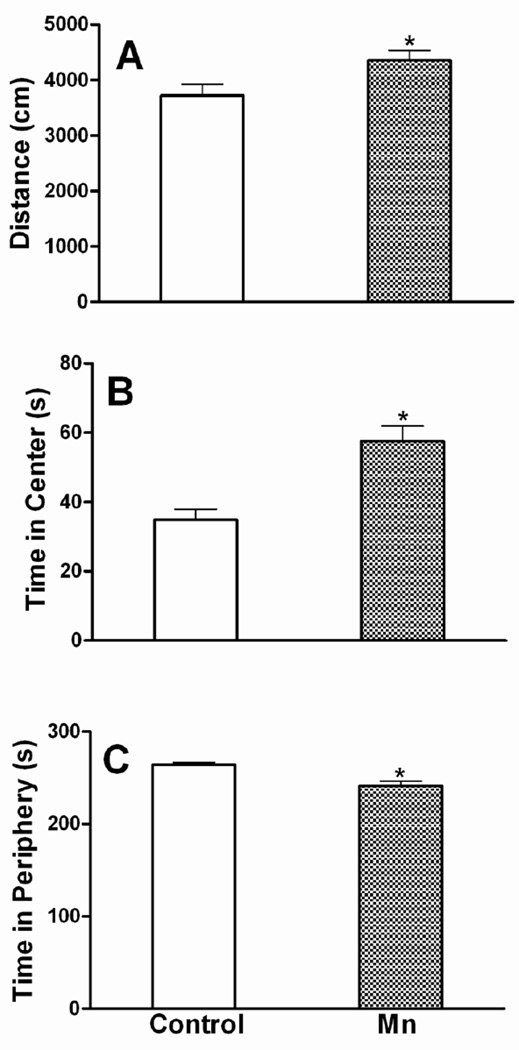
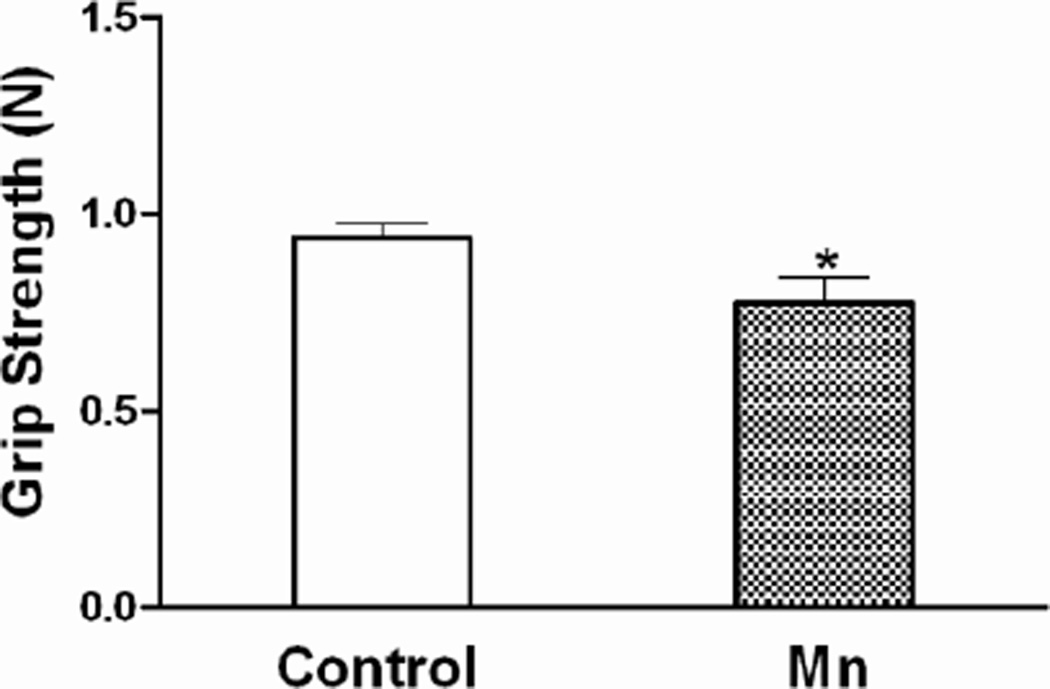
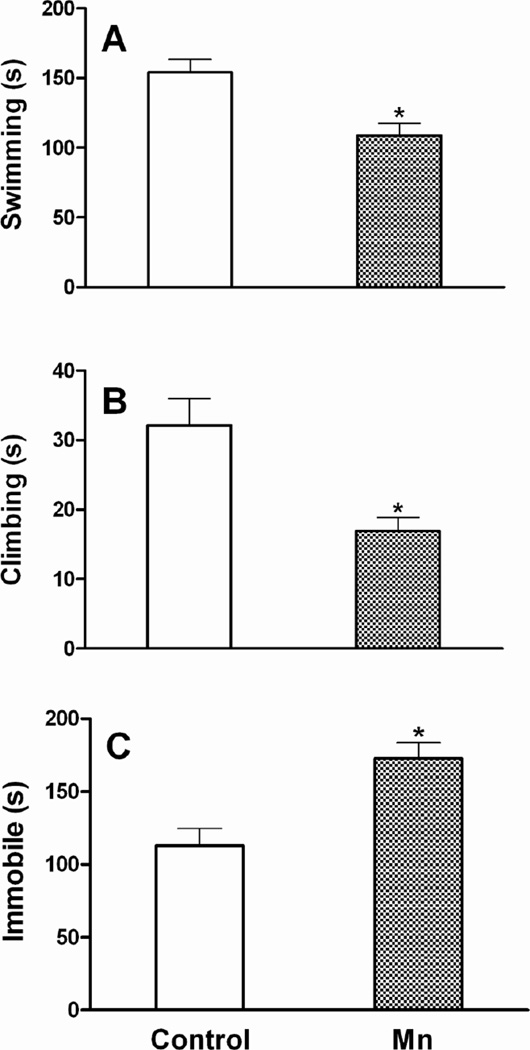
After 6 weeks of Mn DW exposure, mice exhibited a significant increase in locomotor activity during the first five min (interval 1) exploration period of the open field test. Specifically, the total distance traveled (Fig. 5) and the total numbers of grid crossings (data not shown) were increased (p ≤ 0.05). As expected, both control and Mn-treated mice habituated to the arena over time and their overall activity decreased (number of crossings: 185 vs 224 in first 5 min and 140 vs 156 in last 5 min of control and Mn DW groups, respectively); the difference between the two groups was not significant after the first interval (p ≥ 0.10). Two-way ANOVA revealed an overall significant main effect of Mn with respect to the time spent in center versus periphery of the square arena over the entire 30 min of open field testing i.e., Mn-exposed mice spent more time in the center and less time in the periphery (p ≤ 0.001; data not shown). The mean time spent per 5 min interval in the center and periphery, which was also increased (center) and decreased (periphery) significantly (p ≤ 0.001) by Mn DW exposure, is presented in Fig. 5. In addition, Mn decreased the average forelimb grip strength (p ≤ 0.05; Fig. 6). In the forced swim test, Mn-exposed mice exhibited a significant decrease in the mean time spent swimming and climbing per 5 min interval with a concomitant increase in the immobility time (p ≤ 0.05; Fig. 7). Mn-exposed mice also exhibited a tendency toward increased time to turn and total time during the pole test, but these effects did not reach significance (p = 0.116 and p = 0.085, respectively; data not shown).
