Abstract
Enhanced reactive oxygen species production in allergic airways is well described, and correlates with increased airway contractions, inflammatory cell infiltration, goblet cell metaplasia, and mucus hypersecretion. There is also an abundance of interleukin-4/interleukin-13 (IL-4/IL-13) or interleukin-5-secreting cells that are thought to be central to the pathogenesis of allergic asthma. We postulated that dual oxidases (DUOX1 and DUOX2), members of the nicotinamide adenine dinucleotide phosphate oxidase family that release hydrogen peroxide (H2O2) in the respiratory tract, are critical proteins in the pathogenesis of allergic airways. DUOX activity is regulated by cytokines including IL-4 and IL-13, and DUOX-mediated H2O2 influences several important features of allergic asthma: mucin production, IL-8 secretion, and wound healing. The objective of this study was to establish the contribution of DUOX to the development of allergic asthma in a murine model. To accomplish this goal, we utilized a DUOXA-deficient mouse model (Duoxa−/−) that lacked maturation factors for both DUOX1 and DUOX2. Our results are the first to demonstrate evidence of DUOX protein and DUOX functional activity in murine airway epithelium. We also demonstrate that DUOXA maturation factors are required for airway-specific H2O2 production and localization of DUOX to cilia of fully differentiated airway epithelial cells. We compared wild-type and Duoxa−/− mice in an ovalbumin exposure model to determine the role of DUOX in allergic asthma. In comparison to DUOX-intact mice, Duoxa−/− mice had reduced mucous cell metaplasia, and lower levels of TH2 cytokine levels in bronchoalveolar fluid. In addition, increased airway resistance in response to methacholine was observed in Duoxa+/+ mice as expected, but was absent in Duoxa−/− mice. Surprisingly, Duoxa−/− mice had decreased influx of neutrophils in bronchoalveolar fluid and lung tissue sections associated with a lower level of the chemotactic cytokine interleukin-6. These findings suggest that DUOX-derived H2O2 has an important role in signaling neutrophils into allergic airways.
Keywords: dual oxidase, DUOX, asthma, lung, neutrophil, murine
Introduction
Allergic asthma is characterized by airway hyperresponsiveness, eosinophilic inflammation, an increase in smooth muscle mass, abnormal deposition of extracellular matrix, and goblet cell metaplasia [1]. There is compelling evidence in animal models that a predominance of interleukin-4 (IL-4)/interleukin-13 (IL-13) or interleukin-5 (IL-5)-secreting CD4+ T lymphocytes are characteristic of the allergic asthma phenotype [2]. Recently, the respiratory tract epithelium, in conjunction with dendritic cells, has been recognized to be primarily responsible for driving this skewed TH2 milieu [3]. Importantly, there is direct evidence that TH2 cytokines can promote all the primary features of allergic asthma [2], and that the severity of asthma correlates directly with the number of TH2 cells in the airway [4]. The mechanisms responsible for altered cytokine expression in allergic asthma have not been clearly established, but the pattern recognition receptors of the Toll-like family (TLRs), in particular TLR-3, -4, and -5, have been implicated in epithelial-derived allergic sensitization [5-8].
In parallel, reactive oxygen species (ROS) in airway epithelium are thought to exacerbate the response to allergen challenge [9-11]. Enhanced ROS production has long been noted in allergic airways, and correlates with increased airway contractions, inflammatory cell infiltration, goblet cell metaplasia, and mucus hypersecretion [12-14]. There are several potential sources for the ROS which affect asthma symptoms, but there is mounting evidence that two epithelial-specific enzymes, dual oxidase 1 and 2 (DUOX1 and DUOX2) are critical for promoting the allergic airway response. DUOX1 and DUOX2 are members of a seven-member NADPH oxidase family which produce hydrogen peroxide (H2O2) in airway epithelial cells [15-17]. Several recent reports implicate either DUOX1- or DUOX2-mediated H2O2 generation as necessary for TLR signaling in response to house dust mite exposure, bacterial infection, or viral infection [18-21]. DUOX1 has also been implicated to augment mucin expression in the airway, a hallmark feature of allergic asthma [22, 23] and promote epithelial wound healing [24, 25] that may lead to pathologic airway remodeling in response to chronic inflammation and injury. The observation that DUOX1 is regulated by TH2 cytokines IL-4 and IL-13 [26, 27] suggests that DUOX may be part of a positive feedback loop where TLR-induced IL-4/-13 production further enhances DUOX-mediated TLR activation of TH2 cytokines.
To better understand the role of DUOX in allergic asthma, we utilized a Duoxa−/− knockout mouse model that does not express functional DUOX1 or DUOX2 [28]. Because both DUOX1 and DUOX2 have been implicated in models of allergic asthma or inflammatory signaling pathways, it was important to interrogate a model system that lacked both DUOX isoforms. In addition, this model excluded the possibility of one isoform compensating for the loss of the other. Using an ovalbumin exposure model that recapitulates several features of allergic asthma [29], we examined airway hyperresponsiveness, eosinophilic inflammation, and goblet cell metaplasia in Duoxa−/− versus Duox+/+ mice that differentially expressed functional DUOX in the airway. We hypothesized that DUOX was primarily responsible for epithelial activation of allergic responses and that lack of a functional DUOX system will lead to decreased features of allergic disease.
Materials and Methods
Animals
All procedures with mice were performed in accordance with the University of California at Davis Institutional Animal Care and Use Committee. Duoxa−/− knockout mice were generated as described previously [28]. Mice utilized for our experiments were acquired through subsequent breeding of these breeding pairs at the UC Davis facility. All mice were of 129Sv6 background and maintained in HEPA-filtered laminar flow cage racks with a 12-hour light/dark cycle and allowed free access to food (Purina Rodent Chow) and water. Mice were housed and cared for by the veterinary staff of the UC Davis Animal Resource in AALAC-accredited facilities. Genotypes for all mice were verified from tail snips using the REDExtract-N-Amp Tissue PCR Kit (Sigma, St. Louis, MO) per the manufacturer’s protocol. Because Duoxa−/− mice are severely hypothyroid without hormone replacement [28], mice were injected with 40ng L-T4/g body weight from birth to weaning (L-Thyroxine, Sigma). After weaning, Duoxa−/− mice were provided with albuminized drinking water containing 0.26 ng/ul L-T4 to maintain thyroid hormone supplementation. This hormone replacement regimen produced animals with normal growth characteristics and normal adult organ and body weight compared with wild-type animals as previously shown [28]. Mice were euthanized at the end of an experiment with an intraperitioneal (IP) overdose of pentobarbital. Anesthesia and euthanasia procedures were performed according to UC Davis IACUC-approved protocols.
