Abstract
Repeated exposure to drugs of abuse is associated with structural plasticity in brain reward pathways. Rats selectively bred for locomotor response to novelty differ on a number of neurobehavioral dimensions relevant to addiction. This unique genetic animal model was used here to examine both pre-existing differences and long-term consequences of repeated cocaine treatment on structural plasticity. Selectively bred high-responder (bHR) and low-responder (bLR) rats received repeated saline or cocaine injections for 9 consecutive days. Escalating doses of cocaine (7.5, 15 and 30 mg/kg) were administered on the first (day 1) and last (day 9) days of treatment and a single injection of the intermediate dose (15 mg/kg) was given on days 2-8. Motor activity in response to escalating doses of cocaine was compared on the first and last days of treatment to assess the acute and sensitized response to the drug. Following prolonged cocaine abstinence (28 days), spine density was examined on terminal dendrites of medium spiny neurons in the nucleus accumbens core. Relative to bLRs, bHRs exhibited increased psychomotor activation in response to both the acute and repeated effects of cocaine. There were no differences in spine density between bHR and bLR rats under basal conditions or following repeated saline treatment. However, spine density differed markedly between these two lines following prolonged cocaine abstinence. All spine types were decreased in cocaine-treated bHRs, while only mushroom spines were decreased in bLRs that received cocaine. Changes in spine density occurred specifically near the branch point of terminal dendrites. These findings indicate that structural plasticity associated with prolonged cocaine abstinence varies markedly in two selected strains of rats that vary on numerous traits relevant to addiction. Thus, genetic factors that contribute to individual variation in the behavioral response to cocaine also influence cocaine-induced structural plasticity.
Keywords: cocaine, psychomotor sensitization, dendrites, prolonged abstinence, spine density, addiction
1. Introduction
One common theme to various theories of addiction is that the transition from casual drug use to dependence is at least partially attributable to drug-induced changes in neural circuitry, which consequently, leads to changes in behavior (Robinson and Berridge, 1993, Everitt and Wolf, 2002, Wolf, 2002, Everitt and Robbins, 2005, Kalivas and O’Brien, 2008). In rodent models used to study addiction, repeated exposure to psychostimulants results in behavioral sensitization and accompanying neurobiological changes throughout the brain. Specifically, repeated cocaine exposure produces persistent changes in neuroplasticity, including dendritic remodeling (Robinson and Kolb, 1999, Robinson et al., 2001, Kolb et al., 2003, Li et al., 2004, Robinson and Kolb, 2004, Ferrario et al., 2005) and neurogenesis (Yamaguchi et al., 2004, Dominguez-Escriba et al., 2006, Garcia-Fuster et al., 2010, Lloyd et al., 2010, Noonan et al., 2010), that extend well beyond the period of active drug administration.
Our recent work has focused on examining individual differences associated with addiction vulnerability in the selectively bred lines of high-responder (bHRs) and low-responder (bLRs) rats (Flagel et al., 2010, Flagel et al., 2012a). Similar to studies using outbred rats (Piazza et al., 1989, Hooks et al., 1991, Piazza et al., 2000), the selectively bred high- and low-responder rats differ with respect to locomotor activity in a novel environment (Stead et al., 2006), susceptibility to drug-taking behavior (Davis et al., 2008), and responsivity to cocaine (Garcia-Fuster et al., 2010, Cummings et al., 2011, Clinton et al., 2012). The selectively bred lines are unique, however, in that they allow us to parse the predisposing factors that influence addiction-like behavior. For example, relative to bLRs, bHRs show increased aggression (Kerman et al., 2011), increased impulsivity (Flagel et al., 2010), increased responsiveness to drug-associated cues (Flagel et al., 2010) and a hypersensitive dopamine system (Flagel et al., 2010). Furthermore, Garcia-Fuster et al., recently reported distinct cocaine-induced effects on neurogenesis in the hippocampus of bLR vs. bHR rats, and suggested that these effects might underlie differences in psychomotor sensitization (Garcia-Fuster et al., 2010). Thus, the selectively bred lines are a highly advantageous model for studying individual differences in addiction liability and uncovering the neurobiological mechanisms that may underlie these differences.
The current studies determined whether these genetically bred lines known to differ on a constellation of traits relevant to addiction, also exhibit differences in a form of drug-induced structural plasticity. Since cocaine treatment regimens that elicit behavioral sensitization have been shown to produce persistent alterations in dendritic morphology (Ferrario et al., 2005), morphological changes associated with neural plasticity were examined within the classical reward circuitry by quantifying the density of dendritic spines on medium spiny neurons in the nucleus accumbens core (AcbC) subregion. As an important mediator of drug-seeking behavior and relapse (Kelley, 2004, Everitt and Robbins, 2005, Kalivas and Volkow, 2005), the AcbC is an ideal target for examining individual differences since behavioral responses to cocaine and cocaine-induced neuroplasticity occur through mechanisms requiring this structure (Parkinson et al., 1999, Di Ciano et al., 2001, Phillips et al., 2003, van Dongen et al., 2005, Marie et al., 2012). Moreover, there is a strong association between dopamine signaling and spine density regulation (Ingham et al., 1989, Meredith et al., 1995, Garcia et al., 2010, Gonzalez-Burgos et al., 2010). Thus, this study determined whether individual differences in cocaine responsivity influenced either the magnitude or nature of spine density changes in the AcbC following prolonged cocaine abstinence (28 days). This time point was chosen because changes in both brain and behavior are known to persist for a month or longer (Robinson et al., 2001, Grimm et al., 2003, Ferrario et al., 2005 , Lee et al., 2006, Ferrario et al., 2012) following cocaine exposure, and these long-lasting changes are thought to underlie the propensity to relapse (see (Nestler et al., 1993, Robinson, 1993, Robinson and Berridge, 1993, Weiss et al., 2001)). Based on our previous work demonstrating heightened behavioral response to cocaine (Davis et al., 2008, Flagel et al., 2010, Garcia-Fuster et al., 2010, Cummings et al., 2011, Clinton et al., 2012) and evidence for a hypersensitive dopamine system in bHRs relative to bLRs (Flagel et al., 2010), we hypothesized that bHR rats would also exhibit a greater degree of cocaine-induced neuroplasticity following prolonged abstinence.
2. Experimental Procedures
2.1 Subjects
Adult male Sprague-Dawley rats (n=28) from the 22nd generation of our in-house bHR/bLR selective-breeding colony were used. A detailed description of the breeding paradigm and characterization of these bred lines has been published previously (Stead et al., 2006, Flagel et al., 2010, Garcia-Fuster et al., 2010, Clinton et al., 2011). Rats weighed 300-500g and were pair-housed (bHR-bLR) under a 12-hour light-dark cycle with ad libitum access to standard chow and water. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Committee on the Use and Care of Animals.
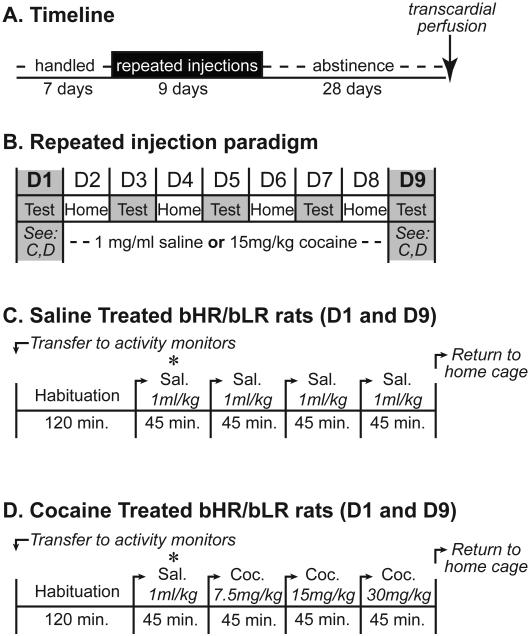
All rats from the 22nd generation of our selective breeding colony were tested for locomotor response to a novel environment around 50-60 days of age for confirmation of phenotype. As previously described (Stead et al., 2006), locomotor response to novelty was assessed in clear acrylic testing boxes (43 × 21.5 × 24.5 cm high). Photocell panels coupled to computerized analysis software determined the number of horizontal locomotion and rearing events every 5 minutes for 60 minutes. Horizontal and rearing activity counts were combined to generate the total locomotor score for each rat. For a given generation, high-responders (bHRs) and low-responders (bLRs) are properly classified with ~99% success based on their lineage from bHR-bHR or bLR-bLR breeding pairs, respectively, with bHRs reliably exhibiting significantly greater locomotor activity compared to bLRs. A subset of rats from the 22nd generation that were correctly identified as either bHRs or bLRs were used in the current study and assigned to saline- (bHR=5, bLR=5) or cocaine-treated (bHR=9, bLR=9) groups that were counterbalanced based on locomotor activity scores. Rats were acclimated to the housing room and briefly handled for 7 days prior to the start of treatment (Fig. 1A).