Fig. 5. Locomotor activity.
Effect of Mn after 6 weeks of DW (0.4 g/l) exposure on distance traveled (first 5 min) during open field testing (a) and on time spent per 5 min interval in the center (b) or the periphery (c) of the open field arena. Graphical representations are mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Fig. 6. Grip strength.
Effect of Mn after 6 weeks of DW (0.4 g/l) exposure on forelimb grip strength (recorded in newtons; N) and presented as mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Fig. 7. Forced swim test.
Effect of Mn after 6 weeks of DW (0.4 g/l) exposure on total time spent swimming (a), climbing (b), or immobile (c) per 5 min interval in a forced swim test. Graphical representations are mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Neurochemistry
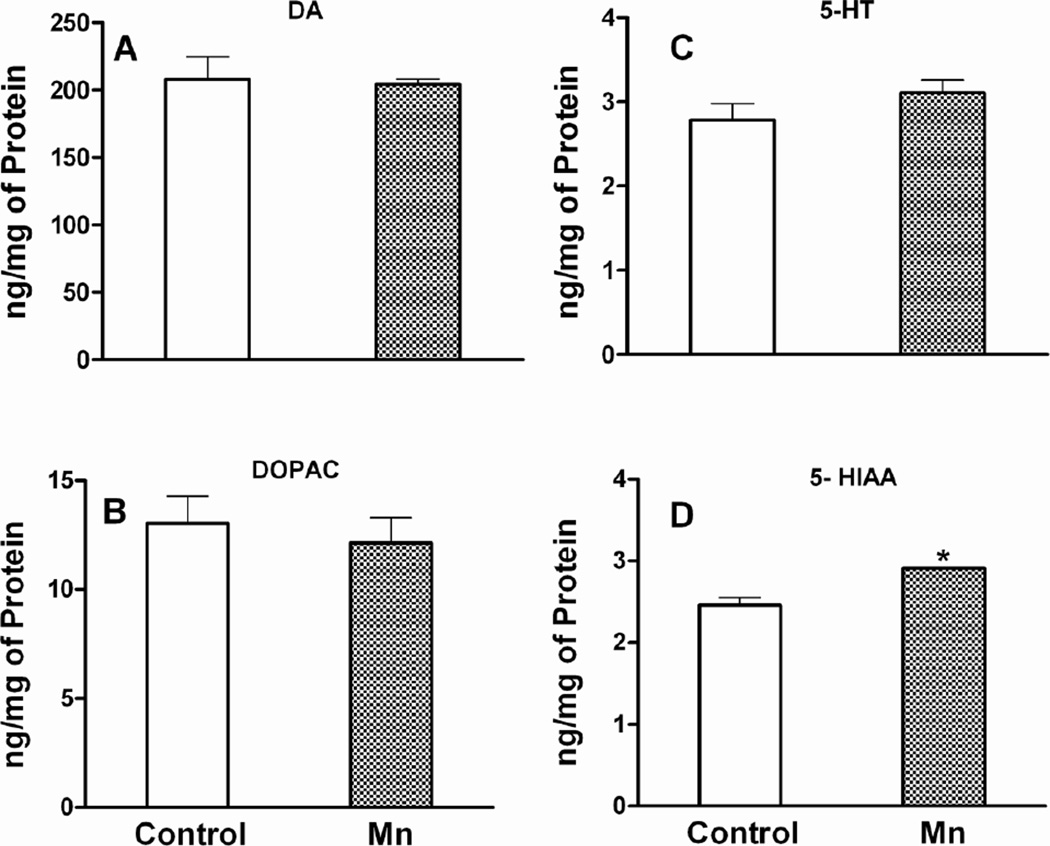
Mn DW exposure for 8 weeks did not affect striatal concentrations of DA and its metabolites DOPAC and HVA (DA and DOPAC data presented in Fig. 8; HVA data not shown). Mn treatment resulted in a small, but significant increase (p ≤ 0.01) in the concentration of the 5-HT metabolite 5-HIAA, without affecting the parent neurotransmitter 5-HT, although a numerical trend towards an increase was observed (Fig. 8).
Fig. 8. Striatal neurochemistry.
Effect of Mn after 8 weeks of DW (0.4 g/l) exposure on striatal dopamine (DA; a), its metabolite 3, 4-dihydroxyphenylacetic acid (DOPAC; b), serotonin (5-HT; c) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA; d). Neurotransmitter/metabolite concentrations are normalized on a per mg protein basis and presented as mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Immunohistochemistry (IHC)
Eight-week Mn DW exposure did not affect striatal expression of TH (Fig. 9). Similarly, GFAP expression in the striatum and Snpc was not different between the two groups (Fig. 9). However, compared to control mice, Mn-exposed mice showed a highly significant increase in GFAP staining in the Snpr (p ≤ 0.01; Fig. 9).
Fig. 9. TH and GFAP Immunoreactivity.
Effect of Mn after 8 weeks of DW (0.4 g/l) exposure on striatal expression of tyrosine hydroxylase (TH; a), striatal expression of glial fibrillary acidic protein (GFAP; b), GFAP expression in the substantia nigra pars compacta (Snpc) and substantia nigra pars reticulata (Snpr; c). Also shown are representative images of striatal TH (a′), striatal GFAP (b′) at 4× magnification and GFAP immunohistochemistry from substantia nigra (c′) at 4× and 10× magnifications. Solid black arrows in images a′ and b′ indicate striatum and images c′ point to Snpr; dashed black arrows in images c′ point to Snpc. Striatal/nigral mean integrated pixel density was used to analyze the intensity of TH and GFAP staining and data are presented as mean ± SEM. * Indicates a significant effect of Mn (p ≤ 0.05).