H2O2 measurements
Duoxa+/+ and Duoxa−/− mouse tracheas were isolated and perfused with 0.2% protease (Sigma, P5147-1G) overnight. Tracheas were then flushed with 0.0125% fetal bovine serum (FBS, Gemini, 100-106) in Opti-MEM (Gibco, 11058-021). Isolated epithelial cells were then centrifuged at 1200G and the cell pellet was washed three times in Opti-MEM to ensure removal of protease and FBS. After the third wash, cell pellets were resuspended in 150 uL of Opti-MEM and H2O2 production was measured after 30 min using the Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, Inc. Eugene Oregon) as previously described [26]. Diphenyleneiodonium (DPI)--1uM for ten minutes prior to H2O2 measurements—was used to determine flavin protein-specific H2O2 production. Relative fluorescence units (RFU), were converted to uM H2O2 using a standard curve generated in parallel. Horseradish peroxidase and 10-acetyl-3.7- dihydroxyphenoxazine were added in excess to ensure H2O2 was the limiting substrate. Eight tracheas from Duoxa+/+ or Duoxa−/− mice were isolated separately and divided into two tubes to measure H2O2 from DPI-treated and DPI-untreated samples. Results for each treatment condition represent the mean and SEM for three measurements from one experiment.
Western Blots
A novel anti-DUOX monoclonal antibody was designed to recognize the amino acid sequence (DTDPPQEIRR) between the EF-hand and second transmembrane domain of murine DUOX1 (Abmart, Shanghai, China). Cell pellets used for H2O2 measurements from the airway epithelium were lysed in 4°C RIPA buffer (Pierce, Rockford, IL) supplemented with Sigma Protease Inhibitor Cocktail and PMSF (Sigma-Aldrich) on ice. The lysate was centrifuged for 10 min at 14,000 rpm at 4°C, and the supernatant was transferred into a fresh tube and stored at 80°C. Total protein concentration was determined using the Bio-Rad DC Protein Assay Kit. Samples were combined with Laemmli buffer (2% SDS, 100 mM dithiothreitol), and heated to 65°C for 10 min and placed on ice. Samples—20 g protein/lane--were resolved on a 7% NuPAGE Novex Tris-Acetate gel (Invitrogen) and then transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked in 5% fat-free dry milk in TBS and incubated with mouse anti-DUOX primary antibody (1:1000; Abmart, Shanghai) at 4°C for 24 h and secondary goat-anti mouse horseradish peroxidase (HRP)-coupled antibodies (1:8000; R&D Systems) at 25°C for 1 h. Detection was achieved by ECL (HyGlo, Denville Scientific). The intensity of DUOX protein on the Western blot was normalized to β-tubulin using MultiGauge v2.3 software (Fujifilm, Cypress, CA).
Ovalbumin asthma model
We generated an ovalbumin allergic asthma model as previously described [30]. Briefly, 9-11 week-old male and female knockout, heterozygous, and wild-type mice were sensitized via intraperitoneal injection of 10ug/0.1 mL ovalbumin (grade V, 98% ≥ pure; Sigma) with alum adjuvant at days 0 and 14. Mice were then challenged with 10mL aerosolized 1% ovalbumin (10mg/mL) in phosphate buffered saline (PBS) for 2 weeks with 6 exposures every other day starting from day 28. Aerosolized exposures of approximately 45 minutes were conducted with a side-stream nebulizer (Invacare Corporation, Elyria, OH) and Passport Compressor (Invacare, Stanford, FL). Matched controls received an intraperitoneal injection of ovalbumin and alum and were provided filtered air while the experimental animals received OVA aerosolization.
Lung function evaluation
Immediately following the last aerosolized ovalbumin challenge on day 42, lung function was evaluated as previously described [30]. Using a plethysmograph for restrained mice (Buxco Inc., Troy, NY) dynamic compliance and resistance were measured while mice were anesthetized and sedated with medatomidine, 0.75mg/kg (Domitor, Orion Pharma, Finland) and tiletamine/zolpidem, 37.5mg/kg (Telazol, Fort Dodge Laboratories, Fort Dodge, IA). Mice were ventilated with a volume of 125uL at 150 strokes per minute (MiniVent, Harvard Apparatus, Cambridge, MA). Nebulized saline and methacholine (0.1-2.0 mg/mL) were administered for 3 minutes with 3 minute recovery periods after each dose. Dynamic compliance (Cdyn as mL/cm of H2O) and resistance (Rrs, cm H2O·s/ml) measurements were made at baseline and immediately after each administration of saline or methacholine.
Bronchoalveolar lavage sample collection and processing
Following lung function testing, mice were euthanized via an overdose of pentobarbital and dilantin. The lung was then lavaged two times with 1mL sterile PBS (pH=7.4) to collect bronchoalveolar lavage fluid (BALF). BALF was centrifuged at 2000 rpm for 10 minutes and supernatant was collected and stored at −80°C. The resulting BALF cell pellet was resuspended in ACK/RBC lysis buffer to remove red blood cells and re-centrifuged at 2000 rpm for 10 minutes. The resulting pellet was resuspended in PBS for WBC counts. Live cell concentrations were estimated by counting trypan-blue-excluding cells on a hemacytometer. To determine BALF cell differentials, cytocentrifuge preparations were stained with a Hema3 kit as described in the manufacturer’s instructions (Fisher Scientific, Kalamazoo, MI), and sealed using Cytoseal 60 (Richard-Allen Scientific, Kalamazoo, MI). Cell percent differentials were calculated by counting 10 fields at 400× magnification and classifying cell types as alveolar macrophage, neutrophil, eosinophil, lymphocyte, or “other” based upon standard morphological characteristics and staining profiles. Totals of each cell type per ml of bronchoalveolar lavage fluid were calculated using the differential percentages.
Lung Histology
Following BALF collection, lungs were inflated at 30cm H2O with 1% paraformaldehyde (PFA) in PBS for approximately 30 minutes and then fixed in 1% PFA for at least 24 hours. Trachea, left lung and right lung were then separated, placed in 70% ethanol, and embedded in paraffin wax. The left lungs were cut into 5 m-thick sections, placed on glass microscope slides, and dried. Next, the sections were deparaffinized with xylene and rehydrated with ethanol for further processing. To visualize DUOX protein localization in lung sections, paraffin-embedded left lung sections from ovalbumin treated animals were incubated with a mouse anti-DUOX antibody followed by addition of goat anti-mouse secondary antibody conjugated to peroxidase (Vectastain ABC Kit, Catalogue #PK-4002) utilizing 3,3′-diaminobenzidine (DAB) substrate detection.