Figure 1. Timeline for repeated cocaine treatment.
A) Rats were handled for 7 days and then administered repeated injections of saline or cocaine for 9 consecutive days. For the next 28 days, rats remained cocaine abstinent until transcardial perfusion and brain extraction. B) Repeated injections of saline (C) or escalating doses of cocaine (D) were administered on the first (day 1; D1) and last (day 9; D9) days of treatment; single injections of saline or cocaine were given on the intermediate days (D2-D8). On days 2-8, injections were associated with the activity boxes (“test”; days 3,5,7) or home cages (“home”; days 2,4,6,8) on alternating days. Repeated injections on day 1 and day 9 were given in the activity boxes according to the treatment paradigm outlined for saline- (C) and cocaine-treated (D) rats. The asterisk (*) indicates the saline injection administered to all rats.
2.2 Activity Boxes
Behavioral activity during the cocaine treatment paradigm was monitored in custom-built activity boxes (33.02 × 68.58 × 60.96cm tall) constructed from expanded PVC with a woven stainless wire cloth grid floor and underlying catch tray. All surfaces of the activity boxes, grid floor and catch tray were coated with ultra-flat black paint to minimize the reflectivity of the testing apparatus. Cameras were mounted directly above each box to record behavior, which was collected using a Pelco (Clovis, CA) DX9100 digital video recorder. All tests were carried out under red-light conditions. A detailed description of the equipment used to monitor behavioral activity has been published previously (Flagel and Robinson, 2007).
2.3 Drugs
Cocaine hydrochloride (Mallinckrodt, St Louis, MO) was dissolved in 0.9% sterile saline (Hospira, Lake Forest, IL) and administered at doses of 7.5, 15 and 30 mg/kg. All injections were administered at 1ml/kg body weight.
2.4 Cocaine Administration
A sensitizing regimen of cocaine produces a number of neuroadaptations that may contribute to addiction (Robinson and Berridge, 1993) and behavioral sensitization to cocaine has been successfully demonstrated in outbred rats using a treatment regimen similar to that used in the current study (Li et al., 2004, Flagel and Robinson, 2007). Specifically, the paradigm used here was adapted from Flagel and Robinson (2007), which illustrated that repeated administration of a 15 mg/kg dose of cocaine is sufficient to produce psychomotor activating effects indicative of behavioral sensitization (Flagel and Robinson, 2007). The use of an escalating dose regimen for cocaine on the first (day 1) and last (day 9) days of treatment allowed the generation of within-subject dose-effect data which was used to assess behavioral sensitization to cocaine by comparing the dose-effect on day 9 vs. day 1 (Flagel and Robinson, 2007).
bHR and bLR rats received repeated intraperitoneal injections of cocaine or vehicle (0.9% saline) for 9 consecutive days (Fig. 1A,B). On the first and last days of treatment, rats were placed into activity boxes for 120 minutes to allow for habituation to the testing environment (Fig. 1C,D). All rats then received a saline injection, regardless of treatment group. All injections were interspersed with a 45-minute period during which behavior was video recorded. After the first saline injection, the saline-treated controls continued to receive saline for the 3 subsequent injections and the cocaine-treated animals received three escalating doses of cocaine (7.5, 15, and 30 mg/kg; Fig. 1D). Rats were returned to their home cages 45-minutes after the last injection. On days 2-8 (Fig. 1B), saline- and cocaine-treated rats received a single injection of either saline or cocaine (15 mg/kg), respectively. On alternate days, injections were administered in the activity boxes (days 3,5,7) or home cages (days 2,4,6,8) to mitigate context-specificity, thereby increasing the likelihood of detecting individual differences (Ahmed et al., 1993, Stewart and Badiani, 1993, Anagnostaras and Robinson, 1996, Uslaner et al., 2001, Uslaner et al., 2003). When injections were administered in the activity boxes (D3,5,7), rats were allowed to acclimate for 60-minutes prior to the injection and returned to their home cage 60-minutes after the injection. Following this 9-day treatment paradigm, rats remained abstinent from cocaine for the next 28 days, at which point brains were obtained for morphological analyses (Fig. 1A).
2.5 Behavioral Analysis
Behavior was digitally recorded on day 1 and day 9 (for details, see (Flagel and Robinson, 2007)). Clever Sys, Inc. (Reston, VA) Drug Effect Scan software was used to analyze locomotor activity and stereotyped head movements (Flagel and Robinson, 2007, Flagel et al., 2008, Flagel et al., 2010). Activity was analyzed in 5-minute “bins” during habituation and following each injection. The final analyses excluded the first 10 minutes immediately following each injection and the last 5 minutes at the end of the 45-minute testing period due to a high amount of variability, which was likely attributable to increased experimenter activity in the testing room during those times.
2.6 Diolistic labeling
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with chilled saline (0.9%, pH 7.4) followed by cold 1.5% paraformaldehyde (pH 7.3-7.4) (Kim et al., 2007). Brains were removed, post-fixed in 1.5% paraformaldehyde (<2-hours), and immersed in 0.1M sodium phosphate buffer (PB; pH 7.4). A 5-7mm block containing the nucleus accumbens was stabilized using 3% agar and 125μm thick coronal sections were made using a Leica (Buffalo Grove, IL) vibratome. Sections were collected into chilled PB and stored at 4°C until diolistic labeling (<96-hours).
Although the Golgi method has long been the standard for examining changes in neuronal structure and dendritic spine density, a number of reports suggest that the Golgi method may result in underestimation of spine density numbers (Feldman and Peters, 1979, Wallace and Bear, 2004, Shen et al., 2009). In recent years, alternative approaches such as “DiOlistic” labeling, which utilizes fluorescent lipophilic dyes which are integrated directly into the plasma membrane (Seabold et al., 2010), have become increasingly popular. Relative to traditional Golgi methods, which rely on accumulation of chromagen in the neuronal cytoplasm extending into the dendritic spines, the DiOlistic approach offers several advantages including more rapid and complete labeling of neuronal processes (Gan et al., 2000, Shen et al., 2009). Thus, the DiOlistic approach was used here, as we believe it may more accurately reflect the numbers and types of dendritic spines.
DiOlistic methods have been described previously (Gan et al., 2000, Grutzendler et al., 2003, Moolman et al., 2004, O’Brien and Lummis, 2006, Staffend and Meisel, 2011a, b)}. Briefly, tefzel tubing (Bio-Rad, Hercules, CA) was purged with nitrogen and precoated with 10mg/ml polyvinylpyrrolidone (PVP; mol wt=360,000; Sigma-Aldrich). The lipophilic dye 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI; Invitrogen, Carlsbad, CA) was solubilized in dichloromethane (Sigma-Aldrich, St. Louis, MO) and applied to the tungsten particles (1.3μm diameter, Bio-Rad). The DiI-coated tungsten was homogenized, suspended in PVP solution, and drawn into the pre-coated Tefzel tubing. Once the DiI-coated tungsten particles had settled, the PVP solution was purged and the tubing was slowly rotated to evenly distribute the DiI-tungsten along the inner wall. After drying, the tubing was cut into 13mm pieces, or “bullets”, and stored at 4°C in a light-protected container with silica gel desiccant (Sigma-Aldrich, St. Louis, MO).