Western blotting
Similar to the IHC data, Mn DW exposure for 8 weeks did not alter the protein expression of striatal TH (p ≥ 0.4; data not shown). Western blot data for striatal protein levels of D2DR, GAD1, NOS2 and GFAP also failed to reveal any significant effect (p ≥ 0.25) of Mn although levels of all these markers were numerically greater in the Mn-exposed mice (data not shown); protein levels of HO-1 in the striatum were undetectable.
qPCR
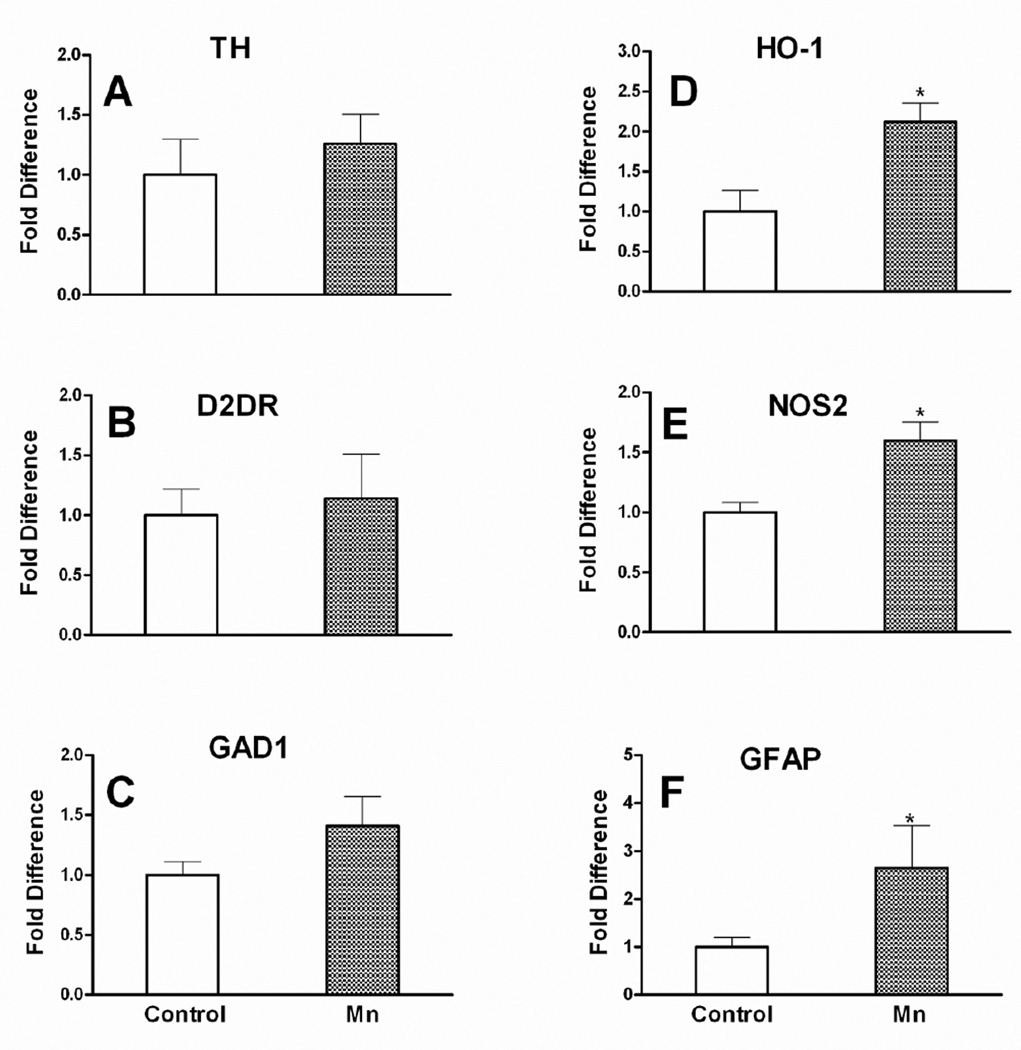
At the end of the 8 week exposure period, HO-1, NOS2 and GFAP mRNA in the substantia nigra of the Mn-exposed mice was significantly upregulated (p ≤ 0.05); the nigral mRNA expression of TH, D2DR and GAD1 was not affected by Mn (Fig.10).
Fig. 10. Nigral mRNA expression.
Effect of Mn after 8 weeks of DW (0.4 g/l) exposure on mRNA expression levels of tyrosine hydroxylase (TH; a), dopamine receptor -2 (D2DR; b), glutamate decarboxylase 1 (GAD1; c), heme oxygenase-1 (HO-1; d), inducible nitric oxide synthase (NOS2; e) and glial fibrillary acidic protein (GFAP; f) in the substantia nigra. mRNA data are β-actin-normalized and are presented as fold change relative to control (mean ± SEM). * Indicates a significant effect of Mn (p ≤ 0.05).
Discussion
The current study was unique in that it utilized a repeated MRI analysis to evaluate brain Mn deposition in adult C57BL/6 mice following DW exposure. The MRI was combined with assessment of neurological effects using behavioral, neurochemical and molecular parameters. The main findings from this work include: 1) subchronic exposure to low-level Mn via DW resulted in significant brain Mn deposition in all ROIs evaluated as evidenced by a decrease in T1 relaxation time and an increase in SNR, apparently reaching a plateau; 2) mice exhibited prominent behavioral deficits following 6 weeks of Mn DW exposure without any significant alteration in striatal DA homeostasis, but in the presence of altered 5-HT homeostasis two weeks later; 3) significant activation of astrocytes and increased expression of oxidative/nitrosative stress markers, HO-1 and NOS2, respectively, was demonstrated in the substantia nigra of Mn-exposed mice with the astrocytic activation being mostly in the pars reticulata of this structure.
To our knowledge, this is the first study which have conjoined and compared all three accepted MRI image analysis parameters, namely, SNR, T1 and PI %, in order to find the best predictor of Mn deposition in the mouse brain following subchronic exposure to Mn via DW. MRI has been used to detect Mn deposition in humans, non-human primates and rats for quite some time (Bock et al. 2008; Dorman et al. 2006b; Fitsanakis et al. 2008; Selikhova et al. 2008; Sen et al. 2011), but reports assessing brain Mn deposition by MRI within the context of Mn neurotoxicity in mice are not available. There are only limited reports where acute systemic Mn administration has been used as a contrast agent for better visualization of brain neuroarchitecture (Lee et al. 2005). Ultimately, our findings suggest that MRI can be used successfully in mice to determine longitudinal brain Mn deposition following DW exposure which will be a useful tool in future analyses of brain Mn deposition dynamics in mice chronically exposed to Mn. Consistent with the findings from other MRI Mn studies, we found that subchronic Mn exposure via DW results in hyperintense T1 weighted signals across different brain regions indicative of a widespread brain Mn deposition (Finkelstein et al. 2008; Fitsanakis et al. 2008; Guilarte et al. 2006b). Even though SNR was significantly increased in all ROIs evaluated after 5 and 6 weeks of Mn treatment, there was no significant difference across the two time points of imaging. Our findings are in accord with rodent and non-human primate studies wherein longitudinal assessment of Mn deposition showed time independence, which could be the result of a plateauing effect (Gallez et al. 1997; Guilarte et al. 2006b). Although Mn levels increased in a region independent fashion, pituitary gland showed the highest degree of Mn deposition in both the Mn DW and the high dose Mn s.c. (positive control) groups. On comparison, both SNR and T1 values changed more drastically in the positive control group than in the Mn DW group, suggesting greater brain Mn deposition after high dose acute s.c. administration of Mn.