Tissue cell differentials were determined from hematoxylin and eosin (HE)-stained tissue slices using a semiquantitative method. Five fields at 1000× magnification were analyzed and cell types were classified as alveolar macrophage, neutrophil, eosinophil, lymphocyte, or “other” based upon standard morphological characteristics and staining profile. Peribronchiolar, vascular, perivascular and alveolar compartments were compared between groups. To visualize neutrophil spatial arrangement in lung sections, paraffin-embedded left lung sections were incubated with a neutrophil-specific ly-6G antibody 1A8 (BD Pharmingen™, Catalogue #551459) followed by a secondary antibody conjugated to peroxidase (Vectastain ABC Kit, Catalogue #PK-4004) utilizing 3,3′-diaminobenzidine (DAB) substrate detection. Tissue sections were sealed using Cytoseal 60 (Richard-Allen Scientific). To visualize mucin production, periodic acid-Schiff base (PAS) staining was utilized and semiquantitative scoring was used to compare groups. PAS stained tissues were examined under light microscopy for PAS positive staining in the upper airway epithelium in five fields at 400× magnification. Percent PAS positive epithelial cells were counted out of total epithelial cells to obtain a percent and compare groups.
ELISA
The supernatant fraction of the BALF was thawed on ice and used in enzyme-linked immunosorbant assays (ELISA). The mouse homologs of human interleukin (IL)-8, Keratinocyte-Derived Cytokine (KC) and Macrophage Inflammatory Protein (MIP)-2, were detected using ELISA (R&D Systems, Product Number MKC00B and MM200, respectively). Sample levels were compared to a standard curve provided by the supplier.
Multiplex
The supernatant fraction of the BALF was used in multiplex analyses (Milliplex Catalogue ID MPXMCYTO-70 K-22) per the manufacturer’s instructions. Analysis was followed per the manufacturer’s protocol and as previously described [31]. Briefly, 50 μL of BALF supernatant was incubated with antibody-coupled beads for analysis. Quantitation of antigen-antibody binding was performed using a flow-based Luminex™ 100 suspension array system (Bio-Plex 200; Bio-Rad Laboratories, Inc.). Known reference cytokine concentrations included in the Milliplex kit were utilized to create a standard curve and sample cytokine concentrations were calculated using the Bio-Plex Manager software. Concentrations found to be below the sensitivity limit of detection (LOD) of the method were calculated as LOD/2 for statistical comparisons.
Statistics
All data was processed using Prism 5 software (GraphPad Software,Inc., San Diego, California). The unpaired student t test was used unless otherwise noted. Males and females were evaluated individually and were not found to be statistically different thus were compiled in results unless otherwise noted. Data was deemed statistically significant at p ≤0.05.
Results
DUOX location and function in mouse airway epithelium
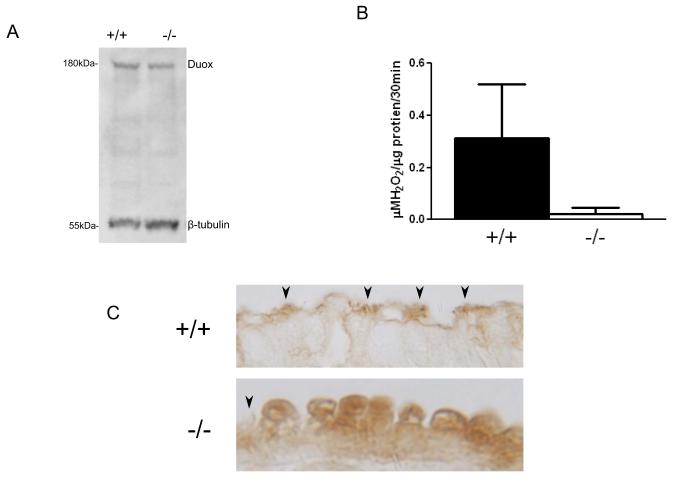
To confirm DUOX was present in mouse airway epithelial cells, we isolated murine airway epithelial cells from Duoxa+/+ and Duoxa−/− mice and identified DUOX using Western Blot analysis (Figure 1A). Densitometry analyses demonstrated that Duoxa+/+ and Duoxa−/− mice had similar levels of DUOX protein (ratio of 0.52 versus 0.39 normalized to β-tubulin, respectively). However, functional DUOX activity was substantially impaired in Duoxa−/− mouse airways. Using Amplex® Red to measure H2O2 production in isolated airway epithelium, Duoxa+/+mice had a 15-fold increase in DPI-inhibitable H2O2 production from unstimulated cells compared to Duoxa−/− mice (0.31 μM H2O2/ μg protein/30 minutes versus 0.02μM H2O2/μg protein/30 minutes, respectively; Figure 1B). These data are consistent with previous observations that DUOX proteins require their respective maturation factors for normal functional activity.
Figure 1.
DUOX expression and activity in isolated mouse airway cells. (A) Western blot analysis confirmed DUOX expression in isolated epithelial cells from Duoxa+/+ and Duoxa−/−mice. (B) Duoxa+/+ and Duoxa−/− mouse airway cells were isolated from tracheas and immediately analyzed for DPI-inhibitable H2O2 production by Amplex® Red analysis. (C) Immunohistochemistry on airway epithelial cells using a mouse anti-DUOX antibody in ovalbumin treated animals (arrowheads indicate cilia).
To further evaluate the effect of Duoxa knockout on DUOX expression, we performed immunohistochemical analysis on lung sections obtained from Duoxa+/+ and Duoxa−/− mice. We found that both genotypes treated with ovalbumin had predominant DUOX expression in the airway epithelium, but the cellular location of DUOX was substantially different between the wild-type and knockout animals. In Duoxa+/+ mice, DUOX was localized to the cilia of the epithelial cell with minimal cytoplasmic staining. Duoxa−/− mice, in contrast, had diffuse protein expression dispersed throughout the cytoplasm without observable staining in cilia (Figure 1C). This suggests an inability of DUOX protein to be properly escorted to the plasma membrane in mice that lack DUOXA.
Duoxa knockout has variable effects on allergic asthma
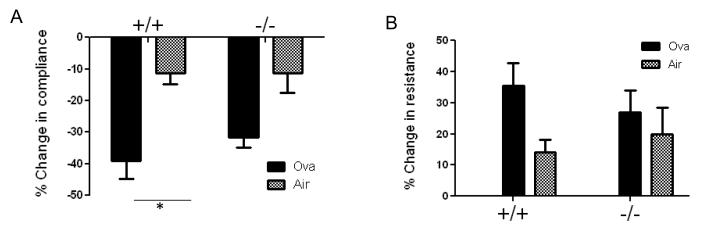
To determine the role of functional Duox in allergic asthma, we first compared changes in airway hyperresponsiveness after ovalbumin exposure in Duoxa+/+ versus Duoxa−/− mice. At the end of sensitization protocol, mice were anesthetized and tracheostomized followed by measurement of dynamic compliance and resistance on a mouse ventilator. Serial administrations of increasing doses of aerosolized methacholine, to a maximum dose of 2 mg/mL, were performed to determine changes in baseline compliance and resistance. Compared to filtered air controls, ovalbumin-exposed Duoxa+/+ mice had a significant reduction in compliance and corresponding increase in resistance after exposure to methacholine, consistent with features of a successful allergic airway model. In contrast, the ovalbumin-exposed Duoxa−/− mice did not demonstrate significantly increased hyperresponsiveness compared to matched air controls even at the highest dose of methacholine tested (Figure 2).