The delivery of DiI-coated particles was empirically tested to optimize neuronal labeling in our samples. Individual sections containing the nucleus accumbens were suspended in PB. Immediately prior to labeling, the PB was removed and one DiI bullet was delivered (80-100 psi, helium; ~20mm above tissue) to each section using a Helios® gene gun (Bio-Rad) with a synthetic membrane filter (3.0μm pore; BD Biosciences, San Jose, CA) attached to the barrel to minimize tissue damage caused by large DiI-tungsten aggregates (Gan et al., 2000, Grutzendler et al., 2003). Labeled sections were immediately resuspended in PB and stored in darkness at room temperature. After 24-hours, DiI-labeled sections were post-fixed (60-minutes) in 4% PFA, coverslipped (ProLong AntiFade; Invitrogen) and stored at −20°C until image capture.
2.7 Morphological Analysis
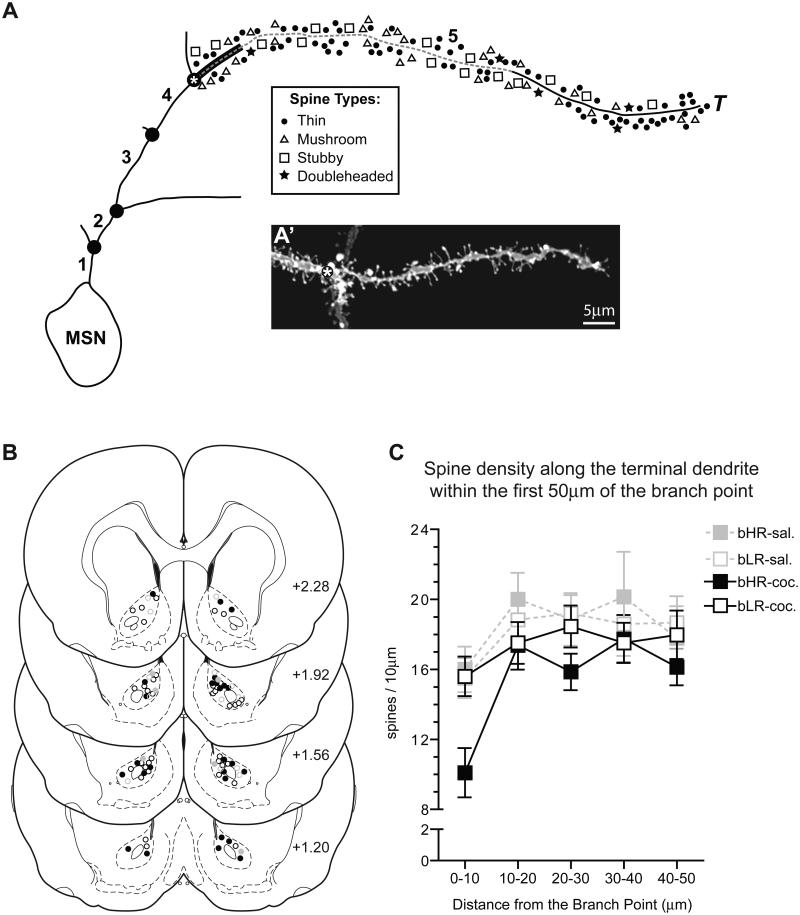
High-quality images of DiI-labeled medium spiny neurons (MSNs) in the AcbC were captured using an Olympus (Center Valley, PA) FV1000 confocal microscope equipped with a 60x oil-immersion objective (PLAPON, Olympus, NA=1.42, WD=0.15 mm) at 2.5x optical zoom using the following parameters: frame size 1024 × 1024 pixels; image field size 83.968 × 83.968 μm and pixel scale 0.082 × 0.082 μm. Branches were scanned along the z-axis at 0.41μm and collected using Kalman averaging at 4X to diminish noise. Neurolucida® (MBF Bioscience, Williston, VT) neuroimaging software was used to create an image montage of each complete branch (soma to terminal tip) using confocal stacks (30-100 images) to trace the dendritic tree, identify branch points, and count/classify dendritic spines.
Selection criteria were established to identify dendritic branches adequate for examining spine density. Dendritic branches included in the analyses were part of a clearly defined dendritic tree originating at the soma, identified as being third order or higher (Coleman and Riesen, 1968, Robinson and Kolb, 1999, Li et al., 2004, Ferrario et al., 2005), and at least 50μm in length measured from the branch point to terminal tip. Terminal tips were visible and distinct on all branches and clearly delineated spines were attached to the dendritic branch. The random pattern of neuronal labeling inherent to the delivery of dye particles via ballistic methods rendered some dendritic branches unusable due to the inability to determine whether these branches met the established criteria, and consequently resulted in their elimination from the final analyses.
Dendritic spines were qualitatively classified as one of the following types: thin, mushroom, stubby, or branched (Jones and Powell, 1969, Peters and Kaiserman-Abramof, 1970, Harris et al., 1992, Lendvai et al., 2000). Filopodia and spines unable to be classified were not included in the analyses. Psychostimulant-induced alterations in spine density have been reported on distal dendrites that exhibit a terminal tip (Robinson and Kolb, 1999, Li et al., 2004), but not proximal dendrites lacking a terminal tip located closer to the soma (Li et al., 2003). Thus, the total number of spines and spine type were quantified along the length of the terminal branch in 10μm increments starting at the branch point and ending at the terminal tip (see Fig. 4A). Spine density was expressed as the number of spines per 10μm dendritic segment. Consistent with our previous spine density analyses (Robinson and Kolb, 1997, 1999, Robinson et al., 2001, Robinson et al., 2002, Kolb et al., 2003, Li et al., 2004, Ferrario et al., 2005), spine density from up to five neurons per hemisphere was averaged to give a single value per hemisphere per rat, making “hemisphere” the unit of analysis for each rat (saline-treated bHRs, n=7; saline-treated bLRs, n=6; cocaine-treated bHRs, n=14; cocaine-treated bLRs, n=17). In some cases, data from only one hemisphere per rat was obtained due to the lack of neurons available that met the established criteria for inclusion in the final analysis.
Figure 4. Selection and analysis of dendritic spines in the AcbC.
Dendritic spines were examined along the entire terminal branch of MSNs in the AcbC (A) in 10μm increments starting at the terminal branch point (asterisk) and extending to the terminal tip (T). Both the branch order (bold numbers) and dendritic branch points (•) are indicated. A representative image of a dendritic branch ending in a terminal tip (A’), typical of those included in the analyses, is shown; as in the schematic, an asterisk marks the branch point preceding the terminal tip. The distribution of MSNs in the AcbC that were included in the quantitative analyses was mapped onto 4 representative coronal sections (B) at 360μm intervals (+2.28, +1.92, +1.56 and +1.20mm bregma (Paxinos and Watson, 2005) for saline-treated bHRs (closed gray circles), cocaine-treated bHRs (closed black circles), saline-treated bLRs (open gray circles) and cocaine-treated bLRs (open black circles). Spine density along terminal branches was analyzed in 10μm increments starting at the branch point (A) and extending toward the terminal tip for 50μm (dashed line). Spine density on terminal branches was impacted by the distance from the branch point (C; effect of distance: p<0.001) and the 10μm segment adjacent to the branch point (A; thick black line) had fewer spines than any other segment.
2.8 Statistics
Statistical analyses were carried out using SPSS® Statistics (IBM, Somers, NY) and differences in behavior or spine density were considered significant at p<0.05. Independent t-tests were used to compare bHR/bLR differences during locomotor screening. Following habituation or injections, the mean ± SEM of the behavioral variable of interest was calculated for each group and linear mixed-effects models (Verbeke and Molenberghs, 2000, West et al., 2006) were used to assess differences (i.e., across doses or days). For each dependent variable, the covariance structure was explored and modeled appropriately. Bonferroni post-hoc comparisons were made when significant main effects or interactions were detected.
The mean ± SEM spine density for each group was obtained from the average spine density per hemisphere within each rat. Spine density differences were evaluated using analysis of variance and Bonferroni post-hoc comparisons were conducted when appropriate. Independent t-tests were used to assess the effect of phenotype (bHR, bLR) on spine density within individual spine types. To evaluate within-phenotype treatment effects, spine density in cocaine-treated rats was expressed relative to the average of the saline-treated group, or “%-saline”, and analyzed using a one-sample t-test (hypothesized value=100). The “%-saline” values were not directly compared between bHR and bLR rats.