The highest degree of Mn deposition in the pituitary gland is likely due to the absence of a BBB and is in line with a rat study wherein the pituitary gland signal intensity, compared to other brain regions, doubled following an acute s.c. administration of MnCl2 (Eschenko et al. 2010). With respect to the T1 relaxation time, increasing order of the degree of T1 reduction following Mn DW exposure was cortex < medulla < globus pallidus < hippocampus < hypothalamus < striatum < pons < substantia nigra < olfactory bulb < cerebellum < pituitary gland. In this regard, another study, which investigated the dose and temporal dependence of brain contrast enhancement after intravenous (i.v.) administration of varying doses of MnCl2, also found a similar trend with the largest and the smallest reduction in T1 value in the pituitary gland and cortex, respectively (Lee et al. 2005). Similarly, significant shortening of the T1 time was observed in the pituitary gland 15 min following a single i.v. or intraperitoneal administration of MnCl2 in C57BL/6 mice (Kuo et al. 2005). After 24 h of high dose Mn s.c. administration in the positive control group, the percentage (%) change (between pre and 24 h post Mn s.c.) in T1 and SNR values in different ROIs was calculated. As compared to SNR, T1 values showed a greater % change in almost all regions evaluated, which also correlated well with the hyperintense signals (indicative of brain Mn deposition) in the T1 weighted images. Taken together, these results suggest that T1 is a better predictor of Mn deposition in the brain than SNR. Consistent with our results, Dorman et al. have also demonstrated the superiority of T1 relaxation time in estimating the regional brain Mn concentration in rats exposed to Mn via inhalation over the other MRI image measurement parameters which led them to suggest that the T1 relaxation time can be used as a potential biomarker of Mn exposure (Dorman et al. 2006b). Evidence from human cases of Mn poisoning and from non-human primates exposed to Mn via inhalation or systemic administration have demonstrated significant Mn accumulation in multiple brain regions, including globus pallidus (Guilarte et al. 2006b; Sen et al. 2011; Struve et al. 2007), the basal ganglia region primarily involved in regulation of voluntary movement. In this respect, it is worth mentioning that the much lower Mn absorption route used in our study, i.e. DW exposure, also succeeded in producing measurable increase in pallidal Mn concentration in mouse brain, evidenced by the significant increase in PI %, SNR and a decrease of T1 relaxation time. Given the significant changes in the extent of brain Mn deposition assessed by SNR and T1 values across different brain ROIs, we conclude that the low level Mn DW exposure adopted in this study produced a widespread Mn deposition in mouse brain, much similar to the pattern of brain Mn distribution demonstrated by MRI studies in rats (Chaki et al. 2000), non-human primates (Guilarte et al. 2006b), and humans (Sen et al. 2011) exposed to Mn by different routes, but at relatively low concentrations.
Contrasting results are reported in the literature regarding changes in locomotor activity following Mn exposure in rodents. For example, studies have demonstrated an increase (Salehi et al. 2003; St-Pierre et al. 2001), decrease (Avila et al. 2010; Schneider et al. 2006; Witholt et al. 2000) and even an absence of a change in locomotor activity after Mn exposure (Dorman et al. 2000). These variations could be due to difference in doses, exposure routes, types of tests and, especially, duration of treatment and timing of testing employed by the different studies. In the present study, Mn-exposed mice were hyperactive during the first five min of exploratory behavior in open field testing. The locomotor activity during the first five min of novel open field testing in rodents is generally considered as an indicator of emotional response to an unfamiliar environment, with an assumption that reduction in activity correlates with increased anxiety (Umezawa et al. 1999). Given the hyperactivity demonstrated in the first five min in our study, it could be inferred that Mn DW exposure produced emotional alterations, specifically an anxiolytic effect. Moreover, the increased time spent in the center versus periphery of the open field arena indicates a decreased anxiety level induced by Mn DW exposure. DW Mn-exposed mice also exhibited a significant decrease in muscle function, particularly muscle strength as demonstrated elsewhere (Bagga and Patel 2012; Bowler et al. 2006). The significantly increased immobility time with a concomitant decrease in the total time spent swimming and climbing in the forced swim test suggest impairment in locomotion or emotional alterations, specifically depression (Deak et al. 2005), induced by Mn DW exposure. The decreased anxiety in the face of increased depressive behavior exhibited by the Mn-exposed mice is likely related to the Mn-caused serotonergic perturbations (Mosienko et al. 2012), as we discuss in more detail later.
It is noteworthy that the neurobehavioral alterations induced by Mn occurred in the absence of a measurable effect on striatal DA homeostasis (measured two weeks post behavioral testing). A very similar result was observed by Witholt et al. who demonstrated significant impairment of neurobehavioral functions without any change in striatal DA following sub-chronic exposure to a cumulative Mn dose comparable to our study (Witholt et al. 2000). Our findings are also consistent with several other studies that reported significant neurobehavioral deficits in the absence of a measurable effect on striatal DA level or its metabolites (Guilarte et al. 2006a; Kim et al. 2012; Pappas et al. 1997). The observed neurobehavioral alterations without overt striatal dopaminergic changes at the whole tissue level and the lack of Mn effect on both mRNA (nigral) and protein (striatal) levels of TH and D2DR (markers of DA terminal integrity) in this study further substantiate the hypothesis that at lower levels of exposure rather than altering the structural integrity of dopaminergic terminals, Mn causes functional deficits of the nigrostriatal dopaminergic system (Guilarte et al. 2006a; Guilarte et al. 2008a). In this regard, dopaminergic dysfunction was only revealed after challenging the system; marked decrease in amphetamine-induced DA release from striatal tissue slices derived from Mn-exposed animals, indicative of the adverse effect of Mn on the protein function associated with vesicular neurotransmitter release at the dopaminergic synapse was reported (Guilarte et al. 2006a). It is also worth mentioning that in our study the concentration as well as turnover of striatal DA was not altered despite significantly increased brain Mn deposition; such lack of correlation between brain Mn and change in striatal catecholamines has been reported by others (Chandra and Shukla 1981; Shukla and Chandra 1981; Struve et al. 2007). However, it can also be that the Mn-induced neurochemical alterations involving dopaminergic pathways might be present in a region-dependent fashion and might have occurred in brain regions other than the striatum. For example, out of the three brain regions (prefrontal cortex, striatum and hippocampus) assessed, significant neurochemical changes characterized by depletion of DA were observed only in the hippocampus following intranasal Mn administration (0.8 mg/kg BW) in rats (Blecharz-Klin et al. 2012). Alternatively, the neurochemical changes in DA systems, if at all present, might be too subtle to be detected, especially when measuring neurotransmitter/metabolite tissue levels and not in vivo release of DA by microdialysis or other sensitive means.