Figure 2.
DUOX is necessary for increased airway hyperresponsiveness. Percent change in compliance (A) or resistance (B) was determined in Duoxa+/+ (+/+) and Duoxa−/− (−/−) mice exposed to two weeks of either filtered air or ovalbumin. Percent change values were calculated by dividing the remainder of compliance or resistance determined at the highest dose of methacholine ( 2mg/mL) minus compliance or resistance determined after exposure to aerosolized saline (baseline value) by the baseline value times 100. Data are shown as mean±SEM from six mice in each group; * = p< 0.05.
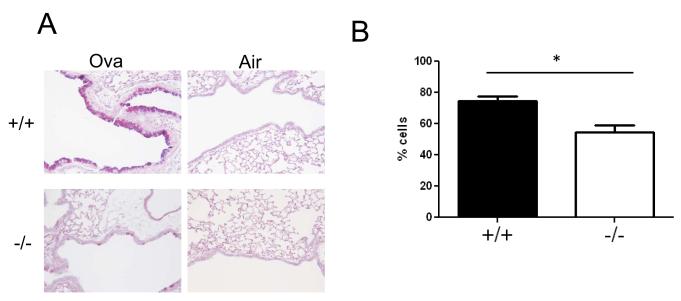
We next analyzed lung tissues for mucin production by PAS staining and found a significant reduction in mucin-positive cells in ovalbumin-exposed Duoxa−/− mice compared with Duoxa+/+ mice (Figure 3A). Animals exposed to filtered air alone had negligible PAS staining. To minimize biased sampling and quantify our results, we determined the number of PAS-positive epithelial cells normalized to the total number of epithelial cells in randomly selected airway sections and found similar results (Figure 3B).
Figure 3.
DUOX induces mucin expression in mouse lung epithelium after allergen challenge. (A) Periodic acid-Schiff (PAS) staining of paraffin-embedded lung sections obtained from Duoxa+/+ (+/+) and Duoxa−/− (−/−) male mice after two weeks of aerosolized ovalbumin exposure in sensitized animals. Similar airway levels are shown to ensure equivalent representation of hyperplastic potential between groups (200x) (B) Quantification of PAS-positive staining was determined by counting the number of PAS-positive epithelial cells normalized to total epithelial cells for Duoxa−/− (-/-) and Duoxa+/+ (+/+) mice. Five fields incorporating the upper airway were randomly chosen for counting, *=p<0.05.
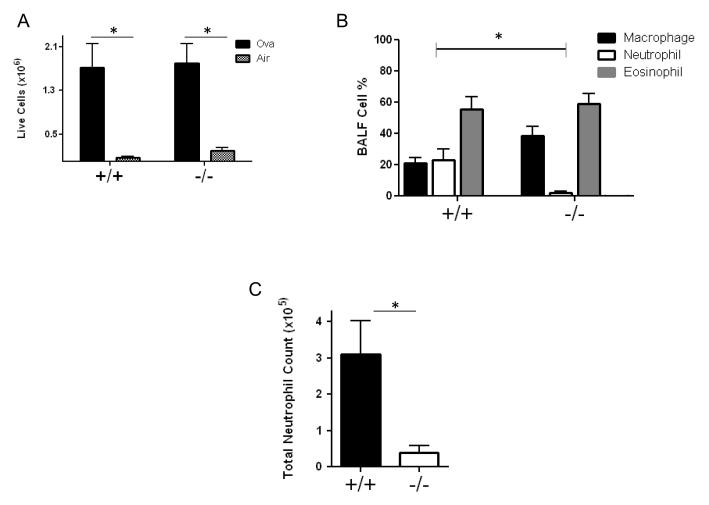
However, the lack of functional DUOX protein had minimal effects on eosinophilic inflammation. After the last challenge with aerosolized ovalbumin or filtered air, we lavaged the lungs of Duoxa+/+ and Duoxa−/− mice to determine total cell counts and cell differentials. In both Duoxa+/+ and Duoxa−/− mice, animals that received nebulized ovalbumin had significant elevations in total live cell counts compared to filtered air-exposed animals (Figure 4A). Sex or genotype had no significant impact on total cell counts or number of dead cells recovered from BALF for each treatment group (data not shown). The predominant cell type in the Duoxa+/+ mice was eosinophils, consistent with the expected allergic asthma phenotype as shown previously (Figure 4B) [32, 33]. Unlike the effect of functional DUOX protein on airway hyperresponsiveness and mucus hyperplasia, however, both Duoxa+/+ and Duoxa−/− mice demonstrated predominant eosinophil infiltration (approximately 60%) and similar numbers of eosinophils between the two groups after ovalbumin exposure (Figure 4B). Together, these data suggest that DUOX plays a significant role in two major features of allergic asthma, airway hyperresponsiveness and mucus hyperplasia, but that eosinophilic inflammation is due to a separate, DUOX-independent mechanism.
Figure 4.
DUOX affects neutrophilic, but not eosinophilic, airway inflammation after allergen challenge. (A) Leukocytes were collected from the airway compartment by bronchioalveolar lavage (BAL) and the number of live cells was determined by trypan blue exclusion. The number of live cells was compared between ovalbumin-exposed (Ova) versus filtered air-exposed (Air) Duoxa−/− (-/-) and Duoxa+/+ (+/+) mice. (B) Leukocytes were collected from the airway compartment by bronchoalveolar lavage after two-weeks of aerosolized ovalbumin exposure and cell differentials were determined visually based on cell morphology. The percent of each cell type was compared between ovalbumin-exposed Duoxa−/− (-/-) and Duoxa+/+ (+/+) mice. (C) Absolute neutrophil counts in ovalbumin-exposed Duoxa−/− (-/-) and Duoxa+/+ (+/+) mice was calculated by multiplying neutrophil percentage with total cell number from BAL. Data are shown as mean±SEM from six mice in each group; * = p< 0.05 using two-way ANOVA and Bonferroni post-test correction.
DUOX mediates neutrophil influx during allergic inflammation
Although neutrophils are not a predominant feature of allergic inflammation, our model induced approximately 20% neutrophil influx into the airways of Duoxa+/+ mice. Duoxa−/− mice, in contrast, had minimal neutrophilic inflammation in BALF after ovalbumin exposure (Figure 4B). When we corrected for the total cell numbers between the two groups of mice, absolute neutrophil counts in the alveolar compartment between Duoxa+/+ and Duoxa−/− mice remained statistically significant (Figure 4C ).