3. Results
3.1 Locomotor Activity in a Novel Environment
The locomotor phenotype of bHR and bLR rats was confirmed by testing for locomotor activity in a novel environment 50-60 days following birth. Consistent with previous generations, locomotor activity was significantly higher in bHR rats compared to bLR rats (bHRs, 1304.50 ± 59.45; bLRs, 93.79 ± 10.46; t(26)=20.06, p<0.001).
3.2 Effects of acute and repeated cocaine treatment on locomotor activity in bHR and bLR rats
Relative to bLR rats, bHR rats were more active during the habituation period and in response to saline injections (data not shown). Because of these differences in “baseline” activity, saline-treated control groups were excluded from the behavioral analyses and when appropriate, cocaine-induced effects were compared to that of the initial saline injection (i.e., within subject control).
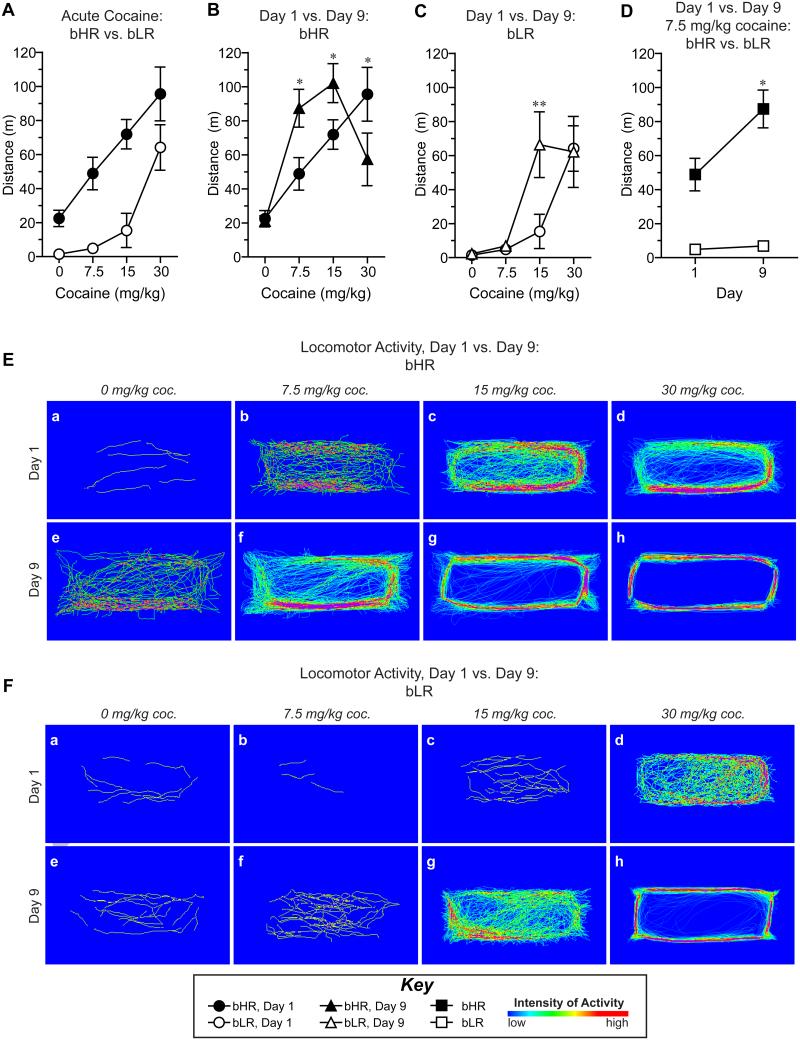
On day 1, cocaine produced dose-dependent increases in locomotor activity in both phenotypes (Effect of Dose: F(3,14)=11.94, p<0.001). However, cocaine-induced locomotor activity was greater in bHRs (Effect of Phenotype: F(1,16)=28.96, p<0.001) as illustrated by a shift in the dose-effect curve upwards and to the left for bHRs relative to bLRs (Fig. 2A). Thus, acute exposure to cocaine (i.e., day 1) increased locomotor activity to a greater extent in bHRs.
Figure 2. The locomotor response to both acute and repeated cocaine treatment is enhanced in bHR rats relative to bLRs.
A) Locomotor activity on day 1 was increased with escalating doses of cocaine and enhanced in bHR rats (closed circles) compared to bLR rats (open circles). B) Following repeated cocaine injections (day 9; black triangles), locomotor activity in bHRs was increased at the lowest (7.5mg/kg) and middle (15mg/kg) doses and decreased at the highest dose (30mg/kg) compared to the acute response on day 1 (black circles). C) In bLRs, locomotor sensitization to cocaine was apparent only at the middle cocaine dose (15mg/kg) on day 9 (open triangles) relative to activity on day 1 (open circles). D) Locomotor activity in response to the lowest dose of cocaine (7.5 mg/kg) was increased in bHR rats (black squares), but not bLR rats (open squares), on day 9 relative to day 1. Representative density maps obtained from individual cocaine-treated bHR (E) and bLR (F) rats on day 1 (a-d) and day 9 (e-h) illustrate all locomotor activity following injections of saline (“0 mg/kg cocaine”, a,e) and escalating doses of cocaine (“7.5 mg/kg”, b,f; “15 mg/kg”, c,g; “30 mg/kg”, d,f). Significant differences between day 1 vs. day 9 (B,C,D) are indicated: **p<0.01, and *p<0.05.
The effects of repeated cocaine treatment were examined separately in bHR (Fig. 2B) and bLR (Fig. 2C) rats due to statistical findings that indicated a significant effect of phenotype (effect of phenotype, F(1,25.66)=18.25, p<0.001) and multiple interactions (phenotype × dose interaction, F(3,92.78)=5.91, p<0.001; phenotype × day × dose interaction, F(3,93.08)=2.52, p=0.06). The degree of psychomotor sensitization to cocaine was assessed within each phenotype by comparing the effects of cocaine after 9 days of treatment to the acute effects of cocaine on day 1 of treatment. Although there was not a significant effect of day for either phenotype, repeated cocaine treatment produced dose-dependent effects on locomotor activity for both bHR (F(3,48.05)=19.36, p<0.001) and bLR (F(3,44.74)=13.51, p<0.001) rats and there was a significant day × dose interaction for both phenotypes (Fig. 2B, bHR, F(3,48.25)=8.05, p<0.001; Fig. 2C, bLR, F(3,44.86)=3.80, p=0.017). On day 9, bHRs exhibited increased locomotor activity at the two lowest doses of cocaine relative to day 1 (Fig. 2B, 7.5mg/kg, p=0.013; 15mg/kg, p=0.049) and a significant decrease in locomotion in response to the highest dose (30mg/kg, p=0.014). For bLRs, the day 9 response was significantly different than the day 1 response only at the intermediate dose (Fig. 2C, 15mg/kg, p=0.003). Figure 2D further illustrates this point, showing that even at the lowest dose of cocaine, bHRs exhibit pronounced sensitization on day 9 relative to day 1, whereas bLRs do not. In agreement, relative to saline, bHRs exhibited increased locomotor activity in response to lower doses of cocaine than bLRs on both days 1 and 9 (Fig. 2). Thus, bHRs exhibit increased responsivity to lower doses of cocaine, suggesting enhanced sensitization relative to bLRs.
These differences in cocaine-induced activity in bHRs vs. bLRs are illustrated by the activity maps (Clever Sys., Inc. software) shown in Figure 2E and F. Escalating doses of cocaine increased the intensity of locomotor activity in specific areas of the activity boxes. Notably, activity patterns became more stereotyped with increasing dose and in response to lower doses following repeated treatment. These findings are consistent with previous reports describing a phenomenon of “stereotyped locomotor activity” wherein locomotor hyperactivity becomes linearized (i.e., darting behavior) in response to either high doses (i.e., see Fig. 2Ed) or with repeated psychostimulant treatment (i.e., behavioral sensitization, see Fig. 2Eh, 2Fh) (Lat, 1965, Schiorring, 1971, Segal, 1975b, Schiorring, 1979). In agreement with the quantitative data (Figs. 2B and 2C), the activity maps indicated that bHRs were more sensitive to both the acute and repeated effects of cocaine relative to bLRs.