The absence of alterations in DA homeostasis in the face of a significant increase in striatal 5-HT metabolite concentrations, indicative of elevated 5-HT utilization or 5-HT turnover, suggest that the serotonergic signaling appears to be more sensitive to subchronic Mn DW exposure than is the dopaminergic signaling. Generally, DA is considered the predominant neurotransmitter controlling locomotor and emotional functions and alterations in DA homeostasis are usually associated with locomotor and emotional abnormalities (Missale et al. 1998; Sotnikova et al. 2005). However, the role of other monoamines, namely 5-HT, in regulating locomotor and emotional functions has been widely studied as well. Specifically, low levels of extracellular, including synaptic levels of 5-HT, are associated with depression and reduced anxious behavior (Belmaker and Agam 2008; Mosienko et al. 2012; Wurtman and Wurtman 1995). Hence, the initial hyperactivity and emotional alterations demonstrated by Mn-exposed mice in the current study could be attributed to the alterations in serotonergic (increased 5-HT utilization or 5-HT turnover), but not dopaminergic neurotransmission. Previous studies have demonstrated that Mn can target both dopaminergic and serotonergic neurons in basal ganglia and the latter has been found relatively less sensitive to the effects of Mn when relatively high level of Mn was administered via DW for a chronic period (Bonilla and Prasad 1984), by direct unilateral intranigral injection (Parenti et al. 1986), or by intragastric gavage (Moreno et al. 2009). However, our results indicate that it is the 5-HT signaling in the striatum that is either more sensitive or is affected earlier than DA signaling following subchronic low level Mn exposure via the DW. Given the relatively low cumulative dose employed in our study in contrast to the high dose acute exposure/high cumulative dose used by the above-mentioned studies, it could be inferred that the toxic effects of Mn on the basal ganglia neurochemistry are dose-related, with impairments in 5-HT signaling appearing at lower cumulative exposures than impairments in DA signaling.
Multiple studies have demonstrated the effect of Mn overexposure on other neurotransmitter systems, namely γ-amino butyric acid (GABA; Burton et al. 2009; Erikson and Aschner 2003). Hence, we assessed the effect of Mn DW exposure on the GABAergic neuronal marker GAD1 (key enzyme for GABA synthesis) and found no significant effect of Mn at both mRNA and protein levels in substantia nigra and striatum, respectively. These findings lend more support to the notion that the subtle serotonergic imbalance may be the major driving force for the Mn-induced behavioral impairments observed in this study. However, we cannot exclude the possibility that Mn DW exposure might have impacted GABAergic signaling independent of GAD1 expression, i.e., at the level of GABA receptors, or at the level of pre-synaptic GABA release.
Meanwhile, Mn has also been found to target and produce pathologic changes characterized by marked neuronal loss and astrocytosis in another basal ganglia structure, the globus pallidus, especially in its medial segment (Aschner 2000; Burton et al. 2009; Liu et al. 2006). In this regard, it is worth mentioning that the neurotoxic effects of Mn evidenced by the significant astrocytic activation in this study was observed in Snpr, the brain region which is functionally analogous to the medial segment of globus pallidus and Mn intoxication associated with neuropathological changes in Snpr has been reported earlier (Perl and Olanow 2007; Verina et al. 2011). In order to further elucidate the effects of Mn DW exposure on the brain, we looked for molecular changes in glial cells considering their activation to be an early event, which typically precedes neuronal death (Zhao et al. 2009). Evidence has mounted in recent years that the neurotoxic effect of Mn is partly mediated by its indirect effect (activation) on glial cells (Filipov and Dodd 2012; Perl and Olanow 2007; Tomas-Camardiel et al. 2002), which in turn leads to neuroinflammation and associated release of a number of inflammatory mediators (Dodd and Filipov 2011; Liu et al. 2009; Zhang et al. 2007). Previous studies have demonstrated significant increase in Mn-induced GFAP immunoreactivity in the basal ganglia (Liu et al. 2006) and hippocamus (Vezer et al. 2007) accompanied by behavioral and neurochemical changes. However, Mn-induced neurological consequences in the absence of significant astrogliosis and associated basal ganglia GFAP activation has also been demonstrated (Calabresi et al. 2001). Intriguingly, Mn DW exposure in the present study resulted in significant astrocytic activation evidenced by more than 2.5 fold nigral GFAP mRNA upregulation and increased GFAP protein expression, specifically in the Snpr. The selective effect of Mn on the Snpr, as compared to the pars compacta has been demonstrated elsewhere (Perl and Olanow 2007; Verina et al. 2011). In comparison to other basal ganglia structures, Snpr is rich in iron content, attributed to the increased concentration of transferrin receptors in the region (Verina et al. 2011). Mn has been shown to alter iron homeostasis and produce iron-mediated oxidative stress in the brain, characterized by increase in reactive oxygen species (ROS) and associated release of inflammatory mediators (Santos et al. 2012; Vezer et al. 2007). This enhanced ROS release either from Mn-exposed neurons or glial cells has been found to activate glial cells, successfully generating a vicious cycle (Filipov and Dodd 2012). Hence, the significant astrocytic activation evidenced by the increased GFAP protein expression in the pars reticulata could be attributed, at least in part, to the Mn-induced iron-mediated oxidative stress and associated ROS production.
Several studies have shown increased number of reactive astrocytes and associated excess expression of the nitrosative stress marker, NOS2, following Mn exposure (Liu et al. 2006; Moreno et al. 2011). Also, Mn-potentiated expression of the oxidative stress marker, HO-1, by activated glial cells has been demonstrated in vitro (Dodd and Filipov 2011). Consistent with these findings, Mn-induced significant upregulation of nigral NOS2 and HO-1 mRNA, indicative of nitrosative and oxidative stress, respectively, was revealed in the current study. Altogether, these research results suggest the possibility that the Mn-induced neurotoxic effects can be viewed on a continuum of neurological deficits, with Mn producing early glial activation and associated release of potent reactive nitrogen species and ROS in the basal ganglia, further leading to neuronal dysfunction as well as neurochemical imbalance in distinct signaling pathways (i.e. serotonergic) and eventually culminating in behavioral alterations of locomotor and emotional nature.