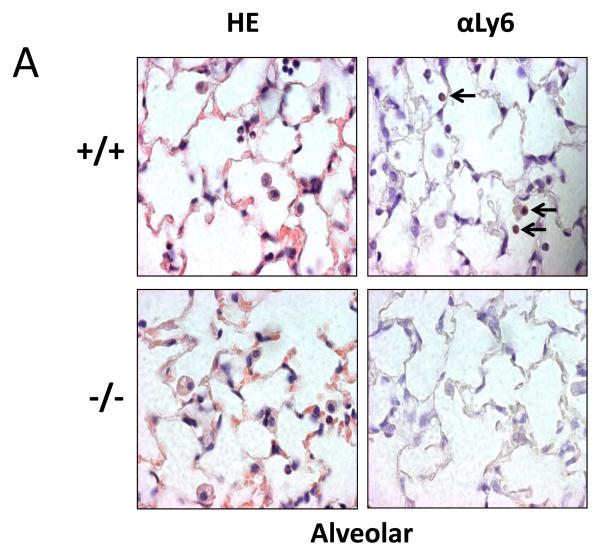
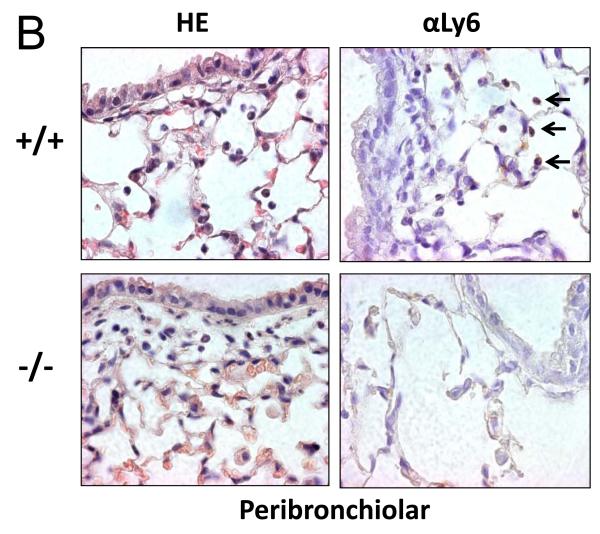
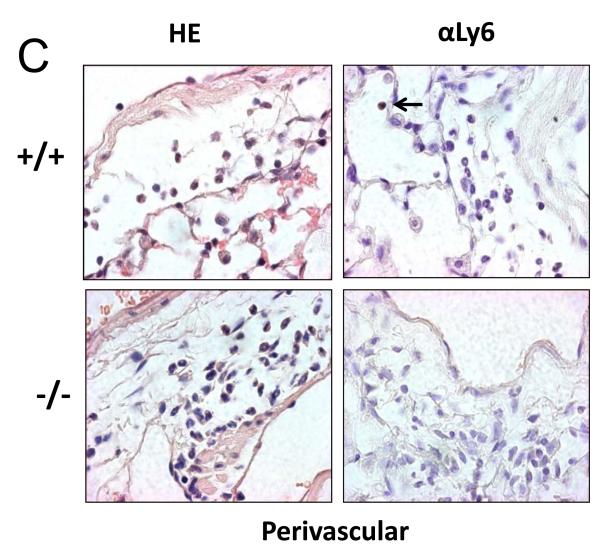
To determine if this difference in neutrophil recruitment correlated with the number of neutrophils in lung tissue, we stained paraffin-embedded lung sections with hematoxalin and eosin to localize neutrophils based on morphology. To ensure only neutrophils were counted, we performed immunohistochemical staining using the 1A8 antibody against the neutrophil-specific marker, ly-6G (Figure 5). Consistent with the BALF data, there was a significant reduction in neutrophils seen in the alveolar compartment of Duoxa−/− mice compared to Duoxa+/+ mice (Figure 5A). Similarly, lower numbers of neutrophils were seen in the peribronchial and perivascular compartments of Duoxa−/− mice (Figures 5B, C). There was no significant difference in neutrophil counts between Duoxa+/+ and Duoxa−/− mice treated with filtered air (data not shown).
Figure 5.
Duoxa−/− mice had lower neutrophil influx into multiple compartments of airway tissue. Paraffin-embedded lung sections were obtained from ovalbumin-sensitized Duoxa+/+ (+/+) and Duoxa−/− (-/-) male mice after two weeks of aerosolized ovalbumin exposure. Lung sections were stained with hematoxylin and eosin (HE) for morphometric analysis of neutrophilic influx. The presence of neutrophils was verified by immunohistochemical staining using with rat anti-Ly6 antibody (αLy6). Representative images (400X) of the alveolar (A), peribronchiolar (B), and perivascular (C) compartments are shown. Black arrows highlight positive neutrophil staining.
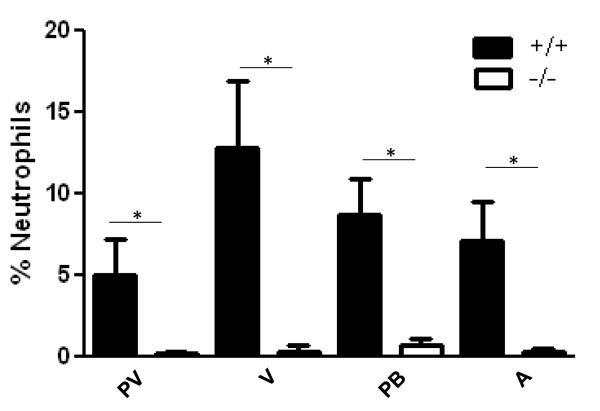
To better quantify the change in neutrophil numbers throughout the lung, we counted cells in the peribronchiolar, vascular, perivascular and parenchyma compartments (see Methods). Cell types were determined based on morphologic appearance and the number of neutrophils for each compartment was determined as a percent of the total cells counted. As shown in Figure 6, neutrophil numbers were reduced in Duoxa−/− versus Duoxa+/+ mice for all four compartments examined in the lung despite similar levels of circulating neutrophils in both groups (Figure S1). These data suggested that DUOX influenced retention of neutrophils in the lung after allergen exposure.
Figure 6.
Neutrophilic inflammation was similarly attenuated in all lung compartments from Duoxa−/− mice. Paraffin-embedded lung sections were obtained from ovalbumin-sensitized Duoxa+/+ (+/+) and Duoxa−/− (-/-) male mice after two weeks of aerosolized ovalbumin exposure. Lung sections were stained with hematoxylin and eosin (HE) to determine the percentage of neutrophils in each of four lung compartments (see Methods). Leukocyte populations in 10 high-powered fields for each compartment were counted and neutrophil percentage was determined by dividing the total neutrophils counted by the total number of leukocytes. Data are shown as mean±SEM for the perivascular (PV), vascular (V), peribronchiolar (PB), and alveolar (A) compartments, * = p=< 0.05.