3.3 Repeated cocaine effects on stereotyped head movements in bHR and bLR rats
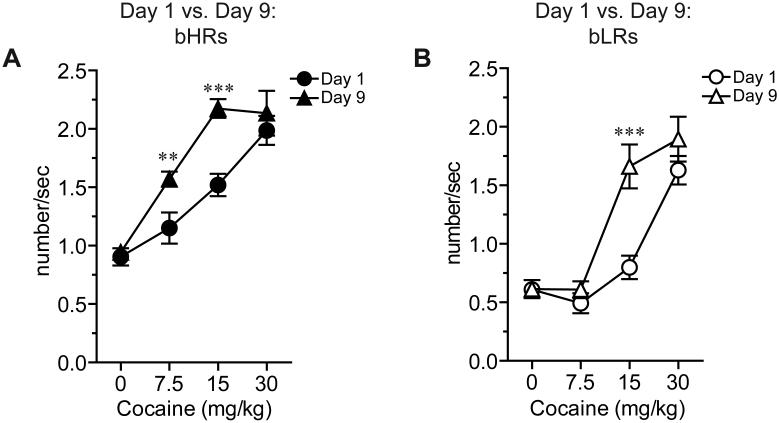
Psychostimulant-induced stereotyped behaviors include not only the linearization of locomotor paths, but also repetitive head movements or head-waving (Randrup and Munkvad, 1967, Lyon and Randrup, 1972, Segal and Mandell, 1974, Segal, 1975a) which occur during periods when the rat exhibits sustained bouts of in-place activity (Segal and Kuczenski, 1987, Ferrario et al., 2005). Here, we used the frequency of head movements as an index of stereotypy (Ferrario et al., 2005, Flagel and Robinson, 2007) and found it to increase with escalating doses of cocaine in both phenotypes (Fig. 3; Effect of Dose: bHR, F(3,24.98)=41.65, p<0.001; bLR, F(3,46.09)=63.29, p<0.001). There was also a significant effect of day, with a leftward shift in the dose-response curve following repeated cocaine (Fig. 3; Effect of Day: bHR, F(1,26.81)=12.48, p=0.002; bLR, F(1,15.75)=11.55, p=0.004) and a significant day × dose interaction for both phenotypes (Fig. 3A, bHR F(3,33.62)=3.72, p=0.021; Fig. 3B, bLR, F(3,41.62)=7.84, p<0.001). Post-hoc analyses confirmed that the frequency of head movements was increased on day 9 relative to day 1 in both bHR (Fig. 3A; 7.5mg/kg, p=0.009; 15mg/kg, p<0.001) and bLR (Fig. 3B; 15mg/kg, p<0.001) rats. However, this effect was most pronounced for bHRs, demonstrating an increase in head movements even at the lowest cocaine dose on day 9 and this was also true relative to saline (i.e., within subject controls). Furthermore, empirically derived threshold criteria (see (Flagel and Robinson, 2007) indicated that a higher percentage of bHR rats were “in stereotypy” compared to bLRs at this lowest dose (data not shown).
Figure 3. Stereotyped head movements induced by repeated cocaine are enhanced in bHR rats compared to bLR rats.
The frequency of lateral head movements is shown for bHR (A) and bLR (B) rats following acute (day 1; circles) and repeated (day 9; triangles) cocaine injections. Repeated cocaine (closed triangles) increased the frequency of head movements at the two lowest doses of cocaine in bHRs (A; 7.5 and 15 mg/kg), but only at the intermediate dose following repeated treatment (open triangles) in bLRs (B; 15 mg/kg). Differences in stereotyped head movements between day 1 and day 9 at the highest dose of cocaine (30 mg/kg) were not observed for either phenotype. Significant differences between day 1 vs. day 9 are shown:***p<0.001, *p<0.01.
At 30 mg/kg there were no significant differences between day 1 and day 9 in the frequency of head movements for either phenotype (Fig. 3). These data suggest that both bHRs and bLRs may have reached their peak response using this metric in response to the highest dose of cocaine, regardless of day. Interestingly, the locomotor response for bLRs on day 9 was also indistinguishable from that on day 1 in response to 30 mg/kg cocaine (Fig. 2C). However, bHRs exhibited a decrease in locomotor response on day 9 relative to day 1 in response to the highest dose of cocaine (Fig. 2B), providing further evidence that their behavioral profile differed from that of bLRs. Decreases in locomotor activity at high drug doses following repeated administration are typical as rats begin to exhibit stereotyped behaviors (Lyon and Robbins, 1975, Segal, 1975a). Although we did not see differences in the frequency of head movements, other measures (e.g. number of head movements or duration of head movements) do reflect enhanced stereotypy in response to the highest dose of cocaine on day 9 relative to day 1 (effect of day for number of head movements, F(1,21.73)=11.62, p=0.003); effect of day for duration of head movements, F(1,28.86)=10.77, p=0.003). In sum, stereotypy was enhanced in bHR vs. bLR rats following repeated cocaine treatment, and together with locomotor activity, indicated that bHR rats exhibited greater behavioral sensitization to the repeated effects of cocaine compared to bLR rats.
3.4 Spine density changes following prolonged cocaine abstinence are phenotype dependent
Following prolonged abstinence from repeated cocaine (or saline) injections, spine density was examined on terminal dendrites of MSNs in the AcbC (Figs. 4A,A’,B). The examination of spines started at the branch point and continued along the terminal dendrite in 10μm increments. Despite the fact that neither phenotype nor treatment significantly altered spine density within the first 50μm of the branch point, there was a tendency for spine density to be reduced in cocaine-treated rats compared to saline-treated controls (F(1,22.48)=3.24, p=0.09). Further, spine density was significantly impacted by distance from the branch point (Fig. 4C; effect of distance, F(4,180.2)=5.88, p<0.001) and post-hoc analyses indicated that the density of spines within the first 10μm of the branch point was decreased compared to all other 10μm segments (vs. 10-20μm, p=0.001; vs. 20-30μm, p=0.003; vs. 30-40μm, p=0.001; vs. 40-50μm, p=0.014). Within this first 10μm region, there was a significant phenotype × treatment interaction (F(1,40)=4.00, p=0.05). Thus, all subsequent analyses were restricted to this 10μm segment of the terminal dendrite nearest the branch point.
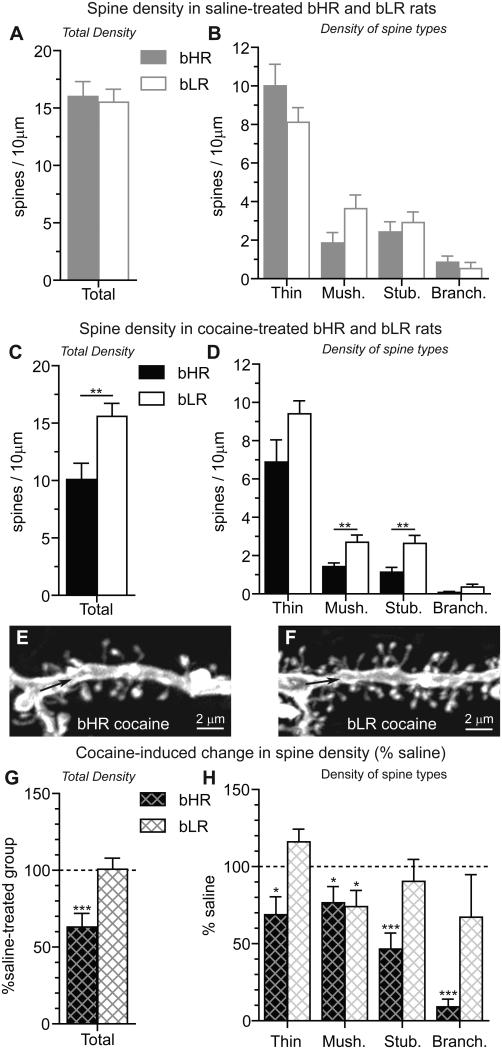
The impact of locomotor phenotype on spine density in the AcbC was evaluated by comparing the density of dendritic spines in control bHR and bLR rats that received repeated saline injections. Spine density was comparable in saline-treated bHRs and bLRs (Fig. 5A) with only a tendency for phenotype differences in mushroom spine density (Fig. 5B, t(11)=-2.03, p=0.07). The lack of significant differences in saline-treated rats is consistent with our findings in untreated bHR and bLR rats from the 15th generation of the selective breeding colony (Hwang et al., 2009).