The Mn dose used in this study lies in the lowest range of fold increase in brain Mn deposition reported in humans after excessive Mn exposure. Daily intake levels of Mn in humans are based on dietary recommendations and not from DW on account of the minimal ingestion of Mn from water. However, the risk of neurotoxicity upon exposure to Mn from contaminated DW may be much higher due to the increased absorption and apparently different metabolism of Mn from water than from food (Bouchard et al. 2011). The current study adds to the existing literature on the potential risks of Mn DW exposure by specifically addresssing the Mn-induced molecular, neurochemical and behavioral alterations in C57BL/6 mice in a DW exposure paradigm. This study provides evidence that low level Mn exposure via DW for a subchronic period causes significant increase in brain Mn deposition (in a region and time-independent manner) and region-specific glial activation in the brain. Our research results also suggest that the astrocyte activation in the Snpr as well as the serotonergic imbalance in the striatum might be associated with the locomotor and emotional perturbations observed in Mn-exposed mice. Collectively, we conclude that the neurobehavioral deficits (emotional and locomotor) and glial activation associated with significant brain Mn deposition are among the early signs of Mn neurotoxicity caused by subchronic DW exposure.
Supplementary Material
Acknowledgements
This project was supported by a grant from the National Institute of Environmental Health Sciences (R01ES016965), awarded to Nikolay M. Filipov.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Ageta H, Murayama A, Migishima R, Kida S, Tsuchida K, Yokoyama M, Inokuchi K. Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS One. 2008;3(4):e1869. doi: 10.1371/journal.pone.0001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29(6):1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Jefferson AM, Billig BK, Felton CM, Hammer MA, Ma F, Frazer DG, O'Callaghan JP, Miller DB. Mild steel welding fume causes manganese accumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology. 2009;30(6):915–925. doi: 10.1016/j.neuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108(Suppl 3):429–432. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35(1):1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11(4):252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB, Soares FA. A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci. 2010;115(1):194–201. doi: 10.1093/toxsci/kfq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga P, Patel AB. Regional cerebral metabolism in mouse under chronic manganese exposure: implications for Manganism. Neurochem Int. 2012;60(2):177–185. doi: 10.1016/j.neuint.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Joniec-Maciejak I, Pyrzanowska J, Widy-Tyszkiewicz E. Effect of intranasal manganese administration on neurotransmission and spatial learning in rats. Toxicol Appl Pharmacol. 2012;265(1):1–9. doi: 10.1016/j.taap.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Bock NA, Paiva FF, Nascimento GC, Newman JD, Silva AC. Cerebrospinal fluid to brain transport of manganese in a non-human primate revealed by MRI. Brain Res. 2008;1198:160–170. doi: 10.1016/j.brainres.2007.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E, Prasad AL. Effects of chronic manganese intake on the levels of biogenic amines in rat brain regions. Neurobehav Toxicol Teratol. 1984;6(5):341–344. [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119(1):138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int J Hyg Environ Health. 2003;206(6):517–529. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27(3):315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Burton NC, Schneider JS, Syversen T, Guilarte TR. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol Sci. 2009;111(1):131–139. doi: 10.1093/toxsci/kfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Ammassari-Teule M, Gubellini P, Sancesario G, Morello M, Centonze D, Marfia GA, Saulle E, Passino E, Picconi B, Bernardi G. A synaptic mechanism underlying the behavioral abnormalities induced by manganese intoxication. Neurobiol Dis. 2001;8(3):419–432. doi: 10.1006/nbdi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27(3):340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chaki H, Furuta S, Matsuda A, Yamauchi K, Yamamoto K, Kokuba Y, Fujibayashi Y. Magnetic resonance image and blood manganese concentration as indices for manganese content in the brain of rats. Biol Trace Elem Res. 2000;74(3):245–257. doi: 10.1385/BTER:74:3:245. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Concentrations of striatal catecholamines in rats given manganese chloride through drinking water. J Neurochem. 1981;36(2):683–687. doi: 10.1111/j.1471-4159.1981.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Saxena DK. Manganese-induced behavioral dysfunction and its neurochemical mechanism in growing mice. J Neurochem. 1979;33(6):1217–1221. doi: 10.1111/j.1471-4159.1979.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Coban A, Filipov NM. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem. 2007;100(5):1177–1187. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- Cordova FM, Aguiar AS, Jr, Peres TV, Lopes MW, Goncalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RD, Erikson KM, Aschner M, Leal RB. In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS One. 2012;7(3):e33057. doi: 10.1371/journal.pone.0033057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DJ, Minoshima S, Anzai Y, Flexman JA, Keogh BP, Kim Y, Maravilla KR. Statistical mapping of functional olfactory connections of the rat brain in vivo. Neuroimage. 2004;23(4):1326–1335. doi: 10.1016/j.neuroimage.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D'Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160(1):125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Filipov NM. Manganese potentiates LPS-induced heme-oxygenase 1 in microglia but not dopaminergic cells: role in controlling microglial hydrogen peroxide and inflammatory cytokine output. Neurotoxicology. 2011;32(6):683–692. doi: 10.1016/j.neuro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal Ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol. 2005;24(6):389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Vitarella D, Byerly FL, Goetz J, Miller R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol. 2000;20(3):179–187. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Wong BA, Dye JA, Robertson ID. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol Sci. 2006b;92(1):219–227. doi: 10.1093/toxsci/kfj209. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43(4–5):475–480. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H, Lenngren S, Heilbronn E. Effect of long-term administration of manganese on biogenic amine levels in discrete striatal regions of rat brain. Arch Toxicol. 1987;59(6):426–431. doi: 10.1007/BF00316209. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Canals S, Simanova I, Beyerlein M, Murayama Y, Logothetis NK. Mapping of functional brain activity in freely behaving rats during voluntary running using manganese-enhanced MRI: implication for longitudinal studies. Neuroimage. 2010;49(3):2544–2555. doi: 10.1016/j.neuroimage.2009.10.079. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Dodd CA. Role of glial cells in manganese neurotoxicity. J Appl Toxicol. 2012;32(5):310–317. doi: 10.1002/jat.1762. [DOI] [PubMed] [Google Scholar]