DUOX-mediated changes in cytokine expression
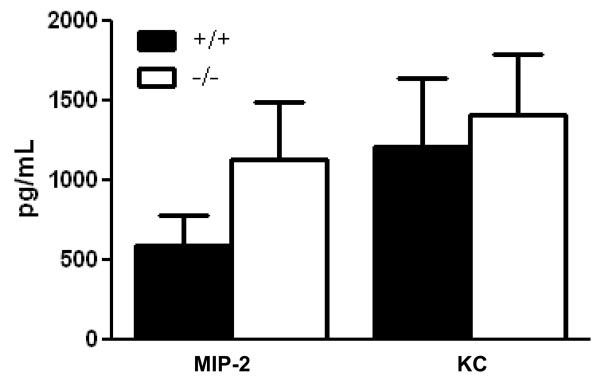
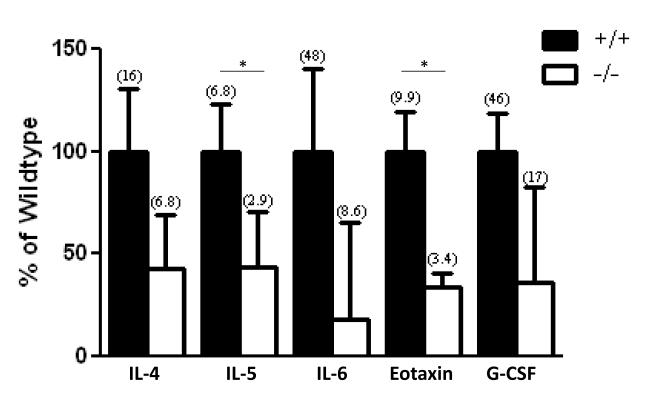
To examine the events driving lower neutrophil infiltration found in Duoxa−/− compared to Duoxa+/+ mice, we examined changes in two major chemokines involved in neutrophil chemotaxis, KC and MIP-2 [34-36]. KC and MIP-2 levels were measured in BALF from Duoxa+/+ and Duoxa−/− mice by ELISA (Figure 7). There was a nonstatistical trend towards higher KC and MIP-2 levels in Duoxa−/− mice, which suggested that KC- or MIP-2-mediated signaling was not the predominant mechanism for neutrophil recruitment in this model. Based on this finding, we utilized multiplex analyses to probe for other potential pro-inflammatory cytokines that were modulated by DUOX during allergic inflammation. As shown in Figure 8, five cytokines were highly expressed in Duoxa+/+ compared to Duoxa−/− mice. Differences between mice for two of these five cytokines, IL-5 and eotaxin, were statistically significant. The remaining 17 cytokines that were measured were either similar in both groups of mice after ovalbumin exposure or undetected (Table S1).
Figure 7.
DUOX does not impact expression of IL-8 homologues after ovalbumin exposure. BALF was obtained from ovalbumin-sensitized Duoxa+/+ (+/+) and Duoxa−/− (−/−) mice immediately after two weeks of aerosolized ovalbumin exposure. ELISA was used to determine levels of MIP-2 and KC in BALF supernatant. Data represent mean±SEM from six animals in each group.
Figure 8.
TH2 cytokine levels are reduced in Duoxa−/− mice. Multiplex analyses were used to determine cytokine levels in BALF supernatant from ovalbumin exposed Duoxa+/+ (+/+) and Duoxa−/− (−/−) mice. For each cytokine, cytokine levels from Duoxa−/− mice are shown as a percent of wildtype levels. Data represent mean±SEM from six animals in each group. Mean cytokine levels in pg/mL from each group are shown in parentheses. * = p<0.05.
Discussion
As previously demonstrated, our ovalbumin exposure model was able to generate several characteristic features of allergic asthma including airway hyperresponsiveness, goblet cell metaplasia, and eosinophilic inflammation [32, 33]. These features correlated with a robust increase in airway expression of IL-4, IL-13, IL-5, and eotaxin consistent with the known role of these cytokines in the generation of allergic asthma [37]. Lack of functional DUOX protein attenuated airway hyperresponsiveness, goblet cell metaplasia, and TH2 cytokine levels in response to ovalbumin exposure. Together, these data suggested DUOX is a critical enzyme that modulates the epithelial response to allergens and the development of several asthmatic features.
Although we cannot exclude contributions from other cell types with potential DUOX activity [42], our data support the notion that airway epithelium-derived DUOX is responsible for our observations. Certainly, differences in PAS staining between wild-type and Duoxa−/− mice suggest direct local effects from lung epithelium consistent with human airway DUOX. Importantly, we provide evidence that functional DUOX exists in the mouse airway epithelium contrary to a previous report [29]. We postulate that the reason for this discrepancy is due to differences in mouse strains, or the use of a mouse-specific anti-DUOX antibody. Additional studies in various mouse backgrounds may shed light on strain differences in airway DUOX expression. Based on our findings, there appear to be several important parallels to human airway DUOX including the requirement of the maturation factor for functional activity [38] and localization to ciliated cells [39]. Using an estimate that airway epithelial cells contain close to 150 g of protein/106 cells [40], we observed H2O2 production rates of approximately 0.70nM H2O2/10min/106 cells from unstimulated murine airway epithelium. This degree of H2O2 production is similar to that seen in unstimulated human airway cultures [41]; although, it is difficult to directly compare without performing parallel experiments in mouse and human airway tissues.
The lack of functional DUOX in the airway epithelium had no impact on eosinophilic inflammation. Both the Duoxa−/− and Duoxa+/+ mice had similar levels of eosinophilic inflammation in response to ovalbumin exposure. This discordant relationship between eosinophilic inflammation and other features of allergic asthma is consistent with previous findings [43], and suggests that DUOX-mediated signaling is a key factor responsible for these differences. Unexpectedly, we observed lower levels of eotaxin in the Duoxa−/− animals despite similar eosinophil numbers between the two groups of mice. This suggests that eotaxin is not primarily responsible for recruiting eosinophils to the lung in our mouse model [44], or that the eotaxin levels expressed in the knockout mice were above a threshold required to amply recruit eosinophils.
The difference we observed in neutrophil infiltration between the Duoxa−/− and Duoxa+/+ mice in response to allergen was unexpected. Although we observed predominant eosinophilic inflammation, over 20% of the cells identified in BAL fluid of DUOX-intact animals were neutrophils. In the absence of functional DUOX, there was greater than an 80% reduction in the number of neutrophils recruited to the lung. Histologic evaluation revealed that this reduced number of neutrophils was observed in the vascular, perivascular peribronchiolar, and alveolar compartments. These data suggest that the primary deficit is due to impaired endothelialneutrophil interactions or reduced numbers of circulating neutrophils in the Duoxa−/− mice. Peripheral blood counts from Duoxa−/− and Duoxa+/+ mice demonstrated similar levels of circulating neutrophils (Figure S1), implying that DUOX-mediated signaling is important for activation of either circulating neutrophils or pulmonary endothelium.