Figure 5. Individual differences in spine density are apparent in the AcbC of cocaine-treated bHR and bLR rats.
All data presented here was obtained from the 10μm segment of the terminal dendrite closest to the branch point (see Fig. 4A; thick black line). Spine density in the AcbC was comparable in bHR and bLR rats treated repeatedly with saline (A). No significant differences between bHR and bLR rats were detected among the different spine types, although there was a tendency for differences in the density of mushroom spines (B) in bHR vs. bLR rats (p=0.07). Differences between bHR and bLR rats in total spine density (C) as well as spine types (D; mushroom and stubby) were apparent following prolonged cocaine abstinence. Although bHR-bLR differences in the density of thin (p=0.06) and branched (p=0.1) spines were not significant, there was a tendency for the density of these spines to differ in cocaine-treated rats, as well. Differences in spine density near the branch point of bHRs (E) and bLRs (F) are clear following cocaine treatment. In bHRs, but not bLRs, cocaine decreased the total density of spines vs. saline-treated controls (G). All spine types were decreased in cocaine-treated bHR rats compared to saline-treated controls (H, thin: t(13)=-2.70, p=0.02; mushroom: t(13)=-2.23, p=0.04; stubby: t(13)=-5.16, p<0.001; branched: t(13)=-18.05, p<0.001). In cocaine-treated bLRs (H), only mushroom spines were decreased by cocaine (t(16)=-2.48, p=0.03), although insignificant increases in thin spines were observed (t(16)=1.94, p=0.07). Significant differences between bHR vs. bLR (A-D) and cocaine vs. saline (G,H) are indicated: ***p<0.001; **p<0.01; *p<0.05.
Dendritic spines were also examined in cocaine-treated bHR and bLR rats following prolonged abstinence. Cocaine-treated bHR and bLR rats exhibited significant differences in spine density (Fig. 5C, F(1,40)=11.33, p=0.002) which were evident on the terminal dendrite near the branch point (e.g., see Fig. 5E-F). Phenotype differences in spine density were specific to the type of dendritic spine in cocaine-treated rats (Fig. 5D). bHR vs. bLR differences in the density of both mushroom (t(29)=-2.79, p=0.009) and stubby (t(29)=-2.94, p=0.006) spines were detected, however, more modest changes that did not reach significance were also noted for thin (t(29)=-1.97, p=0.06) and branched (t(29)=-1.68, p=0.10) spines.
Finally, within-phenotype effects of cocaine treatment on spine density were evaluated by expressing spine density as the percent change in the cocaine-treated group compared to saline-treated group of the same phenotype (i.e., “% saline”). Although cocaine treatment decreased total spine density by 37% in bHRs (Fig. 5G, t(13)=-4.183, p<0.001) and not at all in bLRs (Fig. 5G), the effects on individual spine types were different in bHR and bLR rats (Fig. 5H). All spine types were decreased in cocaine-treated bHR rats, with the most pronounced effects occurring in branched spines (91% decrease) and the smallest effects occurring in mushroom spines (23% decrease), which were equally impacted in bLR rats. All other spine types remained unchanged in cocaine-treated bLR rats, although there was a trend toward increased thin spines (p=0.07). In sum, cocaine differentially impacted spine density in bHR and bLR rats, with robust and consistent decreases in bHRs, specifically in the region of terminal branch nearest the branch point.
4. Discussion
The behavioral, psychological, and neurobiological effects of drugs can vary as a function of genetic background, as has been shown in human studies (for recent reviews, see (Gorwood et al., 2012, Kreek et al., 2012, Levran et al., 2012, Merikangas and McClair, 2012)). Here, we use a unique animal model to show that the psychomotor activating effects of cocaine differ based on genetic background (Figs. 2-3), as does cocaine-induced structural plasticity following prolonged abstinence (Figs. 4-5). We demonstrate that neuroplastic changes in cocaine-treated rats following prolonged abstinence differ markedly between selectively bred high- and low-responder rats. Relative to bLRs, bHR rats appear to be especially sensitive to both the behavioral and neurobiological effects of repeated cocaine treatment. Interestingly, there were no differences in spine density between the lines under baseline conditions or following repeated saline injections suggesting that the differences we saw were specific to repeated cocaine treatment and persisted after a prolonged period of abstinence. These findings support and enhance our previous reports of individual differences in behavioral sensitization to cocaine (Garcia-Fuster et al., 2010, Clinton et al., 2012) and present a unique model in which these differences in sensitization can be related to subsequent changes in neuroplasticity.
The selectively bred lines of high- and low-responder rats are a valuable tool for examining how two “extremes” of a normally distributed population (Stead et al., 2006) with known differences in gene expression (Clinton et al., 2011, Flagel et al., 2012a) differ in response to cocaine at both the behavioral and morphological level. Although the current findings are in agreement with previous studies suggesting enhanced psychomotor sensitization in bHRs relative to bLRs following repeated cocaine treatment (Garcia-Fuster et al., 2010, Clinton et al., 2012), significant differences exist between the current study and our previous work. The current study involved an analysis of within-subject dose-effect functions, which is more sensitive in assessing the psychomotor activating effects of cocaine (Flagel and Robinson, 2007). Further, the psychomotor profile was measured using behavioral analysis software (described in (Flagel and Robinson, 2007)), which allowed the quantification of multiple behaviors (e.g., frequency of lateral head movements) that were not assessed in these earlier studies (Garcia-Fuster et al., 2010, Clinton et al., 2012). In the current study we were able to demonstrate that locomotor activity was enhanced in bHRs compared to bLRs following both acute and repeated cocaine treatment (Fig. 2). We also found that stereotyped head movements were increased in bHRs vs. bLRs in response to repeated cocaine treatment (Figs. 3, S4). Thus, these bHR-bLR differences in the psychomotor activating effects of cocaine provided the framework for examining whether individual differences in behavioral response were related to long-term changes in neural plasticity (i.e., spine density).
Although increased spine density (Norrholm et al., 2003, Li et al., 2004, Ferrario et al., 2005, Lee et al., 2006, Chen et al., 2008, Pulipparacharuvil et al., 2008, Kiraly et al., 2010, Wissman et al., 2011, Marie et al., 2012) and synapse number (Alcantara et al., 2011) have been reported in the AcbC following cocaine treatment, accumulating evidence indicates that behavioral sensitization to psychostimulants and increases in spine density are not inextricably linked (Lee et al., 2006, Shen et al., 2009, Singer et al., 2009, Martin et al., 2011, Dumitriu et al., 2012). Here, we report phenotype-dependent changes in spine density in the AcbC of bHR and bLR rats following prolonged cocaine abstinence, which were specific to the region of the terminal dendrite nearest the branch point. Notably, spine density decreases in cocaine-treated bHRs (Fig. 5) are supported by the recent description of spine density decreases within proximal regions of the terminal dendritic branch of MSNs in the AcbC following prolonged (28 days) cocaine abstinence (Dumitriu et al., 2012). These bHR-bLR differences in spine density following prolonged cocaine abstinence may be suggestive of differences in the propensity to relapse in the selectively bred lines, which is supported by bHR-bLR differences in cue-induced reinstatement (i.e., relapse) after ~1 month of abstinence (Flagel et al., 2012a).
Unfortunately, a clear understanding of the relationship between behavioral activity in response to cocaine and changes in spine density is lacking. Across published studies, inconsistencies in the dendritic region examined for cocaine-induced effects on spine density combined with variations in the duration of the abstinence period following cocaine treatment (i.e., 24h to 3 months) make it difficult, if not impossible, to relate changes in spine density to behavioral activity. One potential explanation, however, is that changes in dendritic spine density are mediated by dysregulation of dopamine-glutamate signaling. In fact, modulation of dopamine and glutamate systems produces changes in both dendritic spine density (Ingham et al., 1989, Jones and Robbins, 1992, Meredith et al., 1995, Testa et al., 1998, Bamford et al., 2004, Day et al., 2006, Deutch, 2006, Garcia et al., 2010, Gonzalez-Burgos et al., 2010) and behavior (Rouillon et al., 2008, Gonzalez-Burgos et al., 2010). Given that GABAergic MSNs in the AcbC receive converging input from both dopaminergic and glutamatergic axon terminals (Sesack and Pickel, 1992), future studies examining dopamine-glutamate interactions following cocaine treatment may help us better understand the relationship between changes in neuroplasticity and behavior.