- Filipov NM, Seegal RF, Lawrence DA. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol Sci. 2005;84(1):139–148. doi: 10.1093/toxsci/kfi055. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Zhang N, Fitsanakis VA, Avison MJ, Gore JC, Aschner M. Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Isr Med Assoc J. 2008;10(11):793–798. [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103(1):116–124. doi: 10.1093/toxsci/kfn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology. 2006;27(5):798–806. doi: 10.1016/j.neuro.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fordahl S, Cooney P, Qiu Y, Xie G, Jia W, Erikson KM. Waterborne manganese exposure alters plasma, brain, and liver metabolites accompanied by changes in stereotypic behaviors. Neurotoxicol Teratol. 2012;34(1):27–36. doi: 10.1016/j.ntt.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallez B, Baudelet C, Adline J, Geurts M, Delzenne N. Accumulation of manganese in the brain of mice after intravenous injection of manganese-based contrast agents. Chem Res Toxicol. 1997;10(4):360–363. doi: 10.1021/tx960194p. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem. 2008a;107(5):1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006a;202(2):381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol Sci. 2006b;94(2):351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ Health Perspect. 2007;115(7):1107–1112. doi: 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Kiuchi K, Nagatsu T. Manganese mimics the action of 1-methyl-4-phenylpyridinium ion a dopaminergic neurotoxin, in rat striatal tissue slices. Neurosci Lett. 2001;311(1):53–56. doi: 10.1016/s0304-3940(01)02144-9. [DOI] [PubMed] [Google Scholar]
- Hogas M, Ciobica A, Hogas S, Bild V, Hritcu L. The effects of the administration of two different doses of manganese on short-term spatial memory and anxiety-like behavior in rats. Arch Biol Sci. 2011;63(4):1031–1036. [Google Scholar]
- Jiao Y, Lu L, Williams RW, Smeyne RJ. Genetic dissection of strain dependent paraquat-induced neurodegeneration in the substantia nigra pars compacta. PLoS One. 2012;7(1):e29447. doi: 10.1371/journal.pone.0029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64(12):2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kim J, Li Y, Buckett PD, Bohlke M, Thompson KJ, Takahashi M, Maher TJ, Wessling-Resnick M. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One. 2012;7(3):e33533. doi: 10.1371/journal.pone.0033533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinawy AA. Impact of gasoline inhalation on some neurobehavioural characteristics of male rats. BMC Physiol. 2009;9:21. doi: 10.1186/1472-6793-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989;44(3):175–178. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- Kontur PJ, Fechter LD. Brain regional manganese levels and monoamine metabolism in manganese-treated neonatal rats. Neurotoxicol Teratol. 1988;10(4):295–303. doi: 10.1016/0892-0362(88)90031-1. [DOI] [PubMed] [Google Scholar]
- Kuo YT, Herlihy AH, So PW, Bhakoo KK, Bell JD. In vivo measurements of T1 relaxation times in mouse brain associated with different modes of systemic administration of manganese chloride. J Magn Reson Imaging. 2005;21(4):334–339. doi: 10.1002/jmri.20285. [DOI] [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20(2–3):433–444. [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Shrairman R, Landau A, Christiani DC, Weisskopf MG. Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology. 2011;32(2):171–179. doi: 10.1016/j.neuro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazrishvili I, Bikashvili T, Shukakidze A, Samchkuashvili K, Shavlakadze O. Effect of short-term manganese chloride intoxicatuion on anxiety and fear of young rats. Georgian Med News. 2011;11(200):102–106. [PubMed] [Google Scholar]
- Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med. 2005;53(3):640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging. 2001;22(6):983–991. doi: 10.1016/s0197-4580(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox Res. 2009;16(1):42–49. doi: 10.1007/s12640-009-9045-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB. Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci. 2006;91(2):521–531. doi: 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007;115(11):1533–1538. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med. 2009;11(4):311–321. doi: 10.1007/s12017-009-8108-8. [DOI] [PubMed] [Google Scholar]
- Malheiros JM, Polli RS, Paiva FF, Longo BM, Mello LE, Silva AC, Tannus A, Covolan L. Manganese-enhanced magnetic resonance imaging detects mossy fiber sprouting in the pilocarpine model of epilepsy. Epilepsia. 2012;53(7):1225–1232. doi: 10.1111/j.1528-1167.2012.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Zhou Y, Ma T, Liu J, Zhang W, Hong JS, Kovacs M, Zhang J. Proteomic analysis of microglial contribution to mouse strain-dependent dopaminergic neurotoxicity. Glia. 2006;53(6):567–582. doi: 10.1002/glia.20294. [DOI] [PubMed] [Google Scholar]
- Messiha FS. Behavioral genetic analysis of regional mouse brain biogenic amines, acidic metabolites and motor activity. Comp Biochem Physiol C. 1990;96(2):389–392. doi: 10.1016/0742-8413(90)90027-7. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240(2):219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Neilan M, Chia R, Gheryani N, Holt N, Charbit A, Wells S, Tucci V, Lalanne Z, Denny P, Fisher EM, Cheeseman M, Askew GN, Dear TN. ENU mutagenesis reveals a novel phenotype of reduced limb strength in mice lacking fibrillin 2. PLoS One. 2010;5(2):e9137. doi: 10.1371/journal.pone.0009137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Hanneman WH, Tjalkens RB. Manganese-induced NF-kappaB activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol Sci. 2011;122(1):121–133. doi: 10.1093/toxsci/kfr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci. 2009;112(2):394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46(2):492–498. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20(2–3):227–238. [PubMed] [Google Scholar]
- Pappas BA, Zhang D, Davidson CM, Crowder T, Park GA, Fortin T. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19(1):17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Parenti M, Flauto C, Parati E, Vescovi A, Groppetti A. Manganese neurotoxicity: effects of L-DOPA and pargyline treatments. Brain Res. 1986;367(1–2):8–13. doi: 10.1016/0006-8993(86)91571-4. [DOI] [PubMed] [Google Scholar]