Reactive oxygen species are known to regulate signaling molecules and proteins [45, 46]. Therefore, it is likely that DUOX-derived hydrogen peroxide or hydrogen peroxide-derived metabolites directly recruit neutrophils to the airway [47-49] or regulates molecule(s) involved in neutrophil chemotaxis [19, 21, 50]. Because DUOX is important for regulating IL-8 in airway epithelial cells [19, 21, 50], we examined differences in mouse homologues of IL-8, MIP-2, KC and LIX, in the BAL fluid of Duoxa−/− and Duoxa+/+ mice and did not observe any significant difference between groups (see Figure 7 and Table S1). This is in contrast to a recent study which demonstrated that Duox2 knockout resulted in lower levels of MIP-2 after nasal flagellin exposure [19]. Given the substantial differences between the two models, it is difficult to speculate on the reason for the discordance between the two studies. However, it will be important to challenge our model with flagellin or bacteria to see what differential effects our double DUOX knockout model may exhibit.
Given that IL-8 homologues were not required for DUOX-mediated neutrophil recruitment, we utilized multiplex analyses to analyze alternative signaling pathways that potentially were responsible for the differences we characterized. We observed decreased levels of IL-6 and GCSF, cytokines increased by activated neutrophils, in the Duoxa−/− mice. Because both cytokines are produced by activated neutrophils, lower levels of both cytokines in Duoxa−/− mice may simply confirm our histologic findings. However, IL-6 is also known to recruit and activate neutrophils as well [51]. Based on the lower level of neutrophils in the vascular compartment of Duoxa−/− mice, we speculate that DUOX-mediated hydrogen peroxide or hydrogen peroxide-derived metabolites from the epithelial compartment activates an IL-6-dependent pathway necessary for endothelial activation and retention of neutrophils. Further study with these mice will determine if this postulate is correct and the mechanisms by which this occurs
In addition, our results confirm mounting evidence that DUOX is essential for signaling events in response to allergen or infection. Ryu et al. found that DUOX2 differentially mediated-glucan-induced TLR2 signaling in the nose and LPS-induced TLR4 signaling in the lungs of knockout mice [18]. Flagellin-mediated activation of TLR-5 induced mucus hypersecretion and IL-8 production occurs through a DUOX2-mediated signaling cascade in vitro [19, 20]. And, DUOX2-mediated signaling is important for TLR-3-mediated soluble TNFR1 shedding in vitro [20]. Similarly, DUOX1 appears to be important for the induction of IL-8 via multiple TLR ligands [21] in vitro, and both DUOX1 and DUOX2 are upregulated in polyp tissue of patients with chronic rhinosinusitis [52]. Because we used a Duoxa−/− knockout model, we are unable to determine which DUOX is predominantly essential for the development of asthma features versus neutrophil influx, but based on previous data both appear to be important for various components of lung immunity. Given the differential regulation of both DUOX1 and DUOX2 [26], it will essential to determine the contribution of each isoform to lung immunity and response to allergen.
Based on our data, it is highly plausible that DUOX activity could worsen asthma symptoms or be partially responsible for severe asthma phenotypes characterized by neutrophilic inflammation. The driving forces behind neutrophil infiltration in an asthmatic lung have not yet been elucidated [53, 54], and it is often associated with more severe forms of asthma, which are less responsive to standard therapeutic strategies [55, 56]. We postulate that further characterization of the mechanisms behind DUOX-mediated neutrophil recruitment after allergen challenge will lead to a deeper understanding of this important clinical problem.
Conclusion
Our study suggests that DUOX has an important role in modulating several features of allergic asthma including airway hyperactivity and mucus metaplasia independent of eosinophilic inflammation. Also, DUOX is necessary to sufficiently target neutrophils into the airways in response to allergic challenge. Based on the known function of DUOX enzymes, it is likely that DUOX-derived hydrogen peroxide is part of important signaling events leading to allergy-initiated inflammation. Specifically, DUOX may be an important target for certain subsets of asthma patients that mechanistically are unique from most allergic asthma patients. It is possible that DUOX activity contributes to the mechanism behind neutrophil infiltration in certain forms of asthma and future study may lead to novel therapeutic strategies for this patient population.
Supplementary Material
Supplemental Figure 1: Circulating neutrophils are similar in Duoxa+/+ and Duoxa−/− mice. Serum samples from Duoxa+/+ (+/+) and Duoxa−/− (−/−) animals were collected via cardiac puncture after IP injection of ovalbumin and two-weeks exposure to aerosolized ovalbumin. A Coulter counter was used to determine total leukocyte population and cell differentials of monocytes, eosinophils, lymphocytes, and neutrophils. Total leukocyte counts were similar in each group (data not shown). Data from six animals from each group are shown with bar and whiskers representing mean±SEM; p>0.05 between groups.
Supplemental Table 1: List of all cytokines measured in BALF supernatant of Duoxa+/+ (+/+) and Duoxa−/− (−/−) mice. Data from ovalbumin-exposed (OVA) and filtered air-exposed (air) animals are shown. Cytokine levels are in pg/mL unless values were below the limit of detection (<LD). All data were measured by multiplex analysis, except for the two denoted by • (signifies cytokines measured by ELISA). Data represent mean±SEM from six animals in each group. Levels are expressed as pg/mL; <LD = value below limit of detection.
Acknowledgements
This work was supported by grants NHLBI R01 HL085311 and NHLBI T32 HL007013 from the National Institutes of Health (Bethesda, MD).