Although the physiological impact of changes in spine density near dendritic branch points is not entirely clear, dendritic branch points have been implicated as sites within the dendritic tree that are important for signal modulation (Shepherd et al., 1985, Branco and Hausser, 2010), Golgi-mediated processes (Horton and Ehlers, 2003, Jan and Jan, 2010), and protein synthesis (Bartlett and Banker, 1984, Tiedge and Brosius, 1996). Converging evidence suggests that molecular substrates implicated in plasticity are localized to dendritic branch points, both in vitro and in vivo (Ferrari et al., 2007, Dayas et al., 2012). In particular, ribosomal protein S6, a downstream target of the mammalian target of rapamycin complex 1 (mTORC1), is enriched at dendritic branch points (Ferrari et al., 2007) and phosphorylated following cocaine treatment (Wu et al., 2011). Interestingly, we have detected bHR-bLR differences in molecules associated with the mTORC1 signaling pathway (unpublished data). Thus, it is plausible that plasticity near dendritic branch points in bHR rats may be particularly sensitive following prolonged cocaine abstinence.
Differences in spine density following cocaine treatment in bHR and bLR rats may be due to the differential regulation of neurotransmitter systems in the selectively bred lines. Numerous genes and signaling pathways associated with addiction are differentially expressed between bHR and bLR rats under basal conditions, both during development and into adulthood (Clinton et al., 2011, Flagel et al., 2012a, Flagel et al., 2012b, Garcia-Fuster et al., 2012). Specifically, neurobiological differences in the dopamine system (Flagel et al., 2010, Flagel et al., 2011), stress systems (Clinton et al., 2008, Kerman et al., 2012), fibroblast growth factor family (Turner et al., 2008, Perez et al., 2009, Turner et al., 2011, Clinton et al., 2012) and hippocampal neurogenesis (Perez et al., 2009, Garcia-Fuster et al., 2010) have been described, all of which undoubtedly contribute to the individual differences in cocaine-induced neuroplasticity in bHR vs. bLR rats. Although the current study did not examine the acute effects of repeated cocaine treatment on spine density in the selectively bred lines, our recent findings indicate that the expression of plasticity-related genes is most different in the selectively bred lines following periods of prolonged cocaine abstinence (Waselus et al., 2013). Specifically, prospective targets under investigation include signaling within the dopaminergic and fibroblast growth factor systems, given their involvement in neuroplasticity (Ingham et al., 1989, Meredith et al., 1995, Kleim et al., 2003, Zaja-Milatovic et al., 2005, Jungnickel et al., 2006, Wang and Deutch, 2008, Garcia et al., 2010, Gonzalez-Burgos et al., 2010, Wolf, 2010, Turner et al., 2012). Taken together, these findings further suggest that the differences in dendritic spine density we report here are not due to cocaine exposure per se. Rather, these bHR-bLR differences are a consequence of the prolonged abstinence following cocaine exposure, a time period during which individual differences in the expression of genes associated with neuroplasticity are most robust (Waselus et al., 2013).
5. Conclusions
In sum, these findings indicate that relative to bLR rats, bHRs are more responsive to both acute and repeated cocaine administration. Furthermore, robust differences in spine density were detected between phenotypes in the nucleus accumbens core following prolonged cocaine abstinence. Similar to the behavioral findings, changes in spine density were more pronounced in bHR rats than bLR rats and these changes were specific to the region of the terminal dendritic branch closest to the branch point. Since bHR-bLR differences in spine density were not detected under basal conditions or following repeated vehicle treatment, it is unlikely that spine density in the AcbC contributes to differences in the initial vulnerability to the psychomotor activating effects of cocaine. However, robust bHR-bLR differences in spine density following prolonged cocaine abstinence may be indicative of differences in vulnerability or resilience following cocaine treatment.
We have a unique genetic animal model to study individual differences in addiction
Both locomotor and stereotyped responses to cocaine are enhanced in bHRs vs bLRs
Spine density was decreased in bHRs, but not bLRs, following prolonged abstinence
Cocaine decreased all spine types in bHRs, but only mushroom spines in bLRs
Spine density decreases were restricted to terminal dendrites near the branch point
Acknowledgements
We are grateful to Sarah Clinton, Ph.D., Peter Blandino, Ph.D., Sue Miller and Kate Mills for overseeing the maintenance of the selective breeding colony. We would also like to thank James Stewart and James Beals for their technical assistance. This study was funded by the Office of Naval Research (ONR) grants N00014-09-1-0598 and N00014-12-1-0366, the National Institute on Drug Abuse (NIDA) grant 5P01DA021633-02, and the Hope for Depression Research Foundation. MW was supported in part by the University of Michigan Substance Abuse Research Center Training Grant T32 DA007267. This work was previously published in part as a scientific abstract (Waselus, et al., Program No. 649.2. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009. Online).
Abbreviations
- AcbC
nucleus accumbens, core subregion
- bHR
selectively bred high-responder rats
- bLR
selectively bred low-responder rats
- DiI
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (lipophilic dye)
- MSN
medium spiny neuron
- PB
phosphate buffer
References
- Ahmed SH, Stinus L, Le Moal M, Cador M. Controlling interindividual differences in the unconditioned response to amphetamine in the study of environment-dependent sensitization. Behav Pharmacol. 1993;4:355–365. [PubMed] [Google Scholar]
- Alcantara AA, Lim HY, Floyd CE, Garces J, Mendenhall JM, Lyons CL, Berlanga ML. Cocaine- and morphine-induced synaptic plasticity in the nucleus accumbens. Synapse. 2011;65:309–320. doi: 10.1002/syn.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci. 1984;4:1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Chen Q, Xiong X, Lee TH, Liu Y, Wetsel WC, Zhang X. Neural plasticity and addiction: integrin-linked kinase and cocaine behavioral sensitization. J Neurochem. 2008;107:679–689. doi: 10.1111/j.1471-4159.2008.05619.x. [DOI] [PubMed] [Google Scholar]
- Clinton S, Miller S, Watson SJ, Akil H. Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psychoneuroendocrinology. 2008;33:162–177. doi: 10.1016/j.psyneuen.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JD, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. Eur J Neurosci. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Turner CA, Flagel SB, Simpson DN, Watson SJ, Akil H. Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacol Biochem Behav. 2012;103:6–17. doi: 10.1016/j.pbb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Riesen AH. Evironmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Smith DW, Dunkley PR. An emerging role for the Mammalian target of rapamycin in “pathological” protein translation: relevance to cocaine addiction. Front Pharmacol. 2012;3:13. doi: 10.3389/fphar.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY. Striatal plasticity in parkinsonism: dystrophic changes in medium spiny neurons and progression in Parkinson’s disease. J Neural Transm Suppl. 2006:67–70. doi: 10.1007/978-3-211-45295-0_12. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ML, Peters A. A technique for estimating total spine numbers on Golgi-impregnated dendrites. J Comp Neurol. 1979;188:527–542. doi: 10.1002/cne.901880403. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Mercaldo V, Piccoli G, Sala C, Cannata S, Achsel T, Bagni C. The fragile X mental retardation protein-RNP granules show an mGluR-dependent localization in the post-synaptic spines. Mol Cell Neurosci. 2007;34:343–354. doi: 10.1016/j.mcn.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Goussakov I, Stutzmann GE, Wolf ME. Withdrawal from cocaine self-administration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines. PLoS ONE. 2012;7:e40898. doi: 10.1371/journal.pone.0040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE. Quantifying the psychomotor activating effects of cocaine in the rat. Behav Pharmacol. 2007;18:297–302. doi: 10.1097/FBP.0b013e3281f522a4. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Kelly R, Watson SJ, Thompson RC, Akil H. Examination of addictive behavior and gene expression profiling in rats selectively bred for locomotor response to novelty; 8th FENS Forum of Neuroscience; Barcelona, Spain: 2012a. p Abstract Number: 2200; Presentation Code: 2136.2225. [Google Scholar]