- Park JD, Chung YH, Kim CY, Ha CS, Yang SO, Khang HS, Yu IK, Cheong HK, Lee JS, Song CW, Kwon IH, Han JH, Sung JH, Heo JD, Choi BS, Im R, Jeong J, Yu IJ. Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal Toxicol. 2007;19(11):965–971. doi: 10.1080/08958370701516108. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66(8):675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19(5–6):566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism--a prospective study. Can J Neurol Sci. 1991;18(3):275–278. doi: 10.1017/s0317167100031814. [DOI] [PubMed] [Google Scholar]
- Royl G, Balkaya M, Lehmann S, Lehnardt S, Stohlmann K, Lindauer U, Endres M, Dirnagl U, Meisel A. Effects of the PDE5-inhibitor vardenafil in a mouse stroke model. Brain Res. 2009;1265:148–157. doi: 10.1016/j.brainres.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Salehi F, Krewski D, Mergler D, Normandin L, Kennedy G, Philippe S, Zayed J. Bioaccumulation and locomotor effects of manganese phosphate/sulfate mixture in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2003;191(3):264–271. doi: 10.1016/s0041-008x(03)00238-2. [DOI] [PubMed] [Google Scholar]
- Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology. 2012;292(2–3):90–98. doi: 10.1016/j.tox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118(1):222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30(3):171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Selikhova M, Fedoryshyn L, Matviyenko Y, Komnatska I, Kyrylchuk M, Krolicki L, Friedman A, Taylor A, Jager HR, Lees A, Sanotsky Y. Parkinsonism and dystonia caused by the illicit use of ephedrone--a longitudinal study. Mov Disord. 2008;23(15):2224–2231. doi: 10.1002/mds.22290. [DOI] [PubMed] [Google Scholar]
- Sen S, Flynn MR, Du G, Troster AI, An H, Huang X. Manganese accumulation in the olfactory bulbs and other brain regions of "asymptomatic" welders. Toxicol Sci. 2011;121(1):160–167. doi: 10.1093/toxsci/kfr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, Perl DP, Olanow W, Calne DB. MRI and PET studies of manganese-intoxicated monkeys. Neurology. 1995;45(6):1199–1204. doi: 10.1212/wnl.45.6.1199. [DOI] [PubMed] [Google Scholar]
- Shukla GS, Chandra SV. Striatal dopamine turnover and L-dopa treatment after short-term exposure of rats to manganese. Arch Toxicol. 1981;47(3):191–196. doi: 10.1007/BF00368679. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Barak LS, Wetsel WC, Caron MG, Gainetdinov RR. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3(8):e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre A, Normandin L, Carrier G, Kennedy G, Butterworth R, Zayed J. Bioaccumulation and locomotor effect of manganese dust in rats. Inhal Toxicol. 2001;13(7):623–632. doi: 10.1080/08958370117066. [DOI] [PubMed] [Google Scholar]
- Staropoli JF, Haliw L, Biswas S, Garrett L, Holter SM, Becker L, Skosyrski S, Da Silva-Buttkus P, Calzada-Wack J, Neff F, Rathkolb B, Rozman J, Schrewe A, Adler T, Puk O, Sun M, Favor J, Racz I, Bekeredjian R, Busch DH, Graw J, Klingenspor M, Klopstock T, Wolf E, Wurst W, Zimmer A, Lopez E, Harati H, Hill E, Krause DS, Guide J, Dragileva E, Gale E, Wheeler VC, Boustany RM, Brown DE, Breton S, Ruether K, Gailus- Durner V, Fuchs H, de Angelis MH, Cotman SL. Large-Scale Phenotyping of an Accurate Genetic Mouse Model of JNCL Identifies Novel Early Pathology Outside the Central Nervous System. PLoS One. 2012;7(6):e38310. doi: 10.1371/journal.pone.0038310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am J Ind Med. 2007;50(10):772–778. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- Subhash MN, Padmashree TS. Effect of manganese on biogenic amine metabolism in regions of the rat brain. Food Chem Toxicol. 1991;29(8):579–582. doi: 10.1016/0278-6915(91)90051-8. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41(1):79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Iba M, Inoue H, Higuchi M, Takao K, Tsukita K, Karatsu Y, Iwamoto Y, Miyakawa T, Suhara T, Trojanowski JQ, Lee VM, Takahashi R. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One. 2011;6(6):e21050. doi: 10.1371/journal.pone.0021050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera EJ, Arcaya JL, Giraldoth D, Suarez J, Bonilla E. Decrease in spontaneous motor activity and in brain lipid peroxidation in manganese and melatonin treated mice. Neurochem Res. 1999;24(5):705–708. doi: 10.1023/a:1021064711866. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Herrera AJ, Venero JL, Cruz Sanchez-Hidalgo M, Cano J, Machado A. Differential regulation of glutamic acid decarboxylase mRNA and tyrosine hydroxylase mRNA expression in the aged manganese-treated rats. Brain Res Mol Brain Res. 2002;103(1–2):116–129. doi: 10.1016/s0169-328x(02)00192-4. [DOI] [PubMed] [Google Scholar]
- Torrente M, Colomina MT, Domingo JL. Effects of prenatal exposure to manganese on postnatal development and behavior in mice: influence of maternal restraint. Neurotoxicol Teratol. 2002;24(2):219–225. doi: 10.1016/s0892-0362(02)00188-5. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Lonnerdal B, Le L, Parker M, Chicz-Demet A, Crinella FM. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology. 2002;23(4–5):645–651. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Uchino A, Noguchi T, Nomiyama K, Takase Y, Nakazono T, Nojiri J, Kudo S. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49(9):715–720. doi: 10.1007/s00234-007-0243-z. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Kogishi K, Tojo H, Yoshimura S, Seriu N, Ohta A, Takeda T, Hosokawa M. High-linoleate and high-alpha-linolenate diets affect learning ability and natural behavior in SAMR1 mice. J Nutr. 1999;129(2):431–437. doi: 10.1093/jn/129.2.431. [DOI] [PubMed] [Google Scholar]
- Verina T, Kiihl SF, Schneider JS, Guilarte TR. Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology. 2011;32(2):215–226. doi: 10.1016/j.neuro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezer T, Kurunczi A, Naray M, Papp A, Nagymajtenyi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am J Ind Med. 2007;50(11):841–852. doi: 10.1002/ajim.20485. [DOI] [PubMed] [Google Scholar]
- Vieregge P, Heinzow B, Korf G, Teichert HM, Schleifenbaum P, Mosinger HU. Long term exposure to manganese in rural well water has no neurological effects. Can J Neurol Sci. 1995;22(4):286–289. doi: 10.1017/s0317167100039482. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt R, Gwiazda RH, Smith DR. The neurobehavioral effects of subchronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol Teratol. 2000;22(6):851–861. doi: 10.1016/s0892-0362(00)00108-2. [DOI] [PubMed] [Google Scholar]
- Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ Health Perspect. 2002;110(6):613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3(Suppl 4):477S–480S. doi: 10.1002/j.1550-8528.1995.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Zhang P, Hatter A, Liu B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol Lett. 2007;173(2):88–100. doi: 10.1016/j.toxlet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Fu J, Zhou Z. In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol In Vitro. 2004;18(1):71–77. doi: 10.1016/j.tiv.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci. 2009;107(1):156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.