Abbreviations
- DUOX1
Dual Oxidase 1
- DUOX 2
Dual Oxidase 2
- DUOXA1
Dual Oxidase A1
- DUOXA2
Dual Oxidase A2
- BALF
brochoalveolar lavage fluid
- L-T4
levothyroxine
- IP
intraperitioneal
- DPI
diphenyleneiodonium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Holgate ST. Innate and adaptive immune responses in asthma. Nature medicine. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- [2].Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nature reviews. Immunology. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66:579–587. doi: 10.1111/j.1398-9995.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- [4].Larche M. Regulatory T cells in allergy and asthma. Chest. 2007;132:1007–1014. doi: 10.1378/chest.06-2434. [DOI] [PubMed] [Google Scholar]
- [5].Reuter S, Dehzad N, Martin H, Bohm L, Becker M, Buhl R, Stassen M, Taube C. TLR3 but not TLR7/8 ligand induces allergic sensitization to inhaled allergen. J Immunol. 2012;188:5123–5131. doi: 10.4049/jimmunol.1101618. [DOI] [PubMed] [Google Scholar]
- [6].Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, Zeldin DC, Kraft M, Garantziotis S, Nakano H, Cook DN. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nature medicine. 2012;18:1705–1710. doi: 10.1038/nm.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature medicine. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 2010;184:3535–3544. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- [9].Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. The Journal of experimental medicine. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ckless K, Hodgkins SR, Ather JL, Martin R, Poynter ME. Epithelial, dendritic, and CD4(+) T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization. Biochimica et biophysica acta. 2011;1810:1025–1034. doi: 10.1016/j.bbagen.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- [13].Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sugiura H, Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxid Redox Signal. 2008;10:785–797. doi: 10.1089/ars.2007.1937. [DOI] [PubMed] [Google Scholar]
- [15].Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- [17].Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1506–1514. doi: 10.1152/ajplung.00029.2007. [DOI] [PubMed] [Google Scholar]
- [18].Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, Choi MK, Joo JH, Kim CH, Lee SN, Lee WJ, Kim J, Shin DM, Kweon MN, Bae YS, Yoon JH. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. The Journal of allergy and clinical immunology. 2012 doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- [19].Joo JH, Ryu JH, Kim CH, Kim HJ, Suh MS, Kim JO, Chung SY, Lee SN, Kim HM, Bae YS, Yoon JH. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxid Redox Signal. 2012;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- [20].Yu M, Lam J, Rada B, Leto TL, Levine SJ. Double-stranded RNA induces shedding of the 34-kDa soluble TNFR1 from human airway epithelial cells via TLR3-TRIF-RIP1-dependent signaling: roles for dual oxidase 2- and caspase-dependent pathways. J Immunol. 2011;186:1180–1188. doi: 10.4049/jimmunol.1001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1068–1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- [22].Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2005;102:767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kuwahara I, Lillehoj EP, Koga T, Isohama Y, Miyata T, Kim KC. The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription. Am J Respir Cell Mol Biol. 2007;37:691–698. doi: 10.1165/rcmb.2007-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- [25].Gorissen SH, Hristova M, Habibovic A, Sipsey LM, Spiess PC, Janssen-Heininger YM, van der Vliet A. Dual oxidase-1 is required for airway epithelial cell migration and bronchiolar reepithelialization after injury. Am J Respir Cell Mol Biol. 2013;48:337–345. doi: 10.1165/rcmb.2012-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [27].Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grasberger H, De Deken X, Mayo OB, Raad H, Weiss M, Liao XH, Refetoff S. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol. 2012;26:481–492. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bratt JM, Franzi LM, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicology and applied pharmacology. 2009;234:273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kenyon NJ, Ward RW, Last JA. Airway fibrosis in a mouse model of airway inflammation. Toxicology and applied pharmacology. 2003;186:90–100. doi: 10.1016/s0041-008x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- [31].Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, behavior, and immunity. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kenyon NJ, Bratt JM, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicology and applied pharmacology. 2008;230:269–275. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kenyon NJ, Liu R, O’Roark EM, Huang W, Peng L, Lam KS. An alpha4beta1 integrin antagonist decreases airway inflammation in ovalbumin-exposed mice. European journal of pharmacology. 2009;603:138–146. doi: 10.1016/j.ejphar.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003;19:358–365. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- [35].Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Wollenberg GK, Remick DG. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock. 2001;15:278–284. doi: 10.1097/00024382-200115040-00005. [DOI] [PubMed] [Google Scholar]
- [36].Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramakrishna L, de Vries VC, Curotto de Lafaille MA. Cross-roads in the lung: immune cells and tissue interactions as determinants of allergic asthma. Immunologic research. 2012;53:213–228. doi: 10.1007/s12026-012-8296-4. [DOI] [PubMed] [Google Scholar]
- [38].Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- [39].Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11:2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Widdicombe JH, Basbaum CB, Highland E. Ion contents and other properties of isolated cells from dog tracheal epithelium. The American journal of physiology. 1981;241:C184–192. doi: 10.1152/ajpcell.1981.241.5.C184. [DOI] [PubMed] [Google Scholar]
- [41].Harper RW. The Role of DUOX Isozymes in the Respiratory Tract Epithelium. In: Valacchi G, Davis P, editors. Oxidants in Biology: A question of balance. Springer; Netherlands: 2008. pp. 267–278. [Google Scholar]
- [42].Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, Leto TL, Williams MS. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Science signaling. 2010;3:ra59. doi: 10.1126/scisignal.2000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yang Y, Loy J, Ryseck RP, Carrasco D, Bravo R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- [45].Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- [46].Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature reviews. Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- [47].Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, Blanchard C, Zirkle D, McDonald D, Pai SY, Serhan CN, Luo HR. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Klyubin IV, Kirpichnikova KM, Gamaley IA. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. European journal of cell biology. 1996;70:347–351. [PubMed] [Google Scholar]
- [50].Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1289–1296. doi: 10.1152/ajplung.00356.2006. [DOI] [PubMed] [Google Scholar]
- [51].Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- [52].Cho DY, Nayak JV, Bravo DT, Le W, Nguyen A, Edward JA, Hwang PH, Illek B, Fischer H. Expression of dual oxidases and secreted cytokines in chronic rhinosinusitis. International forum of allergy & rhinology. 2012 doi: 10.1002/alr.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stirling RG, Chung KF. Severe asthma: definition and mechanisms. Allergy. 2001;56:825–840. doi: 10.1034/j.1398-9995.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- [54].Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. The Journal of allergy and clinical immunology. 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- [55].Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, Corrigan C, de Blic J, Fabbri L, Holgate ST, Ind P, Joos G, Kerstjens H, Leuenberger P, Lofdahl CG, McKenzie S, Magnussen H, Postma D, Saetta M, Salmeron S, Sterk P. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1999;13:1198–1208. doi: 10.1034/j.1399-3003.1999.13e43.x. [DOI] [PubMed] [Google Scholar]
- [56].Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155:542–548. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Circulating neutrophils are similar in Duoxa+/+ and Duoxa−/− mice. Serum samples from Duoxa+/+ (+/+) and Duoxa−/− (−/−) animals were collected via cardiac puncture after IP injection of ovalbumin and two-weeks exposure to aerosolized ovalbumin. A Coulter counter was used to determine total leukocyte population and cell differentials of monocytes, eosinophils, lymphocytes, and neutrophils. Total leukocyte counts were similar in each group (data not shown). Data from six animals from each group are shown with bar and whiskers representing mean±SEM; p>0.05 between groups.
Supplemental Table 1: List of all cytokines measured in BALF supernatant of Duoxa+/+ (+/+) and Duoxa−/− (−/−) mice. Data from ovalbumin-exposed (OVA) and filtered air-exposed (air) animals are shown. Cytokine levels are in pg/mL unless values were below the limit of detection (<LD). All data were measured by multiplex analysis, except for the two denoted by • (signifies cytokines measured by ELISA). Data represent mean±SEM from six animals in each group. Levels are expressed as pg/mL; <LD = value below limit of detection.