- Flagel SB, Waselus M, Sewani S, Kelly R, Watson SJ, Thompson RC, Akil H. Rat selectively bred for locomotor response to novelty differ in addictive behavior and in their gene expression profile in the nucleus accumbens; Neuroscience 2012; New Orleans, LA: Society for Neuroscience; 2012b. p Program No. 418.408 Neuroscience Meeting Planner. [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Neely MD, Deutch AY. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb Cortex. 2010;20:2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Flagel SB, Mahmood ST, Watson SJ, Akil H. Cocaine withdrawal causes delayed dysregulation of stress genes in the hippocampus. PLoS ONE. 2012;7:e42092. doi: 10.1371/journal.pone.0042092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Garcia-Martinez S, Velazquez-Zamora DA, Ponce-Rolon R. Cytoarchitectural impairments in the medium spiny neurons of the Nucleus Accumbens core of hyperactive juvenile rats. Int J Dev Neurosci. 2010;28:475–480. doi: 10.1016/j.ijdevneu.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Le Strat Y, Ramoz N, Dubertret C, Moalic JM, Simonneau M. Genetics of dopamine receptors and drug addiction. Hum Genet. 2012;131:803–822. doi: 10.1007/s00439-012-1145-7. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Tsai J, Gan WB. Rapid labeling of neuronal populations by ballistic delivery of fluorescent dyes. Methods. 2003;30:79–85. doi: 10.1016/s1046-2023(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JM, Flagel SB, Jedynak JP, Clinton SM, Robinson TE, Watson SJ, Akil H. Basal differences in spine density in a selectively bred population of rats with a predisposition for addictive behavior; Society for Neuroscience; Washington, DC: Neuroscience Meeting Planner Online; 2009. p Program No. 239.215. [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969;5:509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- Jungnickel J, Haase K, Konitzer J, Timmer M, Grothe C. Faster nerve regeneration after sciatic nerve injury in mice over-expressing basic fibroblast growth factor. J Neurobiol. 2006;66:940–948. doi: 10.1002/neu.20265. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Bedrosian TA, Abraham AD, Rosenthal DT, Akil H, Watson SJ. High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Res. 2011;1419:34–45. doi: 10.1016/j.brainres.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Clinton SM, Simpson DN, Bedrosian TA, Bernard R, Akil H, Watson SJ. Inborn differences in environmental reactivity predict divergent diurnal behavioral, endocrine, and gene expression rhythms. Psychoneuroendocrinology. 2012;37:256–269. doi: 10.1016/j.psyneuen.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lat J. The spontaneous exploratory reactions as a toold for psychopharmacological studies. In: Mikhelson M, Longo V, editors. Proceedings of the 2nd International Pharmacological Meeting. Vol. 1. Pergamon; London and Praha: 1965. pp. 47–66. [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Levran O, Yuferov V, Kreek MJ. The genetics of the opioid system and specific drug addictions. Hum Genet. 2012;131:823–842. doi: 10.1007/s00439-012-1172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Balest ZR, Corotto FS, Smeyne RJ. Cocaine selectively increases proliferation in the adult murine hippocampus. Neurosci Lett. 2010;485:112–116. doi: 10.1016/j.neulet.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M, Randrup A. The dose-response effect of amphetamine upon avoidance behaviour in the rat seen as a function of increasing stereotypy. Psychopharmacologia. 1972;23:334–347. doi: 10.1007/BF00406736. [DOI] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. The action of central nervous system stimulant drugs: A general theory concerning amphetamine effects. Current developments in psychopharmacology. 1975;2:80–163. [Google Scholar]
- Marie N, Canestrelli C, Noble F. Transfer of neuroplasticity from nucleus accumbens core to shell is required for cocaine reward. PLoS ONE. 2012;7:e30241. doi: 10.1371/journal.pone.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2011;65:490–496. doi: 10.1002/syn.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Ypma P, Zahm DS. Effects of dopamine depletion on the morphology of medium spiny neurons in the shell and core of the rat nucleus accumbens. J Neurosci. 1995;15:3808–3820. doi: 10.1523/JNEUROSCI.15-05-03808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol. 2004;33:377–387. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Lummis SC. Diolistic labeling of neuronal cultures and intact tissue using a hand-held gene gun. Nat Protoc. 2006;1:1517–1521. doi: 10.1038/nprot.2006.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2005. [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia. 1967;11:300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Persistent sensitizing effects of drugs on brain dopamine systems and behavior: implications for addiction and relapse. In: Korenman SG, Barchas JD, editors. Biological Basis of Substance Abuse. Oxford University Press; New York: 1993. pp. 373–402. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rouillon C, Abraini JH, David HN. Prefrontal cortex and basolateral amygdala modulation of dopamine-mediated locomotion in the nucleus accumbens core. Exp Neurol. 2008;212:213–217. doi: 10.1016/j.expneurol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Schiorring E. Amphetamine induced selective stimulation of certain behaviour items with concurrent inhibition of others in an open-field test with rats. Behaviour. 1971;39:1–17. [PubMed] [Google Scholar]
- Schiorring E. An open field study of stereotyped locomotor activity in amphetamine-treated rats. Psychopharmacology (Berl) 1979;66:281–287. doi: 10.1007/BF00428320. [DOI] [PubMed] [Google Scholar]
- Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA. DiOLISTIC labeling of neurons from rodent and non-human primate brain slices. J Vis Exp. 2010 doi: 10.3791/2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS. Behavioral and neurochemical correlates of repeated d-amphetamine administration. Adv Biochem Psychopharmacol. 1975a;13:247–262. [PubMed] [Google Scholar]
- Segal DS. Behavioral characterization of d- and l-amphetamine: neurochemical implications. Science. 1975b;190:475–477. doi: 10.1126/science.1166317. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Brayton RK, Miller JP, Segev I, Rinzel J, Rall W. Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proc Natl Acad Sci U S A. 1985;82:2192–2195. doi: 10.1073/pnas.82.7.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biol Psychiatry. 2009;65:835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling in fixed brain slices: phenotype, morphology, and dendritic spines. Curr Protoc Neurosci. 2011a:13. doi: 10.1002/0471142301.ns0213s55. Chapter 2:Unit 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic Labeling of Neurons in Tissue Slices: A Qualitative and Quantitative Analysis of Methodological Variations. Front Neuroanat. 2011b;5:14. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- Tiedge H, Brosius J. Translational machinery in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16:7171–7181. doi: 10.1523/JNEUROSCI.16-22-07171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr., Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett. 2008 doi: 10.1016/j.neulet.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron. 2012;76:160–174. doi: 10.1016/j.neuron.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-induced c-fos mRNA expression in the caudate-putamen and subthalamic nucleus: interactions between dose, environment, and neuronal phenotype. J Neurochem. 2003;85:105–114. doi: 10.1046/j.1471-4159.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- van Dongen YC, Deniau JM, Pennartz CM, Galis-de Graaf Y, Voorn P, Thierry AM, Groenewegen HJ. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience. 2005;136:1049–1071. doi: 10.1016/j.neuroscience.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer-Verlag; New York: 2000. [Google Scholar]
- Wallace W, Bear MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24:6928–6938. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Flagel SB, Turner CA, Robinson TE, Akil H, Watson SJ. Society of Biological Psychiatry. San Francisco, CA: 2013. Fibroblast Growth Factor 2 Expression in Selectively Bred High-Responder and Low-Responder Rats is Differentially Impacted by the Interaction Between Cocaine and the Environment. [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Chapman & Hall/CRC; Boca Raton, FL: 2006. [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227. doi: 10.1016/j.neuropharm.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, McCallum SE, Glick SD, Huang Y. Inhibition of the mammalian target of rapamycin pathway by rapamycin blocks cocaine-induced locomotor sensitization. Neuroscience. 2011;172:104–109. doi: 10.1016/j.neuroscience.